Abstract
Background: A widely held hypothesis is that interactions between genetic predisposition and Western-type lifestyle contribute to the epidemic of type 2 diabetes (T2D). No study has tested this hypothesis.
Objective: The objective was to assess whether established genetic variants, mainly from genomewide association studies, modify dietary patterns in predicting diabetes risk.
Design: We determined 10 polymorphisms in a prospective, nested, case-control study of 1196 diabetic and 1337 nondiabetic men. A genetic risk score (GRS) was generated by using an allele counting method. Baseline dietary intakes were collected by using a semiquantitative food-frequency questionnaire. We used factor analysis to derive Western and “Prudent” dietary patterns from 40 food groups.
Results: A significant interaction (P = 0.02) was observed between the GRS and Western dietary pattern. The multivariable odds ratios (ORs) of T2D across increasing quartiles for the Western dietary pattern were 1.00, 1.23 (95% CI: 0.88, 1.73), 1.49 (1.06,2.09), and 2.06 (1.48, 2.88) among men with a high GRS (≥12 risk alleles; P for trend = 0.01). The Western dietary pattern was not associated with diabetes risk among those with a lower GRS. In addition, we found that intakes of processed meat, red meat, and heme iron, which characterized the Western dietary pattern, showed significant interactions with GRS in relation to diabetes risk (P for interaction = 0.029, 0.02, and 0.0004, respectively). The diet-diabetes associations were more evident among men with a high GRS (≥12) than in those with a low GRS.
Conclusion: Genetic predisposition may synergistically interact with a Western dietary pattern in determining diabetes risk in men.
INTRODUCTION
The prevalence of type 2 diabetes (T2D) has been increasing alarmingly in the United States and worldwide (1). The global epidemic of T2D is believed to be attributable to the changes in human lifestyle associated with Westernization and their interactions with genetic susceptibility. However, evidence of gene-lifestyle interactions is sparse (2–4).
Adoption of the Western dietary pattern, which is characterized by a high intake of red and processed meats as well as refined foods, was shown in epidemiologic studies to be related to an increased risk of T2D (5, 6). Recently, there have been landmark successes from genomewide association (GWA) studies that identified genetic variants underlying T2D (7–11). In the present study, we sought to examine the potential interactions between the genetic predisposition defined by the established genetic variations and the well-characterized dietary patterns in relation to diabetes risk in a nested, case-control study from a prospective cohort of US men.
SUBJECTS AND METHODS
Study population
The Health Professionals Follow-Up Study (HPFS) is a prospective cohort study of 51,529 US male health professionals aged 40–75 y at study initiation in 1986 (12). Information about health and disease is assessed biennially with a self-administered questionnaire. Between 1993 and 1999, 18,159 men provided blood samples. Subjects for the present study were selected from those who provided blood samples. Diabetes cases were defined as self-reported diabetes confirmed by a validated supplementary questionnaire. For cases before 1998, diagnosis was made on the basis of criteria consistent with those proposed by the National Diabetes Data Group (NDDG). We used the American Diabetes Association diagnostic criteria for diagnosis of diabetes cases after 1998 (13–15). This study included all 1196 T2D cases from the blood cohort (335 cases were diagnosed on or before 1986) by follow-up through 2000. The cases were matched to 1337 nondiabetic control subjects on age, month, year of blood draw, and fasting status. All participants were white of European origin.
Assessment of dietary patterns
The procedure for deriving dietary patterns using food consumption data from the semiquantitative food-frequency questionnaire (FFQ) was described in detail elsewhere (16). Briefly, we conducted factor analysis to derive dietary patterns based on 40 predefined foods or food groups. The factor analysis generated 2 major dietary patterns. The first factor (the Prudent dietary pattern) was characterized by a high intake of vegetables, fruit, legumes, whole grains, fish, and poultry, whereas the second factor (the Western dietary pattern) was characterized by a high intake of processed meat, red meat, butter, high-fat dairy products, eggs, and refined grains. For each participant, the Prudent dietary pattern score and the Western dietary pattern score were calculated by summing the standardized intakes of the component foods, weighted by the factor loadings of the foods. These scores rank participants according to the degree to which they adhere to these dietary patterns. The analyses were conducted by using the FACTOR PROCEDURE in SAS (SAS Institute Inc, Cary, NC).
In a validation study of a subsample of men (n = 127) in the HPFS (17), the 131-item FFQ was administered twice with a 1-y interval, and two 1-wk diet records were collected during that year. The correlations for the factor scores between the 2 FFQs were 0.70 for the Prudent dietary pattern and 0.67 for the Western dietary pattern. The correlations (corrected for week-to-week variations in diet records) between the FFQ and the diet records were 0.52 for the prudent dietary pattern and 0.74 for the Western dietary pattern.
SNP selection and genotype determination
DNA was extracted from the buffy coat fraction of centrifuged blood by using the QIAmp Blood Kit (Qiagen, Chatsworth, CA). DNA samples were genotyped by using the OpenArray SNP Genotyping System (BioTrove, Woburn, MA) according to the manufacturer's instructions. Primers and probes are available on request. We selected 10 single nucleotide polymorphisms (SNPs) that showed a significant association with T2D in recently published GWA studies (7–11) and in our study sample (18): HHEX (rs1111875), CDKAL1 (rs7756992), IGF2BP2 (rs4402960), SLC30A8 (rs13266634), WFS1 (rs10010131), CDKN2A/B (rs564398, rs10811661), TCF7L2 (rs12255372), PPARG (rs1801282), and KCNJ11 (rs5219). Genotyping success rates exceeded 95% for most SNPs. Replicate quality-control samples (10%) were included and genotyped with >95% concordance. All SNPs fit Hardy-Weinberg equilibrium, except for a significant departure for rs7756992 among nondiabetic men (P < 0.05).
Genetic risk score calculation
We calculated the genetic risk score (GRS) using a simple count method, assuming that each SNP is independently associated with T2D risk under an additive genetic model (18). We applied a linear weighting of 0, 1, and 2 to genotypes containing 0, 1, or 2 risk alleles, respectively. This model performs well, even when the true genetic model is unknown or wrongly specified; <5% of genotypes were missing per subject. Scores for individuals with missing genotypes were standardized to those for individuals with complete data, assuming that the missing genotypes were not related to disease status. In sensitivity analyses, we excluded all subjects with missing genotypes. Similar results were observed (18). In addition, the results were similar when SNP rs7756992 was removed from the GRS calculation (data not shown).
Statistical analyses
The geometric means of continuous variables were compared by using general linear models, and the proportions of categorical variables were compared by using chi-square tests. Odds ratios (ORs) and 95% CIs were calculated by using the logistic regression model. Missing genotypes broke some matching pairs. In addition, the matching pairs were further broken in subgroup stratified analyses. Using conditional analysis on matching factors may lead to loss of data. Therefore, we used unconditional logistic regression models in our analysis. In the multivariable analyses, we adjusted for covariates, including age (continuous), body mass index (BMI; in kg/m2: <23, 23–24.9, 25–29.9, 30–34.9, or ≥35), physical activity (<1.5, 1.5–5.9, 6.0–11.9, 12–20.9, or ≥21.0 metabolic equivalent hours/wk), smoking (never, past, or current), alcohol intake (nondrinker and drinker of 0.1–4.9, 5–10, or >10 g/d), family history of diabetes (diabetes in sibling or parent, yes or no), and total energy intakes (in quartiles). Multiplicative interactions between the GRS and dietary intakes were examined by using the likelihood ratio test, with a comparison of the likelihood scores of the 2 models with and without the interaction terms. The SAS statistical package was used for the analyses (version 8.2 for UNIX; SAS Institute). Statistical significance was set at the 0.05 level, and all tests were 2-tailed.
RESULTS
The cases with diabetes were more obese, more likely to be a current smoker, and engaged in less physical activity than were the controls. More cases had a family history of diabetes than did the controls (Table 1). The GRS generated by summing the risk alleles of 10 SNPs ranged from 0 to 20 (median: 11). The GRS was significantly associated with an increased risk of T2D (18). Based on the baseline dietary information, the factor analysis generated 2 major dietary patterns (16). The first pattern was loaded heavily with vegetables, legumes, whole grains, fruit, fish, and poultry; the second pattern was loaded heavily with red meat, processed meat, refined grains, sweets and dessert, French fries, and high-fat dairy products. The first pattern explained 10% of the total variance, and the second pattern explained ≈7% of the total variance. As with our previous study (16), we labeled the first pattern the “Prudent dietary pattern” and the second pattern the “Western dietary pattern.”
TABLE 1.
Baseline characteristics of the diabetic patients and nondiabetic control subjects1
| Nondiabetic | Diabetic | P | |
| No. of participants | 1337 | 1196 | — |
| Age (y) | 55 ± 92 | 56 ± 8 | 0.1 |
| BMI (kg/m2) | 25 ± 2.8 | 27.8 ± 4.1 | 0.22 |
| Obesity (%)3 | 5.3 | 25.5 | <0.0001 |
| Alcohol use (g/d) | 12.2 ± 15.5 | 11.2 ± 16.6 | 0.01 |
| Physical activity (MET/wk) | 21.3 ± 27.4 | 14.6 ± 18.8 | 0.23 |
| Current smoker (%) | 7.0 | 11.3 | 0.0006 |
| Family history of diabetes (%) | 13.0 | 32.4 | <0.0001 |
| Total energy intake (kcal/d) | 2039 ± 634 | 2031 ± 604 | 0.73 |
MET, metabolic equivalent task. The geometric means of continuous variables were compared by using general linear models, and the proportions of categorical variables were compared by using chi-square tests.
Mean ± SD (all such values).
Defined as a BMI ≥ 30.
The baseline characteristics of the nondiabetic men by quartiles of the 2 dietary patterns are shown in Table 2. Participants with a higher Prudent dietary pattern were older, engaged in more physical activity, and less likely to be current smokers. Men in the higher quartiles of Western dietary pattern drank more alcohol and were more likely to be current smokers.
TABLE 2.
Baseline characteristics by quartile (Q) of the major dietary patterns in nondiabetic men1
| Prudent dietary pattern |
Western dietary pattern |
|||||||||
| Q1 (lowest) | Q2 | Q3 | Q4 (highest) | P value | Q1 | Q2 | Q3 | Q4 | P value | |
| No. of participants | 338 | 330 | 335 | 334 | 336 | 333 | 333 | 335 | — | |
| Age (y) | 54 ± 92 | 54 ± 8 | 56 ± 8 | 57 ± 9 | 0.0004 | 56 ± 8 | 55 ± 8 | 55 ± 9 | 55 ± 9 | 0.1 |
| BMI (kg/m2) | 25.4 ± 3.0 | 25.1 ± 2.8 | 24.8 ± 2.7 | 24.8 ± 2.7 | 0.02 | 24.8 ± 2.7 | 25.2 ± 2.9 | 25.1 ± 2.8 | 25.1 ± 2.8 | 0.22 |
| Alcohol use (g/d) | 12 ± 16.6 | 11.5 ± 15.2 | 13.7 ± 16.9 | 11.7 ± 13 | 0.26 | 11.1 ± 13.8 | 11.1 ± 14.7 | 12.4 ± 15 | 14.2 ± 18 | 0.01 |
| Physical activity (MET/wk) | 16.9 ± 31.5 | 19.4 ± 24.7 | 22 ± 22.6 | 26.8 ± 28.9 | <0.001 | 23 ± 26 | 22.4 ± 35.2 | 20 ± 21 | 19.7 ± 25.5 | 0.23 |
| Current smoker (%) | 12.1 | 5.8 | 6.6 | 3.3 | <0.001 | 3.0 | 6.6 | 6.6 | 11.6 | 0.001 |
| Family history of diabetes (%) | 12.4 | 12.1 | 14.9 | 12.6 | 0.69 | 13.7 | 12.9 | 13.2 | 12.2 | 0.95 |
| Food groups (servings/d) | ||||||||||
| Red meat | 0.64 ± 0.43 | 0.63 ± 0.45 | 0.6 ± 0.46 | 0.54 ± 0.46 | 0.05 | 0.27 ± 0.21 | 0.49 ± 0.29 | 0.68 ± 0.36 | 0.98 ± 0.53 | <0.001 |
| Processed meat | 0.47 ± 0.65 | 0.35 ± 0.35 | 0.34 ± 0.38 | 0.28 ± 0.39 | <0.001 | 0.12 ± 0.14 | 0.26 ± 0.23 | 0.35 ± 0.27 | 0.72 ± 0.73 | <0.001 |
| High-fat dairy products | 1.09 ± 1.19 | 1.05 ± 1.07 | 0.93 ± 0.87 | 0.96 ± 0.94 | 0.05 | 0.57 ± 0.57 | 0.8 ± 0.65 | 1.06 ± 0.88 | 1.61 ± 1.44 | <0.001 |
| Low-fat dairy products | 0.68 ± 0.91 | 0.88 ± 0.94 | 1.12 ± 1.21 | 1.11 ± 1.17 | <0.001 | 0.72 ± 0.86 | 0.92 ± 1.03 | 1.04 ± 1.03 | 1.1 ± 1.31 | <0.001 |
| Fish | 0.2 ± 0.15 | 0.32 ± 0.23 | 0.39 ± 0.26 | 0.59 ± 0.75 | <0.001 | 0.48 ± 0.72 | 0.34 ± 0.26 | 0.36 ± 0.31 | 0.32 ± 0.29 | <0.001 |
| Whole grain | 0.67 ± 0.74 | 1.10 ± 1.16 | 1.35 ± 1.21 | 1.82 ± 1.89 | <0.001 | 1.05 ± 1.16 | 1.23 ± 1.23 | 1.23 ± 1.34 | 1.42 ± 1.71 | <0.001 |
| Refined grain | 1.12 ± 1.11 | 1.05 ± 0.96 | 1.09 ± 0.94 | 1.12 ± 1.08 | 0.62 | 0.62 ± 0.58 | 0.82 ± 0.67 | 1.2 ± 0.93 | 1.75 ± 1.35 | <0.001 |
| High-energy drinks | 0.35 ± 0.55 | 0.31 ± 0.45 | 0.25 ± 0.39 | 0.23 ± 0.43 | 0.01 | 0.11 ± 0.22 | 0.21 ± 0.37 | 0.32 ± 0.44 | 0.49 ± 0.63 | <0.001 |
| Nutrient intakes (g/d) | ||||||||||
| Total fat | 69.5 ± 26.8 | 72.9 ± 27.4 | 73.2 ± 26.3 | 75.8 ± 30 | 0.03 | 46.2 ± 14.5 | 61.7 ± 12.8 | 77.4 ± 14.3 | 106 ± 23.7 | <0.001 |
| Saturated fat | 25.2 ± 10.6 | 25.2 ± 9.9 | 24.8 ± 10 | 24.7 ± 11 | 0.95 | 14.9 ± 4.5 | 21 ± 4.6 | 26.7 ± 5.5 | 37.4 ± 9.3 | <0.001 |
| Polyunsaturated fat | 11.4 ± 4.6 | 13.1 ± 5.0 | 13.9 ± 5.2 | 15.6 ± 6.7 | <0.001 | 9.8 ± 5.2 | 11.7 ± 3.8 | 14.2 ± 3.9 | 18.3 ± 5.5 | <0.001 |
| trans Fat | 3 ± 1.56 | 3.12 ± 1.68 | 2.88 ± 1.49 | 2.62 ± 1.53 | <0.001 | 1.49 ± 0.64 | 2.31 ± 0.75 | 3.24 ± 1.13 | 4.61 ± 1.55 | <0.001 |
| Fiber | 16.6 ± 4.8 | 19.8 ± 4.8 | 22.1 ± 5.2 | 28.2 ± 8.9 | <0.001 | 24.6 ± 7.9 | 22.6 ± 7.4 | 20.6 ± 5.6 | 19 ± 7.6 | <0.001 |
| Heme iron | 1.35 ± 0.55 | 1.31 ± 0.48 | 1.27 | 1.19 ± 0.47 | <0.001 | 1.16 ± 0.53 | 1.28 ± 0.5 | 1.31 ± 0.47 | 1.37 ± 0.45 | <0.001 |
MET, metabolic equivalent task. The geometric means of continuous variables were compared by using general linear models, and the proportions of categorical variables were compared by using chi-square tests.
Mean ± SD (all such values).
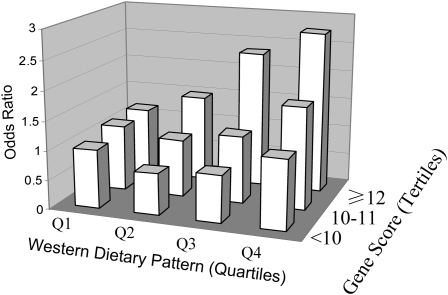
We first tested the interactions between the GRS and the 2 dietary patterns. The GRS significantly interacted with the Western dietary pattern in relation to diabetes risk (P for interaction = 0.02) (Table 3). Adjustment for age, BMI, smoking, alcohol consumption, physical activity, family history of diabetes, and total energy intakes did not appreciably change the results. To perform stratified analysis, participants were grouped into 3 categories according to their GRS: low (<10), median (10–11), and high (≥12). Dietary pattern scores were analyzed in quartiles. A high Western dietary pattern was significantly related to an increased risk of T2D among men with a high GRS (P for trend = 0.01). The multivariable ORs of T2D across increasing quartiles of the Western dietary pattern were 1.00, 1.23 (95% CI: 0.88, 1.73), 1.49 (95% CI: 1.06, 2.09), and 2.06 (95% CI: 1.48, 2.88). The associations between the Western dietary pattern and diabetes risk were not significant among those with a lower GRS. When the joint associations were examined, men with the highest GRS and the highest quartile for the Western dietary pattern had a 2.75-fold (1.56–4.84) higher risk of T2D than did those with the lowest GRS and the lowest quartile for the Western dietary pattern (Figure 1). There were no significant interactions between the GRS and the Prudent dietary pattern.
TABLE 3.
Interactions between dietary patterns and the genetic risk score in relation to diabetes risk1
| Dietary patterns3 |
||||||
| Genetic risk score2 | Q1 (lowest) | Q2 | Q3 | Q4 (highest) | P for trend | P for interaction |
| Western dietary pattern | ||||||
| <10 (n = 503) | 1 | 0.79 (0.46, 1.38) | 0.81 (0.48, 1.37) | 1.07 (0.65, 1.76) | 0.69 | 0.02 |
| 10–11 (n = 904) | 1 | 0.98 (0.67, 1.44) | 1.02 (0.69, 1.49) | 1.40 (0.97, 2.01) | 0.06 | — |
| ≥12 (n = 1126) | 1 | 1.23 (0.88, 1.73) | 1.49 (1.06, 2.09) | 2.06 (1.48, 2.88) | 0.01 | — |
| Prudent dietary pattern | ||||||
| <10 (n = 503) | 1 | 0.85 (0.50, 1.44) | 1.07 (0.65, 1.76) | 1.29 (0.79, 2.11) | 0.24 | NS |
| 10–11 (n = 904) | 1 | 0.75 (0.52, 1.07) | 0.81 (0.56, 1.18) | 0.77 (0.53, 1.11) | 0.21 | — |
| ≥12 (n = 1126) | 1 | 0.81 (0.58, 1.14) | 0.71 (0.51, 0.99) | 0.81 (0.59, 1.13) | 0.16 | — |
The analyses were adjusted for age, BMI, smoking, alcohol consumption, physical activity, family history of diabetes, and total energy intakes. Q, quartile.
Defined by counting the number of risk alleles of 10 single nucleotide polymorphisms from a genomewide association study, including HHEX (rs1111875), CDKAL1 (rs7756992), IGF2BP2 (rs4402960), SLC30A8 (rs13266634), WFS1 (rs10010131), CDKN2A/B (rs564398, rs10811661), TCF7L2 (rs12255372), PPARG (rs1801282), and KCNJ11 (rs5219).
Values are odds ratios (95% CIs) calculated by using an unconditional logistic regression model.
FIGURE 1.
Odds ratios of diabetes risk according to joint classification of Western dietary pattern scores (in quartiles; Q) and genetic risk scores (<10, 10–11, and ≥12). Odds ratios and 95% CIs were calculated by using an unconditional logistic regression model. The analyses were adjusted for age, BMI, smoking, alcohol consumption, physical activity, family history of diabetes, and total energy intakes.
We conducted sensitivity analyses by excluding prevalent cases of T2D or current smokers at baseline (1986) (Table 4). The interactions between the Western dietary pattern and the GRS in relation to diabetes risk remained significant (P = 0.039 and 0.007, respectively), and the associations between the Western dietary pattern and diabetes risk among men with different GRSs were similar to those observed in all the participants.
TABLE 4.
Sensitivity analyses of the stratified associations between the Western dietary pattern and diabetes risk in subpopulations1
| Western dietary patterns2 |
||||||
| Subjects and genetic risk score | Q1 (lowest) | Q2 | Q3 | Q4 (highest) | P for trend | P for interaction |
| Excluding prevalent cases (n = 2198)3 | ||||||
| <10 | 1 | 0.87 (0.46, 1.63) | 0.94 (0.52, 1.69) | 1.22 (0.70, 2.13) | 0.4 | 0.039 |
| 10–11 | 1 | 0.94 (0.62, 1.43) | 0.94 (0.62, 1.44) | 1.40 (0.95, 2.08) | 0.08 | — |
| ≥12 | 1 | 1.15 (0.78, 1.68) | 1.64 (1.13, 2.38) | 2.17 (1.51, 3.13) | <0.0001 | — |
| Excluding current smokers (n = 2305) | ||||||
| <10 | 1 | 0.76 (0.43, 1.36) | 0.74 (0.43, 1.27) | 0.95 (0.56, 1.59) | 0.87 | 0.007 |
| 10–11 | 1 | 0.96 (0.64, 1.42) | 0.94 (0.63, 1.41) | 1.44 (0.98, 2.11) | 0.06 | — |
| ≥12 | 1 | 1.15 (0.81, 1.64) | 1.45 (1.03, 2.05) | 2.13 (1.50, 3.02) | <0.0001 | — |
The analyses were adjusted for age, BMI, smoking, alcohol consumption, physical activity, family history of diabetes, and total energy intakes. Q, quartile.
Values are odds ratios (95% CIs) calculated by using an unconditional logistic regression model.
Prevalent cases at baseline (1986).
We next examined potential interactions between the GRS and the major foods characterizing the Western dietary pattern. Significant interactions were observed between the GRS and the intakes of processed meat (P = 0.029) and red meat (P = 0.02) in relation to diabetes risk (Table 5). Similar to the overall Western dietary pattern, intakes of red meat and processed meat showed significant associations with diabetes risk only among men with a high GRS.
TABLE 5.
Interactions between genetic risk score and individual foods and nutrients characterizing the Western dietary pattern1
| Western dietary patterns2 |
||||||
| Foods and genetic risk score | Q1 (lowest) | Q2 | Q3 | Q4 (highest) | P for trend | P for interaction |
| Red meat | ||||||
| <10 | 1 | 0.54 (0.24, 1.23) | 0.88 (0.39, 1.97) | 0.81 (0.36, 1.80) | 0.55 | 0.02 |
| 10–11 | 1 | 1.27 (0.75, 2.15) | 1.16 (0.68, 1.97) | 1.45 (0.86, 2.44) | 0.23 | — |
| ≥12 | 1 | 1.03 (0.69, 1.54) | 1.32 (0.87, 2.01) | 2.42 (1.58, 3.70) | <0.0001 | — |
| Processed meat | ||||||
| <10 | 1 | 0.91 (0.45, 1.85) | 0.70 (0.38, 1.28) | 1.12 (0.66, 1.92) | 0.76 | 0.029 |
| 10–11 | 1 | 0.98 (0.60, 1.61) | 0.96 (0.62, 1.48) | 1.47 (0.99, 2.20) | 0.06 | — |
| ≥12 | 1 | 1.09 (0.72, 1.67) | 1.88 (1.30, 2.71) | 2.01 (1.41, 2.89) | <0.0001 | — |
| Heme iron | ||||||
| <10 | 1 | 1.06 (0.57, 1.96) | 0.82 (0.45, 1.49) | 0.87 (0.48, 1.56) | 0.47 | 0.0004 |
| 10–11 | 1 | 1.19 (0.77, 1.85) | 1.37 (0.89, 2.10) | 1.85 (1.23, 2.77) | 0.002 | — |
| ≥12 | 1 | 0.71 (0.49, 1.03) | 1.24 (0.86, 1.80) | 2.48 (1.72, 3.56) | <0.0001 | — |
The analyses were adjusted for age, BMI, smoking, alcohol consumption, physical activity, family history of diabetes, and total energy intakes.
Values are odds ratios (95% CIs) calculated by using an unconditional logistic regression model.
We further assessed potential interactions between the GRS and nutrients high in processed and red meats, including total fat, saturated fat, and heme iron. Heme iron intakes showed the strongest and significant interactions with the GRS in relation to diabetes risk (P for interaction = 0.0004; Table 5). High heme iron intakes were significantly related to increased diabetes risk among men with a high (P for trend < 0.0001) or median GRS (P for trend = 0.002). In those with a low GRS, the intakes of heme iron were not significantly associated with diabetes risk.
DISCUSSION
We found significant interactions between the Western dietary pattern, which was characterized by high intakes of red meat, processed meat, and refined foods, and the GRS derived from genetic variants associated with diabetes risk in GWA studies (7–11). Intakes of the Western dietary pattern were significantly associated with increased diabetes risk among men with a higher GRS (≥12 risk alleles), but not among those with a lower GRS.
The fact that T2D is rampant in Western societies and that the incidence has clearly increased more in developing countries that have recently transitioned to a Westernized lifestyle highlights the critical role of a Westernized diet and lifestyle in triggering the epidemic of the disease (1, 2). In addition, it has long been noted that high variability exists among individuals in response to lifestyle changes. Our data suggest that the effects of a Westernized diet on diabetes risk are not homogeneous in people with different genetic backgrounds. High intakes of Westernized diets more likely increase the risk of diabetes among those with a higher genetic susceptibility to this disease.
Our data also indicate that red meat and processed meats may be the major foods driving the interactions between a Western dietary pattern and genetic variation in determining diabetes. High intakes of these foods significantly increased the risk of diabetes among individuals carrying more risk alleles (≥12) of diabetes variants but did not affect the disease risk in those carrying fewer risk alleles.
Intakes of red meat and processed meat and their major components, including saturated fat, cholesterol, and heme iron have been related to insulin resistance and risk of T2D in several human studies (19–21). In addition, preserving, cooking, and processing these foods generate certain types of preservatives, additives, or other chemicals such as advanced glycation and lipoxidation end products that have toxic effect on β cells (22) or induce insulin resistance (23, 24). Available evidence has shown that most diabetes variants might affect insulin secretion (25). Insulin resistance and insulin secretion are closely related, because the dysfunction of one pathway may exacerbate the abnormality of another pathway. Therefore, it is feasible that individuals with high intakes of red or processed meats have a greater risk if they carry more alleles of the risk loci for T2D.
Among the nutrients for which red meat is a major source, heme iron showed the strongest interaction with the genetic variation. High heme iron intakes can result in high body iron stores, which may impair insulin sensitivity and glucose homeostasis (26). Therefore, our data suggest that heme iron is a biological candidate that may act synergistically with genetic factors in affecting diabetes risk.
Several limitations need to be addressed. Population stratification may cause spurious associations. However, our study population was highly homogeneous, because it included only whites with European ancestry, and therefore was less likely affected by population stratification. Statistical methods used to define dietary patterns such as factor analysis are somewhat subjective. However, previous studies have shown reasonable reproducibility over time and comparability between the FFQs and diet records in characterizing dietary patterns in a subsample of the HPFS (17). In addition, the derived Western dietary pattern has been robustly related to the risk of T2D and coronary heart disease (5, 16). Dietary patterns can vary by sex, socioeconomic status, ethnic group, and culture. Thus, it is necessary to replicate the results of our study in other populations. Finally, the genetic variants identified thus far account for a very small portion of the variability in diabetes risk. In addition, individuals with similar GRSs may differ in the specific variants contributing to their GRS. A more comprehensive evaluation of gene-diet interactions will need to include more genetic risk factors when they are identified and perform analyses on the interactions between specific variants and dietary intakes.
In conclusion, we found that the Western dietary pattern interacted with genetic variation in relation to diabetes risk. Our findings suggest that the adoption of a Westernized diet may increase diabetes risk, especially among the genetically high-risk population.
Acknowledgments
The authors' responsibilities were as follows—LQ: contributed to the concept and design, data analysis, statistical support, and manuscript writing and editing; MC, CZ, and RMvD: contributed to the manuscript editing; and FBH: contributed to the funding and manuscript editing. None of the authors had any personal or financial conflicts of interest.
REFERENCES
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–7 [DOI] [PubMed] [Google Scholar]
- 2.Qi L, Hu FB, Hu G. Genes, environment, and interactions in prevention of type 2 diabetes: a focus on physical activity and lifestyle changes. Curr Mol Med 2008;8:519–32 [DOI] [PubMed] [Google Scholar]
- 3.Qi L, Kraft P, Hunter DJ, Hu FB. The common obesity variant near MC4R gene is associated with higher intakes of total energy and dietary fat, weight change and diabetes risk in women. Hum Mol Genet 2008;17:3502–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi L, Meigs J, Manson JE, et al. HFE genetic variability, body iron stores, and the risk of type 2 diabetes in U.S. Women. Diabetes 2005;54:3567–72 [DOI] [PubMed] [Google Scholar]
- 5.van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med 2002;136:201–9 [DOI] [PubMed] [Google Scholar]
- 6.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 2005;82:675–84(quiz 714–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007;316:1331–6 [DOI] [PubMed] [Google Scholar]
- 8.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in U.K. samples reveals risk loci for type 2 diabetes. Science 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 2007;39:770–5 [DOI] [PubMed] [Google Scholar]
- 10.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007;316:1341–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007;445:881–5 [DOI] [PubMed] [Google Scholar]
- 12.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464–8 [DOI] [PubMed] [Google Scholar]
- 13.Qi L, van Dam RM, Meigs JB, Manson JE, Hunter D, Hu FB. Genetic variation in IL6 gene and type 2 diabetes: tagging-SNP haplotype analysis in large-scale case-control study and meta-analysis. Hum Mol Genet 2006;15:1914–20 [DOI] [PubMed] [Google Scholar]
- 14.Qi L, Li T, Rimm E, et al. The +276 polymorphism of the APM1 gene, plasma adiponectin concentration, and cardiovascular risk in diabetic men. Diabetes 2005;54:1607–10 [DOI] [PubMed] [Google Scholar]
- 15.Qi L, Rimm E, Liu S, Rifai N, Hu FB. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care 2005;28:1022–8 [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr 2000;72:912–21 [DOI] [PubMed] [Google Scholar]
- 17.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–9 [DOI] [PubMed] [Google Scholar]
- 18.Cornelis M, Qi L, Zhang C, et al. Joint effects of common genetic variants on the risk of type 2 diabetes in U.S. men and women. Ann Intern Med (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Y, Manson JE, Buring JE, Liu S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: the women's health study. Diabetes Care 2004;27:2108–15 [DOI] [PubMed] [Google Scholar]
- 20.Schulze MB, Manson JE, Willett WC, Hu FB. Processed meat intake and incidence of type 2 diabetes in younger and middle-aged women. Diabetologia 2003;46:1465–73 [DOI] [PubMed] [Google Scholar]
- 21.Jiang R, Ma J, Ascherio A, Stampfer MJ, Willett WC, Hu FB. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: a prospective cohort study. Am J Clin Nutr 2004;79:70–5 [DOI] [PubMed] [Google Scholar]
- 22.LeDoux SP, Woodley SE, Patton NJ, Wilson GL. Mechanisms of nitrosourea-induced beta-cell damage. Alterations in DNA. Diabetes 1986;35:866–72 [DOI] [PubMed] [Google Scholar]
- 23.Hofmann SM, Dong HJ, Li Z, et al. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes 2002;51:2082–9 [DOI] [PubMed] [Google Scholar]
- 24.Peppa M, Goldberg T, Cai W, Rayfield E, Vlassara H. Glycotoxins: a missing link in the “relationship of dietary fat and meat intake in relation to risk of type 2 diabetes in men”. Diabetes Care 2002;25:1898–9 [DOI] [PubMed] [Google Scholar]
- 25.Frayling TM. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet 2007;8:657–62 [DOI] [PubMed] [Google Scholar]
- 26.Tuomainen TP, Nyyssonen K, Salonen R, et al. Body iron stores are associated with serum insulin and blood glucose concentrations. Population study in 1,013 eastern Finnish men. Diabetes Care 1997;20:426–8 [DOI] [PubMed] [Google Scholar]