Abstract
Background: Age-related reductions in serum dehydroepiandrosterone (DHEA) concentrations may be involved in bone mineral density (BMD) losses.
Objective: The objective was to determine whether DHEA supplementation in older adults improves BMD when co-administered with vitamin D and calcium.
Design: In year 1, a randomized trial was conducted in which men (n = 55) and women (n = 58) aged 65–75 y took 50 mg/d oral DHEA supplements or placebo. In year 2, all participants took open-label DHEA (50 mg/d). During both years, all participants received vitamin D (16 μg/d) and calcium (700 mg/d) supplements. BMD was measured by using dual-energy X-ray absorptiometry. Concentrations of hormones and bone turnover markers were measured in serum.
Results: In men, no difference between groups occurred in any BMD measures or in bone turnover markers during year 1 or year 2. The free testosterone index and estradiol increased in the DHEA group only. In women, spine BMD increased by 1.7 ± 0.6% (P = 0.0003) during year 1 and by 3.6 ± 0.7% after 2 y of supplementation in the DHEA group; however, in the placebo group, spine BMD was unchanged during year 1 but increased to 2.6 ± 0.9% above baseline during year 2 after the crossover to DHEA. Hip BMD did not change. Testosterone, estradiol, and insulin-like growth factor 1 increased in the DHEA group only. In both groups, serum concentrations of bone turnover markers decreased during year 1 and remained low during year 2, but did not differ between groups.
Conclusion: DHEA supplementation in older women, but not in men, improves spine BMD when co-administered with vitamin D and calcium. This trial was registered at clinicaltrials.gov as NCT00182975.
INTRODUCTION
Dehydroepiandrosterone (DHEA) is the most abundant adrenal androgen in humans and exists predominately in a sulfated form (DHEAS). Its concentration decreases by ≈70–80% during adulthood (1). Because bone mineral density (BMD) also decreases throughout adulthood, and because low DHEA concentrations are associated with low BMD (2–5) and high fracture risk (6), it is possible that reductions in DHEAS may be partly responsible for age-related bone loss. Therefore, it is of interest to know whether the restoration of DHEA to youthful concentrations in older adults can improve or preserve bone health.
Four randomized controlled trials (RCTs) have assessed the effect of long-term (ie, 12–24 mo) DHEA replacement therapy on BMD in older adults (7–10). These studies reported that DHEA therapy increases or preserves BMD compared with controls, although most of the effects were modest and marginally significant. Two of the trials reported markers of bone turnover; the findings from both studies suggest that DHEA replacement decreases bone resorption in women (8, 9), and results of one study suggest an increase in bone formation in men (9).
It is conceivable that inadequate dietary intakes of vitamin D and calcium could attenuate the beneficial effect of DHEA supplementation on BMD because both are requisite for bone health (11). This is especially relevant in older adults because inadequate intakes of these nutrients may be as prevalent as ≈50% in older adults (12, 13). One of the DHEA replacement trials provided vitamin D, calcium supplements, or both to individuals with apparent inadequate intakes (7) and another reported vitamin D deficiencies in 58% of subjects (9). None of the trials provided vitamin D or calcium supplements to all participants to ensure adequate intakes.
The primary purpose of the present study was to assess the hypothesis that 1 y of DHEA replacement therapy has beneficial effects on BMD in older men and women supplemented with vitamin D and calcium. To gain insights into the mechanisms that contribute to changes in BMD, we measured markers of bone turnover and hormones that may be involved in bone metabolism. An exploratory aim was to determine whether 2 y of DHEA supplementation provides greater benefits than does 1 y of supplementation. Thus, we performed a follow-up study in which subjects who were taking DHEA during the randomized trial continued with DHEA supplementation for a second year and subjects who were taking placebo during the randomized trial took DHEA supplements during year 2.
SUBJECTS AND METHODS
Participants
Sedentary, nonsmoking, 65- to 75-y-old men and women were recruited for the study. A medical history and the results of physical examination, analysis of blood chemistry, hematology, urinalysis, and electrocardiography were used to identify and exclude volunteers with conditions that could interfere with study compliance and that might be considered contraindications for DHEA replacement therapy, including chronic infections, a history or evidence of malignancy within the past 5 y (other than innocuous skin cancer), unstable or occult cardiovascular disease, advanced emphysema, advanced Parkinson's disease, resting blood pressure >170 mm Hg systolic or >100 mm Hg diastolic, or diabetes. Participants taking antihypertensive, antidyslipidemic, or thyroid medications were required to be taking stable doses during the 6 mo before baseline testing. Although volunteers who were taking bisphosphonates were permitted into the study, these subjects were excluded from the outcome analyses for the present study. Written consent was obtained from all participants, and the study was approved by the Human Research Protection Office at Washington University School of Medicine. The protocol is consistent with the principles of the Declaration of Helsinki.
Interventions
Eligible participants were randomly assigned, with stratification for sex, to receive 12 mo of DHEA supplementation (50 mg/d) or placebo. In a previous study from our laboratory (14), this DHEA dose was effective at increasing circulating DHEAS concentrations to those seen in young adults. Participants in both groups received multivitamin and calcium/vitamin D supplements throughout the duration of the yearlong RCT and year 2 extension study. These supplements provided 16 μg/d (650 IU) vitamin D and 700 mg Ca/d. Participants were advised to maintain their usual dietary and physical activity habits throughout the study. On a monthly basis, the participants met with a member of the research team to pick up more DHEA or placebo and vitamin D/calcium. DHEA and placebo pill counts were performed to monitor compliance. During the monthly meetings, the participants were queried about changes in diet, physical activity, medical conditions, and medication use and potential side effects of DHEA supplementation.
Height and weight
Height and weight were measured in the morning, after an overnight fast, while the subjects were wearing only a hospital gown and underwear. BMI was calculated as weight (kg)/height2 (m).
Dietary vitamin D and calcium
Vitamin D and calcium intakes were assessed with 4-d food diaries and computerized nutrient analysis (versions 4.05, 4.06, and 5.0; Nutrition Data System for Research, Nutrition Coordination Center, University of Minnesota, Minneapolis, MN). Participants received detailed instructions from a dietitian on how to measure and record all foods, beverages, and supplements consumed. Daily intake of vitamin D and calcium were quantified in absolute terms and as percentages of “adequate intake” according to the Dietary Reference Intakes (15).
Bone mineral density
Whole-body, anterior-posterior lumbar spine (L2-L4), and proximal femur BMD were measured by using dual-energy X-ray absorptiometry (DXA) (software version 11.2, Delphi 4500-W; Hologic Corporation, Waltham, MA). The CV for these measures at our center is 1.1% for the spine and 1.2% for the femur (16).
Sample analyses
Between 0700 and 0900 and after an overnight fast, blood was collected from an arm vein. Serum was isolated by using standard clinical methods and stored at −20°C. The following analyte concentrations were measured: insulin-like growth factor 1 (IGF-1), insulin-like growth factor binding protein-3 (IGFBP-3), sex hormone–binding globulin (SHBG), total testosterone, and DHEAS were measured by using chemiluminescent assays (Immulite 2000; Diagnostic Products Corporation, San Diego, CA); total estradiol was measured by using an ultrasensitive radioimmunoassay (Diagnostic Systems Laboratories, Webster TX); and C-terminal telopeptide of type I collagen (CTX) and bone-specific alkaline phosphatase (BAP) were measured by using enzyme-linked immunosorbent assays (CTX: CrossLabs, Nordic Bioscience Diagnostics, Herlev, Denmark; BAP: Metra BAP, Quidel Corporation, San Diego, CA).
The molar ratio of IGF-1 to IGFBP-3 was calculated as (IGF-1 × 0.130)/(IGFBP-3 × 36), where IGF-1 is expressed in ng/mL and IGFBP-3 in μg/mL. The free testosterone index was calculated as (total testosterone × 0.0347)/SHBG, where testosterone is expressed in ng/dL and SHBG in nmol/L. The free estradiol index was calculated as (total estradiol × 0.00367)/SHBG, where estradiol is expressed in pg/mL and SHBG in nmol/L.
Monitoring for hormone-sensitive cancer
In men, serum prostate-specific antigen (PSA) concentrations were measured by using a monoclonal antibody assay (Hybritech, San Diego, CA). In women, mammograms and pap smears were performed.
Statistical analyses
On the basis of a previous study of the effects of DHEA supplementation on BMD (14), the mean ± SD increase in spine BMD was expected to be 2.5 ± 3.4%, whereas the placebo group remained unchanged. Thus, given the sample sizes in the present study, 2-tailed tests, and an α of 0.05, the calculated power to detect significant treatment effects was 78% for men and 80% for women.
Comparisons of baseline characteristics between the DHEA and placebo groups were performed by using independent t tests, chi-square tests, and Fisher's exact tests. Outcomes were analyzed with repeated-measures 2-factor (group and test) analyses of covariance, which included baseline values as a covariate; Tukey tests were used for post hoc paired comparisons. Outcomes from the 2-y trial were also analyzed with repeated-measures one-factor analyses of variance to assess within group changes over time, with Tukey tests for post hoc paired comparisons. Data are presented as arithmetic means ± SE. Significance was accepted at P ≤ 0.05. Analyses were conducted with SAS for Windows XP Pro (version 9.1; SAS Institute, Cary, NC).
RESULTS
Participants
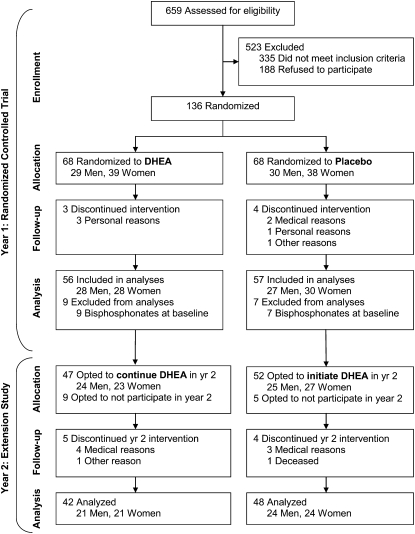
A total of 136 participants were randomly assigned to receive DHEA or placebo and started the yearlong RCT (Figure 1). Retention of study participants was excellent, with year 1 dropout rates of 4% and 6% in the DHEA and placebo groups, respectively (P = 1.0). None of the demographic or baseline characteristics differed between groups (Table 1). A subset of participants agreed to participate in the year 2 extension study, during which all participants underwent open-label DHEA supplementation (50 mg/d) (Figure 1).
FIGURE 1.
Consort diagram indicating sample sizes at each stage during the study. DHEA, dehydroepiandrosterone.
TABLE 1.
Characteristics of participants in the 1-y randomized controlled trial (n = 113)1
| DHEA group (n = 56) | Placebo group (n = 57) | Between-group P value2 | |
| Sex [n (%)] | 0.78 | ||
| Men | 28 (50) | 27 (47) | |
| Women | 28 (50) | 30 (53) | |
| Race [n (%)] | 1.0 | ||
| African American | 1 (2) | 2 (4) | |
| White | 55 (98) | 55 (96) | |
| Education [n (%)]3 | 0.71 | ||
| <College degree | 26 (48) | 23 (41) | |
| College degree | 14 (26) | 15 (27) | |
| Graduate school | 14 (26) | 18 (32) | |
| Age (y) | 70 ± 3 | 70 ± 3 | 0.90 |
| Weight (kg) | |||
| Men | 88.7 ± 13.84 | 85.0 ± 11.5 | 0.29 |
| Women | 77.4 ± 17.6 | 75.4 ± 18.9 | 0.67 |
| All | 83.1 ± 16.7 | 79.9 ± 16.4 | 0.65 |
| BMI (kg/m2) | 29.3 ± 5.9 | 27.7 ± 5.1 | 0.12 |
| Tobacco use [n (%)] | 0.77 | ||
| Never | 30 (54) | 29 (51) | |
| Previous | 26 (46) | 28 (49) | |
| Alcohol use [n (%)] | 0.25 | ||
| <1 drink/d | 43 (77) | 42 (74) | |
| 1–2 drinks/d | 10 (18) | 7 (12) | |
| >2 drinks/d | 3 (5) | 8 (14) |
DHA, dehydroepiandrosterone.
Independent t tests were used for quantitative data, and chi-square tests were used for counts, except for race, which was analyzed by using Fisher's exact test.
Data not available for 3 subjects.
Mean ± SD (all such values).
Compliance
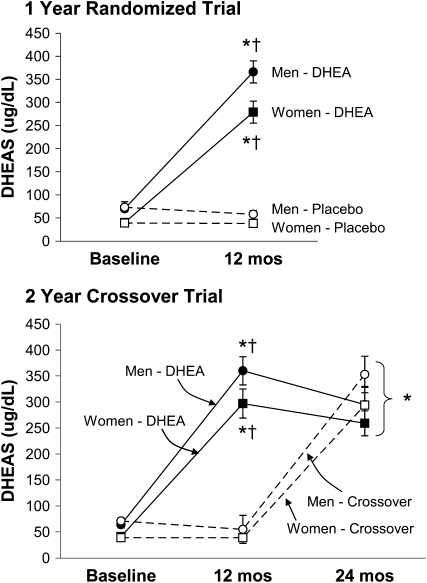
On the basis of the evaluable data (4% of monthly pill counts were missing), pill compliance during the RCT was 94.4 ± 0.4% in the DHEA group and 95.6 ± 0.4% in the placebo group and was similar for men and women. Pill counts were not performed during the extension study. For both men and women, serum DHEAS concentrations increased 5- to 7-fold in the DHEA group and did not change in the placebo group (Figure 2). For both men and women in the 2-y extension study, serum DHEAS concentrations were elevated above baseline at year 1 and 2 in the DHEA group and at year 2 only for participants who crossed over from placebo to DHEA (Figure 2).
FIGURE 2.
Arithmetic mean (±SE) changes in serum concentrations of dehydroepiandrosterone sulfate (DHEAS) during the 1-y randomized placebo-controlled trial (top: n = 55 men and 58 women) and during the 2-y extension study (bottom: n = 45 men and 45 women). During the 2-y extension study, participants took dehydroepiandrosterone (DHEA) supplements for 2 y (DHEA group) or placebo for 1 y and then DHEA supplements for the next year (crossover group). *P < 0.0001 compared with baseline (within group) and †P ≤ 0.0001 for the comparison between the placebo or crossover group at the same time point by 2-factor repeated-measures ANOVA and Tukey-adjusted post hoc paired comparisons (P < 0.0001 for group-by-time interaction in both men and women).
Vitamin D and calcium intake
According to DRI (15) standards, 54% of the participants in the DHEA group had an inadequate vitamin D intake at baseline, and 48% had an inadequate calcium intake. Similarly, in the placebo group, 54% of the participants had an inadequate vitamin D intake, and 44% had an inadequate calcium intake. In response to supplementation, vitamin D intake increased during the 1-y RCT from 12.9 ± 0.9 to 21.3 ± 0.6 μg/d (P < 0.0001) in the DHEA group and from 13.7 ± 1.0 to 22.1 ± 0.6 μg/d (P < 0.0001) in the placebo group. Calcium intake increased from 1301 ± 68 to 1627 ± 56 mg/d (P < 0.0001) in the DHEA group and from 1319 ± 67 to 1646 ± 47 mg/d (P < 0.0001) in the placebo group. As percentages of the DRIs, vitamin D intake increased from 86 ± 6% to 142 ± 4% (P < 0.0001) and calcium intake increased from 108 ± 6% to 135 ± 6% (P < 0.0001) in the DHEA group. The placebo group had similar increases.
Outcomes from the 1-y randomized controlled trial
Bone mineral density
In men, lumbar spine BMD increased by 1.7 ± 0.3% in the DHEA group and by 1.1 ± 0.3% in the placebo group (P = 0.0005 for main effect of time); however, the changes did not differ significantly between groups (Table 2). No changes in hip or whole-body BMD were evident in men (Table 2). In women, lumbar spine BMD increased by 1.8 ± 0.4% (P = 0.0003) in the DHEA group and remained essentially unchanged (+0.7 ± 0.4%; P = 0.96) in the placebo group (Table 2). Hip and whole-body BMD did not change in women.
TABLE 2.
Bone mineral density (BMD) and circulating concentrations of bone turnover markers during the 1-y randomized controlled trial1
| Men (n = 55) |
Women (n = 58) |
|||||||
| Baseline | 6 mo | 12 mo | P for interaction2 | Baseline | 6 mo | 12 mo | P for interaction2 | |
| BMD (g/cm2) | ||||||||
| Lumbar spine | ||||||||
| DHEA | 1.139 ± 0.029 | 1.156 ± 0.030 | 1.157 ± 0.0283 | 1.065 ± 0.029 | 1.083 ± 0.0314 | 1.085 ± 0.0314 | ||
| Placebo | 1.139 ± 0.031 | 1.149 ± 0.031 | 1.153 ± 0.0323 | 0.61 | 1.016 ± 0.029 | 1.029 ± 0.030 | 1.024 ± 0.030 | 0.03 |
| Total hip | ||||||||
| DHEA | 1.031 ± 0.024 | 1.025 ± 0.024 | 1.026 ± 0.024 | 0.896 ± 0.022 | 0.897 ± 0.022 | 0.894 ± 0.022 | ||
| Placebo | 1.015 ± 0.018 | 1.014 ± 0.019 | 1.015 ± 0.019 | 0.42 | 0.881 ± 0.023 | 0.873 ± 0.023 | 0.874 ± 0.023 | 0.09 |
| Femoral neck | ||||||||
| DHEA | 0.805 ± 0.020 | 0.805 ± 0.021 | 0.806 ± 0.023 | 0.749 ± 0.022 | 0.743 ± 0.021 | 0.751 ± 0.023 | ||
| Placebo | 0.812 ± 0.020 | 0.809 ± 0.021 | 0.815 ± 0.022 | 0.81 | 0.727 ± 0.020 | 0.726 ± 0.019 | 0.733 ± 0.023 | 0.74 |
| Trochanter | ||||||||
| DHEA | 0.825 ± 0.023 | 0.817 ± 0.022 | 0.820 ± 0.023 | 0.680 ± 0.016 | 0.683 ± 0.017 | 0.687 ± 0.018 | ||
| Placebo | 0.810 ± 0.018 | 0.812 ± 0.018 | 0.811 ± 0.018 | 0.16 | 0.668 ± 0.021 | 0.665 ± 0.021 | 0.666 ± 0.021 | 0.21 |
| Whole-body | ||||||||
| DHEA | 1.273 ± 0.027 | 1.275 ± 0.027 | 1.275 ± 0.027 | 1.136 ± 0.022 | 1.133 ± 0.023 | 1.148 ± 0.024 | ||
| Placebo | 1.279 ± 0.025 | 1.283 ± 0.024 | 1.287 ± 0.025 | 0.88 | 1.108 ± 0.024 | 1.108 ± 0.024 | 1.107 ± 0.024 | 0.16 |
| Bone turnover markers | ||||||||
| CTX (ng/mL)5 | ||||||||
| DHEA | 0.354 ± 0.022 | — | 0.361 ± 0.032 | 0.422 ± 0.034 | — | 0.357 ± 0.0303 | ||
| Placebo | 0.344 ± 0.027 | — | 0.380 ± 0.046 | 0.52 | 0.550 ± 0.057 | — | 0.477 ± 0.0503 | 0.81 |
| BAP (U/L) | ||||||||
| DHEA | 20.0 ± 1.2 | — | 18.1 ± 1.03 | 24.8 ± 1.7 | — | 20.9 ± 1.33 | ||
| Placebo | 19.6 ± 0.9 | — | 18.7 ± 0.93 | 0.27 | 28.1 ± 2.4 | — | 24.3 ± 1.43 | 0.85 |
All values are arithmetic means ± SEs. CTX, C-terminal telopeptide of type I collagen; BAP, bone-specific alkaline phosphatase; DHEA, dehydroepiandrosterone.
P for group × time interaction from repeated-measures ANCOVA, which included baseline values as a covariate.
P < 0.05 based on main effect of time, which was only tested in the absence of a significant group × time interaction.
Significantly different from baseline (within group and sex), P ≤ 0.05 (Tukey test).
To convert to nmol/L (SI units), multiply values by 7.750.
Bone turnover markers
CTX did not change in men in either group. BAP decreased in men by 7 ± 2% in the DHEA group and by 4 ± 2% in the placebo group (P = 0.003 for main effect of time), with no significant difference between groups (Table 2). In women, CTX decreased by 14 ± 3% in the DHEA group and by 11 ± 4% in the placebo group (P = 0.0002 for main effect of time); however, these changes were not significantly different between groups (Table 2). Furthermore, whereas BAP decreased significantly in both groups of women (DHEA: −12 ± 3%; placebo: −7 ± 3%; P < 0.0001 for main effect of time), the changes were not significantly different (Table 2).
IGF-1 and IGFBP-3
Serum IGF-1 concentrations did not change in men in either group (Table 3). Although there was a tendency for a greater decrease in serum IGFBP-3 in men in the DHEA group as compared with the placebo group, the IGF-1:IGFBP-3 ratio remained unchanged in both groups (Table 3). In women, IGF-1 and the IGF-1:IGFBP-3 ratio increased significantly in the DHEA group and remained unchanged in the placebo group (P ≤ 0.002 for both interactions). IGFBP-3 did not change in either group of women (Table 3).
TABLE 3.
Hormonal responses during the 1-y randomized controlled trial1
| Men (n = 55) |
Women (n = 58) |
|||||
| Baseline | 12 mo | P for interaction2 | Baseline | 12 mo | P for interaction2 | |
| IGF-1 (ng/mL) | ||||||
| DHEA | 135 ± 10 | 132 ± 10 | 114 ± 7 | 128 ± 83 | ||
| Placebo | 126 ± 8 | 127 ± 8 | 0.62 | 109 ± 8 | 105 ± 7 | 0.002 |
| IGFBP-3 (μg/mL) | ||||||
| DHEA | 3.76 ± 0.18 | 3.58 ± 0.18 | 4.17 ± 0.16 | 4.12 ± 0.14 | ||
| Placebo | 3.60 ± 0.15 | 3.58 ± 0.17 | 0.07 | 3.84 ± 0.14 | 3.85 ± 0.14 | 0.48 |
| IGF-1:IGFBP-3 | ||||||
| DHEA | 0.127 ± 0.005 | 0.132 ± 0.006 | 0.096 ± 0.004 | 0.109 ± 0.0053 | ||
| Placebo | 0.126 ± 0.006 | 0.129 ± 0.005 | 0.66 | 0.101 ± 0.005 | 0.098 ± 0.005 | 0.001 |
| SHBG (nmol/L) | ||||||
| DHEA | 33.5 ± 1.9 | 32.9 ± 2.3 | 39.0 ± 2.4 | 32.9 ± 2.13 | ||
| Placebo | 38.1 ± 2.4 | 40.0 ± 2.5 | 0.04 | 42.3 ± 3.8 | 44.3 ± 4.1 | <0.0001 |
| Total testosterone (ng/dL) | ||||||
| DHEA | 420 ± 25 | 491 ± 31 | 25 ± 2 | 57 ± 43 | ||
| Placebo | 420 ± 33 | 448 ± 29 | 0.13 | 24 ± 1 | 25 ± 2 | <0.0001 |
| Free testosterone index (×10−2) | ||||||
| DHEA | 44.8 ± 2.1 | 53.9 ± 2.63 | 2.4 ± 0.2 | 6.5 ± 0.63 | ||
| Placebo | 39.2 ± 2.7 | 39.0 ± 2.3 | 0.0004 | 2.3 ± 0.2 | 2.5 ± 0.3 | <0.0001 |
| Total estradiol (pg/mL) | ||||||
| DHEA | 17.7 ± 1.0 | 21.0 ± 1.03 | 10.0 ± 1.1 | 16.6 ± 0.93 | ||
| Placebo | 16.0 ± 0.9 | 14.7 ± 0.8 | 0.0007 | 10.7 ± 0.7 | 10.1 ± 0.6 | <0.0001 |
| Free estradiol index (×10−4) | ||||||
| DHEA | 21.8 ± 2.0 | 26.2 ± 2.13 | 10.8 ± 1.5 | 20.9 ± 2.13 | ||
| Placebo | 17.0 ± 1.4 | 14.8 ± 1.1 | 0.0002 | 12.2 ± 1.6 | 11.1 ± 1.3 | <0.0001 |
All values are arithmetic means ± SEs. IGF-1, insulin-like growth factor 1; IGFBP-3, insulin-like growth factor binding protein-3; SHBG, sex hormone–binding globulin; DHEA, dehydroepiandrosterone. To convert to SI units, multiply values as follows: by 0.130 to convert IGF-1 to nmol/L, by 36 to convert IGFBP-3 to nmol/L, by 0.0347 to convert total testosterone to nmol/L, and by 3.67 to convert total estradiol to pmol/L.
P for group × time interaction from repeated-measures ANCOVA, which included baseline values as a covariate.
Significantly different from baseline (within group and sex), P ≤ 0.05 (Tukey test).
Sex hormones
Total testosterone increased by 17 ± 3% (P = 0.003) in men in the DHEA group; however, the difference in responses between the DHEA and placebo groups was not statistically significant (Table 3). The free testosterone index also increased in the DHEA group but remained unchanged in the placebo group (Table 3). Total estradiol and the free estradiol index increased in men in the DHEA group and were unchanged in the placebo group (Table 3). In women, SHBG concentrations decreased in the DHEA group and were unchanged in the placebo group (P < 0.0001 for interaction). The concentrations of total testosterone, total estradiol, and their free indexes all approximately doubled in women in the DHEA group and did not change in the placebo group (Table 3).
Outcomes from the year 2 extension study
Bone mineral density
Lumbar spine BMD increased significantly above baseline (≈1.6%) in both the men who took DHEA for 2 y and in those who took placebo during the first year and then crossed over to take open-label DHEA (Table 4 and Figure 3). Hip and whole-body BMD did not change in either group of men. Women who underwent 2 y of DHEA supplementation had an increase of 3.6 ± 0.7% in lumbar spine BMD (Table 4 and Figure 3). In women who were in the control group for 1 y and then took DHEA during the second year, BMD increased by 2.1 ± 0.7% (P = 0.02) during the second year (Table 4 and Figure 3). No changes in hip or whole-body BMD occurred in either group of women (Table 4).
TABLE 4.
Bone mineral density (BMD) and circulating concentrations of bone turnover markers during the 2-y trial1
| Men (n = 45) |
Women (n = 45) |
|||||||
| Baseline | 12 mo | 24 mo | Within-group P2 | Baseline | 12 mo | 24 mo | Within-group P2 | |
| BMD (g/cm2) | ||||||||
| Lumbar spine | ||||||||
| DHEA | 1.146 ± 0.032 | 1.159 ± 0.031 | 1.164 ± 0.0363 | 0.04 | 1.048 ± 0.039 | 1.066 ± 0.0413 | 1.086 ± 0.04234 | <0.0001 |
| Crossover | 1.132 ± 0.034 | 1.141 ± 0.036 | 1.152 ± 0.0363 | 0.04 | 1.011 ± 0.034 | 1.016 ± 0.035 | 1.041 ± 0.03434 | 0.006 |
| Total hip | ||||||||
| DHEA | 1.052 ± 0.025 | 1.045 ± 0.026 | 1.043 ± 0.027 | 0.09 | 0.889 ± 0.031 | 0.886 ± 0.031 | 0.882 ± 0.029 | 0.43 |
| Crossover | 1.012 ± 0.020 | 1.010 ± 0.022 | 1.010 ± 0.021 | 0.88 | 0.875 ± 0.026 | 0.867 ± 0.026 | 0.866 ± 0.025 | 0.11 |
| Femoral neck | ||||||||
| DHEA | 0.817 ± 0.021 | 0.818 ± 0.022 | 0.817 ± 0.024 | 0.99 | 0.739 ± 0.031 | 0.739 ± 0.031 | 0.740 ± 0.027 | 0.74 |
| Crossover | 0.813 ± 0.023 | 0.817 ± 0.024 | 0.816 ± 0.024 | 0.80 | 0.719 ± 0.023 | 0.729 ± 0.027 | 0.724 ± 0.025 | 0.47 |
| Trochanter | ||||||||
| DHEA | 0.840 ± 0.027 | 0.833 ± 0.028 | 0.834 ± 0.028 | 0.21 | 0.678 ± 0.024 | 0.681 ± 0.026 | 0.675 ± 0.023 | 0.58 |
| Crossover | 0.807 ± 0.020 | 0.808 ± 0.021 | 0.808 ± 0.021 | 0.98 | 0.661 ± 0.023 | 0.659 ± 0.022 | 0.653 ± 0.021 | 0.34 |
| Whole-body | ||||||||
| DHEA | 1.303 ± 0.032 | 1.302 ± 0.030 | 1.314 ± 0.033 | 0.33 | 1.120 ± 0.030 | 1.123 ± 0.030 | 1.136 ± 0.031 | 0.08 |
| Crossover | 1.275 ± 0.027 | 1.283 ± 0.028 | 1.275 ± 0.028 | 0.45 | 1.103 ± 0.029 | 1.104 ± 0.028 | 1.101 ± 0.026 | 0.94 |
| Bone turnover markers | ||||||||
| CTX (ng/mL)5 | ||||||||
| DHEA | 0.338 ± 0.025 | 0.330 ± 0.033 | 0.375 ± 0.030 | 0.09 | 0.430 ± 0.042 | 0.366 ± 0.036 | 0.366 ± 0.032 | 0.04 |
| Crossover | 0.343 ± 0.031 | 0.364 ± 0.050 | 0.363 ± 0.051 | 0.84 | 0.604 ± 0.064 | 0.513 ± 0.056 | 0.432 ± 0.0443 | 0.0004 |
| BAP (U/L) | ||||||||
| DHEA | 18.6 ± 1.3 | 16.8 ± 1.0 | 20.2 ± 1.24 | 0.0006 | 25.1 ± 2.2 | 20.8 ± 1.73 | 22.3 ± 1.83 | 0.002 |
| Crossover | 19.5 ± 1.0 | 18.5 ± 1.0 | 22.0 ± 1.434 | 0.0002 | 29.3 ± 2.6 | 24.8 ± 1.43 | 25.5 ± 1.93 | 0.02 |
All values are arithmetic means ± SEs. CTX, C-terminal telopeptide of type I collagen; BAP, bone-specific alkaline phosphatase; DHEA, dehydroepiandrosterone. All outcomes were assessed for between-group differences in change over time (ie, group × time interaction) by using a 2-factor repeated-measures ANOVA; however, none of the interactions was significant.
P values reflect the significance of change over time (within group and sex) by one-factor repeated-measures ANOVA.
Significantly different from baseline (within group and sex), P ≤ 0.05 (Tukey test).
Significantly different from 12 mo (within group and sex), P ≤ 0.05 (Tukey test).
To convert to nmol/L (SI units), multiply values by 7.750.
FIGURE 3.
Arithmetic mean (±SE) percentage changes in lumbar spine bone mineral density (BMD) in response to 2 y of dehydroepiandrosterone (DHEA) replacement therapy (•: n = 21 men and 21 women) or 1 y of placebo followed by 1 y of DHEA replacement therapy (○; n = 24 men and 24 women). *P ≤ 0.05 compared with baseline and †P ≤ 0.05 compared with 12 mo (within group and sex) by one-factor repeated-measures ANOVA and Tukey-adjusted post hoc comparisons.
Bone turnover markers
Serum CTX concentrations did not change during the 2-y study in men in either group (Table 4). The change from baseline to year 1 in serum BAP concentrations was not significant in either group of men who participated in the extension study (DHEA group: −7 ± 3%, P = 0.07; crossover group, −4 ± 2%, P = 0.38). However, BAP concentrations increased by 22 ± 5% in the DHEA group and by 19 ± 4% in the crossover group (P < 0.0001 for both) during year 2. Two-year BAP concentrations were significantly greater than baseline in men in the crossover group (+13 ± 4%; P = 0.008) but not in the DHEA group (+11 ± 4%; P = 0.15).
CTX concentrations decreased significantly in women who underwent 2 y of DHEA supplementation (Table 4), although the post hoc comparisons showed no statistically significant differences (P = 0.07 for baseline compared with year 1 and for baseline compared with year 2). Likewise, CTX decreased significantly in women in the crossover group with a trend (P = 0.06) for lower CTX concentrations at year 1 and significantly (P = 0.0002) lower CTX concentrations at year 2. Serum BAP concentrations decreased by 12 ± 4% during year 1 in women in the DHEA group and remained low (−8 ± 4% compared with baseline) during the second year of DHEA supplementation (Table 4). Similarly, for women in the crossover group, serum BAP decreased by 7 ± 5% during year 1 and remained 9 ± 5% below baseline during year 2.
Safety
Twelve serious adverse events occurred during the study. All were reported to the Washington University Human Research Protection Office and the study's Data Safety Monitoring Board. The serious adverse events in the DHEA group were atrial fibrillation with a prior history of this condition (women) in year 1 and elevated liver enzymes (men), non-Hodgkin's lymphoma (men), chest pain (men), and atrial fibrillation with no prior history (men) in year 2. The serious adverse events in the placebo group were prostate cancer (men), transient ischemic attack (women), swollen tongue with other allergy symptoms (men), pancreatitis (women), and atrial fibrillation with prior history (men) in year 1 and cerebrovascular accident (women) and sudden death (men) in year 2 after crossover to DHEA supplementation.
Serum PSA concentrations in men did not change during the 1-y RCT in the DHEA group (baseline: 1.11 ± 0.13 ng/mL; 1 y: 1.06 ± 0.12 ng/mL; P = 0.52) or in the placebo group (baseline: 1.01 ± 0.14 ng/mL; 1 y: 1.03 ± 0.13 ng/mL; P = 0.86). Likewise, PSA concentrations did not change during the 2-y extension study in the DHEA group (baseline: 1.09 ± 0.15 ng/mL; 1 y: 1.04 ± 0.14 ng/mL; 2 y: 1.06 ± 0.13 ng/mL; P = 0.75) or in the crossover group (baseline: 1.05 ± 0.15 ng/mL; 1 y: 1.07 ± 0.14 ng/mL; 2 y: 1.10 ± 0.16 ng/mL; P = 0.71). On the basis of mammograms and pap smears performed in women at baseline, 1 y, and 2 y, no breast cancer or cervical abnormalities were observed.
During monthly visits to the laboratory in year 1, participants in the DHEA and placebo groups reported 124 minor side effects, the most common of which were symptoms of the common cold (10 reports) or influenza (9 reports), gastrointestinal distress (16 reports), and musculoskeletal stiffness (24 reports). Neither the more common side effects nor rare side effects (including acne and facial hair growth) differed in frequency between the DHEA and placebo groups (P = 0.19–1.0). We also evaluated body weight and circulating lipid concentrations (total, HDL and LDL cholesterol, and triglycerides) to determine potential adverse effects of DHEA. The only adverse effect observed was a decrease in HDL cholesterol of 4.5 mg/dL in women in the DHEA group (P = 0.002 compared with placebo group); however, it is noteworthy that the ratio of total cholesterol to HDL cholesterol was not adversely affected.
DISCUSSION
The results of the present study showed that 50 mg/d of oral DHEA, when co-administered with vitamin D and calcium supplements, caused large and clinically important improvements in lumbar spine BMD in older women. Spine BMD increased by ≈2% during each of the 2 y of DHEA supplementation for a total of an increase of ≈4% from baseline. Similar increases in spine BMD induced by pharmacotherapy are associated with a 30–50% reduction in vertebral fracture risk (17). Furthermore, this 2-y change is similar to or larger than that which results from 2 y of oral estrogen (+2–5%) (18–20), bisphosphonates (+4%) (21–23), and selective estrogen receptor modulators (+1–2%) (24, 25). The robustness of these findings is supported by the increase of ≈2% in spine BMD that occurred when women in the placebo group crossed over to DHEA supplementation in year 2.
The significant improvements in spine BMD in women were accompanied by increases in IGF-1, testosterone, and estrogen. Because these are bone active hormones (26–28), it is possible that some or all of these increases might have mediated the improvements in spine BMD. Furthermore, circulating DHEA might have had a direct effect on bone through a yet-to-be identified DHEA receptor (29) or by conversion to androgens or estrogens within bone cells (ie, an intracrine system) (30). We hoped that measuring bone turnover markers would provide insights about the mechanism for improvements in BMD, ie, whether IGF-1 or testosterone increased bone formation (31, 32) or estrogen suppressed bone resorption (27). However, because the changes in markers of bone turnover were not different between the DHEA and placebo groups, the mechanism for the BMD changes is unclear.
No effect of DHEA supplementation on BMD in men was evident in our study. Although spine BMD increased by 1–2%, it did so in both the DHEA and placebo groups, which suggests that the effect might have been mediated by vitamin D and calcium supplementation, which was provided to all participants. Interestingly, Jankowski et al (7), who provided vitamin D and calcium supplements to participants with apparent deficiencies, reported similar results, ie, a tendency for BMD to increase in men in the placebo and DHEA groups.
Hip BMD did not change in either men or women in response to DHEA supplementation. Greater adaptive responses in the spine than in the hip have also been reported in response to estrogen replacement therapy (33) and exercise training (34); however, the reason for this is not clear. A possible explanation is that the spine contains more trabecular bone, which has a greater rate of turnover than does cortical bone (35), which makes it more responsive to therapeutic interventions. However, this explanation should be interpreted with caution, because few data are available on the proportion of trabecular bone in vertebrae (36). Furthermore, because anteroposterior DXA scans of the spine include the cortical posterior vertebral elements (eg, spinous processes), anteroposterior spine BMD contains a substantial proportion of cortical bone.
Other studies have assessed the effect of DHEA replacement therapy on BMD in older men and women. Although shorter-term trials have yielded mixed results (14, 37), longer-term trials have reported beneficial effects of DHEA supplementation on bone, with more consistent benefits being shown for women. In women, but not men, in the DHEAge study, hip and wrist BMD were preserved or increased slightly during 12 mo of DHEA supplementation, whereas BMD decreased in women in the placebo group (spine BMD was not reported) (9). The DAWN trial showed that spine BMD was preserved (+0.3%) in women undergoing 12 mo of DHEA supplementation, whereas those in the placebo group lost 1.8% of spine BMD (8); no beneficial effect was seen at other skeletal sites or in men. Jankowski et al (7) found that hip and spine BMD increased by ≈2% in women undergoing 12 mo of DHEA supplementation, whereas BMD did not change in men. In a study of women only, Labrie et al (38) reported a 1.9% increase in hip BMD with transdermal DHEA therapy. In the only 2-y DHEA supplementation study performed before the present study, Nair et al (10) reported an increase of ≈3% in wrist BMD in women and of ≈1% in femoral neck BMD in men, but no changes at other skeletal sites.
It is conceivable that inadequate dietary intakes of vitamin D and calcium, as is common in older adults (12, 13), may attenuate the beneficial effects of DHEA supplementation on BMD because these nutrients are important for optimal bone health (11). Indeed, the present study and the study by Jankowski et al (7) showed larger 1-y increases (≈2%) in BMD than did other trials, and these were the only 2 trials that administered vitamin D and calcium supplements. Moreover, after 2 y of DHEA supplementation in the present study, spine BMD increased by nearly 4% in women, whereas Nair et al (10), who did not provide vitamin D and calcium supplements, reported no change in spine BMD after 2 y of DHEA. Although no studies have directly compared the BMD-enhancing effects of DHEA supplementation in individuals with and without vitamin D or calcium deficiencies, it is noteworthy that vitamin D deficiency attenuates the beneficial effects of bisphosphonates on BMD (39).
No adverse effects of DHEA supplementation were evident in the present study; however, our trial was not powered to detect rare or subtle side effects. It should also be noted that because DHEA supplementation resulted in small but significant increases in circulating concentrations of estrogen, testosterone, and IGF-1—all of which may promote tumorigenesis—individuals taking DHEA supplements long term may need to be monitored regularly for hormone-sensitive cancer.
In conclusion, the results of the present study suggest that long-term (1 and 2 y) DHEA supplementation (50 mg/d) in older women has a substantial beneficial effect on spine BMD in combination with dietary vitamin D and calcium supplementation. In light of the possibility that DHEA replacement therapy has other physiologic benefits, such as improvements in glucoregulatory function, immunoregulation, and psychological state (see reference 40 for review), and has no known major side effects, DHEA supplementation may be an attractive option for improving or preserving bone health in older women. In contrast with our findings in women, we saw no evidence of a beneficial effect of DHEA supplementation in men above and beyond the effect of vitamin D and calcium supplementation.
Acknowledgments
The authors' responsibilities were as follows: LF, JOH, and DTV: conception and design; EPW, CPL, JOH, and DTV: data acquisition; EPW, KS, LF, JOH, and DTV: analysis and interpretation of data; EPW: drafting of the manuscript; KS, LF, CPL, JOH, and DTV: critical revision of the manuscript; EPW: statistical analysis; LF, JOH, and DTV: obtaining funding; EPW, KS, and CPL: technical support;: and EPW, CPL, JOH, DTV: supervision;. EPW had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors had any conflict of interest to disclose.
REFERENCES
- 1.Arlt W. Dehydroepiandrosterone and ageing. Best Pract Res Clin Endocrinol Metab 2004;18:363–80 [DOI] [PubMed] [Google Scholar]
- 2.Szathmari M, Szucs J, Feher T, Hollo I. Dehydroepiandrosterone sulphate and bone mineral density. Osteoporos Int 1994;4:84–8 [DOI] [PubMed] [Google Scholar]
- 3.Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 1997;12:1833–43 [DOI] [PubMed] [Google Scholar]
- 4.Haden ST, Glowacki J, Hurwitz S, Rosen C, LeBoff MS. Effects of age on serum dehydroepiandrosterone sulfate, IGF-I, and IL-6 levels in women. Calcif Tissue Int 2000;66:414–8 [DOI] [PubMed] [Google Scholar]
- 5.Clarke BL, Ebeling PR, Jones JD, et al. Predictors of bone mineral density in aging healthy men varies by skeletal site. Calcif Tissue Int 2002;70:137–45 [DOI] [PubMed] [Google Scholar]
- 6.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 2000;15:1526–36 [DOI] [PubMed] [Google Scholar]
- 7.Jankowski CM, Gozansky WS, Schwartz RS, et al. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab 2006;91:2986–93 [DOI] [PubMed] [Google Scholar]
- 8.von Mühlen D, Laughlin GA, Kritz-Silverstein D, Bergstrom J, Bettencourt R. Effect of dehydroepiandrosterone supplementation on bone mineral density, bone markers, and body composition in older adults: the DAWN trial. Osteoporos Int 2008;19:699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baulieu EE, Thomas G, Legrain S, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci USA 2000;97:4279–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 2006;355:1647–59 [DOI] [PubMed] [Google Scholar]
- 11.Gennari C. Calcium and vitamin D nutrition and bone disease of the elderly. Public Health Nutr 2001;4:547–59 [DOI] [PubMed] [Google Scholar]
- 12.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001;22:477–501 [DOI] [PubMed] [Google Scholar]
- 13.Ervin RB, Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third National Health and Nutrition Examination Survey. J Nutr 2002;132:3422–7 [DOI] [PubMed] [Google Scholar]
- 14.Villareal DT, Holloszy JO, Kohrt WM. Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol (Oxf) 2000;53:561–8 [DOI] [PubMed] [Google Scholar]
- 15.Food and Nutrition Board Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: The National Academies Press, 1997 [PubMed] [Google Scholar]
- 16.Napoli N, Villareal DT, Mumm S, et al. Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res 2005;20:232–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cefalu CA. Is bone mineral density predictive of fracture risk reduction? Curr Med Res Opin 2004;20:341–9 [DOI] [PubMed] [Google Scholar]
- 18.Villareal DT, Binder EF, Williams DB, Schechtman KB, Yarasheski KE, Kohrt WM. Bone mineral density response to estrogen replacement in frail elderly women: a randomized controlled trial. JAMA 2001;286:815–20 [DOI] [PubMed] [Google Scholar]
- 19.The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1996;275:370–5 [DOI] [PubMed] [Google Scholar]
- 20.Gallagher JC, Kable WT, Goldgar D. Effect of progestin therapy on cortical and trabecular bone: comparison with estrogen. Am J Med 1991;90:171–8 [PubMed] [Google Scholar]
- 21.Hosking D, Chilvers CE, Christiansen C, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med 1998;338:485–92 [DOI] [PubMed] [Google Scholar]
- 22.Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab 2000;85:1895–900 [DOI] [PubMed] [Google Scholar]
- 23.Välimäki MJ, Farrerons-Minguella J, Halse J, et al. Effects of risedronate 5 mg/d on bone mineral density and bone turnover markers in late-postmenopausal women with osteopenia: a multinational, 24-month, randomized, double-blind, placebo-controlled, parallel-group, phase III trial. Clin Ther 2007;29:1937–49 [DOI] [PubMed] [Google Scholar]
- 24.Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 1997;337:1641–7 [DOI] [PubMed] [Google Scholar]
- 25.Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res 2006;21:1215–23 [DOI] [PubMed] [Google Scholar]
- 26.Tracz MJ, Sideras K, Bolona ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab 2006;91:2011–6 [DOI] [PubMed] [Google Scholar]
- 27.Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA 2003;290:1042–8 [DOI] [PubMed] [Google Scholar]
- 28.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev 2008;29:535–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Wang YD, Wang WJ, Zhu Y, Li DJ. Dehydroepiandrosterone improves murine osteoblast growth and bone tissue morphometry via mitogen-activated protein kinase signaling pathway independent of either androgen receptor or estrogen receptor. J Mol Endocrinol 2007;38:467–79 [DOI] [PubMed] [Google Scholar]
- 30.Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol 2001;22:185–212 [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Eyre DR, Clark R, et al. Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men–a clinical research center study. J Clin Endocrinol Metab 1996;81:3654–62 [DOI] [PubMed] [Google Scholar]
- 32.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev 2008;29:535–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan Y, Tabensky A, DeLuca V, Seeman E. The benefit of hormone replacement therapy on bone mass is greater at the vertebral body than posterior processes or proximal femur. Bone 1997;21:447–51 [DOI] [PubMed] [Google Scholar]
- 34.Villareal DT, Binder EF, Yarasheski KE, et al. Effects of exercise training added to ongoing hormone replacement therapy on bone mineral density in frail elderly women. J Am Geriatr Soc 2003;51:985–90 [DOI] [PubMed] [Google Scholar]
- 35.Seeman E, Wahner HW, Offord KP, Kumar R, Johnson WJ, Riggs BL. Differential effects of endocrine dysfunction on the axial and the appendicular skeleton. J Clin Invest 1982;69:1302–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nottestad SY, Baumel JJ, Kimmel DB, Recker RR, Heaney RP. The proportion of trabecular bone in human vertebrae. J Bone Miner Res 1987;2:221–9 [DOI] [PubMed] [Google Scholar]
- 37.Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf) 1998;49:421–32 [DOI] [PubMed] [Google Scholar]
- 38.Labrie F, Diamond P, Cusan L, Gomez JL, Belanger A, Candas B. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab 1997;82:3498–505 [DOI] [PubMed] [Google Scholar]
- 39.Koster JC, Hackeng WH, Mulder H. Diminished effect of etidronate in vitamin D deficient osteopenic postmenopausal women. Eur J Clin Pharmacol 1996;51:145–7 [DOI] [PubMed] [Google Scholar]
- 40.Genazzani AD, Lanzoni C, Genazzani AR. Might DHEA be considered a beneficial replacement therapy in the elderly? Drugs Aging 2007;24:173–85 [DOI] [PubMed] [Google Scholar]