Abstract
This brief review focuses on the genetic contribution to childhood obesity. Evidence for a genetic component to excess body weight during growth is presented from the perspective of genetic epidemiology studies. Parental obesity is a predictor of childhood excess weight. The familial risk ratio for childhood obesity when a parent is obese reaches >2.5. Birth weight is characterized by a genetic heritability component on the order of 30%, with significant maternal and paternal effects in addition to the newborn genes. About 5% of childhood obesity cases are caused by a defect that impairs function in a gene, and ≥5 of these genes have been uncovered. However, the common forms of childhood obesity seem to result from a predisposition that primarily favors obesogenic behaviors in an obesogenic environment. Candidate gene and genomewide association studies reveal that these obesogenic genes have small effect sizes but that the risk alleles for obesity are quite common in populations. The latter may translate into a highly significant population-attributable risk of obesity. Gene-environment interaction studies suggest that the effects of predisposing genes can be enhanced or diminished by exposure to relevant behaviors. It is possible that the prevalence of childhood obesity is increasing across generations as a result of positive assortative mating with obese husbands and wives contributing more obese offspring than normal-weight parents.
INTRODUCTION
The prevalence of childhood obesity is on the rise around the world. The magnitude of the problem was carefully documented in a special issue of Obesity Reviews, which was devoted entirely to the topic (1). The most recent estimates from the World Health Organization and the International Obesity Task Force of the International Association for the Study of Obesity are that there are >155 million children and adolescents around the world who are overweight, with ≈40 million who are clearly obese (2, 3). A further 22 million children <5 y of age are also showing an excess weight problem according to the same sources. The situation is worst in the United States where a 3-fold increase in the prevalence of childhood and adolescent overweight and obesity cases has been observed over just a few decades. At present, >30% of children 2–19 y of age are classified as overweight or obese with a BMI (in kg/m2) higher than the 85th percentile of the Centers for Disease Control and Prevention growth charts for the year 2000 (4, 5).
Excess weight during the growing years is of great importance for 3 main reasons. First, it constitutes a major risk of overweight and obesity during adult life. Second, childhood obesity correlates with adulthood risk factors for common chronic diseases and with diabetes, cardiovascular disease, and other morbidities. For example, in a recent Danish study in 276,835 schoolchildren, 7–13 y of age, who were monitored after 25 y of age (a total of 5,063,622 person-years of follow-up), the risk of coronary heart disease in adulthood increased in a linear manner with BMI at 7–13 y in boys and at 10–13 y in girls (6). Carotid intima media thickness measured by ultrasound at a mean age of 36 y was associated not only with adulthood BMI but also with childhood BMI in the Bogalusa Heart Study (7). Third, because obesity is a very strong correlate of excess abdominal visceral fat concentration (8) and a low-to-moderate correlate of skeletal muscle and hepatic lipid deposition (9, 10), childhood obesity is likely to set the stage for visceral obesity and, to some extent, for ectopic fat deposition later in life. Dysregulation of fatty acid metabolism is a key feature of type 2 diabetes, and both are more commonly observed in obese individuals than in normal-weight people (11). Although this issue has not been well studied in children, the potential negative consequences of excess adiposity during childhood on later visceral fat concentration and on skeletal muscle and liver ectopic triglyceride deposition warrant a close examination of the determinants of childhood obesity, including genetic factors.
In this article, we will discuss the evidence for the presence of a genetic component to childhood obesity, the potential contributions of genetic differences to variation in birth weight, single genes and genes with small effects contributing to childhood obesity, the evidence for gene-environment interaction effects, and the effect of assortative mating on the magnitude of the problem.
GENETICS AND CHILDHOOD OBESITY
The evidence for a genetic contribution to adult body weight and obesity as derived from genetic epidemiology models has been reviewed in detail elsewhere (12). Briefly, genetic heritability estimates are based on comparisons of pairs of identical and fraternal twins who are generally raised together but in some studies raised apart, comparisons of nuclear family members from 2 generations, and comparisons of adopted offspring raised by foster parents but occasionally with information also on their biological parents. Interestingly, the magnitude of the heritability estimates varies quite substantially among these various designs, with the twin models generating the highest values and the adoption studies the lowest coefficients. Another line of evidence for the presence of a genetic component in adult weight or BMI comes from the computation of the λ coefficient or familial risk ratio (13). Lee et al (14) have computed such a ratio for various levels of BMI in first-degree relatives of probands in the World Health Organization classes of obesity. The familial risk was shown to increase with the severity of obesity, reaching ≥5 with BMIs ≥40. This suggests that an adult with a father, mother, brother, or sister with a BMI of ≈40 is ≈5 times more at risk of becoming obese at the same severity compared with individuals in the population who have only normal-weight first-degree relatives. The first application to obesity of the approach proposed by Risch (13) was by Allison et al (15). In their study based on several data sets, the mother-child and father-child subsamples from the second National Health and Nutrition Examination Survey are of direct relevance to the issue addressed here. When the 85th percentile was used to define obesity, the familial risk level reached ≈1.5 whereas the coefficient reached ≥2.6 if obesity was defined as ≥95th percentile (Table 1).
TABLE 1.
Empirical λ values and 95% CIs for obesity from the second National Health and Nutrition Examination Survey1
| Percentile cutoff for defining obesity | Mothers and children (n = 4427 pairs) | Fathers and children (n = 4351 pairs) |
| ≥50 | 1.07 (1.04, 1.10) | 1.10 (1.07, 1.15) |
| ≥85 | 1.51 (1.34, 1.74) | 1.69 (1.50, 1.91) |
| ≥95 | 2.61 (2.18, 4.11) | 2.80 (2.30, 4.25) |
This table was modified from reference 15.
The key question here is whether there is also a genetic component to the individual differences observed in body weight or adiposity during childhood. This has been addressed in a number of reports that we will not be able to review exhaustively in this article. One recent article based on 501 white infants, birth to 36 mo, from 164 nuclear and extended families of the Fels Longitudinal Study with adulthood BMI and body-composition data showed that the heritability for body weight increased from 0.65 at 12 mo to 0.88 at 24 mo and to 0.95 at 36 mo (16). Another recent article based on 672 twin pairs reported that most of the variance in weight at 5 mo and at 5 y was accounted for by genetic factors, with heritability estimates in the range of 84–88% (17). The previous report and another one based on >5000 twin pairs, with a mean age of 10 y (18), indicate that the heritability estimates based on more recent data collections remain high and are not different from those published many years ago.
These results and many other similar ones are not easily interpreted in light of other types of studies. For example, the strong genetic effects cited above suggest that body weight, BMI, and total adiposity should be very stable over time, implying that lean infants should remain lean and become lean adults, whereas overweight infants should likewise exhibit body-weight stability with age. However, the data available on populations that were monitored from the growing years to adulthood are only partially supportive of this concept (19–21). To illustrate the point, a sample of males and females from the Harvard Longitudinal Studies of Child Health and Development was monitored for ≈50 y (19). Tracking of BMI from childhood to middle age was unimpressive. Thus BMI at 5–7 y of age accounted for ≈17% of the variance in the BMI at 50 y in the same men but for none of the variance in the same women. Tracking across age, however, is more substantial when the extreme cases of fatness or of leanness during childhood are considered (21, 22).
If the genetic component of childhood obesity is as strong as that suggested by current studies, one would expect that the risk of adulthood obesity would be much higher in those who are already overweight or obese during the growing years. Indeed, the data tend to globally support this generalization. A review of 8 prospective studies concluded that almost one-third of obese children became obese adults and that the risk of obesity as an adult was about twice as high for obese children compared with those who had a normal weight during their growing years (23). In the Fels Longitudinal Study, 555 subjects born between 1929 and 1960 with BMI data available from 1 to 18 y and again at 35 y, the odds ratio for being obese (95th percentile) at 35 y was 2 for those who were obese during infancy, varied from 3 to 10 during childhood, and ranged from 5 to 20 for those who were obese during adolescence (24).
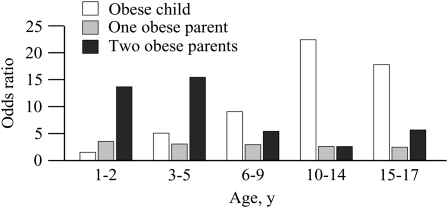
Much can be learned from studies in which the risk of adulthood obesity is evaluated on the basis of body-weight status during childhood, taking into account parental weight. One such study was reported a decade ago (25). Childhood obesity was defined as a BMI ≥85th percentile of appropriate age- and sex-defined distributions, whereas a cutoff BMI of 27.8 for men and of 27.3 for women was used for adult obesity. Odds ratios for adulthood obesity at various age periods during growth according to the obesity status of the parents are shown in Figure 1. Data show that up to ≈5 y of age having 2 obese parents constitutes a much greater risk (≈10-fold) for later obesity than being obese as a child (odds ratio ≤5). However, with increasing age, the weight status of the child becomes a strong predictor of adulthood obesity regardless of parental obesity. There was almost a 20-fold increase in risk when the child was obese after 20 y of age. These results suggest that parental weight status, a surrogate for genetic characteristics in the present design, is particularly informative in the presence of early-onset childhood obesity for the risk of adulthood obesity.
FIGURE 1.
The risk of adult obesity based on the obesity status in childhood and on parental obesity. This figure was constructed from data in reference 25.
GENETICS AND BIRTH WEIGHT
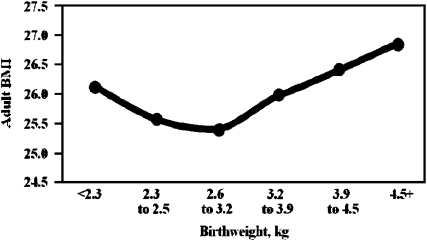
Birth weight is important to consider in any discussion of the role of genetic differences in childhood obesity and even adulthood body-weight regulation. This is well exemplified by the data depicted in Figure 2. Lobstein et al (1) used the results of earlier studies to illustrate the relation between birth weight and age-adjusted BMI (26, 27). The U-shaped relation indicates that a higher prevalence of obesity will eventually be seen among those born with a very low birth weight as well as among those with a birth weight at the opposite end of the distribution. This relation persists when only full-term infants are considered or when data are adjusted for gestational age.
FIGURE 2.
Birth weight and age-adjusted adult BMI (in kg/m2). The figure was drawn from data in references 26 and 27.
The topic of the heritability of birth weight has been addressed for >50 y in the scientific literature. The evidence up to the late 1970s was reviewed by Robson in the 3-volume treatise, Human Growth (28). Her conclusion, which was based on ≈30 y of publications on the topic, was that the fetal genotype played a small role in determining birth weight, probably on the order of 10%, whereas the maternal genotype accounted for ≈25% of the total variance. These estimates were derived from data on full siblings, half-siblings, first cousins, mother-child, father-child, and monozygotic (MZ) and dizygotic twins. Many more studies have been reported on the sources of variance in birth weight since Robson's landmark review. In brief, twin studies have consistently generated significant genetic components for birth weight in the range of 20–40% (17, 29, 30). Interestingly, and in contrast to what one would expect from a score of publications on other traits, several nuclear family studies have generated higher heritability estimates of birth weight than twin studies. For example, in a recent report based on 501 white newborns from 164 families of the Fels Longitudinal Study, heritability estimates for covariate-adjusted birth weight reached 0.81 (16). The coefficient decreased to 0.61 for weight at 1 mo of age. Such high estimates for the genetic component of birth weight have also been reported in prior studies of Norwegian and Mexican American newborns (31–33). However, a more recent report from Norway—in which birth weight was obtained for the mother, father, and up to 3 single births—included data from 101,748 families (34). It concluded that the fetal genetic component of birth weight adjusted for birth order, sex, and generation reached 31% (95% CI: 29.9, 32.0). The heritability estimates also reached 31% for birth length and 11% for variation in gestational age. Given the ample statistical power of the latter study, we believe that 31% is likely to represent the most valid and reliable heritability estimate of the contribution of the fetal genes to birth weight (35). The latter is concordant with the 25% value reported in another large Norwegian study of trios of mother–father–firstborn child (36).
However, it should be appreciated that variation in birth weight is influenced by a number of other factors besides the genes of the newborn. Maternal genes, maternal in utero and placental factors, maternal prepregnancy weight or BMI, maternal birth weight, maternal and paternal smoking, maternal alcohol intake and drug use, exercise during pregnancy, paternal birth weight, paternal genes, and other environmental factors can contribute to weight at birth. Several studies have found a role for the maternal genotype in predicting the weight of the newborn. In the large Norwegian study cited above, maternal genetic factors accounted for 22% of the variation in birth weight (34). Of particular interest is whether there is a paternal genetic component to birth weight. The topic has been controversial for some time.
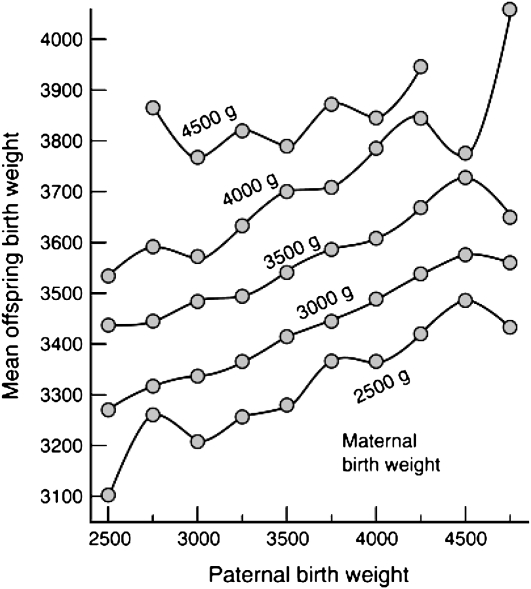
In a study of 6811 white singletons and their natural parents, the effect of parental height and weight on the length and weight at birth was evaluated (37). It was observed that the effects of parental height and weight on birth weight are similar for both parents. However, the influence of the mother's weight on the baby's birth weight was stronger than that of the father. In a report on the role of parental small for gestational age in the familial aggregation of small for gestational age based on 256 cases, it was shown that both parents contributed almost equally to the risk (38). The risk of a small-for-gestational-age baby at birth was 4.7 times greater for mothers and 3.5 times greater for fathers who were themselves small for gestational age compared with those who were average for gestational age. The risk of a small-for-gestational-age baby, however, was 16 times higher when both parents were small for gestational age. The most compelling data for a role for paternal birth weight in influencing the offspring weight at birth come once again from a Norwegian study. A total of 67,795 trios of father–mother–firstborn child was used to plot the birth weight of babies against paternal birth weight by classes of maternal birth weight (36). The results are depicted in Figure 3. The regression of a child's birth weight on the father's birth weight was 0.137, whereas that on the mother's birth weight reached 0.252. As shown in Figure 3, the effect of paternal birth weight is about the same within each category of maternal birth weight, with no significant interaction effects between parental birth-weight levels. Whereas the data were not adjusted for prematurity or gestational age, the authors emphasized that such adjustments could have confounded their data, as gestational age is as much an outcome of pregnancy as birth weight.
FIGURE 3.
Mean offspring birth weight (g) for categories of paternal birth weight in selected strata of maternal birth weight. Families in which the parental birth weight was <2500 g are omitted from the figure. Birth weight was not adjusted for gestational age or prematurity. Reproduced with permission from reference 36.
Evidence for a role for specific genes in determining birth weight is extremely limited. One report has focused on the effect of the A allele [single nucleotide polymorphism (SNP) at −30] at the glucokinase (GCK) gene on birth weight (39). By using data from 2689 mother-child pairs, the A allele in the mother was associated with a 64-g increase in the offspring birth weight. There was no effect of the offspring GCK genotype on birth weight. In another candidate gene study, the effect of a common SNP in the FTO gene was investigated for its relation to weight at birth in 234 full-term, healthy newborns (40). The allelic variant was not associated with birth weight.
Three articles have dealt with genomewide linkages with panels of highly polymorphic markers and birth weight. The first was based on 269 Pima Indians from 92 families and 503 autosomal microsatellite markers. A quantitative trait locus was identified on chromosome 11 [logarithm of odds (LOD) for imprinted locus = 3.4], which suggests that a paternally imprinted gene at map position 88 cM was influencing birth weight in this population (41). Subsequently, a quantitative trait locus on chromosome 6q was shown to be linked to birth weight in Mexican Americans from the San Antonio Family Birth Weight Study (LOD = 3.7) and partially replicated in a European American population (LOD = 2.3) (30). The latest study using this approach also involved Hispanic newborns from Texas (32). Birth weight was available from birth certificates for 629 children from 319 families. Birth weight was highly heritable in this population, and a quantitative trait locus was identified on 10q22 with an LOD score of 2.6.
CONTRIBUTIONS OF SPECIFIC GENES
The shift from quantitative genetic studies of childhood obesity to a more gene- and sequence-variant-focused approach has been characterized by a number of conceptual and technological advances. Some complex segregation analysis studies, but not all, supported the hypothesis that there were one or a few major segregating genes with large effects on body weight and adiposity (12). However, other studies favored a model with oligogenic and polygenic determinants. A large series of candidate gene reports was published, which provided suggestive evidence that many genes were involved. It turned out over time that contradictory evidence was also reported for all genes with positive findings (42). Moreover, it became obvious that even the genes supported by multiple studies had only minor effects and were typically supported by grossly underpowered studies. Technological advances made it possible to scan the whole genome by using panels of highly polymorphic microsatellite markers. Genomewide linkage scans yielded a large number of quantitative trait loci for obesity and related traits, but the individual reports had poor sensitivity and specificity with respect to confirming known obesity genes (43). In this regard, a recent meta-analysis of 37 genome-scan studies with data on 31,000 individuals from >10,000 families could not implicate unequivocally a single genetic locus for obesity or BMI (44). The main lesson from these studies appears to be that genomewide scans, as executed over the past 15 y or so, are not well suited to identifying genes with small effects but may be more appropriate for oligogenes. With recent developments in sequencing techniques, bioinformatics, SNP identification, SNP genotyping technologies, and the progress made in the HapMap project (45), the stage was set for the implementation of a more general approach for the identification of genes with small effects on complex quantitative traits or on the disease state. The approach is referred to as “genomewide association studies” (GWASs). It became clear by early 2007 that GWASs were capable of detecting alleles with relatively small effect sizes.
Here we briefly review the current status of the evidence for obesity that is caused by single genes, followed by a review of the recent advances in finding genes with small effects on body mass or fatness as identified by GWASs.
Single-gene defects
Two types of disorders can be recognized. The first type is known as the Mendelian disorders and causes a number of clinical features, with obesity being one of them. About 50 of these syndromes have been identified to date. The Prader-Willi (prevalence of ≈1:25,000) and the Bardet-Biedl (1:160,000 in whites compared with 1:13,500 in the Middle East) syndromes are among the most common and have received the most attention.
A second class of single-gene defects is characterized by disorders in which obesity appears to be a primary clinical feature (46). Five of these genes are of particular importance because together they account for at least 5% of the early onset, severe childhood obesity cases. They are the leptin (LEP), leptin receptor (LEPR), pro-opiomelanocortin (POMC), melanocortin 4 receptor (MC4R), and prohormone convertase 1 (PCSK1) genes. The laboratory of O'Rahilly and Farooqi at the University of Cambridge (Cambridge, United Kingdom) has been the most active in the investigation of these types of genetic defects, which are best detected in early-onset, severe childhood obesity cases (47, 48). Interestingly, all these genes encode peptides and receptors involved in the regulation of appetite and satiety. Pathogenic mutations in MC4R are by far the most common single-gene defect observed among obese children and adults. For example, in a study of 750 obese Danish men with juvenile-onset obesity and 706 normal-weight controls, 2.5% of obesity cases were associated with mutations in MC4R that diminished its function (49).
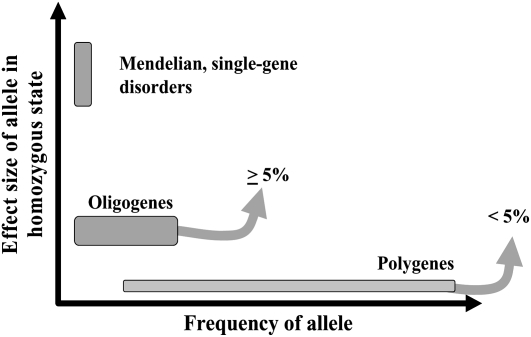
The relation between the frequency of the obesogenic allele at a given gene and its effect size in the homozygous state is depicted in Figure 4. It is well recognized that single-gene disorders are relatively rare compared with obesogenic alleles in genes with small effects. Even though oligogenes have been identified for a number of traits/diseases, they remain less well characterized. In Figure 4, we have arbitrarily set their effect size at 5% in the homozygous state. In the case of obesity, one can argue that oligogenes have not yet been identified unequivocally.
FIGURE 4.
Frequency and effect size of alleles at genes causing obesity or genes that are part of more complex oligogenic or polygenic genotypes associated with a predisposition to obesity.
Genes with small effects
Research on these genes has been based on a number of strategies: candidate gene studies, genomic scans, GWASs, and gene-expression profile difference between normal-weight and obese individuals. Hundreds of candidate gene studies have been reported, and they have generated >20 genes (ACE, ADIPOQ, ADRB2, ADRB3, DRD2, FTO, GNB3, HTR2C, IL6, INS, LDLR, LEP, LEPR, LIPE, MC4R, NR3C1, PPARG, RETN, TNFA, UCP1, UCP2, UCP3, and VDR) with at least 5 positive studies and >12 genes with ≥10 positive reports (50). Many of these genes may turn out to be true contributors to obesity polygenes, but unfortunately the results available thus far come from studies that are seriously underpowered. Genomic scans and gene-expression profiling have been used to identify new candidates; they have indeed generated a few that have been subsequently subjected to more focused investigations. However, in the end, the results for the new candidate genes were affected by the same limitations as the candidate genes identified from our current understanding of the biology of the regulation of energy balance.
GWASs have led over the past 18 mo to exciting new gene discoveries for several common diseases. A few of these reports dealt with obesity and generated one new obesogenic gene plus another locus that is strongly associated with BMI and obesity. Both are succinctly reviewed below.
A number of variants in the fat mass and obesity–associated gene (FTO) showed a very strong association with BMI, obesity, or fat mass in several independent studies (51–56). The relevant SNPs are located in the first intron of the gene (on chromosome 16) and are part of a cluster of ≈40 SNPs that are in strong linkage disequilibrium in a white population (57). On average, homozygotes for the risk allele weigh 3–4 kg more and have a 1.67-fold greater risk of obesity than homozygotes for the nonrisk allele. Each obesogenic allele increases the risk of overweight or obesity by ≈20–30%. Similar risk percentages have been observed in children and adolescents, particularly for total adiposity (53). The risk allele is quite prevalent among whites because ≈16% of them are homozygotes. The risk allele is observed at a much lower frequency in African and Asian populations (57). The population-attributable risk for overweight has been estimated at 13%, whereas that for obesity reaches 20%. It has been shown that FTO encodes for a 2-oxoglutarate–dependent nucleic acid demethylase (58).
In a recent report, common variants located ≈188 kb from the MC4R coding sequence were shown to be associated with fat mass, body weight, and obesity (59). BMI was available in 16,876 persons of European descent in which >359,000 SNPs had been genotyped and had passed quality-control standards. The strongest association signal was with SNP rs17782313 (P = 2.9 × 10−6), which maps 188 kb downstream of MC4R. Replication of the initial observation was sought in 60,352 adults (P = 2.8 × 10−15) and ≈6000 children aged 7–11 y (P = 1.5 × 10−8). In a case control cohort (n = 10,583), the odds for severe childhood obesity reached 1.30 (59). The effect size of each risk allele remains small, amounting to 0.05 BMI z score units in adults and 0.13 units in children. However, the risk allele is not rare with a frequency of ≈20% in white populations.
These genomewide association studies have taught us a number of key lessons. First, the sample size initially required to show an association is much larger than originally predicted. Second, replication is critical and needs to be based on multiple independent samples of substantial sizes. Third, very large panels of SNPs are needed to provide coverage of most of the existing genetic variants. It is expected that a panel of ≈1 million SNPs will allow for capturing ≈80% of human DNA sequence variants. Advances in the HapMap project will lead to further refinement of these estimates and to a population-specific panel of markers designed to maximize the coverage of DNA differences (45). Fourth, GWAS reports have revealed that genes contributing to complex traits tend to be characterized by small effect sizes. Fifth, the first GWAS reports focusing on obesity traits have suggested that there are several other signals to pursue beyond those associated with FTO and rs17782313 on chromosome 18.
GENE-ENVIRONMENT INTERACTIONS
“Gene-environment interaction” refers to a situation in which the response or the adaptation to an environmental agent, a behavior, or a change in behavior is conditional on the genotype of the individual. Of particular interest for our understanding of the etiology of human obesity, including childhood obesity, is the role played by genotype-nutrition and genotype-physical activity interactions. Evidence for the presence of such interaction effects that influence body mass and body composition comes mainly from experimental studies undertaken with pairs of MZ twins. These studies reveal that there are large individual differences in the responsiveness to well-defined energy balance manipulations. Overfeeding as well as negative energy balance protocols indicate that the response to these standardized experimental treatments is strongly influenced by one's genetic background. The genes that are responsible for the individual differences in the sensitivity to alterations in energy balance have not been fully identified. They are likely to be numerous, considering the complexity of the biological systems that are involved in body-weight regulation.
It is generally recognized that some individuals are prone to excessive accumulation of fat, for whom losing weight represents a continuous battle, whereas others enjoy some protection. We investigated whether such differences are inherited. In other words, we asked whether there are differences in the sensitivity of individuals to gaining fat when chronically exposed to positive energy balance and whether such differences are dependent or independent of the genotype.
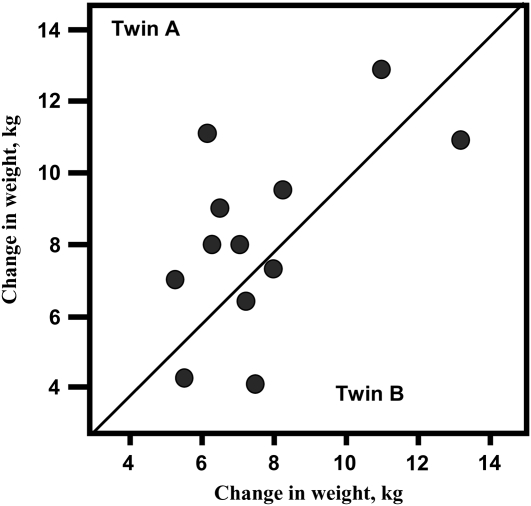
Twelve pairs of male MZ twins ate a 1000-kcal/d caloric surplus, 6 d/wk, during a period of 100 d (60). Significant increases in body weight and fat mass were observed after the period of overfeeding. Data showed that there were considerable interindividual differences in the adaptation to excess calories and that the variation observed was not randomly distributed, as indicated by the significant within-pair resemblance in the response. For example, there was ≥3 times more variance in response between pairs than within pairs for the gains in body weight (Figure 5). These data show that some individuals are more at risk than others to gain fat when energy intake surplus is clamped at the same level for everyone and when all subjects are confined to a sedentary lifestyle. The within-MZ-twin-pair response to the standardized caloric surplus suggests that the amount of fat stored is likely influenced by the genotype.
FIGURE 5.
Body-weight changes in monozygotic twins in response to overfeeding. The F ratio of the between-twins variance in weight gain to the within-pair variance is 3.4 (P < 0.02). Intraclass correlation for the changes is 0.55. Reproduced with permission from reference 60.
Two other experiments tested whether there was a significant within-MZ-twin-pair resemblance in response to a standardized negative energy balance protocol (61, 62). It was concluded from these studies that even though there were large individual differences in response to a negative energy balance protocol, subjects with the same genotype were more alike in responses than subjects with different genotypes.
For the high gainers in our experimental overfeeding studies or for the low losers in the 2 negative energy balance experiments reviewed here, the genotype seems to contribute in no minor way to the continuous battle that the high gainers and low losers must wage to maintain body mass within acceptable limits. It is remarkable that such genotypes were observed in the overfeeding studies reported here despite the fact that the MZ twin pairs were not themselves at risk of obesity or obesity-related complications on the basis of their own weight status (they were quite lean) and their family history (no obesity in the families). The comparison of the between-pairs and within-pairs heterogeneity reveals that members of the same twin pair are generally more alike in their response to variation in energy balance than people who are not related by descent, which supports the notion that genotype–energy balance interaction effects are quite common.
Few genes have been directly implicated in well-defined gene-interaction examples that are relevant to childhood obesity. Candidates for gene-overfeeding interaction effects have been reviewed elsewhere (63). More recently, interactions between the FTO genotype and physical activity level have been reported in 2 independent studies. In both cases, it is only the sedentary homozygotes for the risk allele individuals who are heavier than the other 2 genotypes. The homozygotes for the risk allele are normal weight if they are physically active (51, 64).
The genes that are responsible for those individual differences in the sensitivity to alterations in energy balance have not been fully identified. It would not be surprising if these genes turned out to be quite numerous, considering the complexity of the biological and behavioral systems that are involved in body-weight homeostasis. Many important issues pertaining to gene-behavior interaction effects remain to be investigated, including the following: Are there gene-behavior interaction effects that are time sensitive? Do they vary in their importance with age? Can we take advantage of these gene-behavior interaction effects in designing programs to prevent childhood obesity?
ASSORTATIVE MATING
A few articles have revealed that studies of assortative mating could provide new insights not only into the genetics of obesity but also into the dynamics of the ongoing prevalence increase in a given population (65–67). In a Canadian study, it was observed that spousal correlations were stronger in parents of lean offspring and in parents of obese children but lower in parents of children with average adiposity (67). More recently, a study based on ≈25,000 subjects from 3 cohorts from Sweden [Swedish Obese Subjects (SOS), SOS reference study, and Xenical in the Prevention of Diabetes in Obese Subjects (XENDOS)] and on 8663 husband-and-wife pairs with 4118 adult offspring was reported (66). The overall spouse correlation for BMI reached 0.18 (95% CI: 0.16, 0.20). The prevalence of obese offspring reached 1.4% when both parents were normal weight, 8.2% when only one parent was obese, and 20.1% when both parents were obese. Although they represented 16% of the number of parental pairs, the obese mother and father contributed 35% of all obese adult offspring. In a subset of 209 foster parents and adopted offspring, no association was seen between the BMI status of the foster parents and the prevalence of obesity in the adopted sons or daughters.
Assortative mating can increase homozygosity at loci relevant to a predisposition to obesity. With rates of assortative mating for BMI remaining constant across generations, one would expect a growing prevalence of obese offspring born from obese parents, as shown by simulation studies performed by Jacobson et al (66). The situation would worsen if assortative mating for high BMI was actually on the rise over time. These observations suggest that assortative mating is very relevant to the issue of the growing prevalence of childhood obesity and that much can still be learned by mining parents and offspring databases.
CONCLUSIONS
Childhood obesity is a growing problem not only in developed countries but also in emerging economies of the world. Is the high and increasing prevalence entirely explained by obesogenic behaviors and environments? Is there an inherited biological predisposition that is fueling this trend? Cross-sectional observational studies strongly suggest that there are family lines in which childhood obesity or childhood leanness clusters. Parental weight status is strongly associated with the weight status of offspring up to ≈5 y of age, and it predicts the risk of adulthood obesity as well. Weight at birth is associated with later risk of obesity, with low and high birth weight babies being most at risk. The heritability of birth weight reaches ≈30% with contributions from maternal, parental, and newborn genes. The anatomy of the genetic component of childhood obesity is ill-defined at this time. A few genes have been shown to cause early onset childhood obesity when they carry functional defects. Overall these genes account for ≥5% of the cases. However, most childhood obesity cases appear to result from a genetic predisposition that results from the presence of risk alleles at many genes that may act synergistically in response to obesogenic environmental conditions. The best example to date of such genes is the FTO gene. Its effect size is relatively small, but it is exacerbated by a sedentary lifestyle. Candidate gene and genomewide association studies reveal that typical obesogenic genes have small effect sizes but that the risk alleles for obesity are quite common in populations. Finally, the prevalence of childhood obesity may be increasing as a result of positive assortative mating with obese parents contributing more obese offspring than normal-weight parents. (Other articles in this supplement to the Journal include references 68–75.)
Acknowledgments
The author had no conflicts of interest and no disclosures to declare.
REFERENCES
- 1.Lobstein T, Baur L, Uauy R, IASO International Obesity Taskforce. Obesity in children and young people: a crisis in public health. Obes Rev 2004;5(suppl 1):4–104 [DOI] [PubMed] [Google Scholar]
- 2.International Obesity Taskforce Childhood obesity. Available from: http://www.iotf.org/childhoodobesity.asp (cited 20 Aug 2008)
- 3.World Health Organization Global strategy on diet, physical activity and health. Available from: http://www.who.int/dietphysicalactivity/childhood_what/en/index.html (cited 20 Aug 2008)
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295:1549–55 [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA 2008;299:2401–5 [DOI] [PubMed] [Google Scholar]
- 6.Baker JL, Olsen LW, Sørensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 2007;357:2329–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman DS, Patel DA, Srinivasan SR, et al. The contribution of childhood obesity to adult carotid intima-media thickness: the Bogalusa Heart Study. Int J Obes (Lond) 2008;32:749–56 [DOI] [PubMed] [Google Scholar]
- 8.Bouchard C. BMI, fat mass, abdominal adiposity and visceral fat: where is the ‘beef’? Int J Obes (Lond) 2007;31:1552–3 [DOI] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 2000;49:467–72 [DOI] [PubMed] [Google Scholar]
- 10.Thamer C, Machann J, Bachmann O, et al. Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab 2003;88:1785–91 [DOI] [PubMed] [Google Scholar]
- 11.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002;51:7–18 [DOI] [PubMed] [Google Scholar]
- 12.Bouchard C, Perusse L, Rice T, Rao DC. Genetics of human obesity. Bray GA, Bouchard C, eds Handbook of obesity. New York, NY: Marcel Dekker Inc, 2004:157–200 [Google Scholar]
- 13.Risch N. Linkage strategies for genetically complex traits. I. Multilocus models. Am J Hum Genet 1990;46:222–8 [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Reed DR, Price RA. Familial risk ratios for extreme obesity: implications for mapping human obesity genes. Int J Obes Relat Metab Disord 1997;21:935–40 [DOI] [PubMed] [Google Scholar]
- 15.Allison DB, Faith MS, Nathan JS. Risch's lambda values for human obesity. Int J Obes Relat Metab Disord 1996;20:990–9 [PubMed] [Google Scholar]
- 16.Demerath EW, Choh AC, Czerwinski SA, et al. Genetic and environmental influences on infant weight and weight change: the Fels Longitudinal Study. Am J Hum Biol 2007;19:692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubois L, Girard M, Girard A, Tremblay R, Boivin M, Pérusse D. Genetic and environmental influences on body size in early childhood: a twin birth-cohort study. Twin Res Hum Genet 2007;10:479–85 [DOI] [PubMed] [Google Scholar]
- 18.Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr 2008;87:398–404 [DOI] [PubMed] [Google Scholar]
- 19.Casey VA, Dwyer JT, Coleman KA, Valadian I. Body mass index from childhood to middle age: a 50-y follow-up. Am J Clin Nutr 1992;56:14–8 [DOI] [PubMed] [Google Scholar]
- 20.Braddon FE, Rodgers B, Wadsworth ME, Davies JM. Onset of obesity in a 36 year birth cohort study. Br Med J (Clin Res Ed) 1986;293:299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mossberg HO. 40-year follow-up of overweight children. Lancet 1989;2:491–3 [DOI] [PubMed] [Google Scholar]
- 22.Garn SM, Sullivan TV, Hawthorne VM. Fatness and obesity of the parents of obese individuals. Am J Clin Nutr 1989;50:1308–13 [DOI] [PubMed] [Google Scholar]
- 23.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med 1993;22:167–77 [DOI] [PubMed] [Google Scholar]
- 24.Guo SS, Roche AF, Chumlea WC, Gardner JD, Siervogel RM. The predictive value of childhood body mass index values for overweight at age 35 y. Am J Clin Nutr 1994;59:810–9 [DOI] [PubMed] [Google Scholar]
- 25.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997;337:869–73 [DOI] [PubMed] [Google Scholar]
- 26.Curhan GC, Chertow GM, Willett WC, et al. Birth weight and adult hypertension and obesity in women. Circulation 1996;94:1310–5 [DOI] [PubMed] [Google Scholar]
- 27.Martorell R, Stein AD, Schroeder DG. Early nutrition and later adiposity. J Nutr 2001;131:874S–80S [DOI] [PubMed] [Google Scholar]
- 28.Robson EB. The genetics of birth weight. Falkner F, Tanner JM, eds Human growth. Volume 1, Principles and prenatal growth London, United Kingdom: Baillière Tindall, 1978:285–97 [Google Scholar]
- 29.Vlietinck R, Derom R, Neale MC, et al. Genetic and environmental variation in the birth weight of twins. Behav Genet 1989;19:151–61 [DOI] [PubMed] [Google Scholar]
- 30.Whitfield JB, Treloar SA, Zhu G, Martin NG. Genetic and non-genetic factors affecting birth-weight and adult Body Mass Index. Twin Res 2001;4:365–70 [DOI] [PubMed] [Google Scholar]
- 31.Arya R, Demerath E, Jenkinson CP, et al. A quantitative trait locus (QTL) on chromosome 6q influences birth weight in two independent family studies. Hum Mol Genet 2006;15:1569–79 [DOI] [PubMed] [Google Scholar]
- 32.Cai G, Cole SA, Haack K, Butte NF, Comuzzie AG. Bivariate linkage confirms genetic contribution to fetal origins of childhood growth and cardiovascular disease risk in Hispanic children. Hum Genet 2007;121:737–44 [DOI] [PubMed] [Google Scholar]
- 33.Magnus P, Berg K, Bjerkedal T, Nance WE. Parental determinants of birth weight. Clin Genet 1984;26:397–405 [DOI] [PubMed] [Google Scholar]
- 34.Lunde A, Melve KK, Gjessing HK, Skjaerven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am J Epidemiol 2007;165:734–41 [DOI] [PubMed] [Google Scholar]
- 35.Beaty TH. Invited commentary: two studies of genetic control of birth weight where large data sets were available. Am J Epidemiol 2007;165:753–5 [DOI] [PubMed] [Google Scholar]
- 36.Magnus P, Gjessing HK, Skrondal A, Skjaerven R. Paternal contribution to birth weight. J Epidemiol Community Health 2001;55:873–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffiths LJ, Dezateux C, Cole TJ. Differential parental weight and height contributions to offspring birthweight and weight gain in infancy. Int J Epidemiol 2007;36:104–7 [DOI] [PubMed] [Google Scholar]
- 38.Jaquet D, Swaminathan S, Alexander GR, et al. Significant paternal contribution to the risk of small for gestational age. BJOG 2005;112:153–9 [DOI] [PubMed] [Google Scholar]
- 39.Weedon MN, Frayling TM, Shields B, et al. Genetic regulation of birth weight and fasting glucose by a common polymorphism in the islet cell promoter of the glucokinase gene. Diabetes 2005;54:576–81 [DOI] [PubMed] [Google Scholar]
- 40.López-Bermejo A, Petry CJ, Díaz M, et al. The association between the FTO gene and fat mass in humans develops by the postnatal age of two weeks. J Clin Endocrinol Metab 2008;93:1501–5 [DOI] [PubMed] [Google Scholar]
- 41.Lindsay RS, Kobes S, Knowler WC, Hanson RL. Genome-wide linkage analysis assessing parent-of-origin effects in the inheritance of birth weight. Hum Genet 2002;110:503–9 [DOI] [PubMed] [Google Scholar]
- 42.Rankinen T, Zuberi A, Chagnon YC, et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14:529–644 [DOI] [PubMed] [Google Scholar]
- 43.English SB, Butte AJ. Evaluation and integration of 49 genome-wide experiments and the prediction of previously unknown obesity-related genes. Bioinformatics 2007;23:2910–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saunders CL, Chiodini BD, Sham P, et al. Meta-analysis of genome-wide linkage studies in BMI and obesity. Obesity (Silver Spring) 2007;15:2263–75 [DOI] [PubMed] [Google Scholar]
- 45.International HapMap Consortium, Frazer KA, Ballinger DG, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 2007;449:851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loos RJ, Bouchard C. Obesity: is it a genetic disorder? J Intern Med 2003;254:401–25 [DOI] [PubMed] [Google Scholar]
- 47.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 2003;348:1085–95 [DOI] [PubMed] [Google Scholar]
- 48.Farooqi IS, O'Rahilly S. Monogenic obesity in humans. Annu Rev Med 2005;56:443–58 [DOI] [PubMed] [Google Scholar]
- 49.Larsen LH, Echwald SM, Sørensen TI, Andersen T, Wulff BS, Pedersen O. Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metab 2005;90:219–24 [DOI] [PubMed] [Google Scholar]
- 50.Bouchard C. The biological predisposition to obesity: beyond the thrifty gene scenario. Int J Obes 2007;31:1337–9 [DOI] [PubMed] [Google Scholar]
- 51.Andreasen CH, Stender-Petersen KL, Mogensen MS, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes 2008;57:95–101 [DOI] [PubMed] [Google Scholar]
- 52.Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 2007;39:724–6 [DOI] [PubMed] [Google Scholar]
- 53.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinney A, Nguyen TT, Scherag A, et al. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS One 2007;2:e1361.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Price RA, Li WD, Zhao H. FTO gene SNPs associated with extreme obesity in cases, controls and extremely discordant sister pairs. BMC Med Genet 2008;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 2007;3:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loos RJ, Bouchard C. FTO: the first gene contributing to common forms of human obesity. Obes Rev 2008;9:246–50 [DOI] [PubMed] [Google Scholar]
- 58.Gerken T, Girard CA, Tung YC, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007;318:1469–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loos RJ, Lindgren CM, Li S, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 2008;40:768–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouchard C, Tremblay A, Després J-P, et al. The response to long-term overfeeding in identical twins. N Engl J Med 1990;322:1477–82 [DOI] [PubMed] [Google Scholar]
- 61.Bouchard C, Tremblay A, Despres JP, et al. The response to exercise with constant energy intake in identical twins. Obes Res 1994;2:400–10 [DOI] [PubMed] [Google Scholar]
- 62.Hainer V, Stunkard AJ, Kunesova M, et al. Intrapair resemblance in very low calorie diet-induced weight loss in female obese identical twins. Int J Obes Relat Metab Disord 2000;24:1051–7 [DOI] [PubMed] [Google Scholar]
- 63.Ukkola O, Bouchard C. Role of candidate genes in the responses to long-term overfeeding: review of findings. Obes Rev 2004;5:3–12 [DOI] [PubMed] [Google Scholar]
- 64.Rampersaud E, Mitchell B, Pollin TI, et al. Physical activity modifies the effect of common FTO gene variants on body mass index and obesity. Arch Intern Med 2008;168:1791–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hebebrand J, Wulftange H, Goerg T, et al. Epidemic obesity: are genetic factors involved via increased rates of assortative mating? Int J Obes Relat Metab Disord 2000;24:345–53 [DOI] [PubMed] [Google Scholar]
- 66.Jacobson P, Torgerson JS, Sjöström L, Bouchard C. Spouse resemblance in body mass index: effects on adult obesity prevalence in the offspring generation. Am J Epidemiol 2007;165:101–8 [DOI] [PubMed] [Google Scholar]
- 67.Katzmarzyk PT, Hebebrand J, Bouchard C. Spousal resemblance in the Canadian population: implications for the obesity epidemic. Int J Obes Relat Metab Disord 2002;26:241–6 [DOI] [PubMed] [Google Scholar]
- 68.Atkinson SA. Introduction to the workshop. Am J Clin Nutr 2009;89(suppl):1485S–7S [DOI] [PubMed] [Google Scholar]
- 69.Zeisel SH. Epigenetic mechanisms for nutrition determinants of later health outcomes. Am J Clin Nutr 2009;89(suppl):1488S–93S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koletzko B, von Kries R, Monasterolo RC, et al. Can infant feeding choices modulate later obesity risk? Am J Clin Nutr 2009;89(suppl):1502S–8S [DOI] [PubMed] [Google Scholar]
- 71.Ordovas JM. Genetic influences on blood lipids and cardiovascular disease risk: tools for primary prevention. Am J Clin Nutr 2009;89(suppl):1509S–17S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Symonds ME, Stephenson T, Budge H. Early determinants of cardiovascular disease: the role of early diet in later blood pressure control. Am J Clin Nutr 2009;89(suppl):1518S–22S [DOI] [PubMed] [Google Scholar]
- 73.Carlson SE. Early determinants of development: a lipid perspective. Am J Clin Nutr 2009;89(suppl):1523S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Field CJ. Summary of the workshop. Am J Clin Nutr 2009;89(suppl):1533S–9S [DOI] [PubMed] [Google Scholar]
- 75.Rai D, Larson B. Driving research in infant and children's nutrition: a perspective on industry. Am J Clin Nutr 2009;89(suppl):1530S–2S [DOI] [PubMed] [Google Scholar]