Abstract
Genetic differences in taste preference, food tolerance, and phytochemical absorption and metabolism all potentially influence the effect of plant-based diets on cancer risk. Diet is a mixture of carcinogens, mutagens, and protective agents, many of which are metabolized by biotransformation enzymes. Genetic polymorphisms that alter protein expression or enzyme function can modify risk. Genotypes associated with more favorable handling of carcinogens may be associated with less favorable handling of phytochemicals. For example, glutathione S-transferases detoxify polycyclic aromatic hydrocarbons and metabolize isothiocyanates, which are chemopreventive compounds in cruciferous vegetables. A polymorphism in the GSTM1 gene results in lack of GSTM1-1 protein. Pharmacokinetic studies suggest that lack of GSTM1 enzyme is associated with more rapid excretion of the isothiocyanate sulforaphane; therefore, individuals who have this genetic variation may derive less benefit from consuming cruciferous vegetables. Flavonoids are conjugated with glucuronide and sulfate and are excreted in urine and bile. Polymorphisms in UDP-glucuronosyltransferases and sulfotransferases may contribute to variability in phytochemical clearance and efficacy. Genetic polymorphisms in enzymes that metabolize phytochemicals may account in part for variation in disease risk and also have to be considered in the context of other aspects of human genetics, gut bacterial genetics, and environmental exposures.
INTRODUCTION
Phytochemicals are bioactive nonnutrient chemical compounds found in plant foods, eg, fruits, vegetables, grains, nuts, and seeds. They often are categorized into groups on the basis of their chemical structure (ie, polyphenols, organosulfur compounds, carotenoids, alkaloids, and nitrogen-containing compounds). The polyphenols can be divided further into flavonoids (including flavonols, flavones, catechins, flavanones, anthocyanidins, and isoflavones), phenolic acids, stilbenes, coumarins, and tannins (1).
Many phytochemicals affect biological processes and have the potential to influence disease risk via complementary mechanisms (1–5). Although animal models of carcinogenesis often show protective effects of plant-food constituents (3, 6), the protective effect of a high plant-food diet in relation to cancer risk in humans has not been established conclusively (7). Human genetic variation may be one of the factors modifying response to constituents of plant foods in population-based studies. This variation may contribute in part to the observed heterogeneity across different study populations. Characterizing how genetic factors modify the effects of plant foods or specific components of these foods may enhance understanding of the potential for high plant-food diets to influence cancer risk and help identify populations that may particularly benefit.
PHYTOCHEMICAL DISPOSITION
Phytochemical disposition, like the disposition of drugs and other xenobiotics, involves absorption, metabolism, distribution, and excretion, and each of these aspects may contribute to variability in exposure (8). Many phytochemicals present in plant foods are glycosides or other conjugates and need to be hydrolyzed to be absorbed (9). Hydrolysis can be carried out by brush border membrane-bound β-glucosidases (eg, lactase phlorizin hydrolase) or by gut bacterial β-glucosidases in the lower small intestine and colon. Once absorbed, aglycones undergo extensive first-pass metabolism in the gut epithelium or liver, with many compounds being conjugated with glutathione, glucuronic acid, or, to a lesser extent, sulfate. Conjugation in the intestinal epithelium and liver by UDP-glucuronosyltransferases (UGTs) and sulfotransferases (SULTs) results in conjugates that are excreted in urine and bile. Those that are re-excreted in bile are deconjugated by bacterial β-glucuronidase and can undergo enterohepatic recycling.
The transport of ingested phytochemicals across the intestinal epithelium is another factor determining bioavailability on oral intake. For many phytochemicals and other xenobiotic compounds, transcellular transport is carried out by specific proteins—the membrane-bound, ATP-binding cassette transport proteins, which can translocate a variety of conjugated and unconjugated compounds from the epithelial cells to the submucosal side, facilitating absorption, or back into the intestinal lumen, reducing bioavailability (10). Animal and cell-based studies have shown a role for several of the transporters in regulating the uptake of various flavonoids and other phytochemicals (11, 12).
Some phytochemicals undergo phase I, activating reactions in the liver. Several studies have shown, by using human liver microsomes or in vivo in pharmacokinetic studies, that hydroxylation of lignans, isoflavones, and other flavonoids can occur and produces an array of secondary oxidation products (13–17). Oxidation products seem to be minor metabolites of most polyphenols, however, probably due to rapid conjugation of the would-be phase I substrates in the intestinal epithelium and the liver. In contrast, isothiocyanates (ITCs) derived from cruciferous vegetables have been shown to undergo extensive phase I metabolism in rats (18), although it has not been determined to what extent these reactions occur in humans.
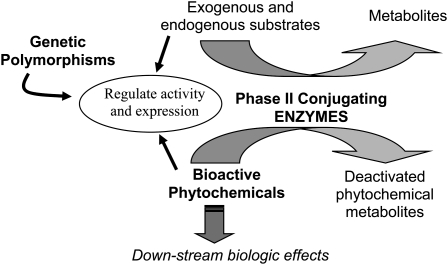
In theory, genetic variation in pathways affecting phytochemical absorption, metabolism, and distribution likely influences exposure at the tissue level (8). Few studies have systematically addressed the factors that contribute to the substantial variation in the metabolism and disposition of phytochemicals in vivo. Expression and activity of many of the enzymes important in phytochemical metabolism are modulated by the substrates they act on and by other xenobiotic compounds (Figure 1). Similarly, genetic variation in the pathways within which of these compounds act can alter biological response. Beyond a few well-recognized conditions (eg, favism: glucose-6-phosphate dehydrogenase deficiency and vicine and covicine ingestion), however, little is known about the biological effects of genetic variation on these gene-phytochemical interactions in humans, especially as they relate to cancer risk. One aspect of phytochemical handling that has received some attention, particularly in relation to specific phytochemicals, is the effect of the genetically polymorphic phase I, conjugating enzymes.
FIGURE 1.
Relation between phytochemicals and polymorphic biotransformation enzymes that metabolize them.
PHYTOCHEMICAL METABOLISM BY POLYMORPHIC PHASE II CONJUGATING ENZYMES
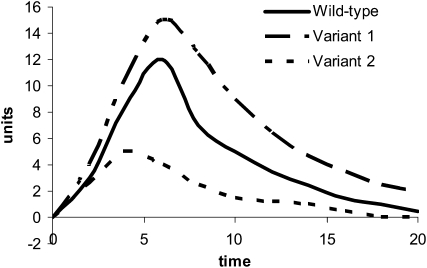
Phytochemicals are conjugated in vivo by biotransformation enzymes in a manner similar to that of other xenobiotics. Many classes of phytochemicals are rapidly conjugated with glutathione, glucuronide, and sulfate moieties and are excreted in urine and bile. Thus, in theory, polymorphisms in biotransformation enzymes, eg, glutathione S-transferases (GSTs), UGTs, and SULTs, have the capacity to affect phytochemical metabolism in the same fashion as they do carcinogens and other xenobiotics. Given that diet is a mixture of carcinogens, mutagens, and protective agents that are all metabolized by the same biotransformation enzymes, genotypes that are associated with more favorable handling of carcinogens (ie, rapid conjugation and clearance of mutagenic substances) may be associated with less favorable handling of phytochemicals (ie, more rapid clearance of potentially beneficial compounds) (see Figure 1). As outlined in Figure 2, genetic polymorphisms that result in proteins with lower enzymatic activity as compared with wild-type protein may result in pharmacokinetic profiles with greater area under the curve (ie, longer exposure), whereas polymorphisms that result in proteins with higher activity may shorten exposure.
FIGURE 2.
Theoretical effect of polymorphic conjugating phase II enzymes on phytochemical pharmacokinetics. Polymorphic enzymes with lower activity may prolong exposure (variant 1), whereas polymorphic enzymes with higher activity shorten exposure (variant 2).
Glutathione S-transferases
To date, probably the most studied group of compounds with regard to effects of genetics on metabolism are the ITCs in cruciferous vegetables. The primary route of in vivo metabolism of ITCs is via the mercapturic acid pathway, a major pathway for elimination of many xenobiotics (19). Thiol conjugates of ITCs are formed by conjugation of ITCs with glutathione; this is catalyzed by GSTs. Subsequently, stepwise cleavage of glutamine and glycine yields l-cysteine–ITC, which is acetylated to produce N-acetyl-l-cysteine ITC conjugates (mercapturic acids); these are excreted in urine. Thus, GSTs play an important role in disposition of ITCs in humans. Relationships between cruciferous vegetable intake and cancer risk may be influenced by genetic polymorphisms in biotransformation enzymes that metabolize ITCs and possibly in receptors and transcription factors that interact with these compounds.
In humans there are 3 major GST families: cytosolic GSTs, mitochondrial GSTs, and microsomal GSTs, referred to as membrane-associated proteins in eicosanoid and glutathione metabolism (20). Cytosolic GST are the largest GST family, containing 7 classes: α, ζ, θ, μ, π, σ, and ω GST. Two studies examined the ITC metabolism with different GST isozymes and showed that GSTM1-1 and GSTP1-1 were the most efficient catalysts, GSTA1-1 was less efficient, and GSTM2-2 and GSTM4-4 were the least efficient (21, 22).
Depending on the population, 27–53% of people are homozygous for a common deletion of the GSTM1 gene (GSTM1*0), which results in lack of GSTM1 activity. Similarly, 20–47% of people in various ethnic groups are homozygous for a deletion of the GSTT1 gene (GSTT1*0) and do not express GSTT1 (23). Investigators have hypothesized that individuals who are null for GSTM1 and GSTT1, and who therefore conjugate and excrete ITCs less readily, would have greater amounts of ITCs at the tissue level and hence experience a greater protective effect of glucosinolate consumption (23). Results of one Chinese population–based study of ITC excretion showed that urinary ITC was higher among GSTT1-positive individuals compared with GSTT1-null individuals but that GSTM1 and P1 genotypes had no effect (24). In contrast, in another Chinese population, Fowke et al (25) evaluated the same GST enzymes and showed a positive association between intake of cruciferous vegetables and urinary ITC excretion only among individuals who had variation in the GSTP1 Ile/Ile genotype.
More recently, a pharmacokinetic study of sulforaphane disposition showed that GSTM1-null individuals, compared with GSTM1-positive individuals, had greater areas under the curve for plasma sulforaphane metabolite concentrations, faster rates of urinary sulforaphane metabolite excretion in the first 6 h after consumption, and higher total excretion of sulforaphane and its metabolites over 24 h (26). In a feeding study of a single meal of broccoli (2.5 g broccoli/kg body weight), urinary ITC concentration did not differ by GSTM1, GSTP1, and GSTA1 genotypes except that there was a tendency toward higher ITC excretion in GSTT1-positive individuals. By using a chi-square analysis, the investigators observed a higher proportion of GSTM1-null individuals who had high urinary ITC excretion compared with the proportion of GSTM1-positive individuals who had high urinary ITC excretion (27). These studies do not support the hypothesis that lack of GST enzyme activity reduces clearance of ITCs. Both studies were conducted with a single serving of broccoli. In contrast, more prolonged feeding studies might reflect habitual dietary practices among populations that routinely consume cruciferous vegetables. Whether or not there are differences between acute and chronic feeding or differences in the varieties of ITCs present in broccoli compared with those present in cruciferous vegetables commonly consumed in China remains to be established (26); nonetheless, these results indicate a need to understand further how genotype influences ITC disposition.
Polymorphisms in ITC-metabolizing enzymes may affect response of other biotransformation enzymes to ITC exposure. In a cross-sectional study of frequent broccoli consumers, GSTM1-null individuals had a 21% higher CYP1A2 activity than did GSTM1-positive individuals (28). This result was not observed in a controlled feeding study designed to test a priori the effect of GSTM1 genotype on response to a diet high in cruciferous vegetables; increased CYP1A2 activity in those individuals on the cruciferous vegetable–containing diet was not altered by GSTM1 genotype (29). In the same feeding study, serum GSTα concentration, which is a surrogate measure of hepatic GSTα and an enzyme also induced by ITCs, increased significantly in response to cruciferous vegetable consumption but only in GSTM1-null individuals (30).
UDP-glucuronosyltransferases
UGTs are a superfamily of enzymes that catalyze the addition of glucuronic acid to a range of endogenous and exogenous compounds, including several classes of phytochemicals. The substrate-binding regions of the UGT genes are highly polymorphic and many result in amino acid changes that alter enzyme activity to varying degrees in in vitro systems (31, 32). The functional effect of an alteration in UGT protein often is substrate specific. In vivo, where multiple UGTs are expressed in the same tissue, the overall effect often is less clear. The UGT1A1*28 polymorphism has been associated most strongly with altered xenobiotic glucuronidation in vivo.
Dietary flavonoids are structurally diverse. This group of compounds includes the flavones and flavonols (eg, apigenin, chrysin, galangin, luteolin, and quercetin), flavanes (eg, catechin, hesperetin, and naringenin), and isoflavonoids (eg, genistein and daidzein). The selectivity of glucuronosyl conjugation of the flavonoids is dependent on the structure of a particular flavonoid and on the UGT enzyme involved in its conjugation. For example, UGT1A1, UGT1A8, and UGT1A9 are especially active in conjugating luteolin and quercetin, whereas UGT1A4, UGT1A10, and UGT2B7 and UGT2B15 in the UGT2B family are less efficient (33). The isoflavone genistein is conjugated by UGT1A3, UGT1A8, and, with less efficiency, UGT1A1 and UGT1A10 but not by UGT2B15 (34, 35).
The effects of UGT polymorphisms on flavonoid clearance have not been examined. Studies showing that polymorphisms affect glucuronidation and clearance of drugs and other xenobiotic compounds suggest that it is possible that similar effects may be seen with dietary flavonoids. For example, the UGT1A1*28 polymorphism, which results in 30–40% lower UGT1A1 gene transcription among homozygous variant individuals, is associated with increased toxicity in patients with colorectal cancer treated with the topoisomerase I inhibitor irinotecan (36). In relation to diet, Peters et al also showed that with exposure to well-cooked red meat (a source of mutagenic compounds) in a controlled feeding study, individuals who had the *1/*28 and *28/*28 genotypes had a higher urinary mutagenicity index than did individuals who had the *1/*1 genotypes (37). The authors hypothesized that greater amounts of the mutagens were being excreted in the free form rather than being glucuronidated and deactivated. Furthermore, Chung et al (38) showed that the UGT2B15 *2/*2 genotype group was associated with 42% lower systemic clearance compared with *1/*1 genotype group for lorazepam in healthy volunteers. Although having this polymorphism may result in adverse responses in the context of exposure to toxic compounds or carcinogens, it may be beneficial in the context of reduced conjugation of phytochemicals. This remains to be studied.
Another UGT polymorphism also has been shown to speed drug clearance, but the effect on phytochemical metabolism remains unknown. The enzyme associated with the UGT1A4 (L48V) variant glucuronidates tamoxifen and its active metabolites at a faster rate than the wild-type protein (39). Women at high risk of breast cancer who take tamoxifen as a chemopreventive agent, in particular those who have the UGT1A4 (L48V) polymorphism, may experience reduced effectiveness of antiestrogen therapy (39).
Sulfotransferases
The majority of flavonoid conjugates in circulation or excreted in urine are glucuronides; however, 2–10% also are conjugated with a sulfate moiety by cytosolic SULTs in the liver and gastrointestinal tract. Because sulfated compounds can be deconjugated in target tissues (40), circulating sulfate conjugates of phytochemicals may act as a source of tissue aglycones. SULT1A1 has shown high sulfating activity toward a variety of flavonoids, isoflavonoids, and other phenolic dietary compounds, and SULT1A3 has high activity toward some flavonoids but not isoflavonoids (41, 42). Genetic variants in SULT genes with associated functional differences in the translated protein have been identified. Single-nucleotide polymorphisms in SULT1A1 and SULT2A1 are common and have been associated with altered response to therapeutic agents and sex steroid concentrations, respectively [reviewed by Nowell and Falany (43)]. Studies in vitro with recombinant SULT1A1 have shown that the SULT1A1*2 variant is less effective than SULT1A1*1 at conjugating resveratrol, apigenin, and epicatechin (44). Similarly, this could influence the in vivo disposition of phytochemicals metabolized by SULT1A1; however, this remains to be evaluated.
SUMMARY
Dietary intake of a phytochemical or its precursor is not guaranteed to equate with exposure at the tissue level. Several factors may contribute to variation in phytochemical metabolism and disposition, including the following: gut microbial community and activity; genetic determinants of biotransformation enzyme expression, stability, and activity; environmental exposures that influence gut microbiota and biotransformation enzymes; and endogenous constituents that modulate biotransformation pathways. Improved characterization of genetic factors that contribute to interindividual differences in phytochemical disposition may clarify the role of these dietary constituents in cancer prevention. (Other articles in this supplement to the Journal include references 45–71.)
Acknowledgments
The author had no potential conflicts of interest.
REFERENCES
- 1.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 2004;134:3479S–85S [DOI] [PubMed] [Google Scholar]
- 2.Fresco P, Borges F, Diniz C, Marques MP. New insights on the anticancer properties of dietary polyphenols. Med Res Rev 2006;26:747–66 [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Kong AN. Dietary cancer-chemopreventive compounds: from signaling and gene expression to pharmacological effects. Trends Pharmacol Sci 2005;26:318–26 [DOI] [PubMed] [Google Scholar]
- 4.Nichenametla SN, Taruscio TG, Barney DL, Exon JH. A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr 2006;46:161–83 [DOI] [PubMed] [Google Scholar]
- 5.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res 2007;55:207–16 [DOI] [PubMed] [Google Scholar]
- 6.IARC IARC handbooks of cancer prevention. Vol 8 Fruits and vegetables. Lyon, France: International Agency for Research on Cancer, 2003 [Google Scholar]
- 7.World Cancer Research Fund and American Institute for Cancer Research Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: American Institute for Cancer Research, 2007 [Google Scholar]
- 8.Lampe JW, Chang JL. Interindividual differences in phytochemical metabolism and disposition. Semin Cancer Biol 2007;17:347–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005;81:230S–42S [DOI] [PubMed] [Google Scholar]
- 10.Brand W, Schutte ME, Williamson G, et al. Flavonoid-mediated inhibition of intestinal ABC transporters may affect the oral bioavailability of drugs, food-borne toxic compounds and bioactive ingredients. Biomed Pharmacother 2006;60:508–19 [DOI] [PubMed] [Google Scholar]
- 11.Sesink AL, Arts IC, de Boer VC, et al. Breast cancer resistance protein (Bcrp1/Abcg2) limits net intestinal uptake of quercetin in rats by facilitating apical efflux of glucuronides. Mol Pharmacol 2005;67:1999–2006 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Cao J, Zeng S. Involvement of P-glycoprotein in regulating cellular levels of Ginkgo flavonols: quercetin, kaempferol, and isorhamnetin. J Pharm Pharmacol 2005;57:751–8 [DOI] [PubMed] [Google Scholar]
- 13.Jacobs E, Kulling SE, Metzler M. Novel metabolites of the mammalian lignans enterolactone and enterodiol in human urine. J Steroid Biochem Mol Biol 1999;68:211–8 [DOI] [PubMed] [Google Scholar]
- 14.Jacobs E, Metzler M. Oxidative metabolism of the mammalian lignans enterolactone and enterodiol by rat, pig, and human liver microsomes. J Agric Food Chem 1999;47:1071–7 [DOI] [PubMed] [Google Scholar]
- 15.Otake Y, Walle T. Oxidation of the flavonoids galangin and kaempferide by human liver microsomes and CYP1A1, CYP1A2, and CYP2C9. Drug Metab Dispos 2002;30:103–5 [DOI] [PubMed] [Google Scholar]
- 16.Piver B, Fer M, Vitrac X, et al. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem Pharmacol 2004;68:773–82 [DOI] [PubMed] [Google Scholar]
- 17.Potter GA, Patterson LH, Wanogho E, et al. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br J Cancer 2002;86:774–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassahun K, Davis M, Hu P, Martin B, Baillie T. Biotransformation of the naturally occurring isothiocyanate sulforaphane in the rat: identification of phase I metabolites and glutathione conjugates. Chem Res Toxicol 1997;10:1228–33 [DOI] [PubMed] [Google Scholar]
- 19.Conaway CC, Jiao D, Kohri T, Liebes L, Chung F. Disposition and pharmacokinetics of phenethyl isothiocyanate and 6-phenylhexyl isothiocyanate in F344 rats. Drug Metab Dispos 1999;27:13–20 [PubMed] [Google Scholar]
- 20.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol 2005;45:51–88 [DOI] [PubMed] [Google Scholar]
- 21.Kolm RH, Danielson H, Zhang Y, Talalay P, Mannervik B. Isothiocyanates as substrates for human glutathione transferases: structure-activity studies. Biochem J 1995;311:453–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Kolm RH, Mannervik B, Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem Biophys Res Commun 1995;206:748–55 [DOI] [PubMed] [Google Scholar]
- 23.Seow A, Vainio H, Yu MC. Effect of glutathione-S-transferase polymorphisms on the cancer preventive potential of isothiocyanates: an epidemiological perspective. Mutat Res 2005;592:58–67 [DOI] [PubMed] [Google Scholar]
- 24.Seow A, Shi C-Y, Chung F-L, et al. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev 1998;7:775–81 [PubMed] [Google Scholar]
- 25.Fowke JH, Shu XO, Dai Q, et al. Urinary isothiocyanate excretion, brassica consumption, and gene polymorphisms among women living in Shanghai, China. Cancer Epidemiol Biomarkers Prev 2003;12:1536–9 [PubMed] [Google Scholar]
- 26.Gasper AV, Al-Janobi A, Smith JA, et al. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr 2005;82:1283–91 [DOI] [PubMed] [Google Scholar]
- 27.Steck SE, Gammon MD, Hebert JR, Wall DE, Zeisel SH. GSTM1, GSTT1, GSTP1, and GSTA1 polymorphisms and urinary isothiocyanate metabolites following broccoli consumption in humans. J Nutr 2007;137:904–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Probst-Hensch NM, Tannenbaum SR, Chan KK, Coetzee GA, Ross RK, Yu MC. Absence of the glutathione S-transferase M1 gene increases cytochrome P4501A2 activity among frequent consumers of cruciferous vegetables in a Caucasian population. Cancer Epidemiol Biomarkers Prev 1998;7:635–8 [PubMed] [Google Scholar]
- 29.Lampe JW, King IB, Li S, et al. Brassica vegetables increase and apiaceous vegetables decrease cytochrome P450 1A2 activity in humans: changes in caffeine metabolite ratios in response to controlled vegetable diets. Carcinogenesis 2000;21:1157–62 [PubMed] [Google Scholar]
- 30.Lampe JW, Chen C, Li S, et al. Modulation of human glutathione S-transferases by botanically defined vegetable diets. Cancer Epidemiol Biomarkers Prev 2000;9:787–93 [PubMed] [Google Scholar]
- 31.Burchell B. Genetic variation of human UDP-glucuronosyltransferase: implications in disease and drug glucuronidation. Am J Pharmacogenomics 2003;3:37–52 [DOI] [PubMed] [Google Scholar]
- 32.Maruo Y, Iwai M, Mori A, Sato H, Takeuchi Y. Polymorphism of UDP-glucuronosyltransferase and drug metabolism. Curr Drug Metab 2005;6:91–9 [DOI] [PubMed] [Google Scholar]
- 33.Boersma MG, van der Woude H, Bogaards J, et al. Regioselectivity of phase II metabolism of luteolin and quercetin by UDP-glucuronosyl transferases. Chem Res Toxicol 2002;15:662–70 [DOI] [PubMed] [Google Scholar]
- 34.Doerge DR, Chang HC, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos 2000;28:298–307 [PubMed] [Google Scholar]
- 35.King CD, Rios GR, Green MD, Tephly TR. UDP-glucuronosyltransferases. Curr Drug Metab 2000;1:143–61 [DOI] [PubMed] [Google Scholar]
- 36.O'Dwyer PJ, Catalano RB. Uridine diphosphate glucuronosyltransferase (UGT) 1A1 and irinotecan: practical pharmacogenomics arrives in cancer therapy. J Clin Oncol 2006;24:4534–8 [DOI] [PubMed] [Google Scholar]
- 37.Peters U, Sinha R, Bell DA, et al. Urinary mutagenesis and fried red meat intake: influence of cooking temperature, phenotype, and genotype of metabolizing enzymes in a controlled feeding study. Environ Mol Mutagen 2004;43:53–74 [DOI] [PubMed] [Google Scholar]
- 38.Chung JY, Cho JY, Yu KS, et al. Effect of the UGT2B15 genotype on the pharmacokinetics, pharmacodynamics, and drug interactions of intravenous lorazepam in healthy volunteers. Clin Pharmacol Ther 2005;77:486–94 [DOI] [PubMed] [Google Scholar]
- 39.Sun D, Chen G, Dellinger RW, Duncan K, Fang JL, Lazarus P. Characterization of tamoxifen and 4-hydroxytamoxifen glucuronidation by human UGT1A4 variants. Breast Cancer Res 2006;8:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasano H, Suzuki T, Miki Y, Moriya T. Intracrinology of estrogens and androgens in breast carcinoma. J Steroid Biochem Mol Biol 2008;108:181–5 [DOI] [PubMed] [Google Scholar]
- 41.Pai TG, Suiko M, Sakakibara Y, Liu MC. Sulfation of flavonoids and other phenolic dietary compounds by the human cytosolic sulfotransferases. Biochem Biophys Res Commun 2001;285:1175–9 [DOI] [PubMed] [Google Scholar]
- 42.Ronis MJ, Little JM, Barone GW, Chen G, Radominska-Pandya A, Badger TM. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J Med Food 2006;9:348–55 [DOI] [PubMed] [Google Scholar]
- 43.Nowell S, Falany CN. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene 2006;25:1673–8 [DOI] [PubMed] [Google Scholar]
- 44.Ung D, Nagar S. Variable sulfation of dietary polyphenols by recombinant human sulfotransferase (SULT) 1A1 genetic variants and SULT1E1. Drug Metab Dispos 2007;35:740–6 [DOI] [PubMed] [Google Scholar]
- 45.Rajaram S, Sabaté J. Preface. Am J Clin Nutr 2009;89(suppl):1541S–2S [DOI] [PubMed] [Google Scholar]
- 46.Jacobs DR, Jr, Gross MD, Tapsell LC. Food synergy: an operational concept for understanding nutrition. Am J Clin Nutr 2009;89(suppl):1543S–8S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobs DR, Jr, Haddad EH, Lanou AJ, Messina MJ. Food, plant food, and vegetarian diets in the US dietary guidelines: conclusions of an expert panel. Am J Clin Nutr 2009;89(suppl):1549S–52S [DOI] [PubMed] [Google Scholar]
- 48.Simon JA, Chen Y-H, Bent S. The relation of α-linolenic acid to the risk of prostate cancer: a systematic review and meta-analysis. Am J Clin Nutr 2009;89(suppl):1558S–64S [DOI] [PubMed] [Google Scholar]
- 49.Pierce JP, Natarajan L, Caan BJ, et al. Dietary change and reduced breast cancer events among women without hot flashes after treatment of early-stage breast cancer: subgroup analysis of the Women's Healthy Eating and Living Study. Am J Clin Nutr 2009;89(suppl):1565S–71S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newby PK. Plant foods and plant-based diets: protective against childhood obesity? Am J Clin Nutr 2009;89(suppl):1572S–87S [DOI] [PubMed] [Google Scholar]
- 51.Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89(suppl):1588S–96S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mangat I. Do vegetarians have to eat fish for optimal cardiovascular protection? Am J Clin Nutr 2009;89(suppl):1597S–601S [DOI] [PubMed] [Google Scholar]
- 53.Willis LM, Shukitt-Hale B, Joseph JA. Modulation of cognition and behavior in aged animals: role for antioxidant- and essential fatty acid–rich plant foods. Am J Clin Nutr 2009;89(suppl):1602S–6S [DOI] [PubMed] [Google Scholar]
- 54.Fraser GE. Vegetarian diets: what do we know of their effects on common chronic diseases? Am J Clin Nutr 2009;89(suppl):1607S–12S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 2009;89(suppl):1613S–9S [DOI] [PubMed] [Google Scholar]
- 56.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Cancer incidence in vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 2009;89(suppl):1620S–6S [DOI] [PubMed] [Google Scholar]
- 57.Craig WJ. Health effects of vegan diets. Am J Clin Nutr 2009;89(suppl):1627S–33S [DOI] [PubMed] [Google Scholar]
- 58.Weaver CM. Should dairy be recommended as part of a healthy vegetarian diet? Point. Am J Clin Nutr 2009;89(suppl):1634S–7S [DOI] [PubMed] [Google Scholar]
- 59.Lanou AJ. Should dairy be recommended as part of a healthy vegetarian diet? Am J Clin Nutr 2009;89(suppl):1638S–42S [DOI] [PubMed] [Google Scholar]
- 60.Sabaté J, Ang Y. Nuts and health outcomes: new epidemiologic evidence. Am J Clin Nutr 2009;89(suppl):1643S–8S [DOI] [PubMed] [Google Scholar]
- 61.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr 2009;89(suppl):1649S–56S [DOI] [PubMed] [Google Scholar]
- 62.Rajaram S, Haddad EH, Mejia A, Sabaté J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr 2009;89(suppl):1657S–63S [DOI] [PubMed] [Google Scholar]
- 63.Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr 2009;89(suppl):1664S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badger TM, Gilchrist JM, Pivik RT, et al. The health implications of soy infant formula. Am J Clin Nutr 2009;89(suppl):1668S–72S [DOI] [PubMed] [Google Scholar]
- 65.Messina M, Wu AH. Perspectives on the soy–breast cancer relation. Am J Clin Nutr 2009;89(suppl):1673S–9S [DOI] [PubMed] [Google Scholar]
- 66.Lönnerdal B. Soybean ferritin: implications for iron status of vegetarians. Am J Clin Nutr 2009;89(suppl):1680S–5S [DOI] [PubMed] [Google Scholar]
- 67.Chan J, Jaceldo-Siegl K, Fraser GE. Serum 25-hydroxyvitamin D status of vegetarians, partial vegetarians, and nonvegetarians: the Adventist Health Study-2. Am J Clin Nutr 2009;89(suppl):1686S–92S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elmadfa I, Singer I. Vitamin B-12 and homocysteine status among vegetarians: a global perspective. Am J Clin Nutr 2009;89(suppl):1693S–8S [DOI] [PubMed] [Google Scholar]
- 69.Marlow HJ, Hayes WK, Soret S, Carter RL, Schwab ER, Sabaté J. Diet and the environment: does what you eat matter? Am J Clin Nutr 2009;89(suppl):1699S–703S [DOI] [PubMed] [Google Scholar]
- 70.Carlsson-Kanyama A, González AD. Potential contributions of food consumption patterns to climate change. Am J Clin Nutr 2009;89(suppl):1704S–9S [DOI] [PubMed] [Google Scholar]
- 71.Eshel G, Martin PA. Geophysics and nutritional science: toward a novel, unified paradigm. Am J Clin Nutr 2009;89(suppl):1710S–16S [DOI] [PubMed] [Google Scholar]