Abstract
Background: A diet high in vegetables, fruit, and fiber and low in fat decreased additional risk of secondary breast cancer events in women without hot flashes (HF−) compared with that in women with hot flashes (HF+), possibly through lowered concentrations of circulating estrogens.
Objective: The objective was to investigate the intervention effect by baseline quartiles of dietary pattern among breast cancer survivors in the HF− subgroup of the Women's Healthy Eating and Living Study.
Design: A randomized controlled trial compared a putative cancer prevention diet with a diet of 5 servings of vegetables and fruit daily in early-stage breast cancer survivors. Participants did not experience hot flashes at baseline (n = 896). We confirmed cancer status for 96% of participants ≈7.3 y after enrollment.
Results: The study intervention achieved a large between-group difference in dietary pattern that, at 4 y, was not significantly different across baseline quartiles of dietary pattern. The intervention group experienced fewer breast cancer events than did the comparison group for all of the baseline quartiles. This difference was significant only in upper baseline quartiles of intake of vegetables, fruit, and fiber and in the lowest quartile of fat. A significant trend for fewer breast cancer events was observed across quartiles of vegetable-fruit and fiber consumption.
Conclusions: The secondary analysis showing the decreased risk in the HF− subgroup was not explained by amount of change in dietary pattern achieved. The difference was strongest in the quartile with the most putatively cancer-preventive dietary pattern at baseline.
INTRODUCTION
The Women's Healthy Eating and Living (WHEL) Study was a large, multiinstitutional, randomized trial designed to test if markedly increasing the consumption of vegetables, fruit, fiber, and carotenoids and decreasing total and saturated fat consumption (a putative cancer-preventive dietary pattern) would lower the risk of breast cancer events (recurrences and new primaries) for women who had been recently diagnosed (<4 y) with early-stage breast cancer (1). Women were randomly assigned to this putative cancer-preventive dietary pattern group or to 5 servings daily of vegetables and fruit, according to recommendations of the National Cancer Institute and many health agencies.
The study intervention was successful in achieving large increases in vegetables, fruit, and fiber intake and decreased fat intake ≤1 mo of study entry (2) and in maintaining these changes for 4 y regardless of a participant's baseline dietary pattern (3, 4). The large self-reported increases in vegetable and fruit intake were validated through a ≥40% increase in plasma carotenoid concentrations observed at 1 and 4 y (5, 6). During a 7.3-y follow-up period (96% participant retention), following the intervention dietary pattern did not reduce additional breast cancer events or deaths in the entire group. Furthermore, no dose-response or threshold effect was observed across baseline quartiles of any component of the dietary pattern (6). However, recently, we have observed that the WHEL intervention improved survival in a subgroup of participants who were at additional risk because they did not have hot flashes at baseline (18).
At the study's inception, we proposed that this putative cancer-preventive dietary pattern may have reduced additional breast cancer events through lowered circulating estrogen concentrations (1). In the WHEL Study, circulating concentrations of estradiol and bioavailable estradiol were higher in women who had recurrences of breast cancer compared with those who did not (7). Furthermore, women who did not have hot flashes after breast cancer treatment (HF−) had significantly higher concentrations of circulating estrogens (7) than did the women who had hot flashes after breast cancer treatment (HF+). Also, 3 studies, including one in the WHEL comparison group (before unblinding the trial), observed that the HF− subgroup had higher recurrence rates (8–10).
Several studies have reported that increasing fiber or decreasing fat consumption can lower circulating hormone concentrations (11–16). In a small subset of early enrollees in the WHEL Study, following the study dietary pattern was associated with 32% lower circulating bioavailable estradiol concentrations (17). In this article, we discuss this intervention effect on breast cancer events. Specifically, we focus on between-group differences in events across baseline quartiles of dietary components. We discuss the change achieved by the intervention within each of these quartiles and look for evidence that the amount of change achieved may have been related to differences in the breast cancer event rate. Although the WHEL Study intervention focused on dietary pattern, physical activity and body mass index (BMI; in kg/m2) also can influence circulating estrogen concentrations (19). Accordingly, in this report we explore whether or not these variables were associated with baseline dietary intake in a way that may partially explain the observed effect on breast cancer events.
SUBJECTS AND METHODS
Study participants
Between 1995 and 2000, the WHEL Study enrolled 3088 women ≤4 y of diagnosis of early-stage breast cancer (American Joint Committee on Cancer, 4th edition: stage I [≥1 cm], II, or IIIA) from 7 clinical sites. Women were randomly assigned to a putative cancer-preventive dietary pattern (daily intake of 5 vegetable servings, 16 oz of vegetable juice or vegetable serving equivalents, 3 fruit servings, 30 g fiber, and 15–20% energy from fat) or to a comparison group that was given print materials recommending 5 servings of vegetables and fruit daily (5-a-day). The study provided intensive telephone counseling to help women in the intervention group incorporate the dietary pattern (4, 20). This counseling included ≈31 telephone contacts for ≥4 y, monthly cooking classes in the first year, and monthly newsletters throughout the study. Institutional review boards at each clinical site approved the protocol, and all of the participants provided written informed consent and consent for medical record review.
HF− subgroup
Baseline hot flash severity in the prior 4 wk (scoring as 0 = none to 3 = severe) was obtained by using the Women's Health Initiative 34-item self-report symptom inventory (21). The current analyses focused on the 896 women who had complete dietary intake data and reported not experiencing hot flashes at baseline (HF− group). Although HF status was not a stratification criterion, 446 (49.8%) of this group were in the intervention group and 450 (50.2%) were in the comparison group. Study participants in the subgroup, similar to those in the total study cohort, were ≈1.98 y past diagnosis of their primary cancer when they were randomly assigned to the study; their mean (SD) age was 52.8 (11) y, and their mean (SD) BMI was 27.4 (6.2). Thirty-nine percent, 38%, and 14% of tumors were poorly, moderately, or well differentiated, respectively, with an unspecified grade for 9% of tumors; in addition, 41%, 54%, and 5% of the cancers were stage I, II, and III, respectively. The study groups were comparable on key demographic and tumor variables except for antiestrogen therapy, which was slightly imbalanced between groups (56% of women in the intervention group and 50% of women in the comparison group received antiestrogen therapy; P = 0.04). Additional breast cancer events during follow-up were reported and verified for 179 (20%) of the HF− subgroup with significantly fewer events in the intervention group than in the comparison group [intervention = 72 (16.1%), comparison = 107 (23.8%); P < 0.01 log-rank test]. Sensitivity analyses showed that these differences do not explain the between-group differences (18).
Dietary assessment
The primary assessment of dietary intake was by 24-h telephone recalls (sets of 4 prescheduled calls conducted by telephone on random days during a 3-wk period). The primary study time points for assessing dietary change were baseline, 1 y (short term), and 4 y (long term). Furthermore, an analysis of plasma carotenoid concentrations was performed to validate the dietary self-report. A measurement error analysis supported the study's decision to use the 24-h recall as the primary dietary assessment measure for the study (22). Dietary assessors conducted recalls on random days during a 3-wk period, stratified by weekend and weekdays, by using the Nutrition Data System for Research software (1994–2006) developed by the Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN. Baseline quartile cutoffs for dietary components were computed by using the entire WHEL Study sample.
Other lifestyle variables
Height and weight were measured at the baseline clinic visit and physical activity was assessed by the Women's Health Initiative 9-item physical activity questionnaire, which was completed by participants at that clinic visit. In a subsample, this physical activity questionnaire was shown to have reasonable validity when compared with the 7-d physical activity recall and accelerometer readings (23). By using Ainsworth's compendium of physical activities (24), we converted activity levels into metabolic equivalents (MET): mild activity was assessed as 3 MET, moderate as 5 MET, and vigorous as 8 MET. For walking, slow, average, fast, and very fast were assessed as 2, 3, 4, and 6 MET, respectively. Total energy expenditure for an activity was estimated as time spent in activity (min) multiplied by MET.
Outcomes
Primary study endpoints were recurrent (local/regional or distant metastasis) or new primary breast cancer events and death from any cause. Carcinoma in situ was not included as an outcome. All of the breast cancer events reported in semiannual telephone interviews between participants and clinic staff were confirmed by medical record review. Outcome ascertainment also included a search of the National Death Index.
Data analysis
We estimated between-group differences in dietary variables at years 1 and 4 postrandomization by quartiles of dietary intake at baseline. To minimize bias, missing data were imputed with a conservative method, described previously (4), that assumed that nonrespondents were following the comparison group dietary pattern. A test for trend in dietary intake differences between groups across baseline quartiles was computed.
Breast cancer event rates were calculated for each quartile of baseline dietary intake of various food components, namely total fruit and vegetable intake (servings/d), fiber intake (g/d), fiber-to-fat ratio [fiber (g/d) divided by fat (g/d)], and percentage of energy from fat. For each baseline dietary quartile, Cox models were used to estimate a hazard ratio (HR) for intervention effect, adjusting for key covariates (ie, stage and grade of the original tumor) that were most strongly associated with breast cancer outcome. In addition, use of antiestrogen therapy was adjusted in the models because of the slight imbalance in this covariate by randomization arm (1). HRs and 95% CIs for the intervention group effect were calculated for each model.
A likelihood ratio test for group by quartile interaction was constructed coding diet quartile as a categorical variable. In addition, a linear trend to examine intervention effect across increasing quartiles of dietary intake was constructed: a Cox model stratified by baseline diet quartile was fitted, and a group-by-quartile score interaction term was included in this model to test for a linear trend in log HRs as quartile score increased. A step-down Bonferroni correction (25) was used to correct the statistical significance level for multiple comparison tests undertaken. These analyses were undertaken by using SAS version 9.2 (SAS Institute, Cary, NC) and R version 2.1.0 (The R Project for Statistical Computing, Vienna, Austria; http://www.r-project.org/).
RESULTS
Dietary change achieved in the HF− subgroup
As reported previously, the intervention and comparison groups reported similar dietary intakes at baseline (1). Among the HF− subgroup, the lowest quartile of vegetable and fruit consumption was ≤4.9 servings/d, with a mean of 3.8 daily servings, and the highest quartile consumed was >8.9 servings/d, with a mean of 11.3 servings/d (intervention) and 11.5 servings/d (comparison) (Table 1). At 1 and 4 y, the comparison group data reflected the expected regression-to-the-mean effect with the lower 2 quartiles having higher intakes than the baseline group and the upper 2 quartiles having lower intakes than the baseline group. At both time points, the intervention was associated with a major increase in vegetable and fruit consumption. Among those in the lowest quartile of consumption at baseline, those in the intervention group consumed 77% more vegetables and fruit at 1 y than did those in the comparison group, and this quantity was still 48% higher at 4 y. At 1 y, there was a significant trend in the amount of change achieved by the intervention to vary by quartile, with the highest change among those who consumed the least at baseline (P = 0.04). By the 4-y time point, however, there was no significant difference in the change observed (P = 0.10), with all quartiles having a ≥40% difference between the intervention and comparison groups.
TABLE 1.
Dietary components at baseline, 1 y, and 4 y by baseline quartiles (Qs) among women without hot flashes in the Women's Healthy Eating and Living Study group1
| Baseline |
Year 1 |
Year 4 |
||||||||
| Intervention (n = 390) | Comparison (n = 377) | Intervention (n = 390) | Comparison (n = 377) | Percentage diff | P for trend2 | Intervention (n = 390) | Comparison (n = 377) | Percentage diff | P for trend2 | |
| Vegetables-fruit (servings/d) | 0.04 | 0.10 | ||||||||
| Q1: ≤4.9 | 3.8 (0.1)3 | 3.8 (0.1) | 8.9 (0.5) | 5.0 (0.2) | 77 | 7.5 (0.4) | 5.1 (0.2) | 48 | ||
| Q2: >4.9–6.7 | 5.9 (0.1) | 5.8 (0.1) | 10.5 (0.4) | 6.6 (0.2) | 60 | 8.6 (0.3) | 6.0 (0.2) | 42 | ||
| Q3: >6.7–8.9 | 7.8 (0.1) | 7.9 (0.1) | 11.7 (0.3) | 7.7 (0.3) | 54 | 10.0 (0.3) | 6.6 (0.2) | 52 | ||
| Q4: >8.9 | 11.3 (0.2) | 11.5 (0.3) | 14.3 (0.4) | 9.8 (0.3) | 46 | 12.2 (0.4) | 7.9 (0.3) | 55 | ||
| Fiber (g/d) | 0.02 | 0.07 | ||||||||
| Q1: ≤15.6 | 12.6 (0.2) | 12.2 (0.3) | 23.7 (0.9) | 15.4 (0.6) | 54 | 20.0 (0.8) | 14.4 (0.5) | 39 | ||
| Q2: >15.6–19.9 | 17.6 (0.2) | 17.5 (0.1) | 26.6 (0.9) | 18.8 (0.6) | 41 | 22.4 (0.7) | 18.0 (0.5) | 29 | ||
| Q3: >19.9–25.2 | 22.1 (0.2) | 22.6 (0.2) | 31.6 (0.8) | 22.7 (0.7) | 39 | 26.7 (0.8) | 21.2 (0.6) | 26 | ||
| Q4: >25.2 | 31.2 (0.6) | 32.0 (0.7) | 33.6 (1.0) | 28.4 (0.9) | 18 | 28.7 (0.8) | 24.0 (0.7) | 20 | ||
| Energy from fat (%) | 0.78 | 0.56 | ||||||||
| Q1: ≤23.8 | 19.5 (0.3) | 19.8 (0.3) | 18.8 (0.6) | 24.2 (0.5) | −23 | 23.2 (0.7) | 27.6 (0.7) | −16 | ||
| Q2: >23.8–28.6 | 26.2 (0.1) | 26.5 (0.2) | 21.9 (0.6) | 26.0 (0.6) | −16 | 26.9 (0.7) | 31.0 (0.8) | −13 | ||
| Q3: >28.6–33.4 | 30.9 (0.1) | 31.1 (0.1) | 25.1 (0.7) | 29.8 (0.7) | −16 | 28.6 (0.7) | 32.1 (0.7) | −11 | ||
| Q4: >33.4 | 37.3 (0.3) | 37.6 (0.3) | 26.2 (0.7) | 32.8 (0.7) | −20 | 32.0 (0.8) | 34.1 (0.8) | −6 | ||
| Fiber-to-fat ratio | 0.17 | 0.54 | ||||||||
| Q1: ≤0.25 | 0.21 (0.004) | 0.20 (0.004) | 0.57 (0.03) | 0.30 (0.02) | 90 | 0.41 (0.03) | 0.28 (0.02) | 46 | ||
| Q2: >0.25–0.36 | 0.30 (0.003) | 0.30 (0.003) | 0.72 (0.04) | 0.42 (0.02) | 71 | 0.54 (0.02) | 0.35 (0.01) | 54 | ||
| Q3: >0.36–0.54 | 0.44 (0.005) | 0.44 (0.005) | 0.80 (0.04) | 0.49 (0.03) | 63 | 0.58 (0.03) | 0.40 (0.02) | 45 | ||
| Q4: >0.54 | 0.80 (0.03) | 0.81 (0.03) | 1.13 (0.06) | 0.67 (0.03) | 69 | 0.78 (0.04) | 0.54 (0.02) | 44 | ||
Quartiles were determined by SAS PROC Univariate (SAS Institute, Cary, NC). diff, difference between groups.
Trends were tested by using general linear models in SAS.
Mean; SEM in parentheses (all such values).
At baseline, the lowest quartile of fiber consumption in the HF− subgroup consumed ≤15.6 g/d, with a mean intake of 12.6 g/d among the intervention arm and12.2 g/d in the comparison arm; the highest quartile had mean intake of 31.2 g/d in the intervention group and 32 g/d in the comparison group. Again, the comparison group intake at 1 and 4 y exhibited a regression-to-the-mean effect. At 1 y, the largest intervention group effect (+54%) occurred in the lowest quartile of baseline intake (compared with +18% in the highest baseline quartile, P = 0.02). By 4 y, however, the difference across quartiles reached only marginal levels of significance (P = 0.07), and a between-group difference of 20–40% was maintained through 4 y.
The lowest baseline quartile of percentage energy from fat was ≤23.8%, with a mean of 19.5% (intervention) and 19.8% (comparison); the highest baseline quartile had a mean of 37.3% (intervention) and 37.6% (comparison). The expected regression-to-the-mean effect for the comparison group was exhibited at 1 y; however, at 4 y, those in the comparison group increased their fat intake across all of the quartiles, with quartile means ranging from 27.6% to 34.1%. The statistically significant between-group difference was equivalent across baseline quartiles of fat intake at 1 y (P = 0.8) and 4 y (P = 0.6). At 1 y, the between-group difference in fat consumption was from 16% to 23%, whereas the maintained between-group difference at 4 y was 6–16%.
For the fiber-to-fat ratio, the lowest baseline quartile had a mean ratio of 0.2 (1 g fiber/5 g fat consumed). The intervention increased this ratio to 0.57 at 1 y, and it was maintained at 0.41 at 4 y, a 46% improvement over the comparison group. The highest baseline quartile had a ratio of 0.8 at baseline (8 g fiber/10 g fat consumed). At 1 y, the intervention had reversed the direction so that more fiber than fat was consumed (1.13); however, by 4 y, the ratio had returned to below its baseline level, although this still represented a 44% improvement over the comparison group. There was no trend for the between-group difference to vary by baseline quartiles at 1 or 4 y.
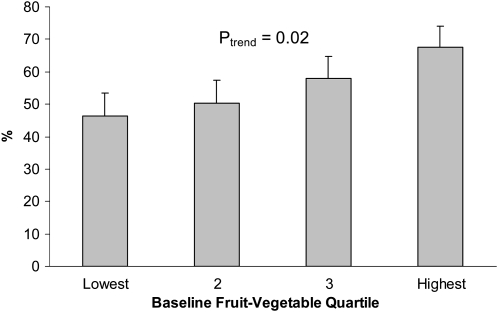
Two-thirds of women in the highest quartile of vegetable and fruit consumption reported physical activity levels >540 MET-min/wk compared with fewer than half of the women (46%) in the lowest quartile (Figure 1). Physical activity levels increased significantly with quartile of vegetable-fruit consumption [P for trend (Ptrend) = 0.02].
FIGURE 1.
Mean (95% CI) rates of physical activity (>540 metabolic equivalents · min · wk−1) by baseline fruit-vegetable quartile.
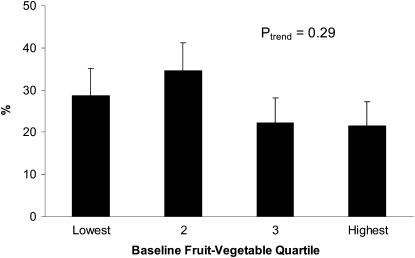
Although obesity was more frequent among those in the lower 2 quartiles of vegetable-fruit consumption than those in the upper 2 quartiles (Figure 2), the trend across quartile was not statistically significant (Ptrend = 0.29), with 29% of women in the lowest quartile being obese compared with 21% of those in the highest quartile. Neither obesity nor physical activity distributions differed significantly, however, between participants in the intervention and comparion groups.
FIGURE 2.
Mean (95% CI) rates of obesity by baseline fruit-vegetable quartile.
Breast cancer events and intervention effect by baseline diet quartiles
HRs for additional breast cancer events (new primaries and breast cancer recurrences) by baseline diet quartiles are listed in Table 2. Although the likelihood ratio test was not significant, we observed meaningful trends across consumption quartiles for several dietary variables. The HF− subgroup was not equally represented in the overall WHEL Study baseline quartiles for vegetable and fruit consumption, with a slight imbalance in favor of the lower quartiles (249 in quartile 1 compared with 215 in quartile 4). The crude event rate in the intervention group was highest in quartile 1 [26/124 (21%)] and lowest in quartile 4 (9.6%), whereas the highest rate in the comparison group was in quartile 1 (28%) and the lowest rate was in quartile 2 (16.6%) but not in quartile 4 (23%). In the time-to-event Cox models, adjusted for tumor stage, tumor grade, and antiestrogen therapy use, and stratified by each baseline quartile, all of the HR estimates for the intervention effect were below unity. A significant intervention group reduction in risk, however, was observed only for women in quartile 4 at baseline (adjusted HR: 0.41; 95% CI: 0.19, 0.86). Furthermore, the linear trend was significant (Ptrend = 0.01), indicating that the intervention increased disease-free survival more with each higher quartile of baseline vegetable and fruit consumption, with the HR estimates in the upper 2 quartiles explaining this trend.
TABLE 2.
Hazard ratios (HRs) and 95% CIs for additional breast cancer events after 7.3 y of follow-up according to baseline dietary quartiles (Qs) in the subgroup of women without hot flashes
| No. of participants (no. of additional breast cancer events) |
|||||
| Dietary component | All | Intervention | Comparison | HR1 (95% CI) | P for trend2 |
| All | 896 (179) | 446 (72) | 450 (107) | 0.7 (0.52, 0.95) | |
| Vegetables–fruit | 0.01 | ||||
| Q1: ≤4.9, servings/d | 249 (61) | 124 (26) | 125 (35) | 0.8 (0.48, 1.35) | |
| Q2: >4.9–6.7 servings/d | 222 (37) | 108 (18) | 114 (19) | 0.99 (0.51, 1.94) | |
| Q3: >6.7–8.9 servings/d | 210 (45) | 110 (18) | 100 (27) | 0.56 (0.31, 1.03) | |
| Q4: >8.9 servings/d | 215 (36) | 104 (10) | 111 (26) | 0.41 (0.19, 0.86) | |
| Fiber | 0.02 | ||||
| Q1: ≤15.6 g/d | 227 (45) | 113 (19) | 114 (26) | 0.82 (0.45, 1.48) | |
| Q2: >15.6–19.9 g/d | 226 (42) | 117 (20) | 109 (22) | 0.79 (0.42, 1.47) | |
| Q3: >19.9–25.2 g/d | 216 (42) | 107 (17) | 109 (25) | 0.99 (0.52, 1.89) | |
| Q4: >25.2 g/d | 227 (50) | 109 (16) | 118 (34) | 0.48 (0.26, 0.87) | |
| Energy from fat | 0.06 | ||||
| Q1: ≤23.8%/d | 229 (34) | 117 (11) | 112 (23) | 0.42 (0.2, 0.88) | |
| Q2: >23.8–28.6%/d | 214 (51) | 106 (25) | 108 (26) | 0.88 (0.5, 1.55) | |
| Q3: >28.6–33.4%/d | 228 (51) | 112 (19) | 116 (32) | 0.69 (0.38, 1.26) | |
| Q4: >33.4%/d | 225 (43) | 111 (17) | 114 (26) | 0.75 (0.4, 1.43) | |
| Fiber-to-fat ratio3 | 0.01 | ||||
| Q1: ≤0.25 | 221 (37) | 104 (15) | 117 (22) | 0.82 (0.42, 1.63) | |
| Q2: >0.25–0.36 | 237 (50) | 119 (22) | 118 (28) | 0.81 (0.45, 1.43) | |
| Q3: >0.36–0.54 | 221 (52) | 123 (24) | 98 (28) | 0.69 (0.39, 1.23) | |
| Q4: >0.54 | 217 (40) | 100 (11) | 117 (29) | 0.38 (0.19, 0.77) | |
Derived from Cox model adjusted for stage and grade of original tumor and antiestrogen therapy.
Linear trend test for intervention effect across quartiles of baseline dietary pattern in multiple Cox model; likelihood ratio tests for group quartile interaction were not significant for any diet component.
Fiber (g/d) divided by fat (g/d).
The HF− subgroup was approximately evenly distributed across the WHEL Study baseline quartiles of fiber consumption. Within the intervention group, the crude event rates varied only from 17% in quartile 1 to 14.6% in quartile 4. In the comparison group, however, these event rates were 22.8% and 28.8% in quartiles 1 and 4, respectively. In the adjusted Cox models, stratified by each baseline quartile, all of the HR estimates were below unity; however, only the highest quartile of fiber consumption showed a significant intervention group effect (adjusted HR: 0.48; 95% CI: 0.26, 0.87). Again there was a significant trend for intervention effect observed across increasing fiber quartiles (Ptrend = 0.02), with the strongest effect observed in the highest quartile.
There were similar proportions of HF−women in each quartile of baseline percentage energy from fat. Again looking at crude unadjusted event rates, within the intervention group the lowest event rate was seen in the quartile with the lowest intake of fat (quartile 1: 9.4%). The event rate was highest in quartile 2 (23.6%), however, before declining across quartiles to 15.3% in quartile 4. Within the comparison group, the event rate was lowest in quartile 1 (20.5%) and highest in quartile 3 (27.6%). In the adjusted Cox model stratified by baseline quartile, the HR estimate was below unity within each quartile; however, only in quartile 1 did we observe a significant intervention group effect (adjusted HR: 0.42; 95% CI: 0.2, 0.88). The bounce seen in event rates across quartiles resulted in a marginally significant linear trend for the effect of the intervention across quartile of increasing fat (Ptrend = 0.06).
For the fiber-to-fat ratio, the crude event rate in the intervention group was lowest in those who consumed the highest ratio of fiber to fat (quartile 4: 11%); however, the highest rate was in quartile 3 (19.5%) not in quartile 1 (14.4%). In the comparison group, the highest event rate was in quartile 3 (28.6%) and the lowest was in quartile 1 (18.8%). Again, in the adjusted Cox models, the HR estimate for the intervention effect was below unity for each quartile, with statistical significance achieved only in the highest quartile (HRadj = 0.38; 95% CI: 0.19, 0.77). The observed statistically significant trend (Ptrend = 0.01) reflected the increasing between-group difference in event rates especially evident in quartiles 3 and 4.
DISCUSSION
Previously, in an a priori secondary analysis, we observed that the WHEL Study intervention was associated with lowered risk of a second breast cancer event among peri- and postmenopausal women who reported no hot flashes at study baseline (18). This article investigates how this effect varied across baseline quartiles of dietary pattern. The intervention achieved significant long-term dietary change across all of the quartiles of baseline intake for each component (vegetable-fruit, fiber, and fat-to- fiber ratio) at 4 y. The change achieved by the intervention was not significantly different across quartiles. Significant trends were observed in the HRs for the intervention across quartiles for vegetable-fruit intake, fiber intake, and fiber-to-fat ratio. Although the trend across quartiles was significant, it was only in the quartile with the most putatively cancer-preventive diet that the reduction in events observed in the intervention group was statistically significant, ie, significantly lower HRs between the intervention and comparison groups were observed only for those in the highest quartiles for vegetable-fruit intake, fiber intake, and fiber-to-fat ratio and in the lowest quartile for percentage energy from fat.
The intervention group in the WHEL Study received more contact than did the comparison group, suggesting that there may have been a possibility that additional social support could be a factor in the observed differences. The total contact in the intervention group, however, was only 31 calls or ≈16 h during 4 y, a minute amount compared with a participant's waking hours during this time period. Social support usually is envisaged as requiring considerable contact to be effective. Additionally, the intervention contact was equivalent in the HF+ subgroup, and there was no effect on study outcomes observed in this subgroup.
Neither the trend across quartiles nor the significant difference only in the most putatively cancer-preventive baseline quartile can be explained by differential response to the study intervention. The study intervention was effective in motivating women to improve their dietary pattern regardless of their dietary intake at baseline. In the HF− subgroup, for vegetable-fruit servings and fiber intake, we observed the largest increases among participants who consumed the fewest vegetable-fruit servings and least amount of fiber at baseline. Even though these differences had diminished by 4 y, considerable between-group differences were observed in nearly all of the baseline quartiles of dietary pattern. Thus, the degree of change achieved by the intervention cannot explain the significant between-group differences observed across baseline quartiles. Furthermore, although BMI or physical activity level may have had an effect on event rates, no baseline between-group differences in these variables were observed and neither changed during the study. This suggests that, contrary to commonly held theory (26, 27), a diet high in fruits and vegetables, ie, typically low in energy density, may need to be accompanied by caloric restriction if it is to be associated with a reduction in weight (28).
We have previously shown that baseline circulating estrogen concentrations were higher in those in the HF− subgroup compared with those in the HF+ subgroup (7). It has been postulated that polymorphisms of estrogen metabolism genes may explain the differences in circulating estrogen concentrations and the reporting of hot flashes (9, 29). Dietary fiber binds unconjugated estrogens, impeding reabsorption from the gut (30); however, the effect of increased fiber on circulating estrogen concentrations seems modest and inconsistent (17). Should this be the mechanism for the dietary effect on a new primary or a breast cancer recurrence, it is possible that significant dietary change may be needed in addition to a high baseline intake to observe a marked effect. A strength of the WHEL Study is that blood samples were collected and stored at 5 intervals throughout the study, thus allowing in-depth analyses to test mechanistic hypotheses.
In summary, the WHEL Study showed that a dietary pattern high in vegetables, fruit, and fiber and low in fat did not lower risk of additional breast cancer events for all of the breast cancer survivors. The intervention did seem to lower the risk in those women in the HF− subgroup, a status suggesting higher circulating estrogen concentrations. Even though this was an a priori secondary analysis, this result needs to be replicated before it can be accepted with confidence. This analysis suggests, however, that although the study intervention may have had an effect across the whole HF− subgroup, it was not the degree of change that was achieved that had the greatest effect on additional events. Instead, the greatest effect occurred among women who already were eating significant amounts of vegetables, fruits, and fiber at baseline. Further study is needed of the biological specimens collected on the WHEL Study to elucidate the mechanism for this apparent differential effect of dietary change on additional breast cancer events in this minority group of breast cancer survivors. (Other articles in this supplement to the Journal include references 31–57.)
Acknowledgments
WHEL Study Group WHEL Study Coordinating Center: University of California, San Diego, Cancer Prevention and Control Program, Moores UCSD Cancer Center, San Diego, CA (John P Pierce, Susan Faerber, Barbara A Parker, Loki Natarajan, Cheryl L Rock, Vicky A Newman, Shirley W Flatt, Sheila Kealey, Linda Wasserman, Wayne A Bardwell, Lisa Madlensky, and Wael Al-Delaimy).
WHEL Study Clinical Sites: Center for Health Research-Portland, Portland, OR (Njeri Karanja and Mark U Rarick); Kaiser Permanente Northern California, Oakland, CA (Bette J Caan and Lou Fehrenbacher); Stanford Prevention Research Center, Stanford University, CA (Marcia L Stefanick and Robert Carlson); University of Arizona, Tucson and Phoenix, AZ (Cynthia Thomson, James Warneke, and Cheryl Ritenbaugh); University of California, Davis, Davis, CA (Ellen B Gold and Sidney Scudder); University of California, San Diego, Moores UCSD Cancer Center, San Diego, CA (Kathryn A Hollenbach and Vicky Jones); University of Texas M D Anderson Cancer Center, Houston, TX (Lovell A Jones, Richard Hajek, and Richard Theriault).
The authors' responsibilities were as follows—JPP and LN: study concept and design; JPP, BJC, EBG, MLS, CAT, and BP: acquisition of data; JPP, LN, BJC, SWF, EBG, MLS, SK, CLR, MP, and NS: analysis and interpretation of data; JPP, LN, BJC, SWF, EBG, and MLS: drafting of the manuscript; JPP, LN, BJC, SK, EBG, RAH, CLR, MLS, CAT, and BP: critical revision of the manuscript for important intellectual content; LN, SWF, MP, and NS: statistical analysis; JPP, BJC, EBG, MLS, and CAT: obtained funding; JPP, SWF, SK, and BBP: administrative, technical, or material support; and JPP, LN, BJC, EBG, MLS, and CAT: study supervision. None of the authors had conflicts of interest to disclose.
REFERENCES
- 1.Pierce JP, Faerber S, Wright FA, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living (WHEL) Study. Control Clin Trials 2002;23:728–56 [DOI] [PubMed] [Google Scholar]
- 2.Madlensky L, Natarajan L, Flatt S, Faerber S, Newman V, Pierce JP. Timing of dietary change in response to a telephone counseling intervention: Evidence from the WHEL study. Health Psychol 2008;27:539–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierce JP, Newman VA, Flatt SW, et al. Telephone counseling intervention increases intakes of micronutrient- and phytochemical-rich vegetables, fruit and fiber in breast cancer survivors. J Nutr 2004;134:452–8 [DOI] [PubMed] [Google Scholar]
- 4.Pierce JP, Newman VA, Natarajan L, et al. Telephone counseling helps maintain long-term adherence to a high-vegetable dietary pattern. J Nutr 2007;137:2291–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierce JP, Natarajan L, Sun S, et al. Increases in plasma carotenoid concentrations in response to a major dietary change in the women's healthy eating and living study. Cancer Epidemiol Biomarkers Prev 2006;15:1886–92 [DOI] [PubMed] [Google Scholar]
- 6.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA 2007;298:289–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rock CL, Flatt SW, Laughlin GA, et al. Reproductive steroid hormones and recurrence-free survival in women with a history of breast cancer. Cancer Epidemiol Biomarkers Prev 2008;17:614–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 2005;23:9312–8 [DOI] [PubMed] [Google Scholar]
- 9.Mortimer JE, Flatt SW, Parker BA, et al. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat 2008;108:421–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuzick J. Hot flushes and the risk of recurrence: retrospective, exploratory results from the ATAC trial. SABCS poster 2069. San Antonio Breast Cancer Symposium, San Antonio, TX, December 2007. Available from: http://www.abstracts2view.com/sabcs07/view.php?nu=SABCS07L_426 (cited 6 March 2009) [Google Scholar]
- 11.Monroe KR, Murphy SP, Henderson BE, et al. Dietary fiber intake and endogenous serum hormone levels in naturally postmenopausal Mexican American women: the multiethnic cohort study. Nutr Cancer 2007;58:127–35 [DOI] [PubMed] [Google Scholar]
- 12.Goldin BR, Woods MN, Spiegelman DL, et al. The effect of dietary fat and fiber on serum estrogen concentrations in premenopausal women under controlled dietary conditions. Cancer 1994;74:1125–31 [DOI] [PubMed] [Google Scholar]
- 13.Heber D, Ashley JM, Leaf DA, Barnard RJ. Reduction of serum estradiol in postmenopausal women given free access to low-fat high-carbohydrate diet. Nutrition 1991;7:137–9 [PubMed] [Google Scholar]
- 14.Barnard RJ, Gonzalez JH, Liva ME, Ngo TH. Effects of a low-fat, high-fiber diet and exercise program on breast cancer risk factors in vivo and tumor cell growth and apoptosis in vitro. Nutr Cancer 2006;55:28–34 [DOI] [PubMed] [Google Scholar]
- 15.Prentice R, Thompson D, Clifford C, Gorbach S, Goldin B, Byar D. Dietary fat reduction and plasma estradiol concentration in healthy postmenopausal women. The Women's Health Trial Study Group. J Natl Cancer Inst 1990;82:129–34 [DOI] [PubMed] [Google Scholar]
- 16.Rose DP, Connolly JM, Chlebowski RT, Buzzard IM, Wynder EL. The effects of a low-fat dietary intervention and tamoxifen adjuvant therapy on the serum estrogen and sex hormone-binding globulin concentrations of postmenopausal breast cancer patients. Breast Cancer Res Treat 1993;27:253–62 [DOI] [PubMed] [Google Scholar]
- 17.Rock CL, Flatt SW, Thomson CA, et al. Effects of a high-fiber, low-fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. J Clin Oncol 2004;22:2379–87 [DOI] [PubMed] [Google Scholar]
- 18.Gold EB, Pierce JP, Natarajan L, et al. Dietary pattern influences breast cancer prognosis in women without hot flashes: the Women's Healthy Eating and Living (WHEL) Trial. J Clin Oncol 2008; 26 Available from: http://jco.ascopubs.org/cgi/reprint/JCO.2008.16.1067v1 (cited 15 December 2008) [DOI] [PMC free article] [PubMed]
- 19.McTiernan A, Wu L, Chen C, et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 2006;14:1662–77 [DOI] [PubMed] [Google Scholar]
- 20.Newman VA, Thomson CA, Rock CL, et al. Achieving substantial changes in eating behavior among women previously treated for breast cancer—an overview of the intervention. J Am Diet Assoc 2005;105:382–91 [DOI] [PubMed] [Google Scholar]
- 21.Matthews KA, Shumaker SA, Bowen DJ, et al. Women's Health Initiative. Why now? What it is? What's new? Am Psychol 1997;52:101–16 [DOI] [PubMed] [Google Scholar]
- 22.Natarajan L, Flatt SW, Sun X, et al. Validity and systematic error in measuring carotenoid consumption with dietary self-report instruments. Am J Epidemiol 2006;163:770–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav 2007;31:193–202 [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–504 [DOI] [PubMed] [Google Scholar]
- 25.Holm S. A simple sequentially rejective multiple test procedure. Scand Stat Theory Appl 1979;6:65–70 [Google Scholar]
- 26.Ledikwe JH, Blanck HM, Kettel Khan L, et al. Dietary energy density is associated with energy intake and weight status in US adults. Am J Clin Nutr 2006;83:1362–8 [DOI] [PubMed] [Google Scholar]
- 27.Bell EA, Rolls BJ. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am J Clin Nutr 2001;73:1010–8 [DOI] [PubMed] [Google Scholar]
- 28.Saquib N, Natarajan L, Rock C, et al. The impact of a long-term reduction in dietary energy density on body weight within a randomized diet trial. Nutr Cancer 2008;60:31–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crandall CJ, Crawford SL, Gold EB. Vasomotor symptom prevalence is associated with polymorphisms in sex steroid-metabolizing enzymes and receptors. Am J Med 2006;119:S52–60 [DOI] [PubMed] [Google Scholar]
- 30.Shultz TD, Howie BJ. In vitro binding of steroid hormones by natural and purified fibers. Nutr Cancer 1986;8:141–7 [DOI] [PubMed] [Google Scholar]
- 31.Rajaram S, Sabaté J. Preface. Am J Clin Nutr 2009;89(suppl):1541S–2S [DOI] [PubMed] [Google Scholar]
- 32.Jacobs DR, Jr, Gross MD, Tapsell LC. Food synergy: an operational concept for understanding nutrition. Am J Clin Nutr 2009;89(suppl):1543S–8S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs DR, Jr, Haddad EH, Lanou AJ, Messina MJ. Food, plant food, and vegetarian diets in the US dietary guidelines: conclusions of an expert panel. Am J Clin Nutr 2009;89(suppl):1549S–52S [DOI] [PubMed] [Google Scholar]
- 34.Lampe JW. Interindividual differences in response to plant-based diets: implications for cancer risk. Am J Clin Nutr 2009;89(suppl):1553S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon JA, Chen Y-H, Bent S. The relation of α-linolenic acid to the risk of prostate cancer: a systematic review and meta-analysis. Am J Clin Nutr 2009;89(suppl):1558S–64S [DOI] [PubMed] [Google Scholar]
- 36.Newby PK. Plant foods and plant-based diets: protective against childhood obesity? Am J Clin Nutr 2009;89(suppl):1572S–87S [DOI] [PubMed] [Google Scholar]
- 37.Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89(suppl):1588S–96S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangat I. Do vegetarians have to eat fish for optimal cardiovascular protection? Am J Clin Nutr 2009;89(suppl):1597S–601S [DOI] [PubMed] [Google Scholar]
- 39.Willis LM, Shukitt-Hale B, Joseph JA. Modulation of cognition and behavior in aged animals: role for antioxidant- and essential fatty acid–rich plant foods. Am J Clin Nutr 2009;89(suppl):1602S–6S [DOI] [PubMed] [Google Scholar]
- 40.Fraser GE. Vegetarian diets: what do we know of their effects on common chronic diseases? Am J Clin Nutr 2009;89(suppl):1607S–12S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 2009;89(suppl):1613S–9S [DOI] [PubMed] [Google Scholar]
- 42.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Cancer incidence in vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 2009;89(suppl):1620S–6S [DOI] [PubMed] [Google Scholar]
- 43.Craig WJ. Health effects of vegan diets. Am J Clin Nutr 2009;89(suppl):1627S–33S [DOI] [PubMed] [Google Scholar]
- 44.Weaver CM. Should dairy be recommended as part of a healthy vegetarian diet? Point. Am J Clin Nutr 2009;89(suppl):1634S–7S [DOI] [PubMed] [Google Scholar]
- 45.Lanou AJ. Should dairy be recommended as part of a healthy vegetarian diet? Counterpoint. Am J Clin Nutr 2009;89(suppl):1638S–42S [DOI] [PubMed] [Google Scholar]
- 46.Sabaté J, Ang Y. Nuts and health outcomes: new epidemiologic evidence. Am J Clin Nutr 2009;89(suppl):1643S–8S [DOI] [PubMed] [Google Scholar]
- 47.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr 2009;89(suppl):1649S–56S [DOI] [PubMed] [Google Scholar]
- 48.Rajaram S, Haddad EH, Mejia A, Sabaté J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr 2009;89(suppl):1657S–63S [DOI] [PubMed] [Google Scholar]
- 49.Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr 2009;89(suppl):1664S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badger TM, Gilchrist JM, Pivik RT, et al. The health implications of soy infant formula. Am J Clin Nutr 2009;89(suppl):1668S–72S [DOI] [PubMed] [Google Scholar]
- 51.Messina M, Wu AH. Perspectives on the soy–breast cancer relation. Am J Clin Nutr 2009;89(suppl):1673S–9S [DOI] [PubMed] [Google Scholar]
- 52.Lönnerdal B. Soybean ferritin: implications for iron status of vegetarians. Am J Clin Nutr 2009;89(suppl):1680S–5S [DOI] [PubMed] [Google Scholar]
- 53.Chan J, Jaceldo-Siegl K, Fraser GE. Serum 25-hydroxyvitamin D status of vegetarians, partial vegetarians, and nonvegetarians: the Adventist Health Study-2. Am J Clin Nutr 2009;89(suppl):1686S–92S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elmadfa I, Singer I. Vitamin B-12 and homocysteine status among vegetarians: a global perspective. Am J Clin Nutr 2009;89(suppl):1693S–8S [DOI] [PubMed] [Google Scholar]
- 55.Marlow HJ, Hayes WK, Soret S, Carter RL, Schwab ER, Sabaté J. Diet and the environment: does what you eat matter? Am J Clin Nutr 2009;89(suppl):1699S–703S [DOI] [PubMed] [Google Scholar]
- 56.Carlsson-Kanyama A, González AD. Potential contributions of food consumption patterns to climate change. Am J Clin Nutr 2009;89(suppl):1704S–9S [DOI] [PubMed] [Google Scholar]
- 57.Eshel G, Martin PA. Geophysics and nutritional science: toward a novel, unified paradigm. Am J Clin Nutr 2009;89(suppl):1710S–6S [DOI] [PubMed] [Google Scholar]