Abstract
Gut bacterial modification of soy isoflavones produces metabolites that differ in biological activity from the parent compounds. Hydrolysis of glycosides results in more active compounds. In contrast, further degradation and transformation of aglycones produce more or less active compounds, depending on the substrate metabolized and the product formed. Bacterial metabolism of soy isoflavones varies among individuals. The predominant daidzein metabolites produced by human intestinal bacteria are equol and O-desmethylangolensin. Among humans, 30–50% have the bacteria capable of producing equol and 80–90% harbor O-desmethylangolensin–producing bacteria. Factors that influence the capacity to produce equol and O-desmethylangolensin are not clearly established; however, gut physiology, host genetics, and diet are reported to contribute to interindividual differences in conversion of daidzein to equol. Effects of these phenotypes on human health are poorly characterized. Some studies in high soy–consuming populations reported an inverse association between urinary and serum equol concentrations and breast and prostate cancer risk. Furthermore, several studies of soy supplementation and bone density suggest that soy products may be more effective in maintaining bone density in equol-producing individuals. Factors that contribute to the phenotypes and the relation of these specific phenotypes to human health need to be further elucidated. The extent to which isoflavone metabolism is key to the efficacy of soy foods remains to be established.
INTRODUCTION
Intake of particular phytochemicals or their precursors does not necessarily equate with exposure at the tissue level. The process of phytochemical disposition, like that of disposition of drugs and other xenobiotics, involves absorption, metabolism, distribution, and excretion, and each of these parts of the process may contribute to overall exposure (1). Interindividual differences in phytochemical metabolism and disposition may be affected by gut microbial identity and activity; genetic determinants of biotransformation enzyme expression, stability, and activity; environmental exposures that influence the gut microbes and biotransformation enzymes; and variation in concentrations of endogenous compounds that modulate biotransformation pathways (1).
Isoflavone metabolism is a familiar example of interindividual differences in effects of host bacteria and the potential effect on human health. Gut bacterial modification of soy isoflavones produces metabolites that differ in biologic activity from the parent compounds. Hydrolysis of glycosides results in more active compounds. In contrast, further degradation and transformation of aglycones produce more or less active compounds, depending on the substrate metabolized and the product formed. Furthermore, bacterial metabolism of soy isoflavones varies among individuals.
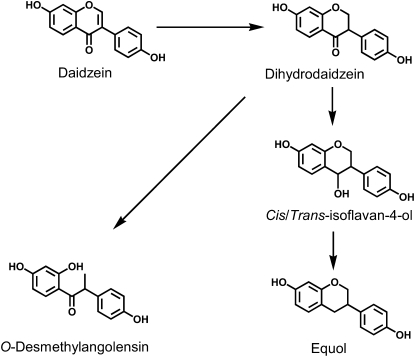
The predominant daidzein metabolites produced by human intestinal bacteria are dihydrodadzein, equol, and O-desmethylangolensin (Figure 1). Among humans, 30–50% have the bacteria capable of producing equol, whereas 80–90% harbor O-desmethylangolensin–producing bacteria. The production of equol in humans is of particular interest for the following reasons: 1) in vitro and in some animal models, equol is more biologically active than its precursor, daidzein, and the alternate metabolite, O-desmethylangolensin and 2) equol production is associated with reduced risk of certain cancers and other diseases in humans (reviewed in reference 2).
FIGURE 1.
Metabolism of the soy isoflavone daidzein to O-desmethylangolensin and equol. Adapted from Reference 31.
DETERMINANTS OF THE EQUOL-PRODUCER PHENOTYPES
Factors that influence the capacity to produce equol are not clearly established; however, gut physiology, host genetics, and diet appear to contribute to interindividual differences in conversion of daidzein to equol. Several aspects of gut function are shown to be associated with the capacity to produce equol. Tamura et al (3) reported that the percentage of equol producers tended to be greater among lactose malabsorbers. Lactase can hydrolyze isoflavone glycosides; thus, among individuals with low lactase activity, presumably more daidzin is reaching the colon and being hydrolyzed and converted to equol (3). Low lactase activity also may affect delivery of other dietary constituents to the colon and further affect the gut bacterial profile. This relation needs to be characterized further. Large intestinal transit also is shown to be associated with the equol-producer phenotype. In a study of 200 premenopausal women, equol producers reported more frequent constipation than nonproducers (4).
The apparent stability of equol production in individuals over time (5) raises the possibility that the phenotype may be under some level of genetic control. Host genetics were shown to influence normal intestinal bacterial populations (6, 7). One family study tested specifically the role of host genetics on daidzein-metabolizing phenotypes (8). Familial correlation and segregation analyses suggested that there may be a genetic component to being an equol producer but that other nongenetic factors also likely were involved. More robust study designs and larger samples are needed to test this hypothesis further.
Comparison of the habitual dietary intakes of equol producers and nonproducers showed some differences; however, the differences appeared to be influenced by the populations under study. Among studies that assessed diet in relation to equol production, positive associations between equol production and intakes of soy, animal products, green tea, and a low-fat, high-carbohydrate diet were reported in some (9–12) but not all of the studies (13–15). Three recently published studies of equol production and diet among women showed few dietary differences by phenotype. Bolca et al (16) reported that in 100 healthy postmenopausal women, the strong equol-producer phenotype (highest of 3 categories of equol excretion with an isoflavone challenge) was associated with higher intakes of polyunsaturated fatty acids and alcohol. Atkinson et al (4) assessed diet with food-frequency questionnaire and a 3-d food record in 200 women but showed few dietary differences between the phenotypes. Associations between intake of vegetables and eggs and equol production were observed, but these were not significant when adjusted for false discovery rate. Nagata et al (17) evaluated urinary equol excretion in relation to diet in 419 Japanese women. In this population, dairy product intake was significantly lower (P = 0.02) in women who excreted detectable equol in urine. The authors suggest that constituents of dairy foods may influence gut bacterial composition and the presence of equol-producing bacteria; however, given the recent observation that the prevalence of equol production is higher among lactose malabsorbers (3), an alternative interpretation of these dietary data is that equol producers are more likely to be lactose malabsorbers and, therefore, tend to avoid dairy foods.
Several studies suggest that the prevalence of equol-producing phenotypes differs between low soy– and high soy–consuming populations (15, 18, 19), although whether this is due to regular soy exposure itself or population differences in other consituents of diet remains to be established. Setchell and Cole (15) reported that in a sample of 41 healthy volunteers (29 vegetarians and 12 nonvegetarians), the prevalence of equol producers among the vegetarians and nonvegetarians was 59% and 25%, respectively. Similarly, Song et al (19) reported that the prevalence of the equol-producer phenotype was significantly higher (51% compared with 36%; P = 0.015) and the O-desmethylangolensin–producer phenotype was significantly lower (84% compared with 92%; P = 0.03) in 91 Korean-American than in 222 white American women, and girls.
Other demographic and lifestyle factors also were evaluated in relation to the equol-producer phenotype. In the United States, equol production was positively associated with education in 2 predominantly white populations (4, 8) but not another (20). Smoking status also is associated with the phenotype in some (20) but not other study populations (4, 8, 17).
ASSOCIATION OF EQUOL-PRODUCER PHENOTYPE WITH HUMAN HEALTH
Effects of the equol-producer phenotype on human health are poorly characterized. In 2005, Atkinson et al (2) reviewed 26 observational and intervention studies that examined the effect of the equol phenotype on human health. At that time, insufficient evidence was available to inform on the potential health effects associated with the ability to produce equol. Limitations of the available observation studies included small sample sizes, insufficient statistical power to stratify by phenotype, and lack of controlled evaluation of equol-producer status, particularly in low soy–consuming populations. Limitations of intervention trials similarly included small sample sizes, which resulted in lack of sufficient power to conduct formal analysis of phenotype-treatment interactions.
Since 2005, additional studies examined the effect of the equol-producer phenotype and reported both significant and null results. In a recent observational study in which postmenopausal women were phenotyped for equol production, Fuhrman et al (21) showed that despite no independent associations of phenotype or soy intake with mammographic density, the interaction between these factors was statistically significant (Pinteraction = 0.01). Among equol producers, those consuming soy at least once per week had lower percentage density, whereas among nonproducers, weekly soy intake was associated with higher percentage density.
In recent intervention studies in which subanalyses by equol-producer status were conducted, there were reports that equol producers showed more favorable responses to soy or soy isoflavone treatment; however, these findings are not always consistent across studies and there is concern that null studies may be underreported. Effects of isoflavones on bone density and bone markers were reported to have a more bone-protective response in equol producers in some recent studies (22, 23) but not in others (24). With regard to markers of cardiovascular risk, Clerici et al (25) showed that, in a randomized placebo-controlled trial of soy-germ supplementation in 62 hypercholesterolemic men and women, reductions in total cholesterol and LDL cholesterol and high-sensitivity C-reactive protein and increases in brachial artery flow-mediated vasodilatation were greatest in the equol producers. In contrast, in 30 postmenopausal women (15 equol producers and 15 nonproducers), 12 wk of isoflavone supplementation had no effect on total cholesterol or adenosine triphosphate-binding cassette A1–dependent cholesterol efflux, irrespective of equol status (26). Another isoflavone intervention in 37 men with a personal or family history of colorectal adenoma showed that circulating insulin-like growth factor I decreased in equol producers but not in nonproducers after 8 wk and that the change in serum insulin-like growth factor I concentrations was inversely associated with serum equol concentrations (27).
Application of gene expression array and other omics platforms to soy isoflavone interventions also may help to guide future studies of the effects of the equol phenotype. For example, Niculescu et al (28) showed a stronger effect of isoflavone supplementation (900 mg/d for 84 d) on estrogen-responsive genes in peripheral lymphocytes among postmenopausal women who were equol producers. Understanding the pathways through which the equol-producer phenotype modifies response to isoflavones may clarify the role of equol itself.
From the latest observational studies and baseline data from intervention studies, it appears that for the most part, in the absence of soy exposure, there are few differences in health and disease-related biomarkers between equol producers and nonproducers (21, 27, 29). Nonetheless, in low soy–consuming populations, there are reports of biomarker differences by equol-producer status (20, 30). These may be chance findings or, alternatively, there may be cases in which host genetics or exposures besides isoflavones modify the effect of equol-producing bacteria and result in measurable differences between equol producers and nonproducers (30).
CONCLUSIONS
Various factors influence the ability to harbor certain bacterial populations, but the specific factors related to the equol-producer phenotype appear to be population dependent and remain unclear. Studies report relationships between the equol-producer phenotype and risk factors for several chronic diseases and differential responses to interventions; however, the evidence to date is inconclusive. More studies are needed that are designed to address a priori the effect of the equol-producer phenotype on disease risk. For intervention studies, sample sizes need to be large enough to allow for formal testing of the interaction of treatment and phenotype. (Other articles in this supplement to the Journal include references 32–58.)
Acknowledgments
The author had no conflicts of interest.
REFERENCES
- 1.Lampe JW, Chang JL. Interindividual differences in phytochemical metabolism and disposition. Semin Cancer Biol 2007;17:347–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 2005;230:155–70 [DOI] [PubMed] [Google Scholar]
- 3.Tamura A, Shiomi T, Hachiya S, Shigematsu N, Hara H. Low activities of intestinal lactase suppress the early phase absorption of soy isoflavones in Japanese adults. Clin Nutr 2008;27:248–53 [DOI] [PubMed] [Google Scholar]
- 4.Atkinson C, Newton KM, Bowles EJ, Yong M, Lampe JW. Demographic, anthropometric, and lifestyle factors and dietary intakes in relation to daidzein-metabolizing phenotypes among premenopausal women in the United States. Am J Clin Nutr 2008;87:679–87 [DOI] [PubMed] [Google Scholar]
- 5.Frankenfeld CL, Atkinson C, Thomas WK, et al. High concordance of daidzein-metabolizing phenotypes in individuals measured 1 to 3 years apart. Br J Nutr 2005;94:873–6 [DOI] [PubMed] [Google Scholar]
- 6.Flatz G, Czeizel A, Metneki J, Flatz SD, Kuhnau W, Jahn D. Pulmonary hydrogen and methane excretion following ingestion of an unabsorbable carbohydrate: a study of twins. J Pediatr Gastroenterol Nutr 1985;4:936–41 [DOI] [PubMed] [Google Scholar]
- 7.Van de Merwe JP, Stegeman JH, Hazenberg MP. The resident faecal flora is determined by genetic characteristics of the host. Implications for Crohn's disease? Antonie Van Leeuwenhoek 1983;49:119–24 [DOI] [PubMed] [Google Scholar]
- 8.Frankenfeld CL, Atkinson C, Thomas WK, et al. Familial correlations, segregation analysis, and nongenetic correlates of soy isoflavone-metabolizing phenotypes. Exp Biol Med (Maywood) 2004;229:902–13 [DOI] [PubMed] [Google Scholar]
- 9.Hedlund TE, Maroni PD, Ferucci PG, et al. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in caucasian men. J Nutr 2005;135:1400–6 [DOI] [PubMed] [Google Scholar]
- 10.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med 1998;217:335–9 [DOI] [PubMed] [Google Scholar]
- 11.Miyanaga N, Akaza H, Takashima N, et al. Higher consumption of green tea may enhance equol production. Asian Pac J Cancer Prev 2003;4:297–301 [PubMed] [Google Scholar]
- 12.Rowland IR, Wisemen H, Sanders TAB, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer 2000;36:27–32 [DOI] [PubMed] [Google Scholar]
- 13.Adlercreutz H, Honjo H, Higashi A, et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr 1991;54:1093–100 [DOI] [PubMed] [Google Scholar]
- 14.Ozasa K, Nakao M, Watanabe Y, et al. Association of serum phytoestrogen concentration and dietary habits in a sample set of the JACC Study. J Epidemiol 2005;15(Suppl 2):S196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr 2006;136:2188–93 [DOI] [PubMed] [Google Scholar]
- 16.Bolca S, Possemiers S, Herregat A, et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J Nutr 2007;137:2242–6 [DOI] [PubMed] [Google Scholar]
- 17.Nagata C, Ueno T, Uchiyama S, et al. Dietary and lifestyle correlates of urinary excretion status of equol in Japanese women. Nutr Cancer 2008;60:49–54 [DOI] [PubMed] [Google Scholar]
- 18.Arai Y, Uehara M, Sato Y, et al. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol 2000;10:127–35 [DOI] [PubMed] [Google Scholar]
- 19.Song KB, Atkinson C, Frankenfeld CL, et al. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J Nutr 2006;136:1347–51 [DOI] [PubMed] [Google Scholar]
- 20.Frankenfeld CL, McTiernan A, Aiello EJ, et al. Mammographic density in relation to daidzein-metabolizing phenotypes in overweight, postmenopausal women. Cancer Epidemiol Biomarkers Prev 2004;13:1156–62 [PubMed] [Google Scholar]
- 21.Fuhrman BJ, Teter BE, Barba M, et al. Equol status modifies the association of soy intake and mammographic density in a sample of postmenopausal women. Cancer Epidemiol Biomarkers Prev 2008;17:33–42 [DOI] [PubMed] [Google Scholar]
- 22.Uesugi S, Watanabe S, Ishiwata N, Uehara M, Ouchi K. Effects of isoflavone supplements on bone metabolic markers and climacteric symptoms in Japanese women. Biofactors 2004;22:221–8 [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Oka J, Higuchi M, et al. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial. Metabolism 2006;55:423–33 [DOI] [PubMed] [Google Scholar]
- 24.Brink E, Coxam V, Robins S, et al. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr 2008;87:761–70 [DOI] [PubMed] [Google Scholar]
- 25.Clerici C, Setchell KD, Battezzati PM, et al. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J Nutr 2007;137:2270–8 [DOI] [PubMed] [Google Scholar]
- 26.Badeau R, Jauhiainen M, Metso J, et al. Effect of isolated isoflavone supplementation on ABCA1-dependent cholesterol efflux potential in postmenopausal women. Menopause 2007;14:293–9 [DOI] [PubMed] [Google Scholar]
- 27.Vrieling A, Rookus MA, Kampman E, et al. Isolated isoflavones do not affect the circulating insulin-like growth factor system in men at increased colorectal cancer risk. J Nutr 2007;137:379–83 [DOI] [PubMed] [Google Scholar]
- 28.Niculescu MD, Pop EA, Fischer LM, Zeisel SH. Dietary isoflavones differentially induce gene expression changes in lymphocytes from postmenopausal women who form equol as compared with those who do not. J Nutr Biochem 2007;18:380–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkinson C, Newton KM, Stanczyk FZ, Westerlind KC, Li L, Lampe JW. Daidzein-metabolizing phenotypes in relation to serum hormones and sex hormone binding globulin, and urinary estrogen metabolites in premenopausal women in the United States. Cancer Causes Control 2008;19:1085–93 [DOI] [PubMed] [Google Scholar]
- 30.Törmälä RM, Appt S, Clarkson TB, Tikkanen MJ, Ylikorkala O, Mikkola TS. Individual differences in equol production capability modulate blood pressure in tibolone-treated postmenopausal women: lack of effect of soy supplementation. Climacteric 2007;10:471–9 [DOI] [PubMed] [Google Scholar]
- 31.Heinonen S, Wähälä K, Adlercreutz H. Identification of isoflavone metabolites dihydrodaidzein, dihydrogenistein, 6′-OH-O-dma, and cis-4-OH-equol in human urine by gas chromatography-mass spectroscopy using authentic reference compounds. Anal Biochem 1999;274:211–9 [DOI] [PubMed] [Google Scholar]
- 32.Rajaram S, Sabaté J. Preface. Am J Clin Nutr 2009;89(suppl):1541S–2S [DOI] [PubMed] [Google Scholar]
- 33.Jacobs DR, Jr, Gross MD, Tapsell LC. Food synergy: an operational concept for understanding nutrition. Am J Clin Nutr 2009;89(suppl):1543S–8S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobs DR, Jr, Haddad EH, Lanou AJ, Messina MJ. Food, plant food, and vegetarian diets in the US dietary guidelines: conclusions of an expert panel. Am J Clin Nutr 2009;89(suppl):1549S–52S [DOI] [PubMed] [Google Scholar]
- 35.Lampe JW. Interindividual differences in response to plant-based diets: implications for cancer risk. Am J Clin Nutr 2009;89(suppl):1553S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon JA, Chen Y-H, Bent S. The relation of α-linolenic acid to the risk of prostate cancer: a systematic review and meta-analysis. Am J Clin Nutr 2009;89(suppl):1558S–64S [DOI] [PubMed] [Google Scholar]
- 37.Pierce JP, Natarajan L, Caan BJ, et al. Dietary change and reduced breast cancer events among women without hot flashes after treatment of early-stage breast cancer: subgroup analysis of the Women's Healthy Eating and Living Study. Am J Clin Nutr 2009;89(suppl):1565S–71S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newby PK. Plant foods and plant-based diets: protective against childhood obesity? Am J Clin Nutr 2009;89(suppl):1572S–87S [DOI] [PubMed] [Google Scholar]
- 39.Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89(suppl):1588S–96S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mangat I. Do vegetarians have to eat fish for optimal cardiovascular protection? Am J Clin Nutr 2009;89(suppl):1597S–601S [DOI] [PubMed] [Google Scholar]
- 41.Willis LM, Shukitt-Hale B, Joseph JA. Modulation of cognition and behavior in aged animals: role for antioxidant- and essential fatty acid–rich plant foods. Am J Clin Nutr 2009;89(suppl):1602S–6S [DOI] [PubMed] [Google Scholar]
- 42.Fraser GE. Vegetarian diets: what do we know of their effects on common chronic diseases? Am J Clin Nutr 2009;89(suppl):1607S–12S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 2009;89(suppl):1613S–9S [DOI] [PubMed] [Google Scholar]
- 44.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Cancer incidence in vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 2009;89(suppl):1620S–6S [DOI] [PubMed] [Google Scholar]
- 45.Craig WJ. Health effects of vegan diets. Am J Clin Nutr 2009;89(suppl):1627S–33S [DOI] [PubMed] [Google Scholar]
- 46.Weaver CM. Should dairy be recommended as part of a healthy vegetarian diet? Point. Am J Clin Nutr 2009;89(suppl):1634S–7S [DOI] [PubMed] [Google Scholar]
- 47.Lanou AJ. Should dairy be recommended as part of a healthy vegetarian diet? Counterpoint. Am J Clin Nutr 2009;89(suppl):1638S–42S [DOI] [PubMed] [Google Scholar]
- 48.Sabaté J, Ang Y. Nuts and health outcomes: new epidemiologic evidence. Am J Clin Nutr 2009;89(suppl):1643S–8S [DOI] [PubMed] [Google Scholar]
- 49.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr 2009;89(suppl):1649S–56S [DOI] [PubMed] [Google Scholar]
- 50.Rajaram S, Haddad EH, Mejia A, Sabaté J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr 2009;89(suppl):1657S–63S [DOI] [PubMed] [Google Scholar]
- 51.Badger TM, Gilchrist JM, Pivik RT, et al. The health implications of soy infant formula. Am J Clin Nutr 2009;89(suppl):1668S–72S [DOI] [PubMed] [Google Scholar]
- 52.Messina M, Wu AH. Perspectives on the soy–breast cancer relation. Am J Clin Nutr 2009;89(suppl):1673S–9S [DOI] [PubMed] [Google Scholar]
- 53.Lönnerdal B. Soybean ferritin: implications for iron status of vegetarians. Am J Clin Nutr 2009;89(suppl):1680S–5S [DOI] [PubMed] [Google Scholar]
- 54.Chan J, Jaceldo-Siegl K, Fraser GE. Serum 25-hydroxyvitamin D status of vegetarians, partial vegetarians, and nonvegetarians: the Adventist Health Study-2. Am J Clin Nutr 2009;89(suppl):1686S–92S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elmadfa I, Singer I. Vitamin B-12 and homocysteine status among vegetarians: a global perspective. Am J Clin Nutr 2009;89(suppl):1693S–8S [DOI] [PubMed] [Google Scholar]
- 56.Marlow HJ, Hayes WK, Soret S, Carter RL, Schwab ER, Sabaté J. Diet and the environment: does what you eat matter? Am J Clin Nutr 2009;89(suppl):1699S–703S [DOI] [PubMed] [Google Scholar]
- 57.Carlsson-Kanyama A, González AD. Potential contributions of food consumption patterns to climate change. Am J Clin Nutr 2009;89(suppl):1704S–9S [DOI] [PubMed] [Google Scholar]
- 58.Eshel G, Martin PA. Geophysics and nutritional science: toward a novel, unified paradigm. Am J Clin Nutr 2009;89(suppl):1710S–6S [DOI] [PubMed] [Google Scholar]