Abstract
Background and Purpose
Chronic infections, including periodontal infections, may predispose to cardiovascular disease. The present study investigates the relationship of periodontal disease and tooth loss with subclinical atherosclerosis.
Methods
We enrolled 711 subjects with a mean age of 66±9 years and no history of stroke or myocardial infarction in the Oral Infections and Vascular Disease Epidemiology Study. Subjects received a comprehensive periodontal examination, extensive in-person cardiovascular disease risk factor measurements, and a carotid scan using high-resolution B-mode ultrasound. Regression models were adjusted for conventional risk factors (age, sex, smoking, diabetes, systolic blood pressure, low- and high-density lipoprotein cholesterol, race-ethnicity, education, physical activity) and markers of cultural background, healthy lifestyle, and psychosocial health.
Results
Measures of both current and cumulative periodontitis became more severe as tooth loss increased. A significant association was observed between tooth loss levels and carotid artery plaque prevalence. Among those with 0 to 9 missing teeth, 46% had carotid artery plaque, whereas among those with ≥10 missing teeth, carotid artery plaque prevalence was ≈60% (P<0.05).
Conclusions
Our data suggest that tooth loss is a marker of past periodontal disease in this population and is related to subclinical atherosclerosis, thereby providing a potential pathway for a relationship with clinical events.
Keywords: atherosclerosis, infection, periodontal disease, tooth loss
Several investigators have suggested that chronic infections may predispose to cardiovascular disease (CVD).1,2 The relationship between oral health, specifically periodontal disease, and CVD has been a subject of mounting research in recent years3–10 and is both biologically plausible and supported by data on transient bacteremia and elevated inflammatory markers.11–13 Studies have reported associations between CVD events and periodontal infections5–7,14–16 or tooth loss.7,17–21
However, recent studies have cast doubt on the relationship.9,10,22–25 The inconsistency may be due to differences in study design, definition of periodontitis, outcome examined, and residual confounding (possibly by smoking).25 Alternatively, including socioeconomic status as a covariate10 may constitute overadjustment because socioeconomic status is a surrogate marker for several characteristics. Age adjustment must also be done carefully because not only is age positively related to both periodontitis and CVD, but tooth loss resulting from treatment for periodontitis may also occur differentially according to age.
The Oral Infections and Vascular Disease Epidemiology Study (INVEST) was designed to study the hypothesis that periodontal infections predispose to accelerated progression of carotid atherosclerosis and incidence of stroke, myocardial infarction, and CVD death. In this report, we hypothesize that in older adults, edentulism and loss of more than a few teeth mark past periodontitis not visible in current observation because tooth removal was the result of either severe infection or the elimination of the site of infection. As a consequence of the dual and conflicting reasons for tooth loss, clinical measures of periodontal disease may be more difficult to interpret as an exposure of significance for systemic diseases. In this article, we present cross-sectional data to study (1) the extent to which tooth loss is concordant with current and cumulative measures of periodontitis and (2) the relationship of the conventional clinical measures of periodontitis (pocket depth and clinical attachment loss [AL]) and tooth loss to the presence of carotid artery plaque. Our study collected extensive data on oral disease, traditional cardiovascular and periodontal risk factors, and markers of healthy behaviors, psychosocial health, and healthcare background; this study was able to include as covariates many variables identified as potential confounders in other studies.21 Particular attention was paid to assessing the confounding role of smoking.25
Materials and Methods
INVEST is a prospective population-based cohort study investigating the relationship between oral infections, carotid atherosclerosis, and stroke. Subjects were randomly selected from northern Manhattan, an area between 145th and 218th streets, bordered on the west by the Hudson River and separated on the east from the Bronx by the Harlem River. Blacks, Hispanics, and whites live together in this area and have access to similar medical care. INVEST participants are also enrolled in the Northern Manhattan Study.26
Eligibility criteria for INVEST are as follows: (1) white, black, or Hispanic resident (>3 months) of northern Manhattan (ZIP codes 10031, 10032, 10033, 10034, and 10040); (2) contacted by random-digit dialing among households with a telephone (all eligible invited); (3) age ≥55 years at the time of the first in-person assessment; (4) no baseline history of stroke, myocardial infarction, or chronic inflammatory conditions such as systemic lupus erythematosus, Lyme disease, gonococcal arthritis, or bacterial endocarditis; and (5) ability to come to the clinic. The planned number of INVEST participants is 1050. We report here on the first 711 enrolled participants. An additional 17 participants were deleted in some adjusted analyses because of missing smoking information. This study was approved by the Institutional Review boards, and all subjects granted informed consent.
Dental History and Oral Examination
At baseline, subjects were interviewed and underwent a complete examination of the oral cavity administered by trained, calibrated dental examiners. Tooth brushing and flossing were recorded as times per week. Edentulous participants reported brushing (gums or dentures) but not flossing (and thus were removed from flossing analyses). Teeth were counted and localized. Gingival crevicular fluid (mesial buccal site in the most posterior tooth per quadrant) and plaque samples (2 most posterior teeth, mesial lingual in the upper and mesial buccal in the lower jaw) were collected before probing. Assessment of periodontal status for all teeth present included presence or absence of dental plaque, probing depth (PD) in millimeters, and location of the gingival margin in relation to the cementoenamel junction at 6 locations for each tooth (mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, distolingual) with a UNC-15 manual probe (Hu-Friedy). A visual check for cavities and tooth mobility was performed. Finally, oral/pharyngeal soft tissue was examined to detect mucosal inflammatory conditions.
In this article, we categorized dentate subjects according to the extent of current (as measured by PD) and cumulative (as measured by AL) periodontal disease. Disease was defined at a particular level of severity, namely ≥5 mm for PD and ≥4 mm for AL. Disease extent was defined by the percent of periodontal sites meeting the above severity criteria for PD and AL. The percent of sites was calculated within each mouth by dividing the number of sites with PD ≥5 mm by the total number of sites measured, and similarly for AL. Tooth loss was categorized as 0 to 9, 10 to 19, and 20 to 31 missing teeth and edentulous. These cutoffs resulted in a balanced distribution of subjects across tooth loss categories (Table 1).
TABLE 1.
Characteristics Across Tooth Loss Categories Adjusted for Age and Sex
| Variable | 0–9 Missing Teeth (n=190) | 10–19 Missing Teeth (n=205) | 20–31 Missing Teeth (n=167) | Edentulous (n=149) |
|---|---|---|---|---|
| Age, y | 63±0.6 | 65±0.6 | 68±0.6 | 70±0.6 |
| Female, % | 44 | 41 | 45 | 39 |
| Presence of carotid artery plaque, % | 47 | 62 | 56 | 57 |
| Completed high school, % | 58 | 47 | 40 | 27 |
| Diabetes, % | 14 | 21 | 27 | 26 |
| Former smokers, % | 45 | 40 | 35 | 38 |
| Current smokers, % | 6 | 15 | 19 | 20 |
| Hypertension, % | 65 | 75 | 73 | 70 |
| Systolic blood pressure, mm Hg | 141±1.5 | 144±1.4 | 144±1.6 | 142±1.7 |
| Diastolic blood pressure, mm Hg | 82±0.85 | 83±0.83 | 83±0.92 | 82±0.99 |
| Serum total cholesterol, mmol/L | 5.2±0.08 | 5.2±0.08 | 5.1±0.07 | 5.1±0.07 |
| HDL-C, mmol/L | 1.2±0.03 | 1.2±0.03 | 1.1±0.03 | 1.1±0.03 |
| LDL-C, mmol/L | 3.3±0.08 | 3.2±0.07 | 3.3±0.07 | 3.3±0.07 |
| Race-ethnicity, % | ||||
| Hispanic | 57 | 60 | 66 | 77 |
| Black | 16 | 25 | 25 | 14 |
| White | 27 | 15 | 9 | 9 |
| Physical activity, % | ||||
| None | 39 | 51 | 46 | 56 |
| Light | 47 | 44 | 45 | 42 |
| Moderate/heavy | 14 | 5 | 9 | 2 |
| Social isolation, % | ||||
| Currently married | 47 | 42 | 38 | 32 |
| Have at least 2 friends | 89 | 88 | 88 | 88 |
| Almost never feel lonely | 54 | 56 | 55 | 54 |
| Had ≥2 visitors in the past week | 57 | 57 | 55 | 51 |
| Oral hygiene, % | ||||
| Brushing at least 1/d | 97 | 99 | 98 | 88 |
| Flossing at least 1/d* | 53 | 41 | 19 | NA |
Values are mean±SE when appropriate.
Patients had missing data for systolic (n=2), lipids (n=26), smoking (n=17), and physical activity (n=9).
Among 562 dentate patients.
Assessment of Carotid Artery Plaque and Maximal Carotid Plaque Thickness
Carotid ultrasound was performed by high-resolution B-mode ultrasound system GE LogIQ 700 with a multifrequency 9/13-MHz linear-array probe. Both right and left carotid arteries were assessed by trained and certified sonologists using previously described27 protocols.
The carotid artery scanning protocol consisted of imaging the carotid arteries in the longitudinal (anterior, lateral, posterior views) and transverse planes. The examination consisted of an initial exploratory transverse and longitudinal scan of the carotid system, followed by a detailed longitudinal examination of the specific arterial segments. Both common (CCA) and internal (ICA) carotid arteries were examined for the presence of atherosclerotic plaque, defined as an area of focal wall thickening. All images were recorded on super-VHS tapes for reading. The carotid plaque reading protocol was performed offline. Measurements of maximal carotid plaque thickness (in millimeters) were performed at the highest plaque prominence in any of the 3 carotid artery segments assessed from the digitized multiangled images. If no carotid plaques were identified, plaque thickness was recorded as zero. For these analyses, the presence or absence (prevalence) of carotid artery plaque and maximal plaque thickness were used. Reading of carotid intima media wall thickness is ongoing and not yet available.
Risk Factor Assessment
Data were collected through interview by trained research assistants, medical record reviews, physical and neurological examination by study physicians, in-person measurements, and fasting (overnight) blood specimens.
All assessments were conducted in English or Spanish. Race-ethnicity was based on self-identification through US census–based interview questions. Subjects were categorized into Hispanic, white non-Hispanic, or black non-Hispanic.
Subjects were interviewed regarding sociodemographic characteristics, stroke risk factors, and other medical conditions. Standardized questions were adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System regarding the following conditions: hypertension, diabetes, hypercholesterolemia, peripheral vascular disease, transient ischemic attack, cigarette smoking, alcohol use, and cardiac conditions such as myocardial infarction, coronary artery disease, angina, congestive heart failure, atrial fibrillation, other arrhythmias, and valvular heart disease. Subjects also completed a comprehensive functional status battery, which included the Bartel Activities of Daily Living, Quality of Well-Being, and Geriatric Social Readjustment Rating scales.
Anthropometric measurements of height and weight were determined with calibrated scales. Research assistants measured blood pressure using a calibrated aneroid sphygmomanometer (Omron), and the average of 2 measurements was used in analysis.26 Hypertension was defined by the patient’s self-report of a history of diagnosed hypertension or use of antihypertensive medications or if mean systolic blood pressure was ≥140 mm Hg or mean diastolic blood pressure was ≥90 mm Hg.
Blood samples were sent for complete blood count on enrollment. Fasting glucose and lipid panels were measured as described.26 Low-density lipoprotein cholesterol (LDL-C) was computed from the Friedewald equation.28 Diabetes mellitus was defined as a history of diagnosed diabetes, use of insulin or hypoglycemic medication, or a fasting glucose >126 mg/dL (7.7 mmol/L). Smoking was assessed both categorically (current, former, or never) and continuously as total pack-years of cigarette smoking. Furthermore, among ever smokers, the following data were also collected: smoking duration (computed as the difference between the age at smoking initiation and the age at smoking cessation) and quantity of cigarettes smoked per day.
Assessment of Cultural, Lifestyle, and Social Isolation Variables
Cultural background was assessed as country of origin and years of residence in northern Manhattan (categorized <5 or ≥5 years).
A physical activity survey was adapted from the National Health Interview Survey of the National Center for Health Statistics, considered reliable in evaluating elderly subjects.29 It records the frequency and duration of 14 recreational activities during the 2-week period before the interview. Physical activity level was categorized as none, light, moderate, or heavy.
Five social isolation variables were examined: (1) marital status (currently married versus not); (2) number of friends known well enough to visit in the subject’s home (0 to 1 versus ≥2); (3) number of visitors in the past week (visiting at the subject’s house or at the friend’s house or going out together; 0 to 1 versus ≥2); (4) feeling lonely (quite often or sometimes versus almost never); and (5) number of members in the household.
Statistical Analysis
All analyses were performed with SAS for Windows, version 8.0. Linear and logistic regression models were used as appropriate. The number of missing teeth per mouth was modeled both continuously and categorically. We estimated the percent of individuals with any carotid plaque and odds ratios between tooth loss categories using logistic regression. The natural logarithm (maximum carotid artery plaque thickness plus 1) was studied with linear regression. We defined age both continuously and categorically by 5-year intervals in preliminary analyses. The 2 definitions of age produced similar results; all models presented here are adjusted for age as a continuous variable.
Results
General Characteristics
Of the 711 participants, 58% were women, and men were younger (65±8 versus 67±9 years) (P=0.007). The study population was triethnic, with 64% Hispanic, 21% black non-Hispanic, and 15% white non-Hispanic. Ninety-five percent of Hispanics were foreign born, with most (64%) from the Dominican Republic. Additional characteristics of the study participants are given in Table 1.
Fifty-five percent of subjects had plaque detected in at least 1 carotid artery: 54% in the ICA and 6% in the CCA. Average plaque thickness size was 0.74±0.86 mm and was similar in both sexes. The age-adjusted carotid artery plaque prevalence was 59% in men versus 53% in women (P=0.06). This sex difference was seen in both the CCA (9% versus 4%, P=0.0009) and ICA (58% versus 51%, P=0.06). Adjusting for age and sex showed that plaque was both more prevalent and of greater size among whites (64%, 0.90 mm) than blacks (58%, 0.84 mm) and Hispanics (53%, 0.67 mm) (P<0.05 for any difference among ethnic groups).
Seventy-nine percent of the participants (n=562) were dentate and had an average of 14±8 missing teeth. Whites had more teeth (17) than Hispanics (14) or blacks (14) (P<0.01). The average number of periodontal sites (±SD) with PD ≥5 mm or AL ≥4 mm was 11±16 and 35±27 respectively. The distribution was similar in both sexes.
Relationship Between Tooth Loss and Clinical Measures of Periodontal Disease
The greater the number of teeth lost, the greater the extent of severe periodontal disease was. Across increasing levels of tooth loss, there was a consistent increase in the age-adjusted proportion of sites with both severe AL (from 28% to 59%) and deep pockets (from 8% to 15%) (Table 2).
TABLE 2.
Age-Adjusted Mean±SE Percent of Sites With Deep Pockets and Severe AL (Measures of Current and Cumulative Extent of Periodontal Disease, Respectively) in Relation to Tooth Loss
| Variable | 0 –9 Missing Teeth (n=190) | 10 –19 Missing Teeth (n=205) | 20 –31 Missing Teeth (n=167) | P for Trend |
|---|---|---|---|---|
| Mean periodontal sites investigated, n | 158±1.1 | 108±1.1 | 45±1.4 | |
| Sites with PD ≥5 mm, % | 8.3±1.2 | 12.2±1.2 | 14.8±1.3 | 0.0003 |
| Sites with AL ≥4 mm, % | 28.4±1.5 | 37.2±1.8 | 58.9±2.4 | <0.0001 |
Conventional Measures of Periodontal Disease, Tooth Loss, and Carotid Artery Plaque
Carotid plaque prevalence was not clearly related to the conventional measures of current and past periodontal disease. The percentage of participants with any carotid artery plaque present was ≈55% at all levels of both current and cumulative measures of periodontal disease extent (data not shown).
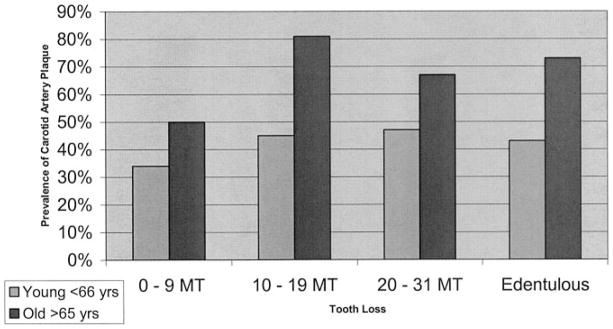
Among those with 0 to 9 missing teeth, 45% had carotid artery plaque, whereas among those with ≥10 missing teeth, the prevalence of carotid artery plaque was ≈60% (Table 3), even after adjustment for standard CVD risk factors. Models adjusting for social isolation, oral hygiene, years of residence, and physical activity barely changed carotid artery plaque prevalence estimates across tooth loss categories. This relationship between tooth loss and carotid plaque prevalence was more pronounced in patients older than the median age of 65 years (the Figure). Carotid artery plaque thickness mirrored plaque prevalence across tooth loss categories; however, this finding was solely reflective of prevalence.
TABLE 3.
Prevalence and Odds Ratio of Carotid Artery Plaque Across Tooth Loss Categories as Predicted by Logistic Regression
| 0–9 Missing Teeth |
10–19 Missing Teeth |
20–31 Missing Teeth |
Edentulous |
|||||
|---|---|---|---|---|---|---|---|---|
| Model | Prevalence, % | OR | Prevalence, % | OR (95% CI) | Prevalence, % | OR (95% CI) | Prevalence, % | OR (95% CI) |
| 1 | 41 | 1.00 | 59 | 2.06 (1.38–3.09) | 60 | 2.13 (1.39–3.26) | 62 | 2.32 (1.50–3.63) |
| 2 | 46 | 1.00 | 62 | 1.93 (1.27–2.94) | 57 | 1.56 (0.99–2.45) | 56 | 1.51 (0.94–2.44) |
| 3 | 45 | 1.00 | 62 | 1.95 (1.25–3.04) | 57 | 1.56 (0.96–2.54) | 57 | 1.58 (0.95–2.63) |
| 4 | 45 | 1.00 | 62 | 1.90 (1.20–3.00) | 56 | 1.50 (0.91–2.47) | 57 | 1.60 (0.93–2.75) |
Model 1, unadjusted; model 2, adjusted for age; model 3, adjusted for age, sex, smoking, diabetes, systolic blood pressure, LDL-C, HDL-C, race-ethnicity, and education; model 4, model 3 plus tooth brushing, social isolation (as described in Materials and Methods), physical activity, and years of residence.
Figure.
Prevalence of carotid artery plaque across tooth loss categories among young and old (P=0.14 for age interaction) adjusted for age, sex, smoking, diabetes, systolic blood pressure, diastolic blood pressure, LDL-C, HDL-C, race, education, tooth brushing, social isolation, and physical activity. MT indicates missing teeth.
Our findings were similar among the subgroup of 326 never smokers: Carotid plaque was present in 39% of those with 0 to 9 missing teeth, 52% of those with 10 to 19 missing teeth, 48% of those with 20 to 31 missing teeth, and 58% of the edentulous. After adjustment for sex, age, diabetes, systolic blood pressure, LDL, high-density lipoprotein (HDL), race-ethnicity, and education, the association was attenuated but retained the same pattern: 42%, 52%, 45%, and 52%, respectively, had carotid plaque. Among the entire population, the percentage with any plaque was effectively unchanged whatever the form of the smoking-adjusting variable: smoking status (never, ex-, current smoker) alone; smoking status and pack-years; or smoking status, time since cessation, cigarettes per day, and smoking duration.
Discussion
We investigated and clarified the relationship between tooth loss and clinical measures of periodontal disease, thus providing a novel epidemiological explanation for conflicting results in the literature linking periodontal disease and CVD. To the best of our knowledge, this is also the first article to identify a relationship between tooth loss and subclinical CVD.
A recent publication showed increased risk of stroke in those with substantial numbers of missing teeth.17 A number of other analyses have reported on tooth loss and CVD previously.7,18–20 All but 1 of these reports17 examined tooth loss exposure simply as a dichotomous variable. These studies have focused primarily on the risk of myocardial infarction, stroke, and prevalent coronary heart disease in relation to tooth loss, whereas we have used subclinical atherosclerosis as the primary outcome in a cohort free of baseline cardiovascular disease. Therefore, our findings shed light on a potential pathway through which oral health may affect vascular diseases.
Here, we report on the nonlinear relationship between tooth loss and carotid plaque. Our analyses reveal that the prevalence of carotid plaque increases substantially and peaks among individuals missing 10 to 19 teeth compared with those missing 0 to 9 teeth before abating somewhat in the 2 groups with the most substantial tooth loss. This subclinical pattern is similar to that observed recently for the clinical stroke end point.17
This curvilinearity may be due to the fact that people lose sets of teeth for various reasons. Although the first teeth might be lost because of caries or orthodontic reasons, we hypothesized that in older adults, edentulism or loss of more than a few teeth would more likely reflect periodontal disease, and our data seem to support that supposition. Tooth loss may be a valid and more telling marker of long-term cumulative periodontal disease in certain populations. However, tooth loss is a difficult marker to grasp epidemiologically because it may mark the result of periodontal treatment, severe disease, or both. In populations in which tooth loss is due primarily to periodontal disease, this may lead to an apparent paradox in which edentulous patients are not significantly different from the dentate patients with the most periodontal disease.9 In other words, when one loses teeth previously affected by periodontal disease, the evidence of the cumulative effect of periodontitis is removed while the systemic damage may partly persist. The possibility that the systemic damage may not be entirely reversible should not be interpreted as evidence of no damage. This varied removal of the proof of periodontal disease exposure may partially explain the discrepancy between epidemiological studies reporting on periodontal-systemic relationships. However, because tooth removal practices vary, we caution that tooth loss may not carry the same relation with periodontal disease and consequently vascular disease in all populations. For example, AL and PD may be better markers of periodontal disease in some possibly younger population groups. This would be consistent with the findings by Mattila et al3 and with our own data in which age was the most important covariate in the relationship between tooth loss and carotid artery plaque. Age plays an epidemiologically confusing role in that it may both increase and decrease risk for CVD: Aging subjects are more likely to develop periodontitis and the ensuing tooth loss but may also be more likely to have their teeth removed for similarly severe periodontal disease compared with younger subjects. Whether age is an explanatory variable or a confounding variable cannot be determined in a cross-sectional design. Thus, we await prospective results from INVEST to make firmer conclusions.
Our analysis was systematic in adjusting for risk factors and healthy behaviors, cultural background, and psychosocial health. None of them removed the relationship of tooth loss with carotid artery plaque prevalence. Furthermore, the nature and scope of the relationship was unaffected by careful assessment of the confounding role of smoking.
We have used cutoffs of 4 mm for AL and 5 mm for PD to measure the severity of clinical periodontal disease. These cutoffs reflected our data and the disease prevalence in our population. Had we chosen a threshold of 3 mm, as done by others,30 90% of our population would have been classified as having severe periodontal disease.
This study shares with others the limitations inherent to cross-sectional data; the relationships reported here, while robust, should not be interpreted as causal. Also, diet data, although collected, were not yet available. This study specifically reports on assessments of carotid artery plaque rather than intima-media thickness (IMT). Beck et al30 have reported on the relationship between intima-media thickness and periodontal measures but not with tooth loss. It is possible that part of the reported relationship by these authors may have been accounted for by plaque because the Atherosclerosis Risk in Communities (ARIC) protocol integrates plaque thickness in the measurement of IMT. Because IMT and plaque are measured separately in INVEST, we will be able to investigate their relative contributions.
Our findings raise the possibility that such a relationship might not be entirely reversible or may require supplemental measures to be reversible. Our results also suggest a threshold effect over which the cumulative effect of periodontal disease may plateau. Further knowledge should be garnered when we report on the progression of atherosclerosis with regard to prospective tooth loss and local microbiological and inflammatory markers of periodontitis. A relationship between periodontitis and subclinical CVD, as reported here, would be of significant public health interest because of the prevalence of periodontitis in the general population.4,31
Acknowledgments
This research is supported by NIH grants DE-13094 and NS-29993. R.D. is supported by T32 HL-07779. T.R. is supported by the Hazel K. Goddess Fund for Stroke Research in Women. We thank George Loo, Dr Mariana Cukier, Dr Shantanu Lal, and Janet DeRosa for their devoted patient care; the INVEST staff, Drs Romel Ramas, Sam Trocio, and Oscar Ramos, for performing the ultrasound scans; and most importantly the patients. Patients were seen at the Columbia University General Clinical Research Center, NIH grant RR-00645.
References
- 1.Kiechl S, Egger G, Mayr M, Wiedermann CJ, Bonora E, Oberhollenzer F, Muggeo M, Xu Q, Wick G, Poewe W, Willeit J. Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation. 2001;103:1064–1070. doi: 10.1161/01.cir.103.8.1064. [DOI] [PubMed] [Google Scholar]
- 2.Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Victor A, Hafner G, Prellwitz W, Schlumberger W, Meyer J. Impact of infectious burden on progression of carotid atherosclerosis. Stroke. 2002;33:2581–2586. doi: 10.1161/01.str.0000034789.82859.a4. [DOI] [PubMed] [Google Scholar]
- 3.Mattila KJ, Asikainen S, Wolf J, Jousimies-Somer H, Valtonen V, Nieminen M. Age, dental infections, and coronary heart disease. J Dent Res. 2000;79:756–760. doi: 10.1177/00220345000790020901. [DOI] [PubMed] [Google Scholar]
- 4.Desvarieux M. Periodontal disease, race, and vascular disease. Compend Contin Educ Dent. 2001;22:34–41. [PubMed] [Google Scholar]
- 5.Syrjanen J, Peltola J, Valtonen V, Iivanainen M, Kaste M, Huttunen JK. Dental infections in association with cerebral infarction in young and middle-aged men. J Intern Med. 1989;225:179–184. doi: 10.1111/j.1365-2796.1989.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 6.Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease: the First National Health and Nutrition Examination Survey and its follow-up study. Arch Intern Med. 2000;160:2749–2755. doi: 10.1001/archinte.160.18.2749. [DOI] [PubMed] [Google Scholar]
- 7.Loesche WJ, Schork A, Terpenning MS, Chen YM, Kerr C, Dominguez BL. The relationship between dental disease and cerebral vascular accident in elderly United States veterans. Ann Periodontol. 1998;3:161–174. doi: 10.1902/annals.1998.3.1.161. [DOI] [PubMed] [Google Scholar]
- 8.Arbes SJ, Jr, Slade GD, Beck JD. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J Dent Res. 1999;78:1777–1782. doi: 10.1177/00220345990780120301. [DOI] [PubMed] [Google Scholar]
- 9.Hujoel PP, Drangsholt M, Spiekerman C, Derouen TA. Examining the link between coronary heart disease and the elimination of chronic dental infections. J Am Dent Assoc. 2001;132:883–889. doi: 10.14219/jada.archive.2001.0300. [DOI] [PubMed] [Google Scholar]
- 10.Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Pre-existing cardiovascular disease and periodontitis: a follow-up study. J Dent Res. 2002;81:186–191. [PubMed] [Google Scholar]
- 11.Ebersole JL, Machen RL, Steffen MJ, Willmann DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997;107:347–352. doi: 10.1111/j.1365-2249.1997.270-ce1162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 13.Papapanou PN, Neiderud AM, Papadimitriou A, Sandros J, Dahlen G. “Checkerboard” assessments of periodontal microbiota and serum antibody responses: a case-control study. J Periodontol. 2000;71:885–897. doi: 10.1902/jop.2000.71.6.885. [DOI] [PubMed] [Google Scholar]
- 14.Mattila KJ, Nieminen MS, Valtonen VV, Rasi VP, Kesaniemi YA, Syrjala SL, Jungell PS, Isoluoma M, Hietaniemi K, Jokinen MJ. Association between dental health and acute myocardial infarction. BMJ. 1989;298:779–781. doi: 10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grau AJ, Buggle F, Ziegler C, Schwarz W, Meuser J, Tasman AJ, Buhler A, Benesch C, Becher H, Hacke W. Association between acute cerebrovascular ischemia and chronic and recurrent infection. Stroke. 1997;28:1724–1729. doi: 10.1161/01.str.28.9.1724. [DOI] [PubMed] [Google Scholar]
- 16.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67(suppl):1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 17.Joshipura KJ, Hung H-C, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34:47–52. doi: 10.1161/01.str.0000052974.79428.0c. [DOI] [PubMed] [Google Scholar]
- 18.DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306:688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paunio K, Impivaara O, Tiekso J, Maki J. Missing teeth and ischaemic heart disease in men aged 45–64 years. Eur Heart J. 1993;14(suppl K):54–56. [PubMed] [Google Scholar]
- 20.Loesche WJ. Periodontal disease as a risk factor for heart disease. Compendium. 1994;15:976–978. 983–985, 986. [PubMed] [Google Scholar]
- 21.Joshipura KJ, Douglass CW, Willett WC. Possible explanations for the tooth loss and cardiovascular disease relationship. Ann Periodontol. 1998;3:175–183. doi: 10.1902/annals.1998.3.1.175. [DOI] [PubMed] [Google Scholar]
- 22.Howell TH, Ridker PM, Ajani UA, Hennekens CH, Christen WG. Periodontal disease and risk of subsequent cardiovascular disease in U.S. male physicians. J Am Coll Cardiol. 2001;37:445–450. doi: 10.1016/s0735-1097(00)01130-x. [DOI] [PubMed] [Google Scholar]
- 23.Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontal disease and coronary heart disease risk. JAMA. 2000;284:1406–1410. doi: 10.1001/jama.284.11.1406. [DOI] [PubMed] [Google Scholar]
- 24.Hujoel PP, Drangsholt MT, Spiekerman C, DeRouen TA. Periodontal disease and risk of coronary heart disease. JAMA. 2001;285:40–41. [PubMed] [Google Scholar]
- 25.Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontitis-systemic disease associations in the presence of smoking: causal or coincidental? Periodontol 2000. 2002;30:51–60. doi: 10.1034/j.1600-0757.2002.03005.x. [DOI] [PubMed] [Google Scholar]
- 26.Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281:53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- 27.Sacco RL, Roberts JK, Boden-Albala B, Gu Q, Lin IF, Kargman DE, Berglund L, Hauser WA, Shea S, Paik MC. Race-ethnicity and determinants of carotid atherosclerosis in a multiethnic population: the Northern Manhattan Stroke Study. Stroke. 1997;28:929–935. doi: 10.1161/01.str.28.5.929. [DOI] [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 29.Sacco RL, Gan R, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;29:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 30.Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. Relationship of periodontal disease to carotid artery intima-media wall thickness: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2001;21:1816–1822. doi: 10.1161/hq1101.097803. [DOI] [PubMed] [Google Scholar]
- 31.Papapanou PN. Periodontal diseases: epidemiology. Ann Periodontol. 1996;1:1–36. doi: 10.1902/annals.1996.1.1.1. [DOI] [PubMed] [Google Scholar]