Abstract
This article reviews evidence for the presence of a compensatory, alternative, neural system and its possible link to associated processing strategies in children and adults with attention deficit hyperactivity disorder (ADHD). The article presents findings on a region by region basis that suggests ADHD should be characterized not only by neural hypo-activity, as it is commonly thought but neural hyperactivity as well, in regions of the brain that may relate to compensatory brain and behavioral functioning. In this context studies from the functional neuroimaging literature are reviewed. We hypothesize that impaired prefrontal (PFC) and anterior cingulate (ACC) cortex function in ADHD reduces the ability to optimally recruit subsidiary brain regions and strategies to perform cognitive tasks. The authors conclude that healthy individuals can recruit brain regions using visual, spatial or verbal rehearsal for tasks as needed. In contrast, individuals with ADHD may be less able to engage higher order executive systems to flexibly recruit brain regions to match given task demands. This may result in greater reliance on neuroanatomy that is associated with visual, spatial, and motoric processing rather than verbal strategies. The authors speculate that this impaired flexibility in recruiting brain regions and associated strategies limits adaptation to new cognitive demands as they present and may require more effortful processing.
Keywords: Brain imaging, Cognitive strategies, Children, Adults, Impulsivity
1. Introduction
Functional neuroimaging provides a unique method for linking how brain activity in living, intact (i.e., non-brain damaged) individuals relates to behavior, symptoms, and cognitive strategies manifested by clinical populations. Through the use of various functional imaging techniques in conjunction with behavioral data and lesion studies we are now able to learn not only about the function of a brain region, but about the use of covert behavioral and cognitive strategies. This article will review evidence from previous functional neuroimaging research in ADHD that suggests children and adults with the disorder engage alternative, compensatory brain regions and concomitant cognitive/behavioral strategies due to a selectively weakened neural system. Specifically, we hypothesize that impaired prefrontal (PFC) and anterior cingulate (ACC) functioning in ADHD individuals reduces their ability to recruit subsidiary brain regions and strategies to optimally perform cognitive tasks. Normal functioning gives flexibility in recruiting subsidiary regions. Thus, healthy individuals can recruit brain regions using either visual–spatial or verbal rehearsal strategies depending on task demands. Impaired flexibility limits adaptation to new cognitive demands as they occur and can result in a rigid, sometimes inappropriate response strategy. This article will present the findings on a region by region basis that suggest ADHD is characterized not only by hypo-activity, but also hyperactivity in regions of the brain, the latter possibly reflecting compensatory mechanisms.
This aim of this manuscript is to review the neuroimaging literature in ADHD, mainly in functional magnetic resonance imaging (fMRI), positron emission tomography (PET), single photon emission tomography (SPECT), but also electrophysiological studies (e.g. event-related potential (ERP)) where they are deemed to be particularly relevant, in a region-by-region basis. These results will then be discussed with respect to the theory of neural and behavioral compensation in individuals with ADHD.
2. Methodological considerations
There are a number of methodological factors that should be considered in any review of the ADHD functional neuroimaging literature. Because there are still relatively few functional imaging studies in ADHD, methodological differences between studies have the potential for substantial impact and may be responsible for some of the contradictory findings among ADHD studies. Variations in task conditions are among the most influential of factors and may include whether subjects are tested under resting state or task conditions, the type of activation paradigm used, behavioral performance levels achieved within the paradigm and the presence or absence of significant task differences between ADHD and control subjects. A number of subject variables can also influence results, including the degree of heterogeneity of group membership. Studies combining ADHD subtypes (e.g., inattentive and combined types) (Vaidya et al., 1998) can potentially reduce the interpretability of the findings (see Table 1 for details regarding fMRI and PET studies addressed in this review).
Table 1.
List of fMRI and PET studies included in this review
| Author | Journal | Modality | Number of subjects |
Developmental level |
Handedness | Diagnosis | ADHD subtypes | LD included |
Comorbidies included (ADHD subjects) |
Gender |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | C | |||||||||||
| Bush et al. 1999 | Biol Psychiatry | fMRI | 16—Total, 8—ADHD | Adults | All right handed | DSM-IV | Not specified | No | Excluded any other current Axis 1 psychiatric disorder, LD, neurologic disorder or pregnancy | 5 male | 5 male | |
| Durston et al., 2003 | Biol Psychiatry | fMRI | 14—Total, 7—ADHD | Children | All right handed | DSM-IV | 3 inattentive 4 combined | Not specified | Excluded any other comorbidity except ODD and CD | 6 male | 6 male | |
| Ernst et al., 2003 | Am J Psychiatry | 22—Total, 10—ADHD | Adults | All right handed | DSM-IV | 4 inattentive 6 combined | Not specified | Excluded current psychopathology, history of other psychiatric disorders. | 5 male | 6 male | ||
| Ernst et al., 1994 | J Am Acad Child Adolesc Psychiatry | PET 18F-fluorodeoxyglucose | 39—Total, 20—ADHD | Adolescents | 2 left-handed ADHD 6 left-handed controls | DSM-III R | Attention deficit disorder with hyperactivity | Yes | Excluded any other Axis I and Axis II disorders or neurological deficits | 15 male | 13 male | |
| Rubia et al., 1999 | Am J Psychiatry | fMRI | 16—Total, 7—ADHD | Adolescents | All right handed | DSM-IV | All hyperactive | No | Excluded any other psychiatric, learning or speech disorder included CD | All male | ||
| Rubia et al., 2000 | Neurosci Biobehavi Rev | fMRI | 24—Total, 7—ADHD | Adolescents/adults | All right handed | DSM-IV | All hyperactive | No | Excluded any other psychiatric or speech disorder included CD | All male | ||
| Schulz et al., 2005 | J Am Acad Child Adolesc Psychiatry | fMRI | 10—Total (ADHD remitters and persisters) 5—ADHD “persiters” | Adolescents | 1 left-handed subject | DSM-IV | 3 inattentive, 1 hyperactive/impulsive*, 1 combined* | Not specified | Excluded schizophrenia, pervasive developmental disorder, major affective disorder, Tourette’s or IQ below 70 included CD and separation anxiety disorder | All male | ||
| Schulz et al., 2004 | Am J of Psychiatry | fMRI | 19—Total, 10—ADHD | Adolescents | 1 left-handed ADHD subject | DSM-III R | Childhood—all combined, adolescence— 3 inattentive*, 1 hyperactive/ impulsive*, 1 combined, 5 partial remission | Not specified | Excluded schizophrenia, pervasive developmental disorder, major affective disorder, Tourette’s or IQ below 70 included childhood CD and separation anxiety | All male | ||
| Schweitzer, Faber et al., 2000 | Am J of Psychiatr | Total—12, 6—ADHD | Adults | All right handed | DSM-IV | All combined | No | Excluded any other Axis I and Axis II disorders | All male | |||
| Schweitzer, Lee et al., 2003 | Neuropsycho-pharmacology | Total—10 (all ADHD) | Adults | All right handed | DSM-IV | All combined | No | Excluded any other Axis I and Axis II disorders | All male | |||
| Schweitzer et al., 2004 | Biol Psychiatry | Total—21, 10—ADHD | Adults | All right handed | DSM-IV | All combined | No | Excluded any other Axis I and Axis II disorders | All male | |||
| Shafritz et al., 2004 | Am J of Psychiatry | fMRI | 41—Total, 15 ADHD/8 RD/4 ADHD+RD/ | Adolescents | Not reported | DSM-IV | All combined | Yes | Included only ODD and CD | 11 male A 6 male RD 4 male A +RD | 7 male | |
| Tamm et al., 2004 | J Am Acad Child Adolesc Psychiatry | fMRI | 22—Total, 10—ADHD | Adolescents | All right handed | DSM-IV | All combined | Not specified | Excluded major depression and family history of bipolar disorder | All male | ||
| Vaidya et al., 1998 | PNAS | fMRI | 16—Total, 10—ADHD | Children | 1 left-handed control subject | DSM-IV | 2 inattentive 8 combined | Yes | Excluded any other Axis 1 psychiatric disorders and those with a high score for comorbid disorders | All male | ||
| Valera et al., 2005 | Biol Psychiatry | fMRI | 40—Total, 20—ADHD | Adults | 1 left-handed ADHD, 1 left-handed control subject | DSM-IV | Not specified | Yes | Excluded any other Axis 1 mood, psychotic, substance-related or generalized anxiety disorders, sensorimotor handicaps or neurological disorders | 12 male | 12 male | |
| Zametkin et al., 1990 | N Engl J Med | PET fluoro-2-deoxy-d-glucose | 75—Total, 25—ADHD | Adults | 5 left-handed ADHD subjects, 5 left-handed controls. | DSM-III | All attention deficit disorder with hyperactivity | Yes | Excluded any other major psychiatric, substance-related or conduct disorders | 18 male | 28 male | |
| Zang et al., 2005 | Brain Dev | fMRI | 18—Total, 9—ADHD | Children/adolescents | All right handed | DSM-IV | 6 inattentive 3 combined | Not specified | Excluded schizophrenia, affective disorder, pervasive developmental disorder or neurological disease included 7 comorbid ODD, 1 comorbid dysthymic disorder | All male | ||
A: ADHD; C: Control; LD: learning disability; fMRI: functional magnetic resonance imaging; PET: positron emission tomography
Authors suggest that these children should really be considered combined type because they had high numbers of both inattentive and hyperactive-impulsive symptoms and experienced a diminution of symptoms with age.
Studies also vary in the rigor used to characterize and diagnose the clinical subjects, including whether comorbidity (e.g., learning disabilities, conduct disorder, oppositional defiant disorder) is assessed and/or represents an exclusion criteria. Equally, the extent of evaluation used to determine inclusion suitability for healthy controls (e.g., interviews, parent and teacher ratings) is also of importance. The presence and extent of pharmacological treatment and age of the subjects are additional factors that tend to vary between studies. The effects of these variables on functional brain imaging data are just beginning to emerge at this time but most likely affect the interpretability and generalizability of the findings. We suggest that future research systematically test the impact of these factors on tests of brain function.
3. Prefrontal cortex
The prefrontal cortex (PFC) has been implicated in higher level cognitive functioning, including attentional processes, working memory, inhibition and planning. The PFC has also been shown to be anatomically and reciprocally connected to practically all sensory and motor systems as well as a wide variety of subcortical structures (Miller, 2000). This makes it an ideal site for learning and adaptation of behavior and goals as well as being able to exert a top-down influence on other brain structures in the facilitation of appropriate behaviors and allocation of attentional resources.
Anatomical and functional variations in PFC as well as deficits in constructs attributed to PFC, such as inhibition and attention, have been implicated in ADHD in a number of behavioral and imaging studies. However, controversy exists as to the degree of each type of deficit in both children and adults. Although a number of studies have suggested an inhibitory deficit in ADHD in ERP (Broyd et al., 2005), imaging (Casey, Castellanos et al., 1997; Casey, Durston, & Fossella, 2001) and behavioral paradigms (Iaboni, Douglas, & Baker, 1995; Konrad, Gauggel, Manz, & Scholl, 2000; Oosterlaan & Sergeant, 1998; Schachar, Mota, Logan, Tannock, & Klim, 2000) some, including some of the aforementioned, authors have pointed out that these differences may be due to other factors than simply an inhibitory deficit alone per se.
Oosterlaan and Sergeant (1998), for example, suggested that a broader deficit underlies ADHD, perhaps a motivational problem or one related to more generally defined executive functions. They suggest that this may be attributable to frontal lobe dysfunction. In a further study, these authors (Kuntsi, Oosterlaan, & Stevenson, 2001) found marginal differences in inhibitory processes between ADHD and control children. The authors support the theory that an inhibitory deficit is not the core deficit in ADHD, rather one of slower information processing and delay-aversion (Sonuga-Barke, Taylor, Sembi, & Smith, 1992). Others again, suggest that adults may display greater deficits in inhibition whereas in children the core problem may be largely attributable to attentional difficulties (Nigg, Butler, Huang-Pollock, & Henderson, 2002). In fact, a very recent review of the STOP inhibitory paradigm supports this view. The authors suggested that from the review it appeared that adults with ADHD experience inhibitory deficits, whereas inhibitory problems experienced by ADHD children are largely accounted for by additional attentional problems (Lijffijt, Kenemans, Verbaten, & van Engeland, 2005).
In a behavioral study by Rubia and coworkers (Rubia, Oosterlaan, Sergeant, Brandeis, & Leeuwen, 1998), the authors found a significant inhibitory deficit and more variable reaction times (RT) in ADHD participants in two different versions of a STOP paradigm and concluded that this may be due to problems either at the level of motor output or at a higher executive level due to inadequate attention and/or motivation. RT differences have also been found between participants with ADHD and normal controls in a number of tasks (Fallgatter et al., 2004; Leth-Steensen, King Elbaz, & Douglas, 2000). Findings of RT differences have been mixed however; some authors suggesting no overall difference but more erratic or variable RT in individuals with ADHD (Berwid et al., 2005; Rucklidge & Tannock, 2002). Others report overall slowing of RT (Fallgatter et al., 2004; van Mourik, Oosterlaan, & Sergeant, 2005) suggesting general difficulties or increased interference in such tasks in ADHD participants, whereas others again suggest slower and more variable reaction times in children at risk for ADHD (Berwid et al., 2005).
Although behavioral tasks have been informative to a certain degree about ADHD, they can only provide limited information about the underlying neural anatomy associated with the behavioral function. For example, many different clinical groups may experience the same patterns of behavioral performance, hence different brain deficits can lead to the same patterns of performance, making them difficult or impossible to distinguish from one another. Occasionally different or hypo-active underlying brain circuitry may be accompanied by normal performance or alternatively performance differences may be too small too detect. Hence potential discrepancies in functioning may not be detected unless behavioral and brain imaging techniques are combined (Eldreth, Matochik, Cadet, & Bolla, 2004; Rubia et al., 2000). Imaging studies can also be a useful tool in increasing our understanding of brain differences in clinical groups. As Rubia et al. (2000) point out, causality is not certain; abnormal brain function or structure may not be the root cause of abnormal behavior. In other words, brain differences may not be causing the deficit but may be caused by years of behaving differently from the norm. Although imaging techniques cannot determine the direction of an effect per se, they may prove useful in addressing the difficult question of causality between brain function and behavior.
In a PET study of adults with ADHD, global glucose metabolism was significantly decreased in participants with ADHD in comparison to controls (Zametkin et al., 1990). This decrease was particularly evident in superior PFC and premotor regions. Imaging studies have also suggested differences in brain volume between ADHD participants and normal controls. With regard to PFC differences, a number of studies have found smaller volumes of PFC in children with ADHD (Castellanos et al., 1996; Durston, Hulshoff Pol et al., 2004; Kates et al., 2002; Mostofsky, Cooper, Kates, Denckla, & Kaufmann, 2002). In fact, Mostofsky et al. (2002) found that reduction in the volume of the PFC in ADHD children accounted for nearly half of the reduction of total cerebral volume. However due to the small sample size in this study (12 ADHD and 12 control subjects) these results should, perhaps, be interpreted with caution. In a study by Filipek et al. (1997), the volume of superior frontal regions was significantly smaller in ADHD subjects, particularly in the right hemisphere. Bilateral inferior frontal regions were smaller in ADHD subjects. This region included caudate head and anterior basal ganglia. Finally, in a more recent study, Sowell et al. (2003) found a decreased bilateral PFC volume (particularly in inferior parts of dorsal prefrontal cortex) in children and adolescents with ADHD when compared to normal controls. This study included a relatively large sample of ADHD and control children (27 children/adolescents with ADHD and 46 controls).
Casey, Castellanos et al. (1997) also used behavioral performance on three tasks, a sensory selection, response selection and response inhibition task in addition to brain volume measurements in order to investigate differences in participants with ADHD and normal controls. Correlations between performance and brain volume in PFC among other areas were seen for both groups in these tasks (patterns of correlations differing between groups). Notably, right PFC volume correlated with performance on the inhibitory task for ADHD subjects. Yeo et al. (2003) also found smaller right DLPFC volumes in ADHD children than control children. In the ADHD group this also correlated with neurometabolite concentrations (creatine and choline-containing compounds and N-acetylaspartate). Interestingly, in this study greater volume in right dorsolateral PFC correlated with poorer performance on the continuous performance task. Hill et al. (2003) also found a decreased superior prefrontal cortex, particularly in right hemisphere, in ADHD participants. In a similar vein to the Yeo et al. (2003) study just discussed, greater volume in this region in ADHD subjects correlated with poorer performance on a task of sustained attention (Conners’ Continuous Performance Test).
ADHD has been associated with a right hemisphere deficit in behavioral (Rubia et al., 1998), functional magnetic resonance imaging (fMRI) (Rubia et al., 1999; Vaidya et al., 1998) and electrophysiological studies (Pliszka, Liotti, & Woldorff, 2000; Steger, Imhof, Steinhausen, & Brandeis, 2000). Executive functions such as sustained attention (Manly et al., 2003), working memory (D’Esposito, Ballard, Aguirre, & Zarahn, 1998) and inhibition (Garavan, Ross, & Stein, 1999; Konishi, Nakajima, Uchida, Sekihara, & Miyashita, 1998) have also been attributed to the right hemisphere, particularly right PFC. It has been suggested that one of the principal deficits experienced in ADHD concerns problems with inhibition (Barkley, 1997). In fact, hypo-activity in ADHD subjects has been observed in right PFC during a classic inhibitory paradigm, the STOP paradigm (Rubia et al., 1999), Hyperactivity has been observed in PFC in adolescents (Schulz et al., 2004) and children (Vaidya et al., 1998) with ADHD during similar GO/NOGO tasks. In both studies functional differences were associated with inhibitory difficulties, as measured by a significant increase in errors of commission, in participants with ADHD.
There were, however, some discrepancies between these studies. For example, as mentioned, some studies have reported hypo-activity in PFC in ADHD when compared to controls (Zang et al., 2005) whereas others report hyperactivity (Vaidya et al., 1998). Whereas Rubia et al. (1999) found reduced activity in right prefrontal regions in the STOP task, Vaidya et al. (1998) noted an increase in activity in bilateral frontal regions, which was interpreted as an extra inhibitory effort in ADHD children. However, the GO/NOGO task employed by Vaidya et al. (1998) used 50:50 GO to NOGO ratio, which may not fully tax the inhibitory systems of, at least, the control group.
Varying the ratio of GO to NOGO stimuli has previously been found to alter “inhibitory” activation patterns (de Zubicaray, Andrew, Zelaya, Williams, & Dumanoir, 2000). When the ratio of NOGO to GO stimuli is low, a prepotent tendency to respond is established, making the stopping process more difficult upon presentation of the NOGO event. However, parametric manipulations of the number of GOs preceding a NOGO stimulus has previously been shown to have no significant effect on children with ADHD (Durston et al., 2003), one preceding GO stimulus being enough to cause inhibitory difficulty on the following trial. Additionally, since inhibitory capabilities have been shown to develop throughout childhood (Rubia et al., 2000), this task may have already been sufficiently demanding on inhibition in this group of age 8 to 13 years. The issue of the potential effect of the prepotency of the GO response should, however be kept in mind. A limitation of the study was that only selected regions were imaged and therefore information on functioning in other regions is not available.
Both hypo- and hyperactivity can be interpreted as being suggestive of inefficiency in underlying brain structures. When comparing a clinical group to normal controls hypo-activity in a certain brain region may suggest an incapability of that particular brain structure to function to the extent required by that particular task. In other words it may be considered to be “underpowered”. Hyperactivity in a region may be suggestive of a very similar problem. In this case it may be that the brain region needs to exert extra energy in order to perform a task to the same degree as the control group. Therefore these regions can be thought of as “inefficient” in that they use more energy than should be necessary to perform a given task. However, extra activity in the clinical group in a region that is not significantly active for the control group may be viewed as compensatory activity or brain regions that the clinical group are enrolling in order to compensate for under-activity in the “appropriate” network of brain areas. This will be discussed in more detail later.
More recently, exploratory analyses (with a very small sample of children) have also suggested increased bilateral prefrontal activity during NOGOs in adolescents with ADHD over normal controls in a GO/NOGO task (Schulz, Newcorn, Fan, Tang, & Halperin, 2005). Furthermore ADHD adolescents were divided into those that displayed a remission of their symptoms in adolescence and those that did not (see Table 1 for details). Adolescents that did not show remission displayed greater activity in ventrolateral PFC then those who did show remission, who in turn showed increased activity in these regions compared to normal control children. This increase in activity was also accompanied by an increase in commission errors in this task across the three groups. However a one-way ANOVA revealed that this trend failed to reach significance, probably due to the very small sample size (5 subjects in each group). As mentioned above, in a previous study by this group, using a similar GO/NOGO paradigm in adolescents, significant differences in performance were found between groups (Schulz et al., 2004). These results may be interpreted as the recruitment of additional compensatory prefrontal regions in the ADHD group in order to perform the task, as normal control activation in this ventrolateral area was more prominent in response to actual GOs than NOGO stimuli.
In an electrophysiological study utilizing the STOP paradigm, differences in wave forms were found between ADHD and control children in right PFC (Pliszka et al., 2000). This wave form was interpreted as reflecting inhibitory processes, although it has also been linked with response conflict monitoring (Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003). Correlations were also found between behavioral performance and amplitude of the wave form in right PFC, particularly for the children with ADHD (Pliszka et al., 2000). In a recent study, Fallgatter and colleagues (2004) noted that the normal pattern of NOGO-anteriorization (NGA) (a significant increase in P3 amplitude at frontal and central electrodes for the NOGO over the GO trial) was not observed in children with ADHD. NGA has previously been pinpointed as an index of prefrontal response control, such as action and conflict monitoring (Fallgatter & Strik, 1999). These authors argue that this suggests a problem with response control in children with ADHD, although this was not reflected by an increase in commission errors in this group (Fallgatter et al., 2004).
Using fMRI, Rubia and coworkers suggested that the functional differences found between ADHD children and normal controls in their imaging study of response inhibition may have been due to an immaturity in the frontal lobes of children with ADHD (Rubia et al., 1999). In order to investigate this further, these authors carried out an additional study which compared the activation patterns of normal adolescents and adults to those of adolescents with ADHD on an inhibitory paradigm and a delay task (2000). In this experiment, the behavioral results and activation patterns of normal adolescents and adults were quite similar, although the activation of adolescents was slightly reduced in prefrontal areas in comparison to adults. However, whereas adults tended to activate bilateral frontal areas, activation in the adolescents was concentrated mainly in right prefrontal cortex. The authors suggested that there is a maturation of the frontal cortex “from a functionally adequate but immature prototype system to a more definitive adult network” (page 18).
The activation patterns of adolescents with ADHD in the study by Rubia et al. were quite different. They tended to activate right pre- and post-central gyrii, right inferior parietal lobe and right caudate. For the delayed response task the ADHD subjects, unlike the comparison groups, did not activate frontal areas, except for a small focus of activation in the supplementary motor area (SMA) (Rubia et al., 2000). The authors suggest that differences in the activation patterns of ADHD subjects and normal controls in the absence of significant behavioral difference may reflect differences in strategies for task performance. It may be that there is some compensatory mechanism at play, in which participants with ADHD, who have an immature prefrontal cortex (Rubia et al., 1999), may compensate with the recruitment of additional cortical areas in order to perform at the same level as that of control participants. For example, Rubia and colleagues argue that lack of activation in “appropriate” task regions (i.e. PFC) may have been compensated for in the ADHD by the enrollment of more posterior prefrontal regions in their study (1999).
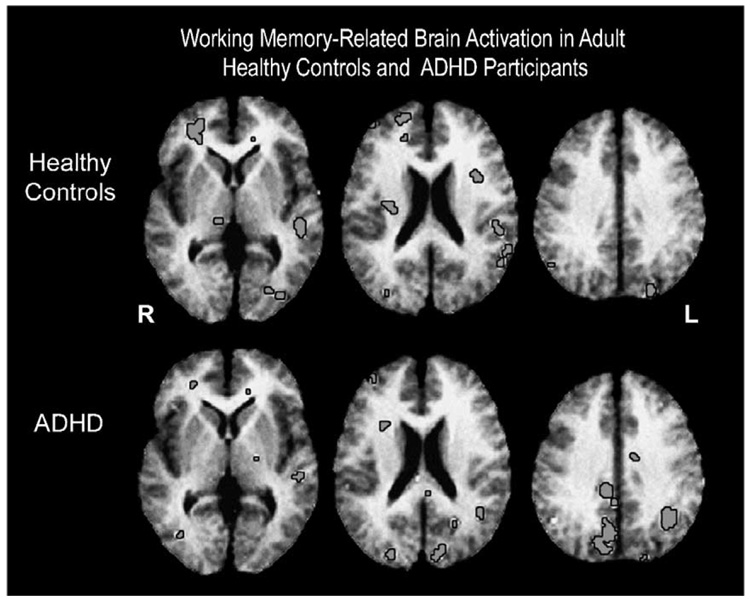
Other studies have supported the argument that individuals with ADHD use compensatory, alternative strategies and brain regions due to impaired functioning of the PFC (Schweitzer, Faber et al., 2000; Schweitzer et al., 2004). In two PET (positron emission tomography) studies of adults with ADHD, participants with ADHD activated a distinctly different network of brain regions during a working memory (WM) task. Both studies utilized a WM paradigm called the Paced Auditory Serial Addition Task (PASAT) (Gronwall, 1977), which also requires participants to inhibit distracting information. Hence it can be seen as a task which taxes executive functioning. These male ADHD subjects did not significantly activate the same right PFC regions that were activated by controls. Instead they tended to activate a more diffuse network of regions that included the parietal, precuneus, and occipital lobe. This was interpreted as a use of more visual strategies in this aurally presented task in the individuals with ADHD (Schweitzer, Faber et al., 2000). This hypothesis was supported by the men’s subsequent verbal testimony, which suggested that they had, in fact, used a visual strategy (see Fig. 1); subjects reported visualizing the task stimuli that were aurally presented via headphones.
Fig. 1.
Brain regions activated during a working memory task (i.e., PASAT) compared to random number generate control task. Images are displayed in radiological space with the right hemisphere displayed on the left side. The top row displays averaged t-maps from healthy control subjects at three horizontal levels with significant (p < 0.005) task-related increases in regional cerebral blood flow (rCBF) of the greatest extent in the right inferior frontal cortex and left superior temporal gyrus. The bottom row displays task-related increases from subjects with ADHD. Task-related increases in rCBF of the greatest extent are in the right precuneus and left inferior parietal lobe.
Visual or more “basic” strategies may be stronger in these subjects, due to a weakened ability to use verbal rehearsal strategies or possibly due to inefficiency of networks underlying WM. DLPFC has been linked to WM and manipulation of information held in WM in a number of studies (D’Esposito et al., 1998; D’Esposito & Postle, 2002; D’Esposito, Postle, Jonides, & Smith, 1999; Postle & D’Esposito, 1999b). A number of authors have also suggested that WM may involve a similar process to that used in sustaining attention (Awh & Jonides, 1998; Coull, Frackowiak, & Frith, 1998; Coull, Frith, Frackowiak, & Grasby, 1996) a system that is also believed to be impaired in ADHD (Carter, Krener, Chaderjian, Northcutt, & Wolfe, 1995). If PFC is, indeed, compromised in ADHD, it may be that subjects are unable to adequately recruit more “efficient” regions and may be forced to rely on alternative, more automatic strategies in order to perform certain paradigms. That is, subjects with ADHD may need to recruit alternative brain regions to boost a weakened neural circuitry.
In a follow-up PET study, the authors (Schweitzer et al., 2004) found that the most common treatment for ADHD, methylphenidate (MPH), led to decreases in activity in the PFC in adults with ADHD. In this study the dose of the medication was individually, clinically determined for each subject. The MPH was interpreted as having “honed” the prefrontal system so that it was able to more efficiently inhibit distracters and boost performance of the task (Schweitzer et al., 2004). In fact, in a previous study by this group on the effects of MPH on the activation patterns of participants with ADHD during rest, it was shown that subjects showed wider and more diffuse patterns of activity during the off-medication period than during the on-medication period (Schweitzer, Lee et al., 2003). Activity during the rest period was primarily associated with cortical motor regions.
As an extension to the WM studies in adults, Schweitzer et al. used fMRI and a visual variant of the PASAT to test WM in children (mean age of 10 years) with ADHD (Schweitzer, Cortes, Gullapalli, Dunning, & Tagamets, 2003). In this preliminary study, control subjects tended to activate the left hemisphere regions significantly more than ADHD children. In contrast, in the ADHD children there were wider spread activations, particularly in the right hemisphere throughout the cortices. Similar to the adult ADHD subjects, the pediatric ADHD subjects activated regions associated with visual processes (e.g., occipital gyrus, cuneus) to a greater extent than the control subjects, once again, suggesting use of a system more reliant on visual strategies and response to visual stimuli.
From the studies just examined it is apparent that PFC may have a central role to play in the deficits associated with ADHD. As mentioned, areas such as DLPFC have been associated with a number of different executive functions such as the maintenance of task set (Frith & Dolan, 1996; MacDonald, Cohen, Stenger, & Carter, 2000; Garavan, Ross, Murphy, Roche, & Stein, 2002; Ruchsow, Grothe, Spitzer, & Kiefer, 2002), inhibition of a prepotent response tendency (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003; Braver, Barch, Gray, Molfese, & Snyder, 2001; de Zubicaray et al., 2000; Garavan et al., 1999, 2002; Kawashima et al., 1996; Konishi et al., 1998), sustaining attention (Coull et al., 1996, 1998; Manly et al., 2003; Sturm et al., 1999; Wilkins, Shallice, & McCarthy, 1987) and WM (D’Esposito et al., 1998). In fact, a substantial number of structural brain imaging studies have reported smaller DLPFC volumes in participants with ADHD (Castellanos et al., 1996, 2002; Durston, Hulshoff Pol et al., 2004; Filipek et al., 1997; Hill et al., 2003; Kates et al., 2002; Mostofsky et al., 2002). Thus, dysfunction in areas of the PFC, such as DLPFC, may account for a large number of the symptoms experienced by individuals with ADHD, such as, trouble inhibiting, maintaining task set and sustaining attention on a task.
It has also been suggested that functional impairment of the PFC in ADHD may be due to catecholamine (e.g., dopamine, noradrenaline) signal transduction defects in this region (Arnsten, 2001; Ernst, Zametkin, Matochik, Jons, & Cohen, 1998; Mehta, Calloway, & Sahakian, 2000). This is, however, beyond the scope of this review. Given that, as mentioned earlier, PFC has interconnections with a vast array of other cortical and subcortical regions, it is unlikely that prefrontal regions work in isolation. This may be particularly important with reference to striatal regions which are thought to play a very important role in ADHD as will be discussed further. Impairment in the PFC is likely to influence the integrity of detection of demands for a given task or situation. An altered PFC has the potential to limit how well it can recruit other brain regions with which it has interconnections to meet task demands.
4. Anterior cingulate cortex
The anterior cingulate cortex (ACC) is another structure implicated in higher-level cognitive functioning, but also to basic, primary stimuli such as reward (Knutson, Fong, Bennett, Adams, & Hommer, 2003; Rogers et al., 2004). A number of authors have implicated ACC in top-down attentional control and inhibition of competing responses to various stimuli (Pardo, Pardo, Janer, & Raichle, 1990; Posner & DiGirolamo, 1998). Casey, Trainor et al. (1997) also found that the size of right ACC in children correlated with performance on an attentional paradigm. There is considerable structural and proposed functional heterogeneity within the ACC. ACC is believed to have two major components: a rostral section that is concerned with emotional or affective processes and a more caudal and dorsal region that is thought to be concerned with cognitive processes (Bush, Luu, & Posner, 2000). Response conflict has been associated with dorsal, caudal areas of ACC (Fassbender et al., 2004; Hester, Fassbender, & Garavan, 2004; Ullsperger & von Cramon, 2001) whereas emotional, pain and mood disorders (Bush et al., 2000; Whalen et al., 1998) and performance monitoring (Garavan, Ross, Kaufman, & Stein, 2003) have often been associated with more rostral and ventral areas. ACC is an area that has been implicated in a wide variety of cognitive tasks such as Stroop tasks (Leung, Skudlarski, Gatenby, Peterson, & Gore, 2000), verb generation tasks (Peterson et al., 1998, 1999) and flanker tasks (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Ullsperger & von Cramon, 2001). It has been included in a network of regions that subserve alertness or arousal (Posner & Petersen, 1990; Sturm et al., 1999) as well as target detection (Posner & Petersen, 1990). It is often seen to be active in conjunction with DLPFC (Duncan & Owen, 2000; Paus, 2001; Posner & DiGirolamo, 1998), another region that has been implicated in cognitive control (Banich et al., 2000; Miller, 2000; Miller & Cohen, 2001). For this reason, it has often been implicated in cognitive control at some level, either as being the direct source of that control (Posner & DiGirolamo, 1998) or as being an indirect source by either detecting errors (Kiehl, Liddle, & Hopfinger, 2000) or potential conflict and signalling to PFC, which then exercises that control (Botvinick et al., 2001). The ACC has also been implicated in motivation and anticipation (Larisch et al., 1999), self-monitoring of motor actions (Luu, Flaisch, & Tucker, 2000), reward-related decision making (Bush et al., 2002) and more recently for being principally involved in autonomic control (Critchley et al., 2003).
Anterior cingulate hypo-activity has been linked to a number of clinical conditions (Alain, McNeely, He, Christensen, & West, 2002; Holcomb, 2004) and has also been observed in cocaine, (Kaufman, Ross, Stein, & Garavan, 2003; Kilts et al., 2001), opiate (Forman et al., 2004) and marijuana users (Eldreth et al., 2004). Not surprisingly, ACC dysfunction has also been associated with ADHD. In a structural imaging study by Filipek et al. (1997), the authors found right anterior-superior frontal differences between ADHD and normal subjects and the cerebral divisions that these analyses were based upon included ACC.
Reduced activity in anterior and posterior cingulate cortex was found for ADHD compared to control subjects in a delay paradigm which required subjects to synchronize their finger movements with stimuli separated by a 5 s delay (Rubia et al., 1999). In the same study, reduced activation was also found in medial PFC for ADHD subjects in a STOP paradigm (Rubia et al., 1999). Reduced activation was also seen in ACC in ADHD adults during performance of a counting Stroop task (Bush et al., 1999).
However, the aforementioned studies utilized block fMRI designs, which can often include task-related confounds such as contamination from error-related processes, vigilance and motivation. As mentioned, these processes have been linked with ACC functioning. Performance differences between groups have previously been shown to significantly affect the number of false positives and negatives and also decrease the number of true positives in activation maps (Murphy & Garavan, 2004). Therefore, it is important to control for performance differences between groups, particularly in clinical groups where performance differences are often expected. In an event-related fMRI study of inhibitory control in ADHD and normal control boys, Tamm, Menon, Ringel, and Reiss (2004) found significantly decreased activation in ACC extending into pre-SMA and SMA, after controlling for the oddball effect of infrequent NOGO events. Although there were performance differences between the groups in this study (ADHD participants making more errors both of commission and omission), event-related analysis allows the experimenter to examine correct events only, therefore excluding error-related processes form activation maps.
Hypo-activity was also noted in children with ADHD in event-related fMRI studies utilizing a GO/NOGO paradigm (Schulz et al., 2004). In the Schultz study, increased activity in ACC was associated with difficulty inhibiting the prepotent response in both ADHD subjects and normal controls. The authors suggested that the increased activity experienced by subjects with ADHD could either be related to difficulties inhibiting the dominant GO response processes or compensatory activation due to inactivity in task-appropriate brain structures (Schulz et al., 2004). Fallgatter et al. (2004) have also found a “significant decrease in electrical activity in the anterior cingulate” (page 979) for ADHD children in the NOGO condition of a continuous performance task in their ERP examination of ADHD; the central NOGO P3 amplitude of ADHD children was significantly diminished in comparison to controls. This finding was supported by a t-test between groups, corrected for multiple comparisons which resulted in significantly diminished NOGO activity in ACC for the ADHD group (Fallgatter et al., 2004).
In a PET study of decision making in adults with ADHD, Ernst et al. (2003) found no significant activation of ACC in the ADHD group in comparison to control subjects. Furthermore, activity in the left ACC was negatively correlated with ADHD severity indexed by the Conner’s Abbreviated Teacher’s Rating Scale. The authors did find one area in right ACC that was activated more for ADHD subjects than controls. Ernst et al. suggested that this area may have been recruited in order to compensate for hypo-activity in left ACC. This suggestion was supported by the observation that there was a negative correlation between activation in this region and performance in normal controls suggesting that in the normal brain activity in this region may be detrimental to performance and may need to be suppressed. In fact, in a previous study of preparatory activation in a GO/NOGO task, suppression of a number of areas including medial PFC was associated with successful inhibition of a prepotent response tendency in healthy controls (Hester et al., 2004).
In a recent fMRI study conducted by this group (Schweitzer, Cortes et al., 2003) we also found ACC hypo-activity in children with ADHD during a WM task. This was previously seen in a PET study of adults with ADHD, irrespective of whether they were medicated or not (Schweitzer et al., 2004). Additionally, in a previous PET examination of activation for a WM paradigm (PASAT), when progressive time-on-task activation was examined, control men showed a significant increase in activation in medial frontal areas over time, whereas men with ADHD showed increases in right lenticulate, left parahippocampal gyrus and in the cerebellum (Schweitzer, Faber et al., 2000). We have suggested that an alternative network, perhaps relying more on motor regions (which were shown to be more active in the ADHD group) was compensating for a lack of activity in ACC and that this alternative network may have been inhibiting responses and monitoring for conflict in place of the ACC (Schweitzer et al., 2004).
In a similar vein to the studies mentioned previously (Rubia et al., 1999; Schweitzer, Anderson, & Ernst, 2000; Schweitzer et al., 2004), we suggest that subjects may be experiencing difficulties in employing PFC to recruit relevant brain networks that are needed to perform paradigms efficiently. It is important to reiterate here, that patterns of activation in ADHD subjects may not always be hypo-activity in areas normally employed by healthy subjects, but may also include hypo-activity in these areas or alternate activations in other, sometimes completely novel, regions of the brain. Harking back to the study of Schweitzer, Faber et al. (2000), we hypothesized that adult subjects were using a visual strategy in this task. Strategies and/or cortical networks employed by ADHD groups may vary due to the population, age and also task requirements. We suggest that due to inefficiency of PFC, subjects with ADHD are required to utilize phylogenetically older, more basic or automatic processes in the performance of particular tasks that require less coordination by PFC. This may be why response inhibition is often seen to be impaired within this group because once a pattern of automatic responding has been established it may be particularly difficult for these participants to override the strong prepotency to respond and employ executive control in order to stop this pattern of responding.
From the studies reviewed it is apparent that there may be structural as well as functional differences between individuals with ADHD and normal controls in ACC. These functional differences are also seen in a variety of different cognitive paradigms and using different imaging techniques. Most studies appear to find hypo-activity in this region in both child and adult participants (Bush et al., 1999; Rubia et al., 1999; Schulz et al., 2004; Schweitzer, Faber et al., 2000; Tamm et al., 2004). A number of these studies suggest the notion of compensatory brain regions or cognitive strategies in individuals with ADHD (Schweitzer, Faber et al., 2000; Schulz et al., 2004; Ernst et al., 2003). ACC is a controversial and complex structure, whose full role in cognitive control has not, as yet, been fully established. Some authors describe it as a structure enforcing top-down control (Pardo et al., 1990; Posner & DiGirolamo, 1998; Tzourio et al., 1997) whereas others believe that it is more indirectly involved in attentional control, detecting errors (Dehaene, Posner, & Tucker, 1994; Falkenstein, Hoormann, Christ, & Hohnsbein, 2000), or the likelihood that an error may occur (Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Carter et al., 1998) and subsequently feeding back this information to other areas in PFC more directly involved in imposing top-down control (Botvinick et al., 2001; Cohen, Botvinick, & Carter, 2000; MacDonald et al., 2000). Thus, dysfunction in the ACC may explain a number of the cognitive deficits experienced by individuals with ADHD. However, deficits in both brain activation patterns and performance may be due to abnormalities in the system either “upstream”, or “downstream” from the ACC.
As mentioned, ADHD is believed to involve a deficit in PFC in general and that PFC is widely considered to be involved in the delegation of brain resources to various aspects of tasks. Part of the PFC’s presumed role is the dynamic adjustment of these resources as task demands change (Miller, 2000; Miller & Cohen, 2001). This may be precisely the problem in ADHD; subjects may be forced to use limited, rigid and inflexible strategies and/or cerebral networks during task performance. Proposed PFC decrements may hamper the ability to enforce top-down control and gate activity appropriately to subserve efficient performance. In fact this may explain a diverse number of ADHD symptoms including difficulties sustaining attention, delaying gratification and inhibiting inappropriate behavior and cognitive sets.
5. Additional cortical regions
In addition to PFC and ACC, it is likely that other areas are involved in the symptomatology of ADHD. Overall reductions in cerebral glucose metabolism (Ernst et al., 1994) and total brain volume have been noted in a number of imaging studies, which has implications for the integrity of the whole brain in this disorder. Evidence for wider spread alterations in brain functioning is also reflected in electrophysiological studies demonstrating differences at various stages of the ERP between participants with ADHD and normal controls, suggesting difficulties at different stages of processing ranging from orienting (Brandeis et al., 1998) or perceptual and cognitive processing to motor preparation and output (Pliszka et al., 2000).
Posterior regions of the brain are relevant, as these regions, including the parietal lobes are implicated in orienting of (Corbetta & Shulman, 2002; Coull, Frith, Buchel, & Nobre, 2000; Coull & Nobre, 1998; Kincade, Abrams, Astafiev, Shulman, & Corbetta, 2005; Le, Pardo, & Hu, 1998) and sustaining (Manly et al., 2001; Pardo, Fox, & Raichle, 1991; Sturm et al., 1999; Sturm & Willmes, 2001) attention. The effect of ADHD on the parietal lobes is unclear at this point, as one study (Filipek et al., 1997) suggests smaller brain volumes in parietal regions and yet another (Sowell et al., 2003) found a 15–30% increase in grey matter in the bilateral inferior parietal lobes of children with ADHD. Although the functional significance of these anatomical findings is not clear, at least two studies found the inferior parietal lobe more active in ADHD subjects during WM (Schweitzer, Faber et al., 2000) and GO/NO-GO paradigms (Durston et al., 2003). Enhanced activity in these regions may reflect compensatory use of these regions, perhaps related to processes subserved by the parietal lobes such as attention and visual spatial processing. Alternatively, hypo-activity in certain brain regions may be linked to a lack of efficiency, leading to over-activation and excessive effort within that region (Vaidya et al., 1998). Conversely, there appear to be relative decrements in activation in regions associated with language or verbal rehearsal, such as the left temporal cortex (Shafritz, Marchione, Gore, Shaywitz, & Shaywitz, 2004).
Additional evidence for enhanced use of posterior regions associated with visual strategies is consistent with activity in the occipital lobe. A preliminary study in children with ADHD suggested (Schweitzer, Faber et al., 2000), that ADHD subjects showed greater activation than controls in the right inferior occipital gyrus and left cuneus in a WM task. Rubia et al. (1999) also found increased activity in adolescents with ADHD in the right extrastriate cortex during an inhibitory task in comparison to normal controls.
Similarly, Durston et al. (2003) found that children with ADHD showed increased activation in precuneus and occipital cortex over normal controls during inhibition in a GO/NOGO task, while displaying decreased activation in prefrontal and ACC. However, this study involved imaging data from a small sample of children (7 with ADHD and 7 controls), which included both inattentive and combined subtypes; therefore some caution is needed in the interpretation of these results. These authors also interpreted the activation of alternate strategies and brain networks in participants with ADHD as a reliance on a more diffuse network of neural systems in the performance of tasks that tax executive control functions. They suggest that the reliance on parietal and posterior networks may reflect a taxing of vigilance or sustained attention systems. Contrary to these findings, a recent study found decreased activity in occipital regions of ADHD subjects relative to normal controls in a GO/NOGO paradigm (Schulz et al., 2005). These authors also found increased activity in parietal regions for participants with ADHD. However, the number of subjects in this study was very small (see Table 1 for details) therefore caution must be exercised in interpreting the results.
6. Basal ganglia
A number of sources of evidence suggest that the basal ganglia are important structures relevant to ADHD research. Firstly, increasing evidence exists of altered performance in the five functionally interconnected subcortical structures of the basal ganglia: the caudate nucleus, putamen, globus pallidus, subthalamic nucleus, and ventral mesencephalon in ADHD. These regions are part of five parallel basal ganglia–thalamocortical circuits (Alexander, Crutcher, & DeLong, 1990; Alexander, DeLong, & Strick, 1986) thought to convey output through specific thalamic zones to the frontal cortex. These circuits contribute to a number of functions thought to be affected in ADHD including motor, somatosensory, oculomotor, executive, emotion, and motivational functions. Second, a mechanism of action of the most common and effective treatment for ADHD, stimulant medication, works within the basal ganglia substructures, via increasing extracellular dopamine in the striatum by inhibiting reuptake by dopamine transporters (Volkow et al., 2001). Third, there is a strong body of findings showing decreased volume and in some instances altered asymmetries in basal ganglia structures in ADHD children in comparison to controls (Castellanos et al., 2002). Reversed caudate asymmetry has been associated with poor executive control as measured by the Stroop task and the Wisconsin Card Sort Task (Semrud-Clikeman et al., 2000). In the same study smaller volume of the head of the left caudate nucleus, associated more with participants with ADHD, was linked to higher scores on the externalizing scale of the Child Behavior Checklist.
Findings within the basal ganglia are arguably the most contradictory of the regions tested in the ADHD functional imaging literature. A number of SPECT studies by Lou and colleagues found decreases in basal ganglia activity in subjects with ADHD relative to controls (Lou, Henriksen, & Bruhn, 1984; Lou, Henriksen, & Bruhn, 1990; Lou, Henriksen, Bruhn, Borner, & Nielsen, 1989). However, there were a number of potential confounds in these studies. Children with comorbidities were included and the three studies utilized a largely overlapping sample of subjects. More recently, two pediatric fMRI studies, one using a GO/NOGO task (Durston et al., 2003) and the other a divided attention paradigm (Shafritz et al., 2004), have found decreases in basal ganglia activation in ADHD participants relative to controls. An fMRI study by Vaidya and co-investigators, however, suggests that task conditions can have a strong influence on the degree of basal ganglia activation in ADHD (Vaidya et al., 1998). In a condition controlling for rate of stimulus presentation, subjects with ADHD showed reduced caudate activation relative to controls. In another condition controlling for rate of responses, subjects with ADHD showed a trend (p=0.08) toward increased caudate activation compared to controls. This study suggests that caudate activation may vary with the type and degree of cognitive demands placed on the subjects.
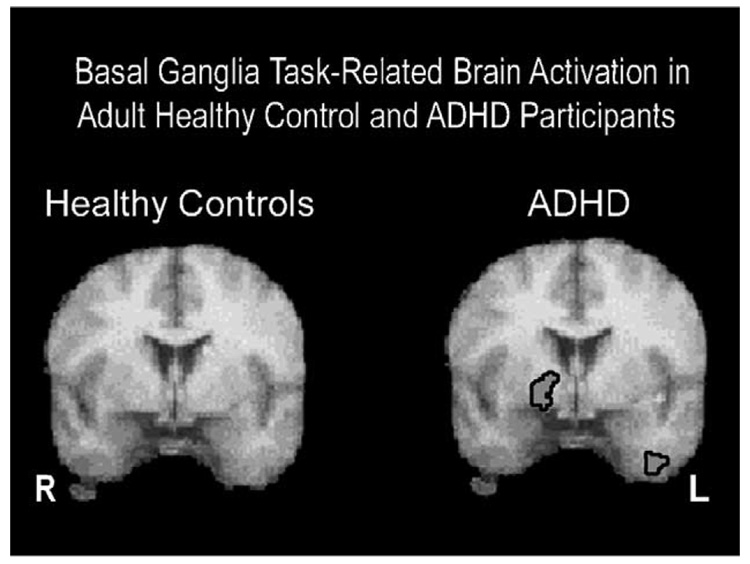
In contrast to the findings in the pediatric literature, studies in adults with ADHD show increased task-related caudate and putamen activity relative to controls (Bush et al., 1999; Flowers, Wood, Price, & Absher, 1997; Schweitzer, Faber et al., 2000; Schweitzer et al., 2004). Schweitzer, Faber et al. (2000) found tasks requiring complex cognitive functions, such as WM paradigms engage the lenticulate nucleus in adults with ADHD (see Fig. 2). This laboratory also found increased right caudate activity (r=0.63, p<0.03) in relation to better task performance on a WM task in adults with ADHD. Activations in this study tended to be on the right side of the basal ganglia, whereas greater caudate activity reported in other studies for healthy pediatric controls appeared to be on the left side (Durston et al., 2003; Shafritz et al., 2004). We speculate that lateralization of basal ganglia activation may be related to differences in how ADHD individuals use this region to perform tasks. Greater basal ganglia activation in ADHD individuals may reflect response preparation and preferential use of visual/spatial strategies in WM tasks (Postle & D’Esposito, 1999, 2003). In addition to task variables, age may also influence basal ganglia activation. Differences between compensatory basal ganglia activity in children and adults may be related to differences in the developmental trajectory of the caudate nucleus, as individuals with ADHD appear to have a smaller caudate nucleus in childhood in comparison to normal controls that normalizes by mid adolescence (Castellanos et al., 2002). Perhaps individuals with ADHD learn to use this structure more flexibly and with increased frequency than normal controls due to the role of the caudate in response preparation, attempts to control motor movement, subtle use of strategies involving motor functioning, and spatial processing (Postle & D’Esposito, 1999).
Fig. 2.
Brain regions activated related to time effects during performance during a working memory task (i.e., PASAT). The right hemisphere is displayed on the left side. The image on the right displays averaged t-maps from subjects with ADHD with significant (p < 0.005) task-related activation in rCBF increasing in the right lenticular nucleus and left insula. The image on the left displays averaged t-maps from healthy control subjects. Note no significant activation in the basal ganglia for the healthy subjects.
The effect of stimulants on basal ganglia activation is also somewhat contradictory. An early PET study in ADHD adults suggested possible MPH-induced decreases in the putamen (Matochik et al., 1994). Studies in ADHD children (Kim, Lee, Cho, & Lee, 2001; Lou et al., 1989; Shafritz et al., 2004; Teicher et al., 2000; Vaidya et al., 1998), however, found MPH increased activity in the basal ganglia. Of note, in the pediatric studies, brain activity was significantly lower in the basal ganglia in ADHD participants than healthy controls during placebo conditions.
7. Cerebellum
Until recently the cerebellum was traditionally thought to be principally involved in motor control. Over the past decade, however, evidence for involvement of this structure in cognitive and emotional functioning has led to the recognition that the cerebellum is involved in more than simply posture and motor control as was previously thought (Daum & Ackermann, 1995; Desmond, Gabrieli, & Glover, 1998; Leroi et al., 2002; Levisohn, Cronin-Golomb, & Schmahmann, 2000; Najib, Lorberbaum, Kose, Bohning, & George, 2004; Schmahmann, 1998; Van Mier & Petersen, 2002). Middleton and Strick (2001) also demonstrated cerebellar–PFC connections, linking portions of the cerebellum to areas such as DLPFC, which is generally associated with a number of cognitive operations.
The cerebellum has also been implicated in a number of developmental disorders, including autism (Acosta & Pearl, 2004; Allen, Muller, & Courchesne, 2004), schizophrenia (Andreasen, Paradiso, & O’Leary, 1998; Ho, Mola, & Andreasen, 2004), and ADHD (see Castellanos et al., 2002 article for further discussion). The exact relationship between this structure and developmental disorders is as yet unknown. However, evidence that the cerebellum is one of the last structures of the brain to fully develop, with development continuing into the 20s (Giedd, personal communication), has implications for how maturation in this structure may relate to the onset of symptoms across disorders and changes in phenotypic markers with development. The cerebellum is also the least genetically determined structure (Giedd, personal communication) and as such is the most sensitive structure to environmental variables.
The cerebellum is of particular interest to ADHD researchers because of the number of functions associated with it that appear impaired in ADHD. Execution of timed movements within a sequence (Van Mier & Petersen, 2002), planning and WM (Middleton & Strick, 2001), verbal rehearsal (Chen & Desmond, 2005) are just a few behaviors linked to cerebellar function that may be implicated in the disorder. Links between the basal ganglia, cerebellum, and the PFC (Middleton & Strick, 1994) suggest that abnormalities found in the cerebellum, basal ganglia, and prefrontal cortex in ADHD may reflect a circuit-wide dysfunction in prefrontal–basal ganglia–cerebellar loops. Similarly, Berquin et al. (1998) proposed that cerebello–thalamo–prefrontal dysfunction may underlie the deficits in motor, inhibition, and executive function typically found in ADHD.
The cerebellum appears to be altered in volume (e.g. Berquin et al., 1998; Castellanos et al., 2002; Mostofsky, Reiss, Lockhart, & Denckla, 1998) and perhaps function (Schweitzer et al., 2004; Valera, Faraone, Biederman, Poldrack, & Seidman, 2005) in ADHD. The vermis of the cerebellum in adults with ADHD shows relatively increased activity in comparison to healthy controls during WM paradigms (Schweitzer et al., 2004). Perhaps increased cerebellar vermis activity in ADHD is in response to suboptimal PFC functioning. It is unclear at this point whether or not increased cerebellar activity is a tonic or phasic response to task demands as these data were acquired using imaging methods with relatively limited temporal resolution (i.e., PET with [15O]H2O). Current studies on the most commonly used pharmacological treatment of ADHD, MPH, suggest however, a tonic response. Consistent evidence indicates MPH increases cerebellar vermis activity, but only in a non-task related fashion (Anderson, Polcari, Lowen, Renshaw, & Teicher, 2002; Mehta et al., 2000; Schweitzer, Lee et al., 2003; Schweitzer et al., 2004; Volkow et al., 2001). Changes in vermal activity also correlate with rates of hyperactivity and predict treatment response (Anderson et al., 2002; Schweitzer, Lee et al., 2003).
The effect of MPH most likely reflects enhanced extracellular concentrations of norepinephrine (Kuczenski & Segal, 2001) as the cerebellum receives rich noradrenergic input (Powers, O’Connor, & Price, 1989), although we cannot rule out MPH effects on dopaminergic (Melchitzsky & Lewis, 2000), or serotonergic systems. These data are consistent with the hypothesis that ADHD may be due to dysregulation of the noradrenergic system or to interactions between the dopaminergic and noradrenergic system (Arnsten, 2000; Biederman & Spencer, 1999). Perhaps the increased cerebellar vermal activation reflects a compensatory attempt of the noradrenergic system to tonically increase response to environmental stimuli due to an overburdened, poorly functioning PFC and ACC.
The over active cerebellar vermis may also be linked to impairments in the noradrenergic system emanating from locus coeruleus (LC) noradrenergic neurons. LC activity is recognized as playing an important role in balancing responses between exploratory behavior and maintenance of task-related attention (Usher, Cohen, Servan-Schreiber, Rajkowski, & Aston-Jones, 1999). Mefford and Potter (1989) hypothesized that ADHD symptoms are consistent with an imbalance in alpha-2 adrenergic receptor number which leads to poor control of inhibition of LC neurons or inhibition of excitatory afferents to the LC. The result is increased exploratory behavior in response to novel environmental stimuli in individuals with ADHD and difficulty maintaining on-task behavior due to high tonic LC activity.
8. Developmental hypotheses and compensatory strategies in ADHD
ADHD is historically labeled as a disorder of developmental delay in regard to behavioral and emotional functioning. Neuroimaging findings support the extension of this label to neural functioning as well. For example, a recent study examining patterns of functional brain activity associated with development in children found brain activation becomes less diffuse and more focal with maturation (Durston, Davidson et al., 2004). Similarly, individuals with ADHD tend to activate a more diffuse, wider system of brain regions to perform a task (Bush et al., 1999; Durston et al., 2003; Schweitzer, Faber et al., 2000, 2004; Tamm et al., 2004). This diffuse pattern may be secondary to an impaired prefrontal and ACC functioning that is less capable of coordinating the recruitment of subsidiary brain regions to match current situational demands.
Developmental factors may also be reflective of an apparent preference for the activation of brain regions associated with visual–spatial and mental imagery versus verbally mediated strategies in individuals with ADHD. Evidence from the developmental literature suggests that eidetic imagery skills decline with maturation (Giray, Altkin, Vaught, & Roodin, 1976; Giray & Barclay, 1977). This decline is hypothesized to be due to the development of verbal abilities interfering with the use of visual/spatial skills (Kosslyn, Margolis, Barrett, Goldknopf, & Daly, 1990). Children with ADHD appear to have a disrupted, immature ability to use internalized speech (Berk & Potts, 1991; Diaz & Berk, 1992). Their immature verbal rehearsal skills may interfere less with visual imagery than the more mature verbal skills of their non-affected peers. A weakened ability to activate brain regions associated with verbal strategies (e.g., temporal cortex) (Schweitzer, Faber et al., 2000; Schweitzer, Lee et al., 2004; Shafritz et al., 2004) may be more consistent with what would be expected from a younger child. Developmental theories are also supported by neuroanatomical evidence suggesting that brain regions impaired in the disorder are phylogenetically late in maturation and some of the last to develop in an individual (e.g., PFC and cerebellum) (Reiss, Abrams, Singer, Ross, & Denckla, 1996). Maturation of fibers that innervate language and association cortices (Thompson et al., 2000) are also fairly late in development. As another reflection of delayed developmental processing, compensation in ADHD may be associated with processes and brain regions that are more proximal to the stimulus input (e.g., visual versus a verbal stimulus) and required response. Thus, there may be less involvement of the higher order processing and associated brain regions (e.g., PFC) to coordinate responding between brain regions. Lack of coordination by these higher order regions may result in more effortful and in some cases, less accurate responding and processing of stimuli. ADHD may not be the only developmental disorder distinguished by compensatory anatomy relying on more rudimentary brain regions and strategies. For instance, there is documentation of similarly increased engagement of posterior (e.g., inferior temporal and occipital) and right-sided regions, in autism (Koshino et al., 2005).
9. Conclusion and clinical implications of a compensatory neuroanatomy in ADHD
Initial functional imaging studies in ADHD (Bush et al., 1999; Rubia et al., 1999; Zametkin et al., 1990) focused on the absence of activity in the frontal cortex, with much less consideration given to concomitant findings regarding over-activity in other regions. As the imaging field evolved, the use of whole-brain techniques became more standard and resulted in the identification of regions with relative over-activity as well. These regions of increased activity may be associated with the use of performance strategies that are more strongly supported by those regions. Indeed, it is possible that there may be relatively enhanced and preferential use of strategies linked to brain regions that are more active in ADHD versus normal controls. This review found evidence to suggest that tasks requiring higher cognitive functioning are associated with greater activation in regions associated with motor and visual, spatial processing in individuals with ADHD (Durston et al., 2003; Kim, Lee, Shin, Cho, & Lee, 2002; Rubia et al., 1999; Schweitzer, Faber et al., 2000; Schweitzer et al., 2004). In contrast, healthy subjects are more likely to engage regions associated with cognitive organization (e.g., PFC, ACC) and phonological strategies.
This review suggests there is a greater need for understanding how the functional imaging “signal” of ADHD relates to behavior, symptoms, and cognitive strategies. Several of the studies reviewed provide clues to guide novel behavioral studies to assess learning styles in ADHD. It is unknown at this point, whether children with ADHD exhibit relative strengths using strategies involving visual, spatial, or motoric processes; however findings from imaging studies support the investigation of compensatory strategies using these mechanisms. We suggest that researchers begin to observe and test whether the behavioral strategies ADHD individuals apply to task performance can be characterized by the use of specific compensatory strategies. Future studies could also explore the relationship between the development and implementation of alternative strategies with level of functioning in academic, social, and career contexts. As we improve our understanding of how the biological substrates of the disorder relate to its clinical presentation, so too will our ability to reliably identify ADHD and develop psychological, educational, and pharmacological treatments that can be tailored to an individual’s “psycho-neuro profile”.
Acknowledgements
This work was supported by a grant from the National Institute for Mental Health (MH 066310). We would like to thank Barbara McGee for technical assistance.
References
- Acosta MT, Pearl PL. Imaging data in autism: From structure to malfunction. Seminars in Pediatric Neurology. 2004;11:205–213. doi: 10.1016/j.spen.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Alain C, McNeely HE, He Y, Christensen BK, West R. Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cerebral Cortex. 2002;12:840–846. doi: 10.1093/cercor/12.8.840. [DOI] [PubMed] [Google Scholar]
- Alexander G, Crutcher M, DeLong M. Basal ganglia–thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in Brain Research. 1990;85:119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Allen G, Muller RA, Courchesne E. Cerebellar function in autism: Functional magnetic resonance image activation during a simple motor task. Biological Psychiatry. 2004;56:269–278. doi: 10.1016/j.biopsych.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Polcari A, Lowen SB, Renshaw PF, Teicher MH. Effects of methylphenidate on functional magnetic resonance relaxometry of the cerebellar vermis in boys with ADHD. American Journal of Psychiatry. 2002;159:1322–1328. doi: 10.1176/appi.ajp.159.8.1322. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical–subcortical–cerebellar circuitry? Schizophrenia Bulletin. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Genetics of childhood disorders: XVIII. ADHD Part 2. Norepinephrine has a critical modulatory influence on prefrontal cortical function. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1201–1203. doi: 10.1097/00004583-200009000-00022. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Dopaminergic and noradrenergic influences on cognitive functions mediated by prefrontal cortex. In: Solanto MV, Arnsten AF, Castellanos FX, editors. Stimulant drugs and ADHD: Basic and clinical neuroscience. New York: Oxford University Press; 2001. [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J. Spatial working memory and spatial selective attention. In: Parasuraman R, editor. The attentive brain. Cambridge, MA: MIT Press; 1998. pp. 353–380. [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, et al. Prefrontal regions play a predominant role in imposing an attentional ‘set’: Evidence from fMRI. Brain research. Cognitive Brain Research. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Berk LE, Potts MK. Development and functional significance of private speech among attention-deficit hyperactivity disordered and normal boys. Journal of Abnormal Child Psychology. 1991;19:357–377. doi: 10.1007/BF00911237. [DOI] [PubMed] [Google Scholar]
- Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL, et al. Cerebellum in attention-deficit hyperactivity disorder: A morphometric MRI study. Neurology. 1998;50:1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- Berwid OG, Curko Kera EA, Marks DJ, Santra A, Bender HA, Halperin JM. Sustained attention and response inhibition in young children at risk for attention deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry. 2005;46:1219–1229. doi: 10.1111/j.1469-7610.2005.00417.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Spencer T. Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biological Psychiatry. 1999;46:1234–1242. doi: 10.1016/s0006-3223(99)00192-4. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychology Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brandeis D, van Leeuwen TH, Rubia K, Vitacco D, Steger J, Pascual-Marqui RD, et al. Neuroelectric mapping reveals precursor of stop failures in children with attention deficits. Behavioural Brain Research. 1998;94:111–125. doi: 10.1016/s0166-4328(97)00174-5. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: Effects of frequency, inhibition and errors. Cerebral Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Johnstone SJ, Barry RJ, Clarke AR, McCarthy R, Selikowitz M, et al. The effect of methylphenidate on response inhibition and the event-related potential of children with attention deficit/hyperactivity disorder. International Journal of Psychophysiology. 2005;58:47–58. doi: 10.1016/j.ijpsycho.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Krener P, Chaderjian M, Northcutt C, Wolfe V. Asymmetrical visual–spatial attentional performance in ADHD: Evidence for a right hemispheric deficit. Biological Psychiatry. 1995;37:789–797. doi: 10.1016/0006-3223(94)00217-Q. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Durston S, Fossella JA. Evidence for a mechanistic model of cognitive control. Clinical Neuroscience Research. 2001;1:267–282. [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, et al. The role of the anterior cingulate in automatic and controlled processes: A developmental neuroanatomical study. Developmental Psychobiology. 1997;30:61–69. [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA: The Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. NeuroImage. 2005;24:332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: Who’s in control? Nature Neuroscience. 2000;3:421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frackowiak RS, Frith CD. Monitoring for target objects: Activation of right frontal and parietal cortices with increasing time on task. Neuropsychologia. 1998;36:1325–1334. doi: 10.1016/s0028-3932(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Buchel C, Nobre AC. Orienting attention in time: Behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38:808–819. doi: 10.1016/s0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: A PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: The neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. Journal of Neuroscience. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Ballard D, Aguirre GK, Zarahn E. Human prefrontal cortex is not specific for working memory: A functional MRI study. NeuroImage. 1998;8:274–282. doi: 10.1006/nimg.1998.0364. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR. The organization of working memory function in lateral prefrontal cortex: Evidence from event-related functional MRI. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. Oxford University Press; 2002. [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum I, Ackermann H. Cerebellar contributions to cognition. Behavioral Brain Research. 1995;67:201–210. doi: 10.1016/0166-4328(94)00144-5. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Andrew C, Zelaya FO, Williams SC, Dumanoir C. Motor response suppression and the prepotent tendency to respond: A parametric fMRI study. Neuropsychologia. 2000;38:1280–1291. doi: 10.1016/s0028-3932(00)00033-6. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Desmond JE, Gabrieli JDE, Glover GH. Dissociation of frontal and cerebellar activity in a cognitive task: Evidence for a distinction between selection and search. NeuroImage. 1998;7:368–376. doi: 10.1006/nimg.1998.0340. [DOI] [PubMed] [Google Scholar]
- Diaz RM, Berk LE. Private speech: From social interaction to self-regulation. Hillsdale, NJ: Lawrence Erlbaum; 1992. [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Spicer J, Galvan A, Fossella JA, et al. Longitudinal functional MRI of the development of cognitive control. Society for neurosciences abstracts. 2004a [Google Scholar]
- Durston S, Hulshoff Pol HE, Schnack HG, Buitelaar JK, Steenhuis MP, Minderaa RB, et al. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. Journal of the American Academy of Child and Adolescent Psychiatry. 2004b;43:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, et al. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, et al. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. American Journal of Psychiatry. 2003;160:1061–1070. doi: 10.1176/appi.ajp.160.6.1061. [DOI] [PubMed] [Google Scholar]
- Ernst M, Liebenauer L, K AC, Fitzgerald G, Cohen R, Zametkin A. Reduced brain metabolism in hyperactive girls. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:858–868. doi: 10.1097/00004583-199407000-00012. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1998;18:5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Ehlis AC, Seifert J, Strik WK, Scheuerpflug P, Zillessen KE, et al. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clinical Neurophysiology. 2004;115:973–981. doi: 10.1016/j.clinph.2003.11.036. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Strik WK. The NoGo-anteriorization as a neurophysiological standard-index for cognitive response control. International Journal of Psychophysiology. 1999;32:233–238. doi: 10.1016/s0167-8760(99)00018-5. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, et al. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain research. Cognitive Brain Research. 2004;20:132–143. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- Flowers DL, Wood FB, Price NJ, Absher JR. Regional cerebral blood flow and brain metabolism in adults with childhood diagnosed with attention deficit disorder. New Orleans, LA: 1997. p. 1837. [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, et al. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biological Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Brain research. Cognitive Brain Research. 1996;5:175–181. doi: 10.1016/s0926-6410(96)00054-7. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. NeuroImage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giray EF, Altkin WM, Vaught GM, Roodin PA. The incidence of eidetic imagery as a function of age. Child Development. 1976;47:1207–1210. [PubMed] [Google Scholar]
- Giray EF, Barclay AG. Eidetic imagery: Longitudinal results in brain-damaged children. American Journal of Mental Deficiency. 1977;82:311–314. [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: A measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Hester R, Fassbender C, Garavan H. Individual differences in error processing: A review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cerebral Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- Hester R, Murphy K, Foxe JJ, Foxe DM, Javitt DC, Garavan H. Predicting success: Patterns of cortical activation and deactivation prior to response inhibition. Journal of Cognitive Neuroscience. 2004;16:776–785. doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- Hill DE, Yeo RA, Campbell RA, Hart B, Vigil J, Brooks W. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology. 2003;17:496–506. doi: 10.1037/0894-4105.17.3.496. [DOI] [PubMed] [Google Scholar]
- Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: Clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biological Psychiatry. 2004;55:1146–1153. doi: 10.1016/j.biopsych.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Holcomb HH. Practice, learning, and the likelihood of making an error: How task experience shapes physiological response in patients with schizophrenia. Psychopharmacology. 2004;174:136–142. doi: 10.1007/s00213-004-1834-6. [DOI] [PubMed] [Google Scholar]
- Iaboni F, Douglas VI, Baker AG. Effects of reward and response costs on inhibition in ADHD children. Journal of Abnormal Psychology. 1995;104:232–240. doi: 10.1037/0021-843X.104.1.232. [DOI] [PubMed] [Google Scholar]
- Kates WR, Frederikse M, Mostofsky SH, Folley BS, Cooper K, Mazur-Hopkins P, et al. MRI parcellation of the frontal lobe in boys with attention deficit hyperactivity disorder or Tourette syndrome. Psychiatry Research. 2002;116:63–81. doi: 10.1016/s0925-4927(02)00066-5. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, et al. Functional anatomy of GO/NO-GO discrimination and response selection—a PET study in man. Brain Research. 1996;728:79–89. [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Archives of General Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kim BN, Lee JS, Cho SC, Lee DS. Methylphenidate increased regional cerebral blood flow in subjects with attention deficit/hyperactivity disorder. Yonsei Medical Journal. 2001;42:19–29. doi: 10.3349/ymj.2001.42.1.19. [DOI] [PubMed] [Google Scholar]
- Kim BN, Lee JS, Shin MS, Cho SC, Lee DS. Regional cerebral perfusion abnormalities in attention deficit/hyperactivity disorder. Statistical parametric mapping analysis. European Archives of Psychiatry and Clinical Neuroscience. 2002;252:219–225. doi: 10.1007/s00406-002-0384-3. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related fMRI. NeuroImage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. European Journal of Neuroscience. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Scholl M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD) Brain Injury. 2000;14:859–875. doi: 10.1080/026990500445691. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Margolis JA, Barrett AM, Goldknopf EJ, Daly PF. Age differences in imagery abilities. Child Development. 1990;61:995–1010. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: Relative roles of dopamine and norepinephrine. Journal of Pharmacology and Experimental Therapy. 2001;296:876–883. [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion or something else? Journal of Child Psychology and Psychiatry. 2001;42:199–210. [PubMed] [Google Scholar]
- Larisch R, Kotter R, Kehren F, Tosch M, Shah NJ, Kalveram KT, et al. Motivation effects in a dichotic listening task as evident from functional magnetic resonance imaging in human subjects. Neuroscience Letters. 1999;267:29–32. doi: 10.1016/s0304-3940(99)00312-2. [DOI] [PubMed] [Google Scholar]
- Le TH, Pardo JV, Hu X. 4 T-fMRI study of nonspatial shifting of selective attention: Cerebellar and parietal contributions. Journal of Neurophysiology. 1998;79:1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- Leroi I, O’Hearn E, Marsh L, Lyketsos CG, Rosenblatt A, Ross CA, et al. Psychopathology in patients with degenerative cerebellar diseases: A comparison to Huntington’s disease. American Journal of Psychiatry. 2002;159:1306–1314. doi: 10.1176/appi.ajp.159.8.1306. [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, King Elbaz Z, Douglas VI. Mean response times, variability and skew in the responding of ADHD children: A response time distributional approach. Acta Psychologia. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the stroop color word interference task. Cerebral Cortex. 2000;10:552–560. doi: 10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: Cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123:1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-Deficit/Hyperactivity Disorder: Deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Lou HC, Henriksen L, Bruhn P. Focal cerebral hypoperfusion in children with dysphasia and/or attention deficit disorder. Archives of Neurology. 1984;41:825–829. doi: 10.1001/archneur.1984.04050190031010. [DOI] [PubMed] [Google Scholar]
- Lou HC, Henriksen L, Bruhn P. Focal cerebral dysfunction in developmental learning disabilities. Lancet. 1990;335:8–11. doi: 10.1016/0140-6736(90)90136-s. [DOI] [PubMed] [Google Scholar]
- Lou H, Henriksen L, Bruhn P, Borner H, Nielsen J. Striatal dysfunction in attention deficit and hyperkinetic disorder. Archives of Neurology. 1989;46:48–52. doi: 10.1001/archneur.1989.00520370050018. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Manly T, Owen AM, Datta A, Lewis GH, Scott SK, Rorden C, et al. ‘Busy doing nothing’: Increased right frontal and parietal activation associated with self-sustained attention to an ‘unchallenging’ task. NeuroImage. 2001;13:S331. [Google Scholar]
- Manly T, Owen AM, McAvinue L, Datta A, Lewis GH, Scott SK, et al. Enhancing the sensitivity of a sustained attention task to frontal damage: Convergent clinical and functional imaging evidence. Neurocase. 2003;9:340–349. doi: 10.1076/neur.9.4.340.15553. [DOI] [PubMed] [Google Scholar]
- Matochik J, Liebenauer L, King C, Szymanski H, Cohen R, Zametkin A. Cerebral glucose metabolism in adults with attention deficit hyperactivity disorder after chronic stimulant treatment. American Journal of Psychiatry. 1994;151:658–664. doi: 10.1176/ajp.151.5.658. [DOI] [PubMed] [Google Scholar]
- Mefford IN, Potter WZ. A neuroanatomical and biochemical basis for attention deficit disorder with hyperactivity in children: A defect in tonic adrenaline mediated inhibition of locus coeruleus stimulation. Medical Hypotheses. 1989;29:33–42. doi: 10.1016/0306-9877(89)90164-3. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Calloway P, Sahakian BJ. Amelioration of specific working memory deficits by methylphenidate in a case of adult attention deficit/hyperactivity disorder. Journal of Psychopharmacology. 2000;14:299–302. doi: 10.1177/026988110001400314. [DOI] [PubMed] [Google Scholar]
- Melchitzsky D, Lewis D. Tyrosine hydrolase- and dopamine transporter-immunoractive axons inthe primate cerebellum: Evidence for a lobular- and laminar-specific dopamine innervation. Neuropsychopharmacology. 2000;22:466–472. doi: 10.1016/S0893-133X(99)00139-6. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews. Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Reiss AL, Lockhart P, Denckla MB. Evaluation of cerebellar size in attention-deficit hyperactivity disorder. Journal of Child Neurology. 1998;13:434–439. doi: 10.1177/088307389801300904. [DOI] [PubMed] [Google Scholar]
- Murphy K, Garavan H. Artifactual fMRI group and condition differences driven by performance confounds. NeuroImage. 2004;21:219–228. doi: 10.1016/j.neuroimage.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Najib A, Lorberbaum JP, Kose S, Bohning DE, George MS. Regional brain activity in women grieving a romantic relationship breakup. American Journal of Psychiatry. 2004;161:2245–2256. doi: 10.1176/appi.ajp.161.12.2245. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective and Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Butler KM, Huang-Pollock CL, Henderson JM. Inhibitory processes in adults with persistent childhood onset ADHD. Journal of Consulting and Clinical Psychology. 2002;70:153–157. doi: 10.1037//0022-006x.70.1.153. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Sergeant JA. Response inhibition and response re-engagement in attention-deficit/hyperactivity disorder, disruptive, anxious and normal children. Behavioural Brain Research. 1998;94:33–43. doi: 10.1016/s0166-4328(97)00167-8. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nature Reviews. Neuroscience. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Archives of General Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word–color interference: Evidence for cingulate subregions subserving multiple distributed attentional systems. Biological Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: Event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biological Psychiatry. 2000;48:238–246. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: Conflict, target detection, and cognitive control. In: Parasuraman R, editor. The attentive brain. Cambridge, MA; London, England: The MIT Press; 1998. pp. 401–424. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. Dissociation of human caudate nucleus activity in spatial and nonspatial working memory: An event-related fMRI study. Brain research. Cognitive Brain Research. 1999;8:107–115. doi: 10.1016/s0926-6410(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. “What–Then–Where” in visual working memory: An event-related fMRI study. Journal of Cognitive Neuroscience. 1999b;11:585–597. doi: 10.1162/089892999563652. [DOI] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. Spatial working memory activity of the caudate nucleus is sensitive to frame of reference. Cognitive, Affective and Behavioral Neuroscience. 2003;3:133–144. doi: 10.3758/cabn.3.2.133. [DOI] [PubMed] [Google Scholar]
- Powers RE, O’Connor D, Price D. Noradrenergic systems in human cerebellum. Brain Research. 1989;481:194–199. doi: 10.1016/0006-8993(89)90504-0. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender, and IQ in children, a volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biological Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rubia K, Oosterlaan J, Sergeant JA, Brandeis D, Leeuwen T. Inhibitory dysfunction in hyperactive boys. Behavioral Brain Research. 1998;94:25–32. doi: 10.1016/s0166-4328(97)00166-6. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: A study with functional MRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Functional frontalisation with age: Mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Reviews. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Grothe J, Spitzer M, Kiefer M. Human anterior cingulate cortex is activated by negative feedback: Evidence from even-trelated potentials in a guessing task. Neuroscience Letters. 2002;325:203–206. doi: 10.1016/s0304-3940(02)00288-4. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, Tannock R. Neuropsychological profiles of adolescents with ADHD: Effects of reading difficulties and gender. Journal of Child Psychology and Psychiatry. 2002;43:988–1003. doi: 10.1111/1469-7610.00227. [DOI] [PubMed] [Google Scholar]
- Schachar R, Mota VL, Logan GD, Tannock R, Klim P. Confirmation of an inhibitory control deficit in attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2000;28:227–235. doi: 10.1023/a:1005140103162. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Dysmetria of thought: Clinical consequences of cerebellar dysfunction on cognition and affect. Trends in Cognitive Sciences. 1998;2:362–371. doi: 10.1016/s1364-6613(98)01218-2. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, et al. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: An event-related FMRI study. American Journal of Psychiatry. 2004;161:1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Newcorn JH, Fan J, Tang CY, Halperin JM. Brain activation gradients in ventrolateral prefrontal cortex related to persistence of ADHD in adolescent boys. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:47–54. doi: 10.1097/01.chi.0000145551.26813.f9. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Anderson C, Ernst M. Attention-deficit hyperactivity disorder: Neuroimaging and behavioral/cognitive probes. In: Ernst M, Rumsey JM, editors. Functional neuroimaging in child psychiatry. New York: Cambridge University Press; 2000. pp. 278–297. [Google Scholar]
- Schweitzer J, Cortes C, Gullapalli R, Dunning C, Tagamets M. Working memory in children with attention deficit/hyperactivity disorder: Use of compensatory neural activity; Organization for human brain mapping conference; 2003. p. 628. [Google Scholar]
- Schweitzer JB, Faber TL, Grafton ST, Tune LE, Hoffman JM, Kilts CD. Alterations in the functional anatomy of working memory in adult attention deficit hyperactivity disorder. American Journal of Psychiatry. 2000;157:278–280. doi: 10.1176/appi.ajp.157.2.278. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Lee DO, Hanford RB, Tagamets MA, Hoffman JM, Grafton ST, et al. A positron emission tomography study of methylphenidate in adults with ADHD: Alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003;28:967–973. doi: 10.1038/sj.npp.1300110. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Lee DO, Hanford RB, Zink CF, Ely TD, Tagamets MA, et al. Effect of methylphenidate on executive functioning in adults with attention-deficit/hyperactivity disorder: Normalization of behavior but not related brain activity. Biological Psychiatry. 2004;56:597–606. doi: 10.1016/j.biopsych.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Steingard RJ, Filipek P, Biederman J, Bekken K, Renshaw PF. Using MRI to examine brain–behavior relationships in males with attention deficit disorder with hyperactivity. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:477–484. doi: 10.1097/00004583-200004000-00017. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. American Journal of Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion: I. The effect of delay on choice. Journal of Child Psychology and Psychiatry. 1992;33:387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Steger J, Imhof K, Steinhausen H, Brandeis D. Brain mapping of bilateral interactions in attention deficit hyperactivity disorder and control boys. Clinical Neurophysiology. 2000;111:1141–1156. doi: 10.1016/s1388-2457(00)00311-4. [DOI] [PubMed] [Google Scholar]
- Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, et al. Functional anatomy of intrinsic alertness: Evidence for a fronto–parietal–thalamic–brainstem network in the right hemisphere. Neuropsychologia. 1999;37:797–805. doi: 10.1016/s0028-3932(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. NeuroImage. 2001;14:76–84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A, Glod CA, Maas LC, Renshaw PF. Functional deficits in basal ganglia of children with attention-deficit/hyperactivity disorder shown with functional magnetic resonance imaging relaxometry. Nature Medicine. 2000;6:470–473. doi: 10.1038/74737. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Massioui FE, Crivello F, Joliot M, Renault B, Mazoyer B. Functional anatomy of human auditory attention studied with PET. NeuroImage. 1997;5:63–77. doi: 10.1006/nimg.1996.0252. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event-related fMRI and ERPs. NeuroImage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Ston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Van Mier HI, Petersen SE. Role of the cerebellum in motor cognition. Annals of the New York Academy of Sciences. 2002;978:334–353. doi: 10.1111/j.1749-6632.2002.tb07578.x. [DOI] [PubMed] [Google Scholar]
- van Mourik R, Oosterlaan J, Sergeant JA. The Stroop revisited: A meta-analysis of interference control in AD/HD. Journal of Child Psychology and Psychiatry. 2005;46:150–165. doi: 10.1111/j.1469-7610.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Folwer JS, Logan J, Gerasinov M, Maynard LDY, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. Journal of Neuroscience. 2001;21:121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, et al. The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–365. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]
- Yeo RA, Hill DE, Campbell RA, Vigil J, Petropoulos H, Hart B, et al. Proton magnetic resonance spectroscopy investigation of the right frontal lobe in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:303–310. doi: 10.1097/00004583-200303000-00010. [DOI] [PubMed] [Google Scholar]
- Zametkin AJ, Nordahl TE, Gross M, King AC, Semple WE, Rumsey J, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. New England Journal of Medicine. 1990;323:1361–1366. doi: 10.1056/NEJM199011153232001. [DOI] [PubMed] [Google Scholar]
- Zang YF, Jin Z, Weng XC, Zhang L, Zeng YW, Yang L, et al. Functional MRI in attention-deficit hyperactivity disorder: Evidence for hypofrontality. Brain and Development. 2005;27:544–550. doi: 10.1016/j.braindev.2004.11.009. [DOI] [PubMed] [Google Scholar]