Abstract
Background and Purpose
More than 47 million individuals in the United States meet the criteria for the metabolic syndrome. The relation between the metabolic syndrome and stroke risk in multiethnic populations has not been well characterized.
Methods
As part of the Northern Manhattan Study, 3298 stroke-free community residents were prospectively followed up for a mean of 6.4 years. The metabolic syndrome was defined according to guidelines established by the National Cholesterol Education Program Adult Treatment Panel III. Cox proportional-hazards models were used to calculate hazard ratios (HRs) and 95% CIs for ischemic stroke and vascular events (ischemic stroke, myocardial infarction, or vascular death). The etiologic fraction estimates the proportion of events attributable to the metabolic syndrome.
Results
More than 44% of the cohort had the metabolic syndrome (48% of women vs 38% of men, P<0.0001), which was more prevalent among Hispanics (50%) than whites (39%) or blacks (37%). The metabolic syndrome was associated with increased risk of stroke (HR=1.5; 95% CI, 1.1 to 2.2) and vascular events (HR=1.6; 95% CI, 1.3 to 2.0) after adjustment for sociodemographic and risk factors. The effect of the metabolic syndrome on stroke risk was greater among women (HR=2.0; 95% CI, 1.3 to 3.1) than men (HR=1.1; 95% CI, 0.6 to 1.9) and among Hispanics (HR=2.0; 95% CI, 1.2 to 3.4) compared with blacks and whites. The etiologic fraction estimates suggest that elimination of the metabolic syndrome would result in a 19% reduction in overall stroke, a 30% reduction of stroke in women; and a 35% reduction of stroke among Hispanics.
Conclusions
The metabolic syndrome is an important risk factor for ischemic stroke, with differential effects by sex and race/ethnicity.
Keywords: epidemiology, ischemic stroke, metabolic syndrome, race/ethnicity, risk factors, sex
The metabolic syndrome is a highly prevalent constellation of vascular risk factors, including elevated blood pressure, elevated blood glucose, obesity, and dyslipidemia. Since Reaven1 first described the metabolic syndrome in 1983, attempts have been made to reach a consensus on its definition.1–3 The National Cholesterol Education Program Adult Treatment Panel III guidelines for the metabolic syndrome have become the most widely used definition because of its ease of use and relation to increased risk of coronary artery disease.3
Data from the National Health and Nutrition Examination Survey III estimate that >47 million persons in the United States have the metabolic syndrome.4 The metabolic syndrome is associated with increasing risk of cardiovascular morbidity and mortality, with risk estimates ranging from 1.4 to 4.5.5–13 The risk of stroke associated with the metabolic syndrome is less well established, with few prospective cohort studies including ischemic stroke as a rigorously defined outcome measure. In the prospective Framingham cohort, both diabetes and the metabolic syndrome were powerful risk factors, with a 10-year risk of ischemic stroke associated with diabetes of 14% in men and of 10% in women compared with 8% and 6% in nondiabetic men and women, respectively, with the metabolic syndrome alone.12 Women and minorities have a high prevalence of the metabolic syndrome and may be particularly vulnerable to vascular risks.13 In the Atherosclerosis Risk in Communities study, the metabolic syndrome was a significantly greater stroke risk factor for women than men.14 However, there remains a paucity of data from prospective population-based cohorts examining the relation between the metabolic syndrome and ischemic stroke by sex and race/ethnicity. The aim of this study was to investigate the relation between the metabolic syndrome and risk of ischemic stroke and vascular events in an urban community–based, multiethnic, prospective cohort.
Subjects and Methods
The Northern Manhattan Study (NOMAS) is a prospective, population-based, cohort study documenting stroke incidence, risk factors, and prognosis in a multiethnic urban community. Based in northern Manhattan, an area of ≈260 000 people with 104 000 ≥39 years of age, this study has a unique race/ethnic distribution of ≈63% Hispanic, 20% black, and 15% white residents. NOMAS is strongly representative of the underlying ethnic mix in this community. Methodology for the NOMAS study has been described previously and will be summarized briefly.15
Selection of Prospective Cohort
A total of 3298 subjects were recruited and enrolled between 1993 and 2001. Participants were eligible if they (1) had never been diagnosed with an ischemic stroke, (2) were ≥40 years old, and (3) resided for at least 3 months in a household with a telephone in northern Manhattan. Subjects were identified by random-digit dialing, and interviews were conducted by trained bilingual interviewers.16 The telephone response rate was 91% (9% refused to be screened). This study was approved by the local governing institutional review board, and written consent was obtained.
Baseline Evaluation
Subjects were recruited from the telephone sample to have an in-person baseline interview and assessment. The enrollment response rate was 75%, with an overall response rate of 68% (telephone response×enrollment response). Standardized questions were adapted from the validated Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System.17 We have published our findings on the validity of these questions in the northern Manhattan cohort.18
Definition of Race/Ethnicity
Race and ethnicity were defined by self-identification based on a series of interview questions modeled after the US census. Race was mutually exclusive and defined by 6 categories: “white, black, Indian (American), Eskimo, Asian or Pacific Islander, and other.” Ethnicity was subdivided as Hispanic or non-Hispanic based on the answer to the question: “Are you of Spanish/Hispanic origin?” Race/ethnic groupings were mutually exclusive. All participants responding affirmatively to being of Spanish origin or identifying themselves as Hispanic were classified as Hispanic.
Definition and Assessment of Components of the Metabolic Syndrome
The Adult Treatment Panel III definition of the metabolic syndrome includes 3 or more of the following: (1) fasting blood glucose ≥100 mg/dL; (2) elevated blood pressure, ie, ≥130/80 mm Hg or a history of hypertension; (3) HDL cholesterol of <40 mg/dL for men and <50 mg/dL for women; (4) triglycerides >150 mg/dL; or (5) a waist circumference of >40 inches for men and >35 inches for women.2 Participants reporting a definite history of diabetes were included if they met the other criteria for the metabolic syndrome. Serum glucose was measured by standard techniques, and HDL and triglyceride measurements were performed on plasma samples as described previously.19
Covariate Definitions
Smoking was categorized as non, former, and current smoker. Moderate alcohol use was defined as current drinking of >1 drink per month and ≤2 drinks per day. Cardiac disease was defined as a history of angina, myocardial infarction (MI), coronary artery disease including surgery, atrial fibrillation, or valvular heart disease. Physical activity was defined as engaging in leisure activity during the past 10 days before enrollment. Social resources were defined by educational level and health insurance status. Health insurance was separated into 3 mutually exclusive groups: (1) individuals who had Medicaid or Medicaid/Medicare or no other insurance, (2) individuals who had private insurance or private/Medicare insurance, and (3) individuals with Medicare only (reference group). We combined the no-insurance group with the Medicaid group based on similar risk ratio behavior in these groups, as well as the very low prevalence (7%) of noninsured persons in this cohort.
Annual Prospective Follow-Up
Subjects were screened annually by telephone to determine any change in vital status, detect neurologic and cardiac symptoms and events, review interval hospitalizations, and review risk factor status, medication changes, and changes in functional status. The telephone interview simple stroke screening tool (The screening question was “Since your last visit, have you been diagnosed with a stroke?”) had a sensitivity of 92% and specificity of 95% when compared with a clinical diagnosis of stroke, which included a review of medical records, physician examination, and imaging when necessary. Subjects and family were continually reminded to notify us in the event of stroke, MI, or death. Persons with positive screens were scheduled for an in-person assessment, including chart review and examination by the study neurologists. Ongoing hospital surveillance of admission and discharge International Classification of Diseases-9 codes provided current data on mortality and morbidity.
Outcome Classifications (Stroke, MI, and Vascular Death)
For these analyses, we had 2 outcome measures: first ischemic stroke and first vascular event, defined as either first ischemic stroke, first MI, or vascular death. Ischemic stroke was defined by World Health Organization criteria. Ischemic stroke subjects underwent standard diagnostic tests, including brain imaging, that were used to confirm ischemic stroke subtype. All hospitalizations were reviewed to verify details of any suspected events. More than 70% of ischemic stroke cases were hospitalized at the Columbia University Medical Center. Two neurologists classified the ischemic strokes independently after review of all data.
Other vascular outcomes included MI and vascular death. MI was defined by criteria adapted from the Lipid Research Clinics Coronary Primary Prevention Trial20 and required at least 2 of the 3 following criteria: (1) ischemic cardiac pain determined to be typical angina, (2) cardiac enzyme abnormalities defined as abnormal creatine phosphokinase-MB fraction or troponin values, and (3) ECG abnormalities. For subjects who died, the date of death was recorded, along with cause of death. Deaths were classified as vascular or nonvascular based on information obtained from the family, physician, medical record, and death certificate. Causes of vascular death included ischemic stroke, MI, heart failure, pulmonary embolus, cardiac arrhythmia, and other vascular causes. Deaths were reviewed and validated by our team of study cardiologists and neurologists.
Statistical Analyses
The prevalence of sociodemographic characteristics, conventional vascular risk factors, and other baseline variables was calculated. Age, sex, race/ethnicity, education, and insurance status were considered sociodemographic factors. We included cardiac disease, smoking, physical inactivity, and alcohol consumption as confounding vascular risk factors. A Kaplan–Meier curve capturing survivors free of ischemic stroke was estimated. Cox proportional-hazards regression models examined the association between the metabolic syndrome and incidence of events after adjusting for other confounding factors. Time to first event was analyzed as outcome with censoring at the time to either a nonvascular event or last follow-up. Stratified analyses were performed by race/ethnicity and sex.
The adjusted etiologic fraction (EF) due to the metabolic syndrome was estimated for outcome events, overall and by sex and race/ethnicity. Statistical analyses were performed with SAS (version 8.2). The EF estimates the proportion of events (ie, stroke, vascular outcomes) that could be reduced if a particular risk factor (ie, metabolic syndrome) could be eliminated. Our goal in estimating the EF for the metabolic syndrome was to provide a public health impact factor for this syndrome with regard to risk of stroke.
Results
A cohort of 3298 community residents was enrolled. The mean age at baseline was 69±10 years; 63% were women; and 21% were white, 24% were black, and 53% were Hispanic (Table 1). More than 44% of the cohort met the criteria for the metabolic syndrome (48% of women vs 38% of men, P<0.0001; Table 2). The metabolic syndrome was more prevalent among Hispanics (50%) than whites (39%) or blacks (37%, P=0.003). Among the components of the metabolic syndrome, women were more likely than men to be obese (defined by waist circumference, P=0.03). Hispanics had lower HDL levels than blacks or whites (P=0.001), and Hispanics and whites had higher triglycerides than blacks (P=0.05).
Table 1.
Baseline Sociodemographic Characteristics of the Cohort: NOMAS
| Prevalence |
||||||
|---|---|---|---|---|---|---|
| Overall, n=3297 | Women, n=2077 | Men, n=1220 | White, n=692 | Black, n=791 | Hispanic, n=1714 | |
| Sociodemographic characteristics | ||||||
| Mean age, y | 69±10 | 70±11 | 68±10 | 74±10+ | 72±10 | 66±9 |
| Men, % | 37 | … | … | 41 | 33 | 37 |
| White, % | 21 | 20 | 23 | … | … | … |
| Black, % | 24 | 26 | 21 | … | … | … |
| Hispanic, % | 52 | 52 | 53 | … | … | … |
| Completed high school, % | 46 | 43* | 50 | 82† | 63 | 22 |
| Other insurance, % | 56 | 52* | 62 | 88† | 74 | 34 |
| Medicaid only, % | 34 | 39* | 25 | 9† | 22 | 50 |
| Other vascular risk factors | ||||||
| History of hypertension, % | ||||||
| History of diabetes, % | 17 | 17 | 17 | 10 | 19 | 20 |
| Mean fasting glucose, mg/dL | 106±10 | 105±9 | 92±9 | 101±9 | 105±9 | 105±11 |
| Mean waist circumference, in. | 36±7 | 36±8 | 38±7 | 36±9 | 37±9 | 37±9 |
| Mean HDL, mg/dL | 47±15 | 41±13 | 50±15 | 44±13 | 52±16 | 49±15 |
| Any physical activity, % | 59 | 57* | 63 | 70† | 67 | 51 |
| Cardiac disease, % | 22 | 22 | 23 | 30† | 21 | 20 |
| Current smoking, % | 15 | 13* | 19 | 12† | 21 | 14 |
| Mild/moderate alcohol intake, % | 32 | 27* | 42 | 42† | 32 | 29 |
P<0.05 between men and women.
P<0.05 between whites, blacks, and Hispanics.
Table 2.
Prevalence of the Metabolic Syndrome and Specific Subcomponents Overall and by Sex and Race/Ethnicity
| Prevalence |
||||||
|---|---|---|---|---|---|---|
| Overall Cohort, n=3297 | Women, n=2077 | Men, n=1220 | White, n=692 | Black, n=791 | Hispanic, n=1714 | |
| Components of the metabolic syndrome | 44 | 48 | 38 | 39 | 37 | 50 |
| Waist, % (woman, >35 in.; man, >40 in.) | 42 | 54 | 22 | 37 | 47 | 43 |
| Hypertension, % (systolic ≥130 or diastolic ≥85 mm Hg or hypertension history) | 86 | 88 | 84 | 81 | 90 | 88 |
| Triglycerides, % >150 mg/dL | 30 | 29 | 31 | 30 | 19 | 35 |
| HDL, % (women, <50; men, <40) | 54 | 56 | 52 | 47 | 41 | 64 |
| Blood glucose, % ≥100 mg/dL | 33 | 21 | 12 | 31 | 33 | 34 |
Incidence of Ischemic Stroke and Vascular Outcomes
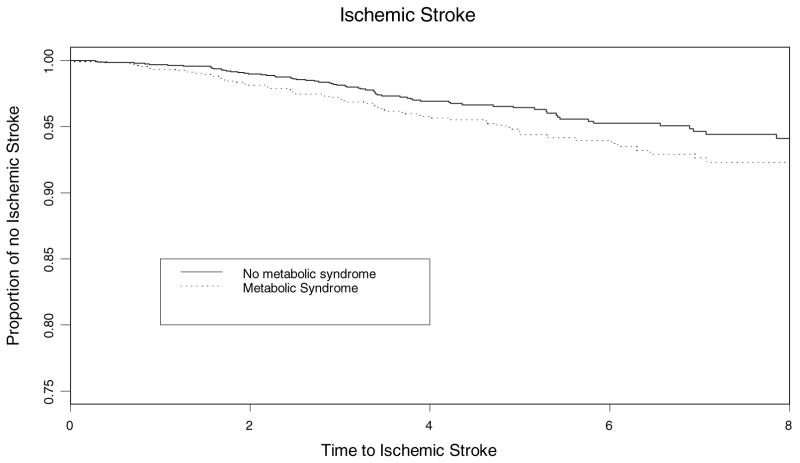
Of the 3298 enrolled in the baseline cohort, 4 were lost to follow-up. The median follow-up was 6.4 years and maximum follow-up was 12 years. We detected 176 incident ischemic strokes, 157 MIs, and 282 vascular deaths. Figure 1 illustrates the Kaplan–Meier survival curve for ischemic stroke and baseline metabolic syndrome. This figure includes only members of the cohort eligible for follow-up. Participants who died or were not yet eligible for a particular follow-up were not included in the “at-risk” numbers. The metabolic syndrome was significantly associated with increased risk of ischemic stroke (HR=1.5; 95% CI, 1.1 to 2.2, P<0.03) as well a vascular event (HR=1.6; 95% CI, 1.3 to 2.0, P<0.005) after adjusting for age, race/ethnicity, sex, education, insurance status, physical inactivity, cardiac disease, and current smoking (Table 3). In the multivariable model stratified by sex, the effect of the metabolic syndrome on ischemic stroke risk was greater among women (HR=2.0; 95% CI, 1.3 to 3.1, P<0.003) than men (HR=1.1; 95% CI, 0.6 to 1.9, P<0.67; sex interaction <0.002; Table 3). In adjusted stratified models, the metabolic syndrome conferred a greater ischemic stroke risk on Hispanics (HR=2.0; 95% CI, 1.2 to 3.4) than either blacks (HR=1.3; 95% CI, 0.7 to 2.3) or whites (HR=1.3; 95% CI, 0.6 to 2.5). The sex×metabolic syndrome interaction was not included in these stratified models as the interaction term was not significant, likely due to decreased power.
Figure 1.
Kaplan–Meier estimates of metabolic syndrome (Met Syn) risk for ischemic stroke.
Table 3.
Prevalence, HRs, and Proportion of Ischemic Strokes and Vascular Events Attributable to the Metabolic Syndrome
| Ischemic Stroke (n=176) |
Stroke, MI, Vascular Death (n=529) |
||||
|---|---|---|---|---|---|
| Prevalence | Adjusted HR (95% CI) | EF | Adjusted HR (95% CI) | EF | |
| Overall | 44% | 1.5 (1.1–2.2) | 0.19 (0.05–0.34) | 1.6 (1.3–2.0) | 0.22 (0.13–0.31) |
| Sex | |||||
| Men (n=1220) | 38% | 1.1 (0.6–1.9) | 0.04 (−0.17–0.24) | 1.4 (1.0–2.0) | 0.15 (0.03–0.28) |
| Women (n=2077) | 48% | 2.0 (1.3–3.1) | 0.30 (0.11–0.49) | 1.8 (1.4–2.4) | 0.27 (0.15–0.39) |
| Race/Ethnicity | |||||
| Whites (n=692) | 39% | 1.28 (0.6–2.5) | 0.04 (−0.23–0.31) | 1.8 (1.2–2.8) | 0.24 (0.08–0.39) |
| Blacks (n=791) | 37% | 1.3 (0.7–2.3) | 0.08 (−0.14–0.30) | 1.4 (0.9–2.0) | 0.12 (−0.03–0.26) |
| Hispanics (n=1714) | 50% | 2.0 (1.2–3.4) | 0.35 (0.12–0.57) | 1.7 (1.2–2.4) | 0.27 (0.12–0.42) |
All models were adjusted for the metabolic syndrome, age, education, insurance status, any physical activity, smoking, moderate alcohol use, and cardiac disease.
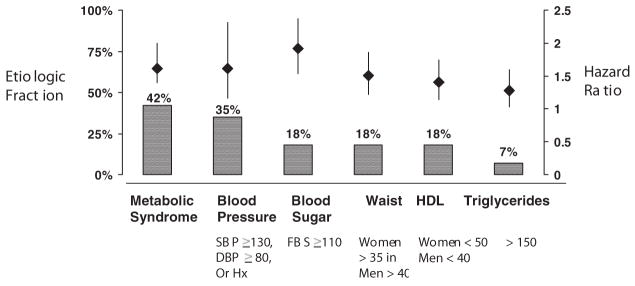
The overall EF of the metabolic syndrome was similar for ischemic stroke (19%) and vascular events (22%, Table 3). The metabolic syndrome accounted for 30% of all ischemic strokes among women compared with 4% among men. The impact of the metabolic syndrome differed by race/ethnicity: the EF for Hispanics was 35% compared with 8% for blacks and 4% for whites. Figure 2 compares the HR and EF for the metabolic syndrome and its components separately.
Figure 2.
A comparison of vascular risk ratios for metabolic syndrome and each of its components.
Discussion
In this multiethnic, prospective, population-based cohort study, we found a significant association between the metabolic syndrome and ischemic stroke risk, independent of other confounding factors including age, education, physical activity, alcohol use, and current smoking. These are important findings, because the relation between the metabolic syndrome and ischemic stroke in multiethnic populations has not been well characterized. In 1 study, the metabolic syndrome was associated with a 2-fold history of stroke.19 In the National Health and Nutrition Examination Survey, the metabolic syndrome was associated with stroke history (odds ratio=2.2; 95% CI, 1.5 to 3.2) among 15 922 subjects.13 Prospective studies including Framingham and the Atherosclerosis Risk in Communities study have recently demonstrated that the metabolic syndrome is associated with ischemic stroke.13,14
Our data demonstrate that the metabolic syndrome may be more potent among women. The Atherosclerosis Risk in Communities data suggest that there may be risk differentials according to sex for the metabolic syndrome as well.15 Possible explanations for these differences include disparities in the prevalence and potency of vascular risk factors. In Framingham, women with 3 or more “metabolically linked factors” had a relative risk of 5.9 (95% CI, 2.5 to 13.7) for coronary artery disease compared with a relative risk of 2.3 (95% CI, 1.6 to 2.4) in men.21 Data from the National Health and Nutrition Examination Survey identified more vascular risk factors among women than men and among ethnic minority women than among white women.4 However, in NOMAS, significant differences by sex in the prevalence of risk factors, including hypertension and diabetes, are less prominent. Alternative explanations for possible sex differences include a greater impact of the metabolic syndrome among postmenopausal women. Our data on menopause and hormone use in NOMAS are incomplete. Clearly this question warrants further investigation.
In this study, we have demonstrated a greater prevalence, stronger HR, and significantly higher EF for ischemic stroke among Hispanics with the metabolic syndrome. Hispanics have greater rates of adiposity and higher prevalence and incidence rate of type II diabetes.22 The greater prevalence rates of low HDL and high triglycerides among Hispanics in our cohort may be contributing to overall race/ethnicity-specific differences in risk with respect to the metabolic syndrome.
Alternatively, sex and race/ethnicity may act as proxies for underlying differences in access to social resources. In NOMAS, women and Hispanics were less likely to have completed high school. Furthermore, these groups were more likely to be uninsured or have Medicaid. Although these 2 indicators of social resource access were adjusted for in our models, continued disparities in stroke by sex and race/ethnicity suggest that there may be a number of other social resources, both at the individual level and population level, which need further exploration.23
We have demonstrated that the HR for the metabolic syndrome is essentially the same as for each of its component factors (Figure 2). However, we argue that risk alone is not the only important criterion in evaluation of the usefulness of the metabolic syndrome as a clinical entity. We have demonstrated, using a measure of EF, that the public health impact is significantly greater for the metabolic syndrome than for each of its components. We suggest that the metabolic syndrome constitutes an important risk factor, as it allows the clinician to identify high vascular risk patients earlier. Identification of persons with the metabolic syndrome provides a broader delineation of high vascular risk patients who may not be identified and treated by conventional vascular risk factor criteria. Furthermore, minority populations with definite risk factors are historically not adequately treated or controlled. The use of criteria for the metabolic syndrome may help to target these underserved population earlier.
The strengths of our study include a prospective population-based design wherein baseline exposures and outcomes were well documented. Our aggressive follow-up strategies resulted in <1% loss to follow-up. Study participants were seen in person at both study entry and follow-up to document outcome events. The inclusion of a large multiethnic, elderly, heterogeneous cohort with similar geographic access to the medical center is generalizable to other multiethnic urban populations and allows for more valid comparisons across race/ethnic categories.
The limitations of our study include the lack of specific data to measure insulin resistance, the older age of our cohort, and the lack of repeated measures of metabolic syndrome components in the entire cohort. Furthermore, we were unable to include only those who were free of diabetes at study entry.
Summary
The metabolic syndrome constitutes a major public health burden as defined by its prevalence, risk, and EF. With the obesity epidemic, the impact of the metabolic syndrome is likely to increase. Greater emphasis needs to be placed on the early diagnosis and treatment of patients at risk for vascular disease. Further understanding of gender and race/ethnic differences in terms of their impact on the metabolic syndrome will help us effectively target populations at increased risk of ischemic stroke.
Acknowledgments
The authors would like to acknowledge the National Institute of Neurological Disorders and Stroke (RO1 29993) for its support of the NOMAS study. The authors would like to thank Janet DeRosa, MPH, NOMAS project manager, and the entire NOMAS research staff for their continued hard work and dedication.
Source of Funding
This work was funded by the National Institutes of Health, National Institute of Neurological Disorders and Stroke NS 29993.
Footnotes
Disclosures
R.L.S. provided consultation to Boehringer Ingelheim.
References
- 1.Reaven G. Banting Lecture 1988: role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Expert panel on detection, evaluation and treatment of high cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on the detection, evaluation, and treatment of high blood cholesterol in adults (ATP III) JAMA. 2001;2001:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation. 2003;108:1541–1545. doi: 10.1161/01.CIR.0000088845.17586.EC. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among us adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Hsia J, Bittner V, Tripputi M, Howard BV. Metabolic syndrome and coronary angiographic disease progression: the women’s angiographic vitamin and estrogen trial. Am Heart J. 2003;146:439–445. doi: 10.1016/S0002-8703(03)00227-8. [DOI] [PubMed] [Google Scholar]
- 6.Folsom AR, Szklo M, Stevens J, Liao F, Smith R, Eckfeldt JH. A prospective study of coronary heart disease in relation to fasting insulin, glucose, and diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 1997;20:935–942. doi: 10.2337/diacare.20.6.935. [DOI] [PubMed] [Google Scholar]
- 7.Lakka H, Laaksonen D, Lakka T, Niskanen L, Kumpusalo E, Tuomilehto J, Salonen J. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 8.Ninomiya J, L’Italian G, Criqui M, Whyte J. Association of the metabolic syndrome with a history of myocardial infarction and stroke in the third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- 9.Hu G, Qiao Q, Tuomilehto J for the DECODE study group. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in non-diabetic European men and women. Arch Intern Med. 2004;164:1066–1076. doi: 10.1001/archinte.164.10.1066. [DOI] [PubMed] [Google Scholar]
- 10.Malik S, Wong ND, Franklin S, Kamath TV, L’Italian G, Pio JR, Williams GR. Impact of metabolic syndrome on mortality from coronary artery heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 11.Milionis H, Liberopoulos EG, Goudevenos J, Bairaktari E, Seferiadis K, Elisaf M. Risk factors for first-ever acute ischemic non-embolic stroke in elderly individuals. Int J Cardiol. 2005;99:269–275. doi: 10.1016/j.ijcard.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Najarian R, Sullivan L, Kannel W, Wilson P, D’Agostino R, Wolf P. Metabolic syndrome compared to type 2 diabetes mellitus as a risk factor for stroke. Arch Intern Med. 2006;166:2106–2111. doi: 10.1001/archinte.166.1.106. [DOI] [PubMed] [Google Scholar]
- 13.Scott CL. Diagnosis, prevention, and intervention for the metabolic syndrome. Am J Cardiol. 2003;92:35i–42i. doi: 10.1016/s0002-9149(03)00507-1. [DOI] [PubMed] [Google Scholar]
- 14.McNeill A, Rosamond W, Girman C, Golden S, Schmidt MI, East H, Ballantyne C, Heiss G. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the Atherosclerosis Risk in Communities Study. Diabetes Care. 2005;28:385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, Paik MC, Shea S. Race-ethnic disparities in the impact of stroke risk factors: the Northern Manhattan stroke study. Stroke. 2001;32:1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 16.Thornberry OTMJ. Proceedings of the Section on Survey Research Methods. Alexandria, Va: American Statistical Association; 1983. Coverage and response in random digit dialing national surveys; pp. 654–659. [Google Scholar]
- 17.Gentry EMKW, Hooelin GC, Jones JT, Gaines KL, Forman MR, Marks JR, Trowbridge FL. The behavioral risk factor surveys, II: design, methods, and estimates from combined state data. Am J Prev Med. 1985;1:9–14. [PubMed] [Google Scholar]
- 18.Kargman DE, Sacco RL, Boden-Albala B, Paik MC, Hauser WA, Shea S. Validity of telephone interview data for vascular disease risk factors in a racially mixed urban community: the Northern Manhattan Stroke Study. Neuroepidemiology. 1999;18:174–184. doi: 10.1159/000026209. [DOI] [PubMed] [Google Scholar]
- 19.Isomma B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 20.Morris DL, Kritchevsky SB, Davis CE. Serum carotenoids and coronary heart disease: the Lipid Research Clinics coronary primary prevention trial and follow-up study. JAMA. 1994:1439–1441. doi: 10.1001/jama.272.18.1439. [DOI] [PubMed] [Google Scholar]
- 21.Wilson P, Kannel W, Silbershatz H, D’Agostino R. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159:1104–1109. doi: 10.1001/archinte.159.10.1104. [DOI] [PubMed] [Google Scholar]
- 22.Haffner SGV, Hazuda HP, Valdez R, Mykkanen L, Stern M. Prevalence of hypertension in Mexico City and San Antonio, Texas. Circulation. 1994;90:1542–1549. doi: 10.1161/01.cir.90.3.1542. [DOI] [PubMed] [Google Scholar]
- 23.Boden-Albala B, Sacco R. Socioeconomic status and stroke: refining the relationship. Stroke. 2002;33:274–276. [PubMed] [Google Scholar]