Abstract
A venovenous bypass for transplantation of the liver was developed and evaluated in dogs and applied clinically, with flows that averaged less than 500 milliliters per minute. Fatal pulmonary emboli were seen in two of 40 experiments. The venovenous flow in the four pediatric recipients was 200 to 1,200 milliliters per minute, and there were no complications.
Although venovenous bypasses without systemic heparinization have revolutionized adult hepatic transplantation, bypasses have not been used for pediatric recipients. Application of the technique has been inhibited by concern that clotting and consequent iatrogenic pulmonary emboli could occur in the low flow bypass circuits of small subjects. In this study, we report our experience with a low flow venovenous bypass that was acquired first with replacement of the liver in small dogs and subsequently with four pediatric hepatic recipients.
METHODS
Dogs
Orthotopic hepatic transplantation was performed upon 40 healthy beagle dogs weighing between 10 and 15 kilograms. General anesthesia was induced with 25 milligrams per kilogram thiopental sodium. The dogs were paralyzed with 0.1 milligram per kilogram pancuronium bromide and ventilated through a cuffed endotracheal tube.
Principles of the transplantation
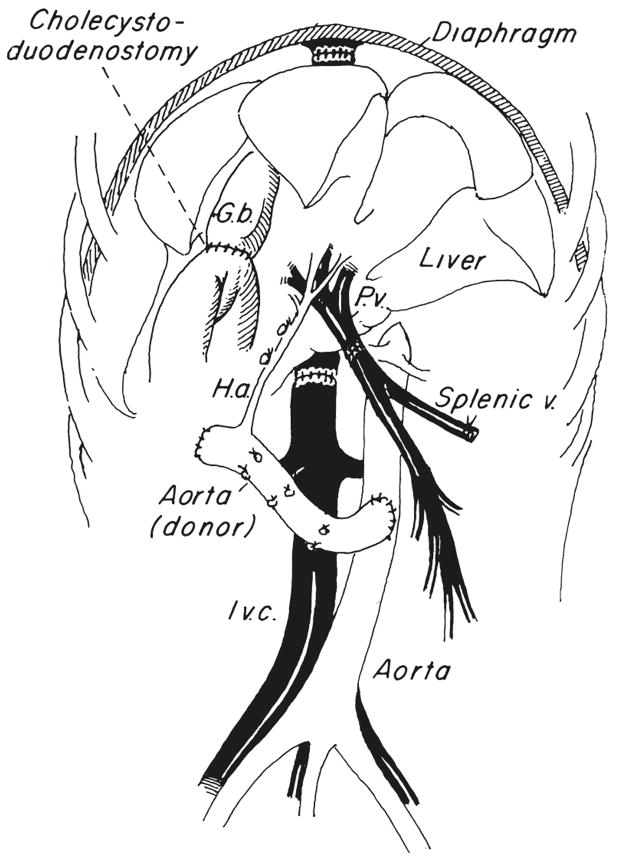
The homograft is cooled by infusing it with cold lactated Ringer’s solution as the donor is exsanguinated. The liver is placed into the fossa which is evacuated by the recipient hepatectomy and it is revascularized with a modification of our originally described technique (1). The vena caval anastomoses above and below the liver are performed as well as a portal venous anastomosis and an aorta to aorta anastomosis (Fig. 1). Reconstruction of the biliary tract was done with cholecystoduodenostomy after ligation of the graft common duct (Fig. 1). Transfusions usually are limited to the donor blood collected during terminal exsanguination and hepatic cooling.
Fig. 1.
Completed orthotopic hepatic transplantation in dogs. G.b., Gallbladder; I.v.c., inferior vena cava; H.a., hepatic artery, and P.v., portal vein.
The bypass
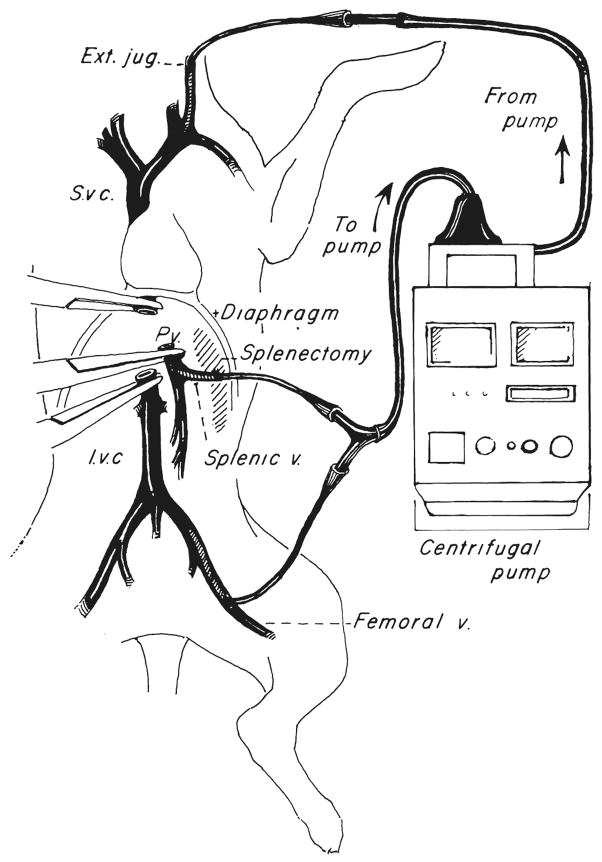
The technique was similar to that described by Denmark and colleagues (2). The inferior vena caval system was drained through the proximal femoral vein after ligation of the distal vessel. Splenectomy was performed and the central splenic vein was cannulated to drain the splanchnic bed (Fig. 2). Venous reentry was through the external jugular vein (Fig. 2). All of the cannulas were standard No. 12 chest tubes (Argyle Division of Sherwood Medical).
Fig. 2.
Venovenous bypass used in dogs. Note entry of splanchnic cannula through the splenic vein after splenectomy. S.v c., Superior vena cava; I.v.c., inferior vena cava, and Ext. jug., external jugular vein.
The extracorporeal bypass consisted of a three-eighth inch (internal diameter) Tygon (Norton Industrial Plastics) tubing interrupted with a centrifugal pump (Bio-Medicus, Inc.) and primed with 250 milliliters of Plasmalyte (Fig. 2). An electromagnetic probe on the venous return side was used to measure flow.
The venous bypass time was that required to complete the recipient hepatectomy, obtain hemostasis in the hepatic fossa and perform the venous anastomoses. Usually, the suprahepatic caval, infrahepatic caval and portal anastomoses were carried out While on bypass before revascularizing the portal vein. In a few dogs, the portal vein was revascularized after only the suprahepatic caval and portal anastomoses. Although this modification reduced the cold ischemia time, it necessitated a period of even lower venovenous bypass flow since only the vena caval bed was being drained while the third anastomosis was performed. The aortic anastomosis was carried out after the bypass was terminated, after all cannulas had been removed and not until good hemostasis had been obtained.
The human experience
Four children, each of whom had a different diagnosis, were two and one-half to nine years old and weighed between 13 and 24 kilograms (Table I). The reasons for resorting to the venovenous bypasses are listed in Table I.
TABLE I.
CLINICAL FEATURES OF PEDIATRIC HEPATIC RECIPIENTS
| OT*, No. | Age, yrs. | Weight, kgm. | Diagnosis | Duration bypass, mins. | Caval plus portal bypass ml./min. | Caval bypass alone ml./min. | Reason for bypass |
|---|---|---|---|---|---|---|---|
| 452 | 6 | 24 | Drug induced acute liver failure | 95 | 1,000 | 600 | Hypotension caused by venous occlusion |
| 478 | 5 | 15.5 | Biliary atresia | 130 | 1,200 | 400 to 200 | Hepatectomy impossible otherwise |
| 475 | 9 | 21 | Familial intrahepatic cholestasis | 108 | 1,200 | 600 | Profound metabolic depletion. Hypotension caused by venous occlusion |
| 494 | 2½ | 13 | Postnecrotic cirrhosis | 232 | 800 | 500 | Profound metabolic depletion |
OT, Orthotopic transplantation.
The equipment was the same as in the canine studies except that the chest tubes used for cannulas ranged from No. 12 to 16. When the chest tubes were inserted, they were flushed and filled with heparin diluted to 1/500, a precaution that was not exercised in dogs. The portal cannula was placed down the transected portal vein (3). The vena cava was decompressed through a cannula inserted at the saphenofemoral junction and venous return was to the axillary vein (3). Both the splanchnic and systemic venous systems were decompressed during most of the bypass, but during performance of the portal anastomosis, caval flow only was being bypassed.
RESULTS
In dogs
Bypass flow features
In the 40 dogs (Table II), the flow range was 200 to 1,500 milliliters per minute during the bypass periods of 53.1 ± 12.9 (S. D.) minutes, a range of 34 to 82. The average peak flow as 575.0±229.2 (S. D.) milliliters per minute. The mean minimum flow was 312.3±107.9 (S. D.) milliliters per minute, an average which was kept artifactually high by our policy of discontinuing bypasses when flows fell to less than 200 milliliters per minute. Such discontinuance near the end of the run was necessary in ten of the 40 experiments.
TABLE II.
DATA FROM 40 VENOVENOUS BYPASSES IN DOGS UNDERGOING LIVER REPLACEMENT, PLUS OR MINUS VARIATIONS ARE STANDARD DEVIATION
| No. dogs | 40 |
| Mean weight, kgm | 12.3±1.5 |
| Mean maximum flow, ml./mins. | 575±229 |
| Mean minimum flow, ml./mins. | 312±108 |
| Bypass time, mins. | 53±13 |
The experiments were part of a program to evaluate the new cyclosporine analogue, Cyclosporin G. Herein, it need only be noted that 80 per cent of the test dogs survived the operation for at least six days postoperatively. If a bypass was maintained during the venous occlusions, the exact amount of flow did not obviously influence either survival or the rate of complications.
Thromboembolic complications
The results of extensive clotting studies on six of the dogs were normal before and during the bypass. Two of the 40 dogs died suddenly of pulmonary emboli. In both instances, the bypass was discontinued for several minutes because of technical problems. When pump-driven flow was resumed, clots in the stagnant blood in the tubing were advanced into the right atrium, right ventricle and pulmonary artery. In two other experiments, fibrin strands were found in the pump head but these had not caused complications. When the same bypass system was tested under systemic heparinization of 3 milligrams per kilogram, the heparin effect could not be reversed and the dogs bled to death.
In humans
The bypass flows in the pediatric recipients ranged from 200 to 1,200 milliliters per minute (Table I). The lower flows always were at the end of the run when the portal anastomosis was being performed and when only vena caval blood was entering the bypass system. The duration of the bypasses was one and one-half to four hours (Table I). At their conclusion, fibrin or clots were not found in the extracorporeal circuits. The difficult technical or metabolic situations posed by these patients were dealt with easily, and all of the recipients are alive after one to four months.
DISCUSSION
The threat of pulmonary emboli from the extracorporeal circuit has been known since the first clinical trials of hepatic transplantation in which passive bypasses were used (4). The risk was well recognized by others (2, 3) who described precautions to prevent this complication. Other authors have speculated that bypass flows of less than 1,000 milliliters per minute would be unsafe (5).
In actuality, the safe level of flow is probably much lower than this. In the laboratory experiment in which the dogs had normal clotting, bypass flows were almost always below 1,000 milliliters per minute, and the average flow was less than one-half of this. Evidence of extracorporeal clot formation was uncommon, occurring in four of 40 dogs.
Pulmonary emboli in two of these four dogs were precipitated by technical misadventures that caused nonflowing blood to be left stagnant in the extracorporeal circuit after which pumping was resumed. The pump presumably propelled the clots in these two dogs.
In spite of the probable role of the pump in the embolization, the danger of thromboembolic complications has seemed to be less in the efficient pump driven circuits than it was with the passive bypasses that were used many years ago (4). To our knowledge, pump driven bypasses were first described in dogs (6). Others (6, 7) described a single pump driven femorojugular bypass after putting the splanchnic and systemic venous systems into communication with a temporary portacaval shunt (1).
In the clinical situation, the risk of emboli should be substantially less than with normal dogs. Almost all hepatic recipients, including the four children reported upon herein, came to the operating room with complex abnormalities of coagulation that are caused by end stage hepatic disease. In the four pediatric patients we studied, low flow bypasses were used for one and one-half to almost four hours without a trace of fibrin in the circuit.
In the original report of Denmark and coauthors (2), as in our laboratory and clinical experience reported herein, heparin bonded Gott shunts were not used. In one study (3), heparin bonded cannulas were added in the first clinical trials as a safety hedge. However, these cannulas are expensive, bulky and often of inappropriate size.
In 1984, chest tube cannulas of varying sizes were often used in adults as well as in children without any clotting difficulties. If this method is as good as with the heparin bonded cannulas and tubing, the expense of the venovenous bypasses will be substantially reduced. The question of need of the heparin bonded equipment remains to be resolved.
SUMMARY
A low flow venovenous bypass was used during the anhepatic phase of orthotopic transplantation of the liver in 40 dogs and in four pediatric recipients who were two and one-half to nine years old and who weighed between 13 and 24 kilograms. In these selected human recipients, the advantages of venovenous bypasses were the same as previously described for adults.
Acknowledgments
Supported by Research Grants from the Veterans Administration; by Project Grant No. AM-29961 from the National Institutes of Health and by Grant No. RR-00084 from the General Clinical Research Centers Program of the Division Research Resources, National Institutes of Health, Bethesda, Maryland.
References
- 1.Starzl T, Kaupp H, Brock D, et al. Reconstructive problems in canine liver homotransplantations with special reference to postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733. [PMC free article] [PubMed] [Google Scholar]
- 2.Denmark SW, Shaw BW, Jr, Griffith BP, Starzl TE. Venovenous bypass without systemic anticoagulation in canine human and liver transplantation. Surg Forum. 1983;34:380–382. [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith BP, Shaw BW, Jr, Hardesty RL, et al. Veno-venous bypass without systemic anti-coagulation for human liver transplantation. Surg Gynecol Obstet. 1985;160:270–272. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE. Experience in Hepatic Transplantation. With the assistance of C. W. Putnam. Philadelphia: W. B. Saunders Co; 1969. pp. 122–125.pp. 166–167. [Google Scholar]
- 5.Shaw BW, Jr, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–534. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutropia JC, Coratolo F, Spinetta A, et al. Trasplante hepatico ortotopico experimental. Rev Esp Enferm Apar Dig. 1972;38:533–570. [PubMed] [Google Scholar]
- 7.Toledo-Pereyra LH, Mackenzie GH. Orthotopic liver transplantation with the assistance of an external shunt and a nonpulsatile perfusion pump. Transplantation. 1983;35:102–104. [PubMed] [Google Scholar]