Abstract
BACKGROUND
After the successful evolution of hepatic transplantation during the last decade, small bowel and multivisceral transplantation remains the sole elusive achievement for the next era of transplant surgeons. Until recently, and for the last thirty years, the results of the sporadic attempts of intestinal transplantation worldwide were discouraging because of unsatisfactory graft and patient survival. The experimental and clinical demonstration of the superior therapeutic efficacy of FK 506, a new immunosuppressive drug, ushered in the current era of small bowel and multivisceral transplantation with initial promising results.
STUDY DESIGN
Forty-three consecutive patients with short bowel syndrome, intestinal insufficiency, or malignant tumors with or without associated liver disease, were given intestinal (n=15), hepatic and intestinal (n=21), or multivisceral allografts that contained four or more organs (n=7). Treatment was with FK 506 based immunosuppression. The ascending and right transverse colon were included with the small intestine in 13 of the 43 grafts, almost evenly distributed between the three groups.
RESULTS
After six to 39 months, 30 of the 43 patients are alive, 29 bearing grafts. The most rapid convalescence and resumption of diet, as well as the highest three month patient survival (100 percent) and graft survival (88 percent) were with the isolated intestinal procedure. However, this advantage was slowly eroded during the first two postoperative years, in part because the isolated intestine was more prone to rejection. By the end of this time, the best survival rate (86 percent) was with the multivisceral procedure. With all three operations, most of the patients were able to resume diet and discontinue parenteral alimentation, and in the best instances, the quality of life approached normal. However, the surveillance and intensity of care required for these patients for the first year, and in most instances thereafter, was very high, being far more than required for patients having transplants of the liver, kidney or heart.
CONCLUSIONS
Although intestinal transplantation has gone through the feasibility phase, strategies will be required to increase its practicality. One possibility is to combine intestinal transplantation with contemporaneous autologous bone marrow transplantation.
Although the intestine was one of the first organs to be transplanted experimentally (1, 2), it was the last to be grafted successfully in humans (3–6). It was considered to be a “forbidden” organ for nearly three decades (7). After demonstrating the superior therapeutic index of the new immunosuppressive drug FK 506 for experimental intestinal and multivisceral transplantation (8, 9), these procedures were used frequently at the Pittsburgh Transplant Institute (10–14). We report herein our experience with 43 consecutive patients who were given 45 intestinal grafts, either alone, in continuity with the donor liver, or as part of a multivisceral composite of abdominal splanchnic organs.
MATERIALS AND METHODS
Patient population
The 43 patients were treated between May 2, 1990 and April 15, 1993. Twenty-one of the 43 patients were adults 19 to 58 years of age and 22 were children who were 0.5 to 15.5 years of age. All patients, except two who were given multivisceral grafts, were on total parenteral nutrition (TPN) for one to 132 months before transplantation and all had had more than one episode of sepsis, damage to the liver or other TPN-related complications (Table I). Two additional adult patients bled to death as a result of massive venous collaterals during preliminary dissection of the native organs, and they were excluded from the analyses. Both were candidates for multivisceral transplantation because of extensive thrombosis of the splanchnic and hepatic venous systems concomitant with hepatic failure. The causes of intestinal failure and indications for the three different operations are given in Table II. The state of the liver was the most critical factor in the choice of procedure.
TABLE I.
CLINICAL FEATURES OF THE INTESTINAL RECIPIENTS
| Total population, n=43 | Isolated intestine, n=15 | Intestine plus liver, n=21 | Multivisceral, n=7 | |
|---|---|---|---|---|
| No. of patients | ||||
| Children | 22 | 5 | 15 | 2 |
| Adults | 21 | 10 | 6 | 5 |
| Sex | ||||
| Male | 22 | 7 | 11 | 4 |
| Female | 21 | 8 | 10 | 3 |
| Age, y* | ||||
| Children | 3.5±3.7 | 5.1±4.4 | 3.0±3.7 | 3.2±1.9 |
| Adults | 33.3±10.2 | 36.6±11.4 | 27.9±9.1 | 33.2±7.4 |
| Duration of TPN, mo* | 37±34 | 52±47 | 28±21 | 26±20 |
| Serum bilirubin, mg/dL* | 11±13 | 1.0±0.6 | 19±14 | 5±9 |
| Graft | ||||
| Cold ischemia time, h* | 7.4±2.2 | 6.5±2.0 | 7.9±2.0 | 7.8±2.5 |
| Positive cytotoxic cross-match | 2 (4.7) | 0 (0) | 2 (9.5) | 0 (0) |
| Small intestine | ||||
| Without colon | 30 | 10 | 17 | 3 |
| With colon | 13 | 5 | 4 | 4 |
| Follow-up, mo* | 15±10 | 14±7 | 16±12 | 11±8 |
Mean plus or minus standard deviation.
Multivisceral: stomach, duodenum, pancreas, intestine, and liver.
TPN, Total parenteral nutrition; y, year; h, hour, and mo, month.
Numbers in parentheses are percentages.
TABLE II.
CAUSES OF INTESTINAL FAILURE AND INDICATIONS FOR INTESTINAL TRANSPLANTATION
| Cause | Intestine | Intestine plus liver | Multivisceral |
|---|---|---|---|
| ADULTS, N=21 | |||
| Crohn’s disease | 5 | 1 | 0 |
| Abdominal trauma | 2 | 2 | 0 |
| Celiac artery occlusion | 0 | 0 | 3* |
| S.M.A. thrombosis | 0 | 2 | 0 |
| Desmoid tumor | 1 | 1 | 0 |
| Surgical adhesions | 2 | 0 | 0 |
| Metastatic gastrinoma | 0 | 0 | 1 |
| Budd-Chiari syndrome | 0 | 0 | 1 |
| CHILDREN, N=22 | |||
| Gastroschisis | 1 | 5 | 0 |
| Necrotizing enterocolitis | 0 | 4 | 0 |
| Volvulus | 1 | 3 | 0 |
| Intestinal atresia | 1 | 2 | 0 |
| Microvillus disease | 1 | 1 | 0 |
| Pseudo-obstruction | 1 | 0 | 1 |
| Multiple polyposis | 0 | 0 | 1 |
These patients had short-gut syndrome due to concomitant superior mesenteric artery thrombosis from protein S deficiency, n=1; antithrombin III deficiency, n=1, or from unknown cause, n=1.
Multivisceral: stomach, duodenum, pancreas, intestine, and liver.
S.M.A., Superior mesenteric artery.
Intestine only (n=15)
The isolated intestinal recipients were free of jaundice, although most had minor hepatic function abnormalities. Frequent central line sepsis and increasing difficulty in obtaining central access for intravenous nutrition had been frequent problems.
Liver plus intestine (n=21)
All patients undergoing hepatic and intestinal transplantation were jaundiced and had other evidence of advanced liver disease (Table I).
Multivisceral transplantation (n=7)
Six of these seven allografts included all of the usual multivisceral constituents. The liver was removed from the seventh multivisceral graft and used for another recipient. In two of the five adult patients, deficiency of protein S and antithrombin III underlay previous thrombosis of both the superior mesenteric artery and celiac axis. Because the liver is the source of these factors, its inclusion in the graft was mandatory although hepatic failure was not present.
Transplantation procedures
All of the cadaveric donors were ABO blood group identical with the recipients. Human leukocyte antigen matching was random and uniformly poor. The lymphocytotoxic cross-match was positive in two patients (Table I). No attempts were made to alter the graft immunologic tissue with irradiation, antilymphocyte preparations, or other modalities. An effort was made to eradicate fungal and bacterial contamination of the donor alimentary tract.
The principles (15) as well as details (8–14, 16, 17) of both the donor and recipient operations have been described elsewhere. The isolated intestinal grafts were arterialized from the aorta with superior mesenteric venous drainage into the native portal system (Fig. 1) when technically feasible (n=14 of 15). In 14 of the 21 intestinal and hepatic transplantations, the residual portal venous drainage from the host pancreas and other retained native organs was directed into the graft portal vein by portal-to-portal anastomosis (Fig. 2). In the other seven, the residual recipient splanchnic venous bed was decompressed with a portacaval shunt (Fig. 2, right inset). Six of the multivisceral operations consisted of resection of the remaining host intra-abdominal viscera and the insertion of a full set of viscera minus the spleen (Fig. 3). In the seventh instance, the host liver was retained.
Fig. 1.
Isolated intestinal transplantation including one-half of the colon (main figure) or the small intestine only (left insert) is shown. Graft venous outflow is drained end-to-side (main figure) or end-to-end (right insert) into the host portal system. IVC, Inferior vena cava.
Fig. 2.
Hepatic and intestinal transplantation including part of the colon (main figure) or with small intestine only (left insert) are shown. The host portal vein is drained into the graft portal vein when possible, but in one-third of the instances, this blood was diverted into the vena cava (right insert). CA, Celiac artery; PV, portal vein; IVC, inferior vena cava, and SMA, superior mesenteric artery.
Fig. 3.
Depicted is a full multivisceral operation including the ascending and right transverse colon. Note that pyloroplasty or pyloromyotomy was performed and that bile flow was temporarily decompressed in all instances.
In most instances with all three procedures, the colon was discarded. However, in 13 patients, the ascending colon, with or without the transverse colon, was included with the small bowel graft with a nearly equal frequency in the three cohorts (n=4 or 5 in each) (Table I). The cold ischemia times after initial flushing with University of Wisconsin solution were 7.4 ± 2.2 (standard deviation [SD]) hours (range of 2.8 to 11.1 hours) with no significant difference (p=0.1) between the three different types of visceral grafts (Table I).
Reconstruction of gastrointestinal continuity was with conventional techniques. Four different types of enterostomies were used for early decompression and monitoring of the intestinal allograft. Initially, both ends of the graft were exteriorized by the chimney method. After the first five cases, the chimney was used only at the distal end (Figs. 1 and 2) and a tube jejunostomy, with or without gastrostomy, was used proximally for decompression as well as tube feeding as soon as possible (Figs. 1, 2, and 3). In the first ten of the 13 patients who received colon as well as small bowel, an end transverse colostomy was constructed of the allograft colon, but the last three patients had a distal ileal loop exteriorized by the Bishop-Koop principle plus a distal colo-colostomy (Figs. 1, 2, and 3). The various enterostomies were taken down with an extraperitoneal technique two to 11 months after transplantation.
Biliary reconstruction for the recipients of hepatic and intestinal transplantation was with choledochojejunostomy (Fig. 2). The bile flow was temporarily decompressed with a cannula in the graft common bile duct in the multivisceral recipients in an attempt to minimize the risk of postoperative pancreatitis. The cannula was placed through the cystic duct (Fig. 3). Cholecystectomy was performed in all native and allograft livers.
Management
Immunosuppression
Intravenous FK 506, steroids, and Prostin®, Upjohn, Kalama-zoo, MI (prostaglandin E1 [PGE1]) were begun intraoperatively as previously described (11, 18, 19). Enteral administration of FK 506 was begun one to two weeks after transplantation, with several days of overlap with intravenous treatment. The 12 hour plasma FK 506 trough levels and the doses necessary to maintain these levels are illustrated in Figure 4.
Fig. 4.
FK 506 plasma trough levels and FK 506 and prednisone doses in the three patient cohorts. Values are mean plus or minus standard error of the mean.
Except for the first eight recipients, PGE1 was begun intraoperatively at a beginning dose of 0.2 mg per kg per hour and gradually increased to 0.6 to 0.8 mg per kg per hour and continued until intravenous FK 506 was stopped. High perioperative steroid doses were gradually reduced (Fig. 4) or stopped when possible, particularly in the pediatric recipients. A low dose of Imuran®, Burroughs Wellcome, Research Triangle Park, NC (azathioprine) (1 to 2 mg per kg per day) was given to 22 patients at some time during the postoperative period.
Augmented immunosuppressive therapy was initiated during rejection episodes, based on their severity. A steroid bolus was given and FK 506 dose was increased when this was possible without nephrotoxicity. A steroid recycle for five days or a seven day course of OKT3, or both, were backup options.
Prophylaxis of infection
Selective intestinal decontamination was used for four to six weeks postoperatively and resumed later during moderate to severe rejection episodes (20). Systemic antibiotics were given prophylactically for the first five days, as well as subsequently, if indicated by the results of blood and body fluid cultures. Frequent cultures of blood, ostomy discharge, wound, urine, stool, and sputum were done to monitor changes in flora, and to obtain direct evidence of active infection or translocation.
Chronic viral and protozoal prophylaxis was with Zovirax®, Burroughs Wellcome (acyclovir) for cytomegalovirus (CMV) and Bactrim®, Roche Laboratories, Nutley, NJ (timethoprim and sulfamethoxazole) for pneumocystis carinii. Because of the high incidence of CMV enteritis in recipients who received CMV positive grafts, Cytovene®, Syntex, Palo Alto, CA (gancyclovir) was given prophylactically for three months after transplantation in the last cases. If severe CMV infection occurred despite prophylaxis, Foscavir®, Astra USA, Inc., Westboro, MA (foscarnet) or CMV immunoglobulin “cytogam,” or both, were added to the gancyclovir treatment.
Nutrition
The preoperative TPN was continued postoperatively. After confirming the integrity of the gastrointestinal anastomoses and partial recovery of the intestinal motility by the appropriate gastrointestinal contrast studies (usually at seven to ten postoperative days), enteral feeding with a jejunal tube was commenced with peptamen (Clintec Nutrition Co., Deerfield, IL) and TPN was tapered gradually. Peptamen is an isotonic elemental diet that contains peptide-based protein, medium-chain triglycerides, and glutamine. Four to six weeks after transplantation, peptamen was converted in children to compleat B (Sandoz, East Hanover, NJ), a lactose and gluten-free diet that contains dietary fibers to promote normalization of intestinal motility function. Enteral feedings were gradually decreased with a reciprocal increase in oral intake. Opiates, loperamide, and kaolin-pectin mixture were used for patients who had high stomal output or diarrhea. Prokinetic agents were given to patients with gastrointestinal dysmotility.
Graft monitoring
Rejection
Graft rejection was diagnosed from clinical observations, endoscopic findings, and histopathologic studies of endoscopically-guided mucosal biopsies. Protocol graft endoscopy with random multiple mucosal biopsies (particularly of the ileum) was accomplished at least once per week for the first three months, monthly for the next three months, and every three to six months thereafter, and whenever it was clinically indicated. The clinical, endoscopic, and histologic criteria adopted for the diagnosis of intestinal allograft rejection were comprehensively described elsewhere (12). Immunosuppression was adjusted accordingly.
Function
Standard hepatic and pancreatic function tests were used to track the functional status of these organs. Contrast examinations of the gastrointestinal tract were performed to determine the duration of gastric emptying, intestinal transit time, and changes of mucosal foldings (21). Oral FK 506 pharmacokinetic studies provided a direct measure of absorption after discontinuation of intravenous FK 506 doses. Absorption of d-xylose and fat was measured with the methods of Breiter and associates (22) and Amenta (23), respectively. Serum concentrations of protein, albumin, vitamins, minerals, and trace elements were measured frequently. Anthropometric measures included weight and upper arm measurement of fat and muscle.
Statistical analysis
Data were collected for the total population, pooled at first, and then stratified according to the type of the transplanted graft. Values usually were expressed as the mean and SD. Survival time for patients was defined as the time that elapsed from the transplantation date until death, or the date of the last follow-up evaluation. For calculating graft survival, the date of graft removal was also considered. The patient and graft survival rate for the total and three different recipients was estimated by the Kaplan-Meier method (24) and the comparison was done by the log-rank test (25).
The median values for the daily FK 506 and Deltasone®, Upjohn (prednisone) doses as well as the measured 12-hour FK 506 trough plasma levels were calculated for each recipient for 30 days at one, two, three, six, 12, and 24 months after transplantation. These median values were pooled in the three cohorts and the means (plus or minus standard error of the mean) were calculated.
Single variable comparison for qualitative (non-survival) data was made by chi-square analysis. Kruskald Wallis’ one-way analysis of variance was used for three-way comparison. The Mann-Whitney U-Wilcoxon test was used for independent variable analysis.
RESULTS
Patient survival
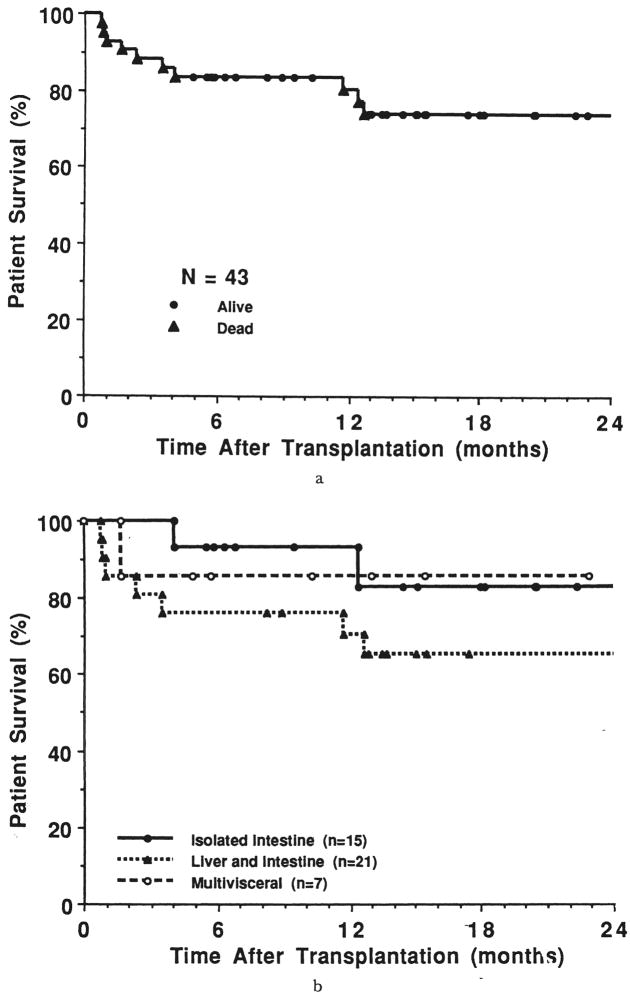
The follow-up period for the current survivors (n=30, 29 bearing grafts) is 17±9 SD months (range of six to 39 months). The actuarial survival rate for the 43 patients at three, six, 12, and 24 months is 88, 84, 81, and 74 percent, respectively (Fig. 5a). The survival rate for the three cohorts is illustrated in Figure 5b, with a mean follow-up period that is similar for all patients (p=0.7) (Table I). At three months, the survival rate for the isolated intestinal recipients was 100 percent; 81 percent for those with combined hepatic and intestinal transplantation, and 86 percent for the multivisceral recipients. At one year after transplantation, these estimates were 93, 71, and 86 percent, respectively. At two years, the actuarial survival rate for the isolated intestinal recipients was 83 percent, with 65 and 86 percent for those who received combined hepatic and intestinal and multivisceral grafts, respectively. The isolated intestinal recipients had a significantly better survival time at two years than the recipients of liver and intestine (p=0.02), but this actuarial projection overstated the quality of the results.
Fig. 5.
a, Patient survival rate, all 43 recipients, and b, patient survival rate according to procedure.
Graft survival
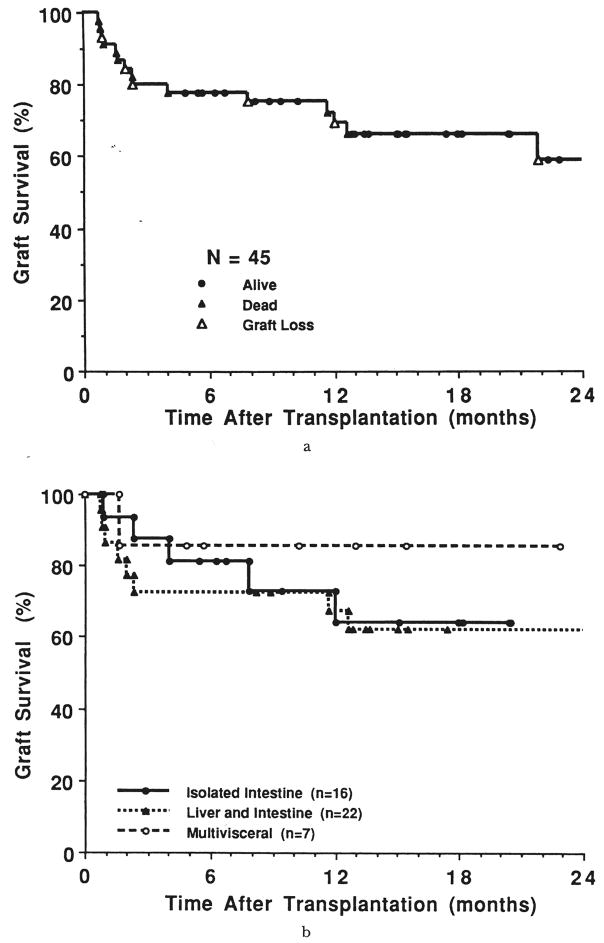
The estimated actuarial survival rate for all of the grafts (n=45) was 80, 78, 72, and 59 percent at three, six, 12, and 24 months, respectively (Figure 6a). Graft survival was highest at three months for the isolated intestine (88 percent) versus liver plus intestine (73 percent) and multivisceral cases (86 percent). However, by six months and at all subsequent times, the highest graft survival rate was in the multivisceral recipients. At 18 months, the rate was 86 percent compared with 64 percent in recipients of isolated intestines, and 62 percent in recipients of combined hepatic and intestinal grafts (Fig. 6b).
Fig. 6.
a, Graft survival rate, all 45 attempts including two retransplantations, and b, graft survival rate according to procedure.
Causes of patient death
During potential follow-up periods of six to 39 months, 13 (30 percent) of the 43 patients died: four of 15 receiving isolated intestine, eight of 21 receiving combined liver and intestine, and one of seven multivisceral. The deaths were the result of either technical complications, opportunistic infections, uncontrolled graft rejection, or disseminated post-transplant lymphoproliferative disease (PTLD). Seven of these deaths occurred during, or shortly after, the first three months postoperatively, but six more were at or well beyond one year (Table III).
TABLE III.
TIME AND CAUSE OF DEATH
| Recipient | Case | Time, d | Cause |
|---|---|---|---|
| Isolated intestine, n=15 | |||
| 1 | Adult | 123 | Adult respiratory distress syndrome |
| 2 | Adult | 376 | Sepsis after removal of the graft |
| 3 | Adult | 440 | Pulmonary embolism due to caval thrombosis* |
| 4 | Adult | 776 | Sepsis after removal of the second graft |
| Intestine plus liver, n=21 | |||
| 1 | Child | 23 | Enteric leak; graft versus host disease |
| 2 | Child | 26 | Respiratory syncytial viral pneumonia |
| 3 | Child | 29 | Biliary and enteric leak; sepsis† |
| 4 | Child | 70 | Biliary leak; sepsis |
| 5 | Child | 107 | Acute rejection of the second graft |
| 6 | Adult | 356 | Disseminated coccidioidomycosis |
| 7 | Child | 385 | Disseminated lymphoproliferative disease |
| 8 | Adult | 752 | Chronic rejection; hepatorenal failure |
| Multivisceral, n=7 | |||
| 1 | Adult | 49 | Disseminated lymphoproliferative disease |
Occurred due to central line insertion 201 days after the removal of the rejected intestinal graft on postoperative day 239.
Required liver replacement due to hepatic artery thrombosis.
d, Day.
The four patients who died after isolated intestinal transplantation were adults. In three of these patients, an attempt was to remove the rejected grafts and return to TPN, whereas the fourth patient, who had been extremely debilitated before transplantation with active Crohn’s disease, died as a result of respiratory failure with a functioning graft.
In contrast, six of the eight mortalities after combined hepatic and intestinal transplantation were children. Enteric leaks, with or without biliary leaks, were responsible for three deaths. The other three were caused by respiratory syncytial viral pneumonia, refractory acute rejection, or PTLD (one each). One of the two adult recipients of hepatic and intestinal transplantation died as a result of hepatorenal failure combined with chronic rejection and the other died as a result of disseminated coccidioidomycosis. The latter was a California resident who contracted the disease during a coccidioidomycosis epidemic in her community, which also claimed three previously well non-transplant victims. The death that occurred in the multivisceral series was caused by PTLD, which was diagnosed at autopsy 49 days post-transplantation, after having been misdiagnosed as rejection while the patient was alive, resulting in over treatment.
Causes of graft loss
Sixteen (six isolated intestine, nine liver and intestine and one multivisceral) of the 45 implanted grafts were lost either because of death (n=10) (Table III) or graft removal (n=6). The times, causes, and outcomes of graft removal or replacement are listed in Table IV.
TABLE IV.
TIME, CAUSE AND OUTCOME OF GRAFT REMOVAL OR REPLACEMENT
| Graft | Time, d | Cause | Procedure | Outcome |
|---|---|---|---|---|
| Isolated intestine, n=16 | ||||
| 1 | 27 | Acute rejection* | Enterectomy | Alive, on TPN |
| 2 | 239 | Acute rejection* | Enterectomy | Death, pulmonary embolism |
| 3 | 366 | Acute rejection* | Enterectomy | Death, sepsis |
| 4† | 667 | Chronic rejection* | Enterectomy | Retransplant |
| 5† | 71 | Acute rejection* | Enterectomy | Death, sepsis |
| Intestine plus liver, n=22 | ||||
| 1 | 11 | Hepatic artery thrombosis | Retransplantation, liver only | Death, sepsis |
| 2 | 47 | Acute rejection | Retransplantation, liver and intestine | Death, rejection |
| Multivisceral graft, n=7 | ||||
| 1 | 11 | Severe pancreatitis | Pancreatoduodenectomy | Alive |
Reduction or withdrawal of immunosuppression precipitated rejection (see text).
Same recipient.
TPN, Total parenteral nutrition.
Grafts removed
Refractory rejection was the reason for removal of all six grafts (five isolated intestine in four patients, one liver and intestine) (Table IV). Two of the isolated intestinal grafts were received by the same adult. The first graft was lost at 667 days from chronic rejection after sporadic drug noncompliance, and the second was lost to acute rejection and concomitant CMV enteritis 71 days after retransplantation. This patient died as a result of multiple organ failure 20 days after the second enterectomy.
The rejected intestinal graft that was removed 239 days after transplantation was in a recipient in whom immunosuppression was stopped because of a demyelinization neurologic syndrome. The patient fully recovered, only to die 29 weeks later as a result of a pulmonary embolism during an operation to replace a TPN line.
An isolated intestinal graft was lost at 366 days because of a delay in treating acute rejection that was initially misdiagnosed as recurrent Crohn’s disease. This patient died ten days after enterectomy as a result of severe necrotizing pancreatitis and systemic infection.
The intestinal graft that was lost from rejection at 27 days was in a child who received small bowel plus half of the colon. Aggressive immunosuppression was not possible because of a concomitant respiratory syncytial viral pneumonia and the graft was rejected. The patient is currently alive seven months after returning to TPN.
The only composite graft removed at reoperation was a liver and intestine transplanted to a child across a strong positive cytotoxic cross-match. Although graft removal and retransplantation after 47 days was technically successful, refractory rejection of the second set of organs caused death after another 60 days. Thus, graft removal or replacement rescued only one of five patients in whom this was attempted.
Grafts lost as a result of death
The causes of death were invariably multiple, but the principal factors are given in Table III.
Salvage of intestine by removal of other organs
Loss of part of the composite graft occurred in two patients because of either severe preservation injury of the pancreas necessitating pancreatectomy, or hepatic artery thrombosis necessitating hepatectomy (Table IV). The multivisceral graft recipient in whom the pancreas was lost is alive more than six months later, whereas the liver and intestine recipient who underwent total hepatectomy and hepatic replacement from another donor died 18 days later (Tables III and IV).
Rejection
Only two patients (both with combined liver and intestine) were spared clinical or histopathologic diagnosis of intestinal graft rejection at some time postoperatively (Table V). The follow-up period in the two exceptional instances was 23 days (when the patient died) and 242 days (the patient is well). Although the clinical diagnosis of rejection was made in 95 percent, this was confirmed histopathologically in only 31 patients (72 percent). The diagnostic limitation of intestinal mucosal biopsies was underscored in some instances by finding rejection in the whole graft after its removal or at autopsy, after earlier inconclusive biopsy reports.
TABLE V.
HEPATIC AND INTESTINAL ALLOGRAFT REJECTION AMONG INTESTINAL RECIPIENTS
| Incidence |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intestine |
Liver |
Episode/graft |
Steroid recycle* |
OKT3* |
|||||||
| Graft | No. of grafts | No. | Percent | No. | Percent | Intestine | Liver | No. | Percent | No. | Percent |
| Isolated Intestine | 16 | 16 | 100 | – | – | 4.0 | – | 10 | 63 | 5 | 31 |
| Intestine plus liver | 22 | 20 | 91 | 10 | 45 | 3.8 | 0.86 | 10 | 45 | 1 | 5 |
| Multivisceral | 6† | 6 | 100 | 2 | 33 | 4.5 | 0.33 | 3 | 50 | 2 | 33 |
| Total | 44 | 42 | 95 | 12 | 43 | 4.1 | 0.60 | 23 | 52 | 8 | 18 |
Number of grafts that required treatment.
The multivisceral graft without a liver was excluded.
Multivisceral: stomach, duodenum, pancreas, intestine, and liver.
Although the FK 506 doses, plasma trough levels, and the prednisone doses were similar in the three types of allograft recipients (Fig. 4), the incidence of pathologically confirmed rejection was higher in the isolated intestine cases (93 percent) than in the liver and intestine (62 percent) and multivisceral (57 percent) cases. Most of the rejections were graded mild to moderate. The mean postoperative time to the first episode in all instances was 18.8±28 days (range of three to 138 days); 10.7±6 for the isolated intestine 22±34 for the combined liver and intestine, and 15±7 for the multivisceral allografts.
Beyond three post-transplant months, about 50 percent of the patients in each transplant group had rejection of the intestinal graft. This often was associated with attempts to reduce immunosuppression because of CMV enteritis, PTLD, or other opportunistic infections. Statistically, there was no significant difference between the three groups of intestinal recipients in the incidence, frequency, and severity of rejection, or the need for augmented steroids or OKT3 (Table V).
Five (36 percent) of the 14 intestinal grafts, which included colon, showed histologic evidence of colonic rejection at some time. None of the multivisceral grafts had histopathologically proved gastric rejection, but one had two episodes of pancreatitis that responded to augmented steroid therapy.
On 88 occasions in which both liver and small bowel biopsies were taken simultaneously or closely together, 47 (53 percent) of the dual specimens had no signs of rejection in either organ, 12 (14 percent) had rejection in both, 15 (17 percent) had rejection only in the liver, and 14 (16 percent) had rejection in the intestine only. The histopathologic examination of full thickness sections of the six resected grafts showed chronic rejection in two of the isolated intestinal grafts. In addition, one combined liver and intestine recipient with strong positive cross-match had chronic rejection of both organs. This patient died as a result of hepatorenal failure with the graft in place.
Infectious complications
Bacterial
Complications caused by enterococcus, Streptococcus fecium and faecalis, Enterobacter cloacae, coagulase negative and positive staphylococcus, Clostridium difficile and perfringens, Streptococcus viridans, Klebsiella, and Acinetobacter anitratum were recorded on 114 occasions in 38 (88 percent) of the 43 recipients (Table VI). The episodes included line sepsis, wound and intra-abdominal infections, pneumonia, arthritis, colitis, urinary tract infection and bacteremia of unknown source.
TABLE VI.
INFECTIOUS COMPLICATIONS AFTER INTESTINAL TRANSPLANTATION
| Bacterial |
Fungal |
Cytomegaloviral |
Lymphoproliferative disease, | |||||
|---|---|---|---|---|---|---|---|---|
| Recipient | No. of pts. | No. | Episode/pt. | No. | Episode/pt. | No. | Episode/pt. | No. |
| Isolated Intestine | 15 | 12 | 1.8 | 6 | 1.0 | 9 | 1.8 | 0 |
| Intestine plus liver | 21 | 20 | 3.7 | 8 | 1.3 | 5 | 1.6 | 3 |
| Multivisceral | 7 | 6 | 3.7 | 5 | 1.2 | 2 | 1.0 | 1 |
| Total | 43 | 38 | 3.1 | 19 | 1.2 | 16 | 1.5 | 4 |
Fungal
Nineteen (44 percent) of the 43 recipients had fungal infection of the esophagus, paranasal sinuses, trachea, lung, and peritoneum (Table VI), the highest incidence being in the multivisceral recipients (71 percent). The fungi were: Torulopsis glabrata, Candida albicans, Trichoderma koningii, and Coccidioides immitis.
Translocation
The diagnosis of bacterial or fungal translocation, or both, was made in patients who had the same microorganisms in the intestinal lumen and blood simultaneously with a quantitative stool culture count of more than 1×109 cfu per mL. Thirteen episodes of translocation occurred in 11 recipients. Four (two adults and two children) of these were isolated intestinal recipients and seven (two adults and five children) were combined liver and intestine recipients. Incongruously, there were no examples in the multivisceral cohort. The isolated microorganisms were enterococcus, S. fecium and faecalis, C. perfringens, coagulase negative staphylococcus, Klebsiella, K cloacae, C. albicans, and T. glabrata and in different combinations. Ten (77 percent) of these episodes were associated with rejection.
Cytomegaloviral
Sixteen (37 percent) intestinal recipients had at least one episode of new or reactivated CMV infection (Table VI) 72±63 days after transplantation (range of 21 to 128 days), enteritis (n=11), hepatitis (n=2), pneumonitis (n=2), gastritis (n=1), retinitis (n=1), and CMV syndrome (n=1). Ten of these 16 morbid instances involved seronegative recipients who received seropositive grafts. Eight of these ten seropositive recipients were isolated intestinal recipients. The other two were combined liver and intestine and multivisceral recipients. Five of the CMV infected isolated intestinal recipients had graft rejection after reduction of immunosuppression and three of them had graft removal. In contrast, there were no episodes of CMV infection in CMV seronegative recipients who received CMV seronegative grafts.
Reactivation of CMV occurred in the remaining six patients. Four patients received seronegative and two received seropositive grafts. Four patients were combined liver and intestine recipients, one was isolated intestine, and the other was multivisceral.
Post-transplant lymphoproliferative disease
The occurrence of PTLD was histologically documented in four intestinal recipients (three children and one adult). The complication was diagnosed at 49, 252, 287, and 383 days after transplantation and was fatal in two of the four patients. The PTLD involved both the native and transplanted organs. Treatment was attempted in the three pediatric patients with reduction of immunosuppression and initiation of antiviral therapy. In the adult, the disease was diagnosed at the time of autopsy.
Acute Epstein-Barr viral (EBV) lymphadenitis occurred in another two patients, one combined liver and intestine pediatric recipient and one multivisceral adult recipient. Interestingly, the adult patient was successfully treated with triple antiviral therapy (gancyclovir, foscarnet, and alpha-interferon) despite augmentation of immunosuppression because of simultaneous graft rejection. None of the isolated intestinal graft recipients had PTLD or EBV infections.
Other viral infections
The pediatric recipients were more prone to other viral infections, which occurred in 12 patients on 17 occasions. The viral agents isolated were adenovirus, influenza and parainfluenza viruses, respiratory syncytial virus, rotavirus, and herpes simplex virus.
Graft function
Nutrition
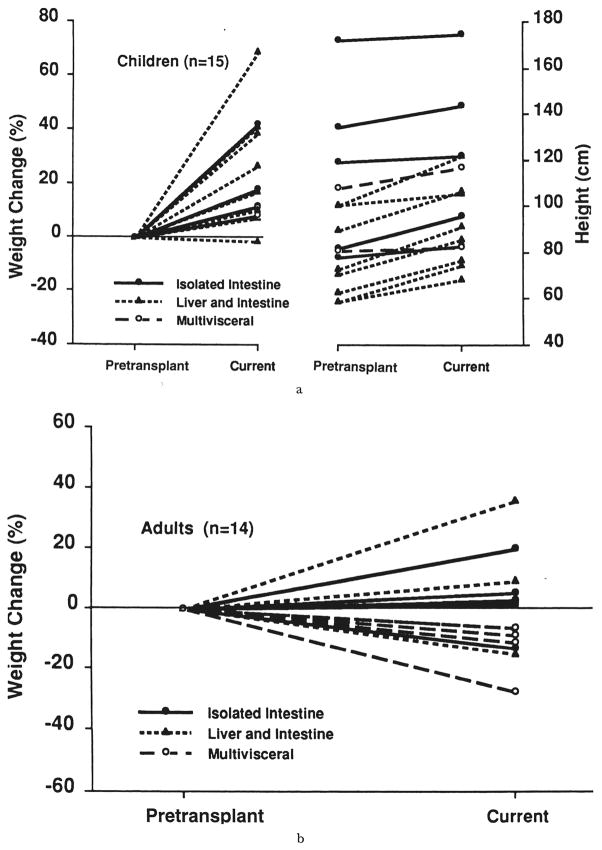
Total parenteral nutrition was discontinued for all recipients after 18 to 210 days (59±49 days) and tube feeding begun at three to 54 days (16±10 days) after transplantation. The average overlap time between initiation of enteral feeding and discontinuation of TPN was usually four weeks. The difference in timing between the three different recipients is given in Table VII. Interestingly, the isolated intestinal recipients tolerated enteral feeding significantly earlier than the combined liver and intestine (p=0.0001) and multivisceral recipients (p=0.005). Most pediatric patients had failed to learn (or had forgotten) to eat. Oral feeding had to be taught. Few patients still prefer tube feeding to eating despite intensive rehabilitation. Except for four patients (one isolated, one combined liver and intestine, and two multivisceral recipients), the other 25 current survivors with graft in place are supported nutritionally solely with their functioning grafts. The four exceptional patients intermittently have been on partial TPN because of CMV enteritis, (n=1), gastric atony because of progression of the primary gastrointestinal disease, “pseudo-obstruction” (n=1), or unexplained gastrointestinal dysmotility (n=2). The 30th patient had graft enterectomy. To date, all of the children except one gained body weight and had an increase in height (Fig. 7a). Seven adult recipients (four multivisceral, two combined liver and intestine, and one isolated intestine), lost 3 to 27 percent of their pretransplant body weight (Fig. 7b). The current values, however, are within the calculated ideal body weight for each individual.
TABLE VII.
POSTOPERATIVE COURSE AND CURRENT STATUS OF INTESTINAL RECIPIENTS
| Total population, n=43 | Isolated intestine, n=15 | Intestine-liver, n=21 | Multivisceral, n=7 | |
|---|---|---|---|---|
| Course* | ||||
| ICU stay, d | 29±51 | 13±18 | 36±67 | 48±39 |
| Enternal feeding started, d | 16±10 | 8±4 | 20±9 | 24±18 |
| TPN stopped, d | 59±49 | 43±28 | 76±64 | 54±21 |
| Hospital stay, wk | 12±8 | 11±6 | 12±9 | 16±7 |
| Readmission, times | 4±4 | 3±3 | 5±4 | 4±4 |
| Current status, survivors† | n=29 | n=10 | n=13 | n=6 |
| Total parenteral nutrition | ||||
| Free | 25 (86) | 9 (90) | 12 (92) | 4 (67) |
| Partial | 4 (14) | 1 (10) | 1 (8) | 2 (33) |
| Total | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Location | ||||
| Home | 26 (90) | 9 (90) | 13 (100) | 4 (66.7) |
| Hospitalized | 3 (10) | 1 (10) | 0 (0) | 2 (33.3) |
Mean ±SD.
Graft in place.
Numbers in parentheses are percentages.
ICU, Intensive care unit; TPN, total parenteral nutrition; d, day, and wk, week.
Fig. 7.
a, Weight and height performance of children with intestinal graft in place, and b, weight performance of adult recipients.
Gastrointestinal motility
Gastric emptying in the early postoperative period was delayed from two to 24 hours in 17 (85 percent) of the 20 adult recipients studied, which was slight (more than three hours) in six and marked in 11 (more than six hours). Gastric hypoperistalsis frequently was associated with use of narcotic analgesics during the early postoperative period. Recovery of the gastric motility was spontaneous and complete in all patients who were evaluated four to six months after transplantation. The mean intestinal transit time for the roentgenographically studied transplanted intestine (n=21) was 4.1 ± 5.8 SD hours (range of 0.3 to 24 hours). Of these, 13 (62 percent) had an abnormality of the intestinal transit time that was markedly accelerated (less than one hour) in six instances or prolonged (more than three hours) in the other seven. The mean intestinal transit time was 0.5 ± 0.2 hours (range of 0.33 to 0.83 hours) and 9.4 ± 7.2 hours (range of 3.25 to 24 hours) for each abnormality, respectively. Grafts with abnormal transit time were seven isolated intestine, four combined liver and intestine, and two multivisceral. When studied later, these abnormalities were significantly improved.
In nine adult recipients, the myoelectric activity of the native and transplanted intestine was characterized by measuring the migrating motor complex (MMC). The antral motility was abnormal in all of the patients studied exhibited by decrease amplitude or frequency of contraction. Transmission of contraction waves from native intestine to transplanted intestine occurred, although it was not always coordinated. Multivisceral transplantation seemed to increase the likelihood of propagated MMC activity, as opposed to small bowel or liver and small bowel transplantation.
Absorption
Complete oral administration of the standard FK 506 dose (0.3 mg per kg per day) was able to maintain the desired therapeutic plasma trough level of the drug for all patients by the end of the fourth postoperative week or earlier (Fig. 4). D-xylose was adequately absorbed by the intestinal graft in most of the patients studied (n=37) in the absence of significant preservation injury or graft rejection. Vitamin E absorption was slightly depressed in most of the patients (n=10). The amount of the total fecal lipids was usually high in the early postoperative period, and fat absorption was still abnormal in some patients who were evaluated one year after transplantation.
Disease recurrence
With follow-up periods ranging from eight to 22 months, none of the 13 recipients who had chronic primary disease of the native intestine (including six patients with Crohn’s disease) showed any clinical or histopathologic evidences of disease recurrence in the visceral graft.
Graft versus host disease
Using standard histologic methods and in situ hybridization techniques that allow distinction of donor from recipient cells (26), graft versus host disease (GVHD) was unequivocally diagnosed in only one combined liver and intestine pediatric recipient. Because of Pneumocystis carinii pneumonia and an intestinal anastomotic leak, light immunosuppression was attempted early in the postoperative course of this child who had preexisting IgA deficiency. The skin lesions appeared ten days after transplantation. The overall clinical signs and symptoms simulated life-threatening sepsis. The immunosuppression was reduced significantly, and 13 days later, the patient died as a result of multiple organ failure.
Hospitalization
The early convalescence of most of the recipients was prolonged and complicated. With a median hospitalization period of 11 weeks (range of three to 45 weeks) after transplantation, recipients required staying in the intensive care unit (ICU) ranging from two to 300 days (median of 11 days). The mean values for the total population and each group are given in Table VII. The isolated intestinal recipients required less time in the ICU compared with the combined liver and intestine (p=0.1) and multivisceral recipients (p=0.004). During the study period, the median number of readmissions for all patients was three (range of zero to 14). The differences in mean values for each of the transplanted groups were not statistically significant (Table VII). The primary causes for prolonged hospitalization and frequent readmission were CMV enteritis, rejection, dehydration, postoperative pulmonary insufficiency, EBV infections and PTLD. Twenty-six (90 percent) of the 29 current survivors with graft in place are home and fully active, except one child who is permanently paraplegic after a lumbar puncture.
DISCUSSION
Long-term survival after human intestinal transplantation has been accomplished only in the last six years. The index transplantations were of a multivisceral graft in November 1987 (3), a combined liver and intestine in November 1988 (4), a small intestinal segment from a living related donor in 1988 (5), and a cadaveric small bowel in 1989 (6). These sporadic achievements using cyclosporine based immunosuppression became commonplace with all three procedures after the demonstration by Murase and associates of the superior potency of FK 506 in rat intestinal transplantation models (8, 9), and the prompt confirmation of these experimental results by others (26–28). The ensuing clinical trials have been described previously by Todo and Tzakis and co-workers (10–13), for which the present report has provided follow-up periods of six to 39 months.
Survival of most patients treated with each of the three intestinal transplant operations is justification for optimism. However, in this report, we have concentrated on the limitations and complications of these procedures, which we believe preclude their widespread application without further improvements in management. The problems have stemmed from the need for perfect and continuous control of rejection to maintain the barrier to alimentary contamination and to prevent translocation of bacteria and fungi. The necessarily continuous heavy immunosuppression is potentially self-defeating. This was exemplified by the fact that more than one-half of the deaths occurred within the first 120 days postoperatively. However, the most disappointing observation was that, despite heavy immunosuppression, the threat of rejection remained exorbitantly high long after three months. Infectious and other complications that are related to rejection per se, as well as infections independent of the grafts, continued thereafter in the increasingly vulnerable recipients. Six more of the 13 deaths occurred at or well beyond one year.
The effort and expense to care for this small collection of pioneer patients was prodigious, but without the sense of eventual security that often is achieved in the long surviving recipients of the liver alone. Having reached this conclusion, we have instituted a revised treatment strategy of perioperative bone marrow transplantation that would have been unthinkable at the time this program was begun because of the historic fear of GVHD associated experimentally with intestinal transplantation (29–31). However, in actual clinical experience, GVHD has been seen in only one recipient who had an anastomotic (enteric) leak necessitating temporary discontinuance of immunosuppression. This patient also had preexisting IgA deficiency.
In addition to these empiric observations of a minimal GVHD threat, much evidence has been uncovered in the last 18 months that the basis of acceptance of all kinds of grafts is the ubiquitous migration of passenger leukocytes of bone marrow origin from transplanted organs with the occurrence of systemic chimerism and the gradual induction of donor specific nonreactivity (32–34). Thus, instead of causing GVHD under the therapeutic circumstances of whole organ transplantation, the chimeric cells are apparently sufficiently tolerogenic to be able to allow eventual drug weaning if they are present in sufficient quantities, as is the situation with the liver (33, 35).
Our revised protocol for future intestinal, liver and intestine, and multivisceral recipients is to augment this passenger leukocyte traffic with supplementary bone marrow from the intestinal donor, using the same perioperative immunosuppressive protocol as was created in the first 43 cases. It remains to be seen if this approach will result in greater stability of the intestinal graft and its recipient, early and late after transplantation.
Acknowledgments
Supported, in part by and grant from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Lillehei RC, Goott B, Miller VA. The physiologic response of small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg. 1959;150:543–560. doi: 10.1097/00000658-195910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Kaupp HA., Jr Mass and homotransplantation of abdominal organs in dogs. Surg Forum. 1960;11:28–30. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Rowe M, Todo S, et al. Transplantation of multiple abdominal viscera. J A M A. 1989;261:1449–1457. [PMC free article] [PubMed] [Google Scholar]
- 4.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181–184. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 5.Deltz E, Schroeder P, Gebhardt H, et al. Successful clinical small bowel transplantation: report of a case. Clin Transpl. 1989;3:89–91. [Google Scholar]
- 6.Goulet O, Revillon Y, Brosse N, et al. Successful small bowel transplantation in an infant. Transplantation. 1992;53:940–943. doi: 10.1097/00007890-199204000-00046. [DOI] [PubMed] [Google Scholar]
- 7.Kirkman R. Small bowel transplantation. Transplantation. 1984;37:429–433. doi: 10.1097/00007890-198405000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Murase N, Kim D, Tod̄o S, et al. Induction of liver, heart, and multivisceral graft acceptance with a short course of FK 506. Transpl Proc. 1990;22:74–75. [PMC free article] [PubMed] [Google Scholar]
- 9.Murase N, Demetris AJ, Matusuzaki T, et al. Long survival in rats after multivisceral versus isolated small bowel allotransplantation under FK 506. Surgery. 1991;110:87–98. [PMC free article] [PubMed] [Google Scholar]
- 10.Todo S, Tzakis AG, Abu-Elmagd K, et al. Cadaveric small bowel and small bowel-liver transplantation in humans. Transplantation. 1992;53:369–376. doi: 10.1097/00007890-199202010-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzakis AG, Todo S, Reyes J, Starzl TE. Liver and small bowel transplantation for short gut syndrome in a child. Transpl Sci. 1991;1:27–33. [Google Scholar]
- 12.Todo S, Tzakis AG, Abu-Elmagd K, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223–234. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todo S, Tzakis A, Reyes J, et al. Small in testinal transplantation in humans with or without colon. Transplantation. 1994;57:840–848. doi: 10.1097/00007890-199403270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzakis AG, Todo S, Starzl TE. Intestinal transplantation. In: Coggins CH, Hancock EW, editors. Annual Review of Medicine: Selected topics in the clinical sciences. Vol. 45. Annual Reviews Inc; Palo Alto, CA: 1994. pp. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335–344. [PMC free article] [PubMed] [Google Scholar]
- 16.Casavilla A, Selby R, Abu-Elmagd K, et al. Logistics, technique for combined hepatic-intestinal retrieval. Ann Surg. 1992;216:605–610. doi: 10.1097/00000658-199211000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzakis AG, Todo S, Reyes J, et al. Piggyback orthotopic intestinal transplantation. Surg Gynecol Obstet. 1993;176:297–298. [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Elmagd K, Fung JJ, Reyes J, et al. Management of intestinal transplantation in humans. Transpl Proc. 1992;24:1243–1244. [PMC free article] [PubMed] [Google Scholar]
- 19.Takaya S, Iwaki Y, Starzl TE. Liver transplantation in cytotoxic crossmatch cases using FK 506, high dose steroids, and prostaglandin E1. Transplantation. 1992;54:927–929. doi: 10.1097/00007890-199211000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reyes J, Todo S, Tzakis A, et al. Transplantation of the small bowel and other abdominal organs in humans. In: Makowka L, Sher L, editors. Intra-abdominal Organ Transplantation 2000. Georgetown, TX: R. G. Landes Co; 1994. pp. 165–181. [Google Scholar]
- 21.Campbell WL, Abu-Elmagd K, Federal MP, et al. Contrast examination of the small bowel in patients with small-bowel transplants: findings in 16 patients. A J R. 1993;161:297–300. doi: 10.2214/ajr.161.5.8273638. [DOI] [PubMed] [Google Scholar]
- 22.Breiter HC, Craig RM, Levee G, Atkinson AJ. Use of kinetic methods to evaluate d-xylose malabsorption in patients. J Lab Clin Med. 1988;112:533–543. [PubMed] [Google Scholar]
- 23.Amenta JS. Lipodol absorption and urinary Iodide excretion as a screening test for steatorrhea. Clin Chem. 1969;15:295–306. [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Mathews DE, Farewell VT. In: Using and Understanding Medical Statistics. Matthews DE, Farewell VT, editors. Basel, New York: Karger; 1985. pp. 67–87. [Google Scholar]
- 26.Hoffman AL, Makowka L, Banner B, et al. The use of FK 506 for small intestine allotransplantation: inhabitation of acute rejection and prevention of fatal graft versus host disease. Transplantation. 1990;149:483–490. doi: 10.1097/00007890-199003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee K, Stangl MJ, Todo S, et al. Successful orthotopic small bowel transplantation with short term FK506 immunosuppressive therapy. Transpl Proc. 1990;22:78–79. [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimi F, Nakamura K, Zhu Y, et al. Canine total orthotopic small bowel transplantation under FK 506. Transpl Proc. 1991;23:3240–3242. [PMC free article] [PubMed] [Google Scholar]
- 29.Starzl TE, Kaupp HA, Jr, Brock DR, et al. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219–229. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monchik GJ, Russell PS. Transplantation of small bowel in the rat: technical and immunological considerations. Surgery. 1971;70:693–702. [PubMed] [Google Scholar]
- 31.Murase N, Demetris AJ, Woo J, et al. Graft versus host disease (GVHD) after BN to LEW compared to LEW to BN rat intestinal transplantation under FK 506. Transplantation. 1993;55:1–7. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 34.Starzl TE, Demetris AJ, Murase N, et al. Donor cell chimerism permitted by immunosuppressive drugs. Immunol Today. 1993;14:326–332. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes J, Zeevi A, Ramos H, et al. Frequent drug free state after orthotopic liver transplantation. Transpl Proc. 1993;25:3315–3318. [PMC free article] [PubMed] [Google Scholar]