Abstract
Supplemental choline in the maternal diet produces a lasting enhancement in memory in offspring that resists age-related decline and is accompanied by neuroanatomical, neurophysiological and neurochemical changes in the hippocampus. The present study was designed to examine: 1) if prenatal choline supplementation alters behaviors that contribute to risk or resilience in cognitive aging, and 2) whether, at old age (25 months), prenatally choline supplemented rats show evidence of preserved hippocampal plasticity. A longitudinal design was used to look at exploration of an open field, with and without objects, at 1 and 24 months of age in male and female rats whose mothers were fed a diet supplemented with choline (SUP; 5 mg/kg choline chloride) or not supplemented (CON; 1.1 mg/kg choline chloride) on embryonic days 12–17. Aging caused a significant decline in open field exploration that was more pronounced in males but interest in novel objects was maintained in both sexes. Prenatal choline supplementation attenuated, but did not prevent age-related decline in exploration in males and increased object exploration in young females. Following behavioral assessment, rats were euthanized to assess markers of hippocampal plasticity. Aged SUP males and females had more newly proliferated cells in the hippocampal dentate gyrus and protein levels of vascular-endothelial growth factor (VEGF) and neurotrophin-3 (NT-3) were significantly elevated in female SUP rats in comparison to all other groups. Taken together, these findings provide the first evidence that prenatal cholinesupplementation causes changes in exploratory behaviors over the lifespan and preserves some features of hippocampal plasticity that can be seen even at 2 years of age.
Keywords: Bromodeoxyuridine, Vascular endothelial growth factor, Neurotrophin-3, Corticosterone, Open field, Object exploration
Introduction
Choline is an essential nutrient important for a variety of biological processes in humans and other mammals (for reviews see Blusztajn, 1998; Zeisel, 2004; 2006). In addition to its importance as the precursor to the neurotransmitter, acetylcholine, choline also contributes to the construction of cellular membranes and lipid transport as a constituent of phosphatidylcholine. Via its conversion to betaine it becomes a methyl group donor altering gene and histone methylation processes, and as a component of the platelet aggregating factors, lysosphosphatidylcholine and sphingosylphosphorylcholine, it participates in cell signaling. An abundant literature clearly reveals that the choline content during early development has a profound impact on the developing organism with measurable, often marked, effects on behavior and neural function that persist well into adulthood (see Hohmann and Berger-Sweeney, 1998; Meck and Williams, 2003; Meck et al., 2008).
In rodents, supplementation of the maternal diet with choline on embryonic days (ED) 11–17, significantly enhances spatial memory of the adult offspring (e.g., Meck and Williams, 1997; Meck et al., 1988; 1989; also see Schenk and Brandner, 1995; Tees and Mohammadi, 1999) and prevents the memory decline that normally occurs with advanced age (Meck et al., 2008; also see Meck and Williams, 2003). This long-term enhancement in cognitive function is accompanied by facilitation of hippocampal long-term potentiation (LTP) in young adult and middle-aged rats (Jones et al., 1999; Pyapali et al., 1998) and is positively correlated with the acetylcholine (ACh) content of hippocampal slices following stimulated release in aged rodents (Meck et al., 2008). To date, the precise behavioral and neural mechanisms mediating the life-long persistence of prenatal choline supplementation effects on memory and on hippocampal function are not known. The present study was designed to examine: 1) if prenatal choline supplementation alters behaviors that contribute to risk or resilience in cognitive aging, and 2) whether, at 25 months of age, prenatally choline supplemented rats show evidence of preserved hippocampal plasticity. A longitudinal design was used examine activity, exploration and plasma corticosterone responses to acute stress in prepubertal male and female rats. At 24 months of age, these rats were retested for their tendencies to exploration and their general activity levels. Markers of hippocampal plasticity (i.e. cell proliferation and levels of several growth factors) were determined in all rats once behavioral assessments were complete, at approximately 25 months of age.
There is considerable evidence that enriched environments, activity/exercise, and stress modulate hippocampal plasticity and cognitive aging. Enriched environments increase rats’ exploratory behavior as measured in an open field (Fernandez et al., 2004), and improve cognitive function in aged rodents (Lores-Arnaiz et al., 2006). Exercise has dramatic effects on hippocampal plasticity and improves memory function (see Cotman and Berchtold, 2002). And, it is well known that stress-induced activation of the hypothalamic-pituitary-adrenal axis causes loss of hippocampal spines, inhibition of hippocampal cell proliferation, and cognitive impairment (Lupien et al., 1998; McEwen, 1999), while reduction of corticosterone in old age reverses these effects (see Cameron and McKay, 1999). Therefore, we reasoned that prenatal choline supplementation might modify cognitive aging by altering rats’ activity, exploratory behavior and/or stress reactivity. If this hypothesis is correct, then prenatally choline supplemented rats may have life-long changes in the way in which they interact with their environment compared to control rats and this behavioral change over the lifespan may contribute to the maintenance of neural plasticity and might help to explain why they show attenuated cognitive decline during aging (Meck et al., 2008; Meck and Williams, 2003).
One age-related change in hippocampal plasticity is greatly diminished neurogenesis in the dentate gyrus (DG), which begins during middle age in the rodent (Kuhn et al., 1996; Nacher et al., 2003; Rao et al., 2005). Moreover, it has been suggested that this loss in plasticity may contribute to age-related learning and memory impairments (Drapeau et al., 2003). While the causal factor or factors underlying decreased hippocampal neurogenesis beginning in middle age is not known, a number of proliferation/growth factors also show reduced concentrations in the hippocampus as rats age, including fibroblast growth factor-2 (FGF-2), insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF) (Shetty et al. 2005), and brain derived neurtrophic factor (BDNF) (Bimonte-Nelson et al., 2008, Hattiangady et al., 2005, but see also Katoh-Semba et al., 1998). On the other hand, nerve growth factor (NGF) and neurotrophin 3 (NT-3) show varied patterns with aging, rising or staying constant at least through 24 months of age in the rat (see Bimonte-Nelson et al., 2008; Katoh-Semba et al., 1998). All these factors have been shown to modulate the proliferation of stem/progenitor cells in the subgranual zone of the dentate gyrus and/or the survival of new neurons (Lichtenwalner et al., 2001; Jen et al., 2003; Lee et al., 2002; Cao et al., 2004; Shimazu et al., 2006; Frielingsdorf et al., 2007).
These data are of particular interest because prenatal choline supplementation to rat mothers leads to enhanced basal levels of hippocampal neurogenesis in adult offspring that is accompanied by increased BDNF (Glenn et al., 2007; Wong-Goodrich et al., 2008a), NGF (Sandstrom et al., 2002; Wong-Goodrich et al., 2008a), and IGF-1 (Wong-Goodrich et al., 2008a; 2008b) and -2 (Napoli et al., 2008) levels. These data are strongly suggestive of a plasticity mechanism underlying the cognitive effects of prenatal choline supplementation. As has been suggested by Kempermann (2008), organisms with an increased capacity for hippocampal plasticity may have a “neurogenic reserve” that may make them more resilient to age-related cognitive decline. This provocative idea was tested in the present study by examining whether markers of plasticity, hippocampal cell proliferation and growth factor content (e.g., BDNF, NGF, VEGF, NT-3, and IGF-1), are elevated in rats aged to 25 months that were treated in utero with choline supplementation compared to rats fed a control diet. We hypothesized that the aged brains, and specifically the hippocampi, of prenatal choline supplemented rats would show more features consistent with enhanced plasticity when compared to control rats.
2. Results
2.1. Behavioral measures in young and old rats
2.1.1. Open field exploration and activity
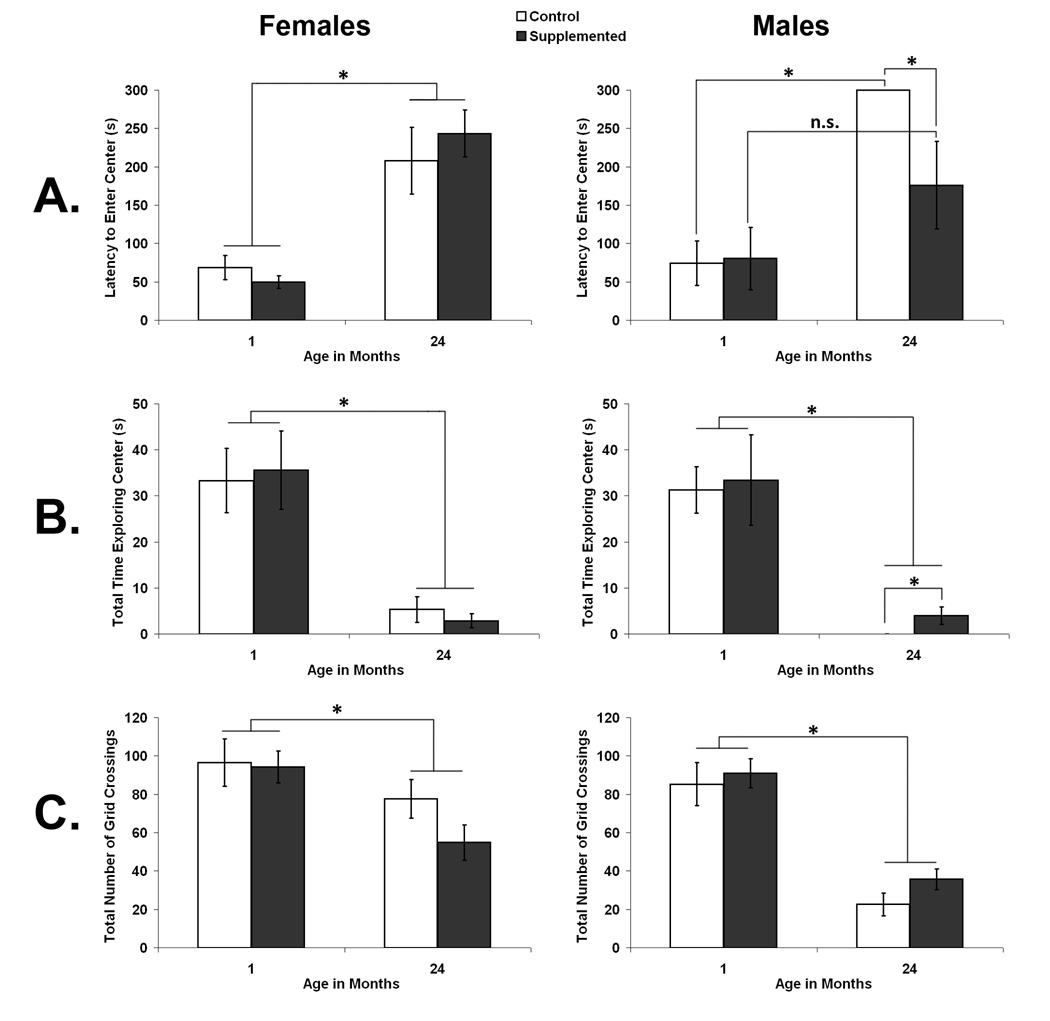
The behavior of female and male rats in a large open field was assessed at 1 and again at 24 months of age. Latency to enter the center area of the field and the total amount of time rats spent in the center of the open field were used as indices of exploration. The total number of crossings rats made into each of 16 squares created by overlaying a 4×4 grid over the arena during video scoring was used as index of general activity levels. The results for each of these three measures of open field behavior are illustrated in Fig. 1. Overall, there was a clear decline in general activity and in open field exploration with age and the decline in exploration, but not activity, was attenuated by prenatal choline supplementation in male, but not female rats.
Fig. 1.
Open field behavior as a function of prenatal diet and age in females (left panel) and males (right panel). A. shows the latency of rats to enter the center area of the open field; B. shows the total time rats spent exploring the center area; and C. shows the total numbers of grid crossings made in the field. Error bars are SEM and *p < 0.05.
Latency to enter the center of an open field was used as an indication of fearfulness versus exploratory tendencies. A 2×2×2 mixed factorial analysis of variance (ANOVA) on latency to enter the center of the open field with the between factors of Sex (Female and Male) and Diet (CON and SUP) and the repeated measure of Age (1 and 24 months) revealed a main effect of Age, F[1,22] =57.617, p < 0.01 and a trend towards a 3-way interaction, F[1,22] = 3.506, p = 0.074. The main effect of age reflected an overall increase in the latency of rats to go into the center of the field at 24 months compared to 1 month of age. Planned comparisons within each sex revealed that while aged female CON and SUP rats did not differ in latency to the center of the field (p > 0.05), aged male SUP rats had a significantly shorter latency to enter the center than aged male CON rats (p < 0.05). In fact, no aged male CON rats entered the center of the field at all while 4 of 7 aged male SUP rats did so. Aged male CON rats showed the expected increase in latency to enter the center of the open field compared to young CON rats (p < 0.001). In contrast, prenatal choline supplemented male rats did not show a significant increase in latency to enter the center of the open field in old age (p < 0.05). As well, both female CON and SUP rats showed a significant increase in latency to enter the center with age (p < 0.05 and p < 0.01, respectively).
Time spent in the center of the open field was used as a measure of fearfulness versus exploratory tendencies. A 2×2×2 ANOVA (Sex × Diet × Age) conducted on the total time spent in the center of the open field during the 5 minute trial also revealed a significant main effect of Age, F[1,22] = 61.410, p < 0.001, showing that all groups spent less time in the center area at 24 months of age than when they were 1 month of age (see Fig. 1B). No other main effects or interactions reached significance. However, a planned test showed that aged male SUP rats spent significantly more time in the center than aged male CON rats (p < 0.05), none of whom went into the center area. The difference in total center time between aged female CON and SUP rats was not significant (p > 0.05).
Total number of grids crossed during the 5 min trial was used as a measure of rats’ general activity. A 2×2×2 ANOVA (Sex × Diet × Age) conducted on the total number of grid crossings revealed a significant main effect of Sex, F[1,22] = 13.624, p < 0.01, indicating that females were, overall, more active than males, which is consistent with previous studies (Renner et al., 1992a). There was also a significant Age × Sex interaction, F[1,22] = 5.977, p < 0.05; see Fig. 1C. As expected based on previous studies (see Altun et al., 2007), both male and female rats were less active at 24 months of age compared to 1 month of age, (p < 0.05 and p < 0.01, respectively). However, when we calculated the mean ± standard error (SEM) change in number of grids crossed from 1 month of age to 24 months of age in female (29.9 ± 10.6 crossings) and male (58.7 ± 6.5 crossings) rats, a post-hoc test revealed that, overall, male rats had a more substantial decline in activity with age than female rats (p < 0.05).
2.1.2. Object exploration
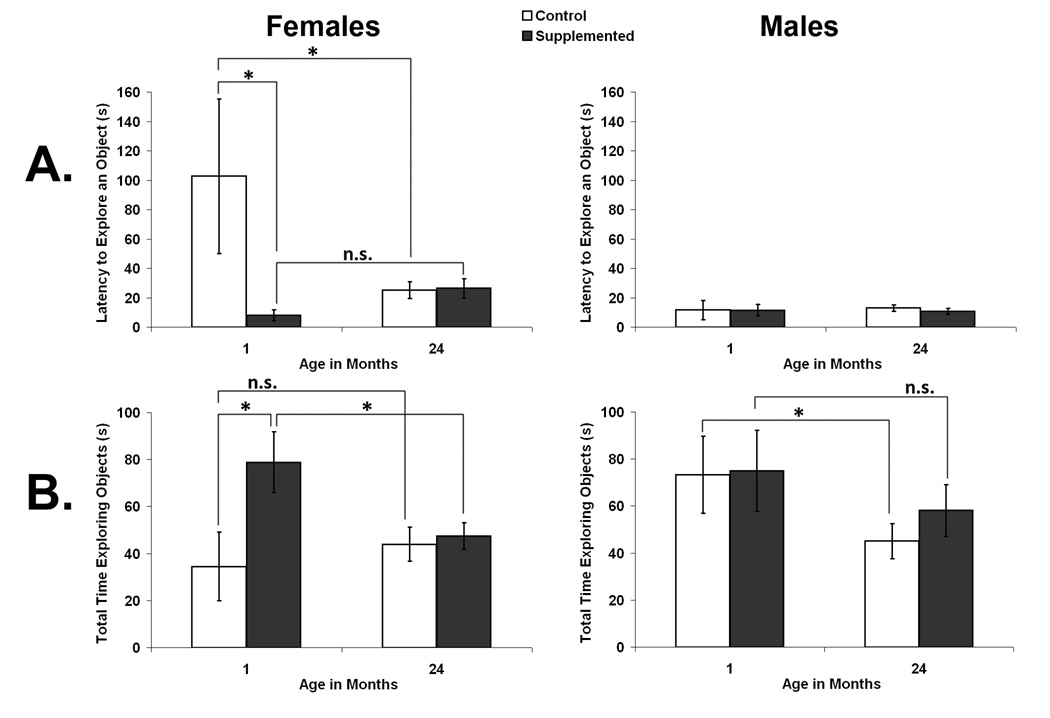
After rats had experience in the open field, their response to novelty was gauged by placing 4 unfamiliar objects in the field and examining rats’ latency to investigate an object and the total duration (out of 5 min) spent exploring all objects (see Fig. 2). In 1 month old rats, prenatally choline supplemented females approached novel objects more rapidly and spent more time investigating novel objects than control females and males, although this difference was not maintained as female rats aged. In males, total time spent exploring objects declined with age and this modest decline was attenuated by prenatal choline supplementation.
Fig. 2.
Object exploration as a function of prenatal diet and age in females (left panel) and males (right panel). A. shows the latency of rats to explore an object and B. shows the total time rats spent exploring objects. Error bars are SEM and * p < 0.05.
Latency to approach and contact the first object was used as a measure of fearfulness versus exploratory tendencies (see Fig. 2A).. A 2×2×2 ANOVA (Sex × Diet × Age) on latency to approach objects revealed no significant main effects of Sex, Age, or Diet and no significant interactions (p’s > 0.05). However, because of the very different patterns shown by male and female rats, planned comparisons within each sex were performed. Overall, young female SUP rats had a shorter latency to explore an object than young female CON rats (p < 0.05) or old female SUP rats (p < 0.05), but there were no significant effects of diet in old rats or in males (p’s > 0.05).
Time spent in contact with the 4 novel objects was used as a measure of exploration (see Fig. 2B). A 2×2×2 ANOVA on total time spent exploring objects revealed a significant main effect of Age, F[1,23] = 4.690, p < 0.05, but no interactions were significant (p’s > 0.05). Again, planned tests within each sex showed that young female SUP rats spent significantly more time exploring objects than young female CON rats (p < 0.05) and they also showed a significant decline with age from their high level of exploration when young (p < 0.05), a decline not evident in female CON rats (p > 0.05). Male SUP and CON rats were not significantly different at either age (p’s > 0.05); however, male CON, but not SUP, rats showed a significant decline in their total time spent with objects with age (p = 0.050 and p > 0.05, respectively).
2.2 Corticosterone response to acute stress in young rats
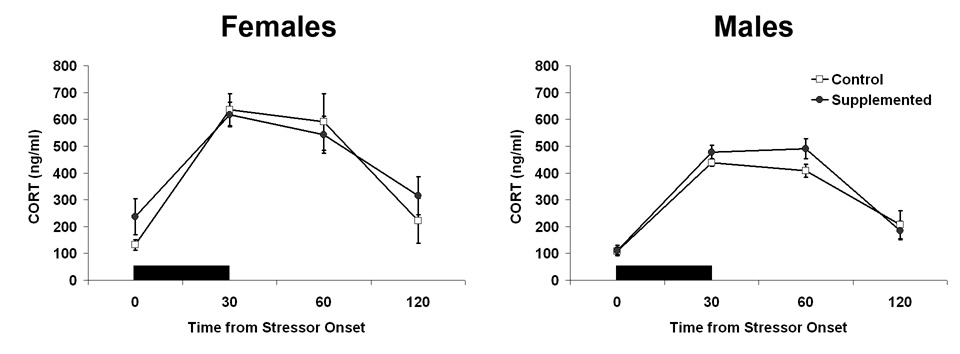
To determine whether prenatal choline supplementation altered rats basal corticosterone levels and/or showed attenuated reactivity to an environmental stressor, we measured corticosterone (CORT) in blood over the course of 2 hrs beginning with 30 min of restraint stress in young male and female, CON and SUP rats. We chose this measure of environmental reactivity because considerable prior work has characterized normative changes in CORT following restraint stress (e.g., Paris et al., 1987). Because it is known that circulating gonadal hormones influence the reactivity of the hypothalamic-pituitary-adrenal axis (Carey et al., 1995; Helmer et al., 2007; also see Critchlow et al., 1963; Kitay et al., 1971), CORT was measured in prepubertal rats. The levels of CORT at each of the 4 time points (i.e., at the beginning of restraint, at the end of restraint, and 30 and 60 min following restraint) in each group are shown in Fig. 3. No significant effects of diet were seen in rats’ basal corticosterone levels nor in their HPA response to restraint stress.
Fig. 3.
Corticosterone response to 30 minutes of acute restraint stress in young rats as a function of sex and diet. Data shown are relative to stressor onset; black bar indicates the duration of the acute stressor and its presentation relative to collection time points. Error bars are SEM.
To characterize the corticosterone response to stress, we examined the effects of Time from first restraint and the effects of Sex and prenatal Diet. A 2×2×4 ANOVA (Sex × Diet × Time) revealed significant main effects of Time, F[3,75] = 62.627, p < 0.05 and Sex, F[1,25] = 12.363, p < 0.05. The significant main effect of Time reflects a significant increase in CORT in all groups in response to the 30 min of restraint and a significant decline from the elevated levels to baseline levels by the end of the 2 hrs (90 min after the cessation of the acute stress). The significant effect of Sex reflects an overall higher level of CORT in female than male rats. As can be seen in Fig. 3, females tended to have slightly higher levels of basal CORT and a larger increase in CORT in blood with stress. No other main effects or interactions were significant.
2.3. Hippocampal plasticity measures
2.3.2. BrdU-labeling in the hippocampus as a marker of new cell proliferation.
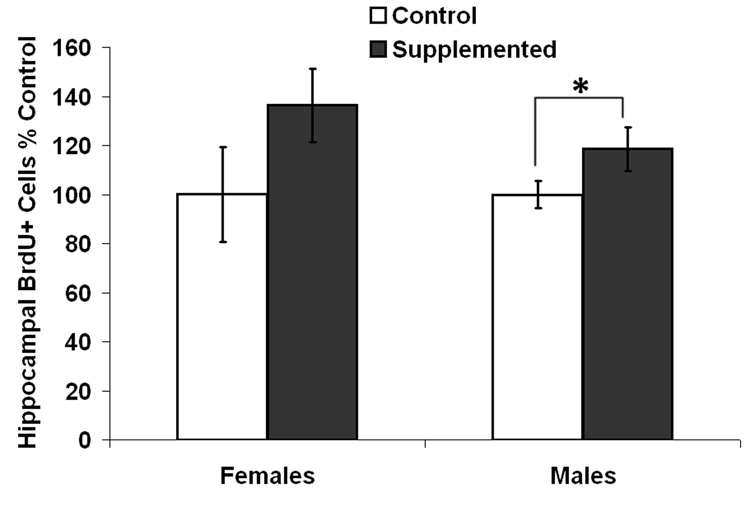
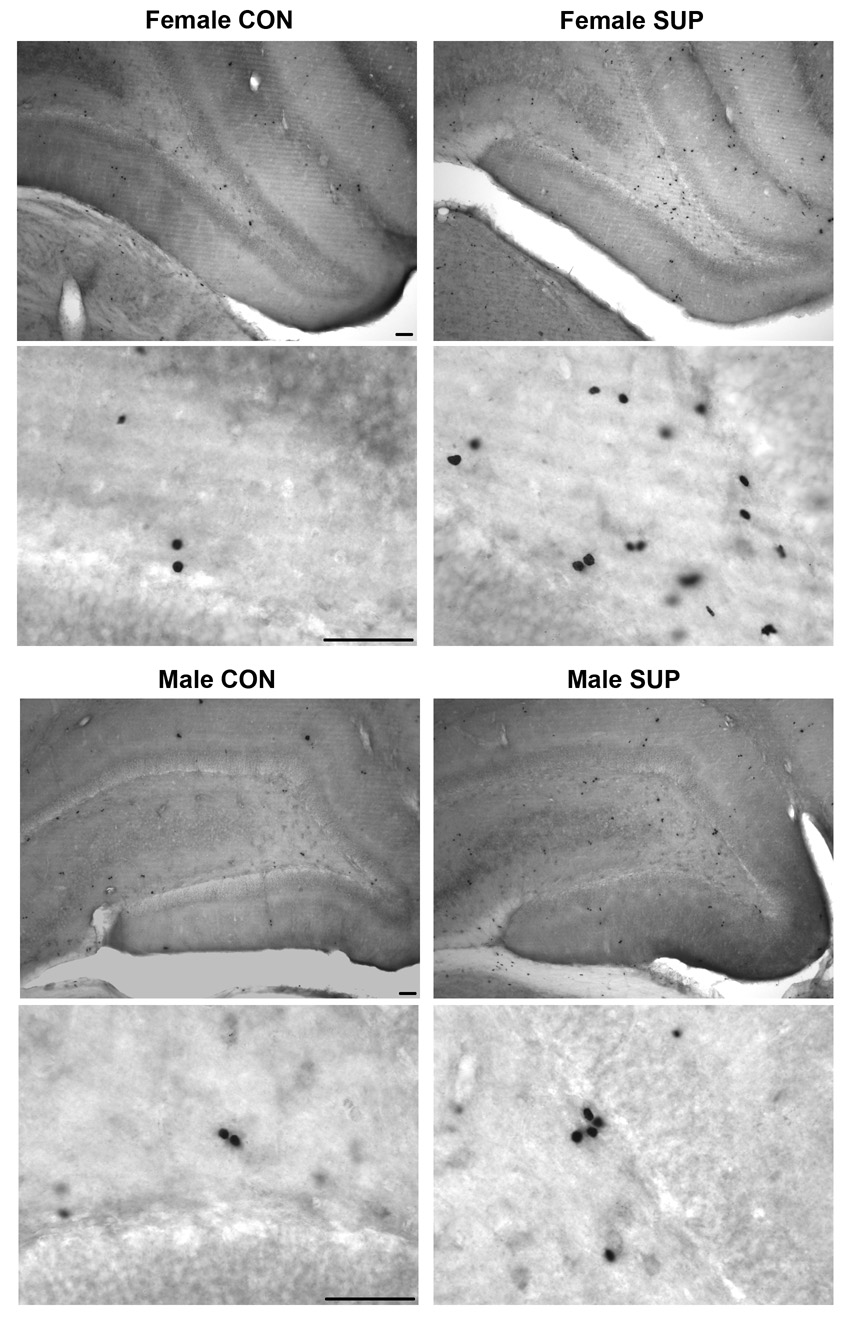
The levels of BrdU-labeling, relative to each control group, in the hippocampal dentate gyrus of 25 month old rats and the associated photomicrographs of the staining are shown in Fig. 4 and Fig. 5, respectively. Overall, there was a significant effect of Diet on cell proliferation, with SUP rats showing more BrdU+ cells than CON rats, F[1,19] = 4.249, p < 0.05. For this measure, there was no significant effect of Sex (F < 1) and the interaction between Sex and Diet was also not significant (F < 1). Consistent with the overall increase in BrdU+ cells in SUP rats, planned tests showed that male SUP rats had significantly more BrdU+ cells than male CON rats (p < 0.05) and although BrdU counts in female SUP rats were not significantly different from those in female CON rats, female SUP rats tended to have more BrdU+ cells than female CON rats (p = 0.08).
Fig. 4.
Levels of hippocampal cell proliferation in old rats expressed as percent control and as indexed by numbers of BrdU+ cells in the dentate gyrus as a function of sex and diet. Error bars are SEM and *p<.05.
Fig. 5.
Photomicrographs showing BrdU staining in the dentate gyrus in old female (top 4 images) and male (bottom 4 images) CON (left panels) and SUP (right panels) rats. Top 2 images in each section were taken at 10x magnification and bottom 2 images in each section were taken at 60x magnification with oil immersion. Scale bars are 25 µm.
2.3.1. Growth factor protein levels in hippocampus.
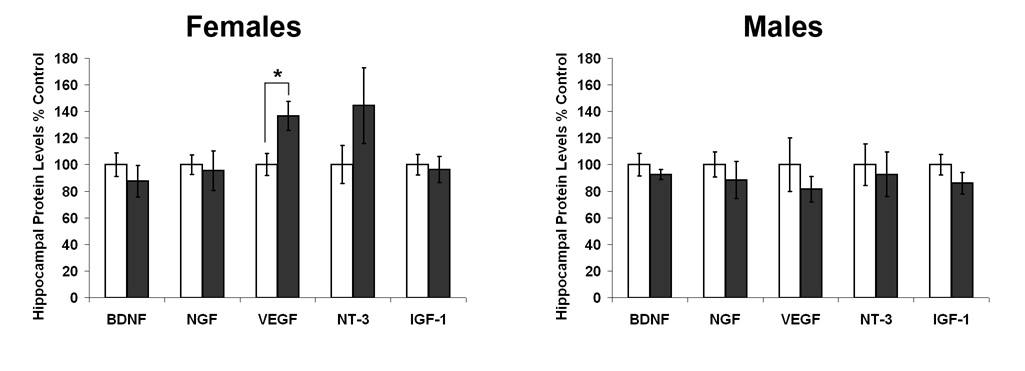
The levels of growth factor protein in the hippocampus of 25 month old rats, relative to each control group, for BDNF, NGF, VEGF, NT-3, and IGF-1 in male and female, CON and SUP rats are shown in Fig. 6. ANOVAs (Sex × Diet) on percent control values, revealed a significant main effect of Sex, F[1,22] = 4.990, p < 0.05, and a significant Sex × Diet interaction, F[1,22] = 4.959, p < 0.05 for VEGF levels in the hippocampus. These significant statistical results reflect an increased level of VEGF in female SUP rats. There were no other significant main effects or interactions for any other growth factors (all p’s > 0.10), however, there was also a tendency for NT-3 to also be elevated in female SUP rats compared to female CON rats (p = 0.09).
Fig. 6.
Levels of growth factor protein in the hippocampus in old rats expressed as percent control as a function of sex and diet. Error bars are SEM and *p<.05.
3. Discussion
The present study was designed to evaluate the hypotheses that prenatal choline supplementation may alter rats’ responses to novel environments/objects and may enhance hippocampal plasticity in adulthood, preserving it into old age. We utilized a within-subjects longitudinal design to investigate these questions in male and female rats. Behavioral measures were first collected before the onset of puberty when rats were about 1 month of age and again when rats were 24 months of age. Neural measures were collected at approximately 25 months of age. The results of this study showed a clear difference in the way prenatal choline supplementation affects male and female rats over the lifespan. Prenatal choline supplementation caused a robust increase in object exploration in prepubertal females, but not in aged females nor in males, whereas prenatal choline supplementation appeared to attenuate the decline in open field and object exploration seen in control rats. On the other hand, we found no effects of prenatal diet on general activity level of young or old, male or female rats, nor on stress reactivity as assessed by examining plasma corticosterone changes before, during and after restraint stress, suggesting that prenatal choline supplementation may be altering brain mechanisms for exploration of novel environments and objects without having broad effects on locomotion or the responsiveness of the HPA axis. We also found evidence of long-lasting effects of prenatal choline supplementation on markers of hippocampal plasticity: aged male and female SUP rats had higher basal levels of hippocampal cell proliferation at 25 months of age and female SUP rats, but not males, also had higher levels of VEGF and perhaps IGF-1 in the hippocampus. Together, these findings suggest that prenatal choline supplementation: 1) may cause alterations in rats’ responses to novelty starting before puberty that last into old age, and 2) may initiate brain and behavioral changes that lead to an aged brain with enhanced neural plasticity.
3.1. Differential effects of prenatal choline supplementation on exploratory behavior over the lifespan in male and female rats
The longitudinal design used for this part of the study allowed us to examine behavior in the same animals with a 2-year long interval between test sessions. The first behavioral measures were taken in prepubescent rats in an effort to collect data before the activational influences of gonadal hormones at puberty and again at 2 years of age, when gonadal hormone levels in both sexes are quite low. It is well known that rats show large sex difference in open field behavior, with adult males being less active and exploratory than females (Blizard et al., 1975). All of the prepubescent rats in our study were very active in our open field and there were no significant diet or sex differences observed. All rats entered the center area early in the test session and spent a substantial portion of the 5-min test exploring that area. Because of the vigorous activity and exploration at this age, it may have been difficult to detect an increase in exploration caused by the prenatal choline supplementation. In contrast, we found that prepubescent males were quicker to explore novel objects than females and spent more time investigating them. Previous work using a larger open field, a longer observation period and different types of objects have reported no sex differences in object exploration at 30 days of age, with differences emerging after puberty (Renner et al., 1992a). Interestingly, the effect of prenatal choline supplementation in our study was to increase females’ interest in objects and to make them approach objects more rapidly and to investigate objects for a longer duration, much like the pattern seen in our young males.
At 24 months of age, the most marked effects of prenatal choline supplementation on behavior were observed in the males. Overall, there was a tremendous decline in rats’ general activity and their exploration of the open field from 1 to 24 months of age, findings that are consistent with previous studies (Altun et al., 2007). In fact, our aged male rats whose mothers consumed a control diet during pregnancy showed very little exploration and failed to enter the center of the field during the test. In contrast, just 7 days of a choline supplemented diet during embryonic development led to significant preservation of exploratory behavior in aged male rats. In contrast, we saw no effect of prenatal choline supplementation in aged female rats in open field exploration, though the aged female rats were more active and exploratory than aged male rats. Again, the effect of prenatal choline supplementation was to increase exploratory behavior in the animals with low levels up to the level obtained by the most exploratory rats. Both male and female aged rats, showed a high level of interest in novel objects, and compared to open field exploration, there was only a modest decline in object exploration with age. These findings are consistent with previous reports of aging effects on object exploration (Altun et al., 2007). Again, we saw some savings of novel object exploration in aged males that were prenatally supplemented with choline, in contrast with a decline in time spent with objects in aged control rats.
Taken together, these findings suggest that, overall, prenatal choline supplementation increases exploratory activity in young and old rats and a protective effect of prenatal choline supplementation is most evident in males when exploratory behaviors that are normally compromised with age are examined. Given the differences between male and female rats that we observed in some of our behavioral measures, it is not surprising that effects of the diet manipulation emerge for different behaviors in male and female rats, and at different points in the lifespan. There are a number of potential reasons why prenatally choline supplemented and control rats’ exploratory responses to a novel environment and novel objects across their lifespan might be different. There is considerable evidence to suggest that the hippocampus is central to emotional behaviors, especially those relating to anxiety and arousal (Delacour, 1995; Gray, 1982; Hasselmo, 1995). And, while we have characterized our behaviors as “exploration” there is no doubt that fearfulness and arousal may be components of an animal’s tendency to explore a novel environment or object. Simple exploration of a novel environment activates ACh release to frontal cortex and hippocampus (Bianchi et al., 2003; Giovannini et al., 2001), while pharmacologic inhibition of ACh muscarinic receptors in the hippocampus causes decreases in exploration (Renner et al., 1992b). These data suggest that prenatal choline supplemented rats, which have larger basal forebrain cholinergic neurons (Williams et al., 1998), a larger presynaptic ACh pool leading to increased depolarization-evoked acetylcholine release, and slow ACh recycling (Cermak et al., 1998), may explore more than control rats because their hippocampal cholinergic system has adapted to their prenatal choline supply.
The sex differences in exploration and the effects of prenatal choline on exploration in young and old male and female rats are likely due to a combination of early organizational and later activational actions of gonadal steroids. Sex steroids modulate the function of dentate neurons under normal conditions, and can directly alter regulation of dentate gyrus neurogenesis and synaptic remodeling (eg. Tanapat et al., 1998; Woolley, 1998). Gonadal hormones can also influence the hippocampus indirectly, via subcortical hormone The sex differences in exploration and the effects of prenatal sensitive structures such as the cholinergic septohippocampal system (see Hajszan et al., 2007). Further work using gonadectomized animals will be needed to tease apart how and in what fashion gonadal hormones contribute to the sex differences reported here.
3.2. General Activity and Stress Reactivity
It is important to emphasize that we found no evidence that prenatal choline supplementation modifies general activity of male or female rats or changes the pattern of activity decline seen as rats age. In addition, alteration of prenatal choline availability did not modify basal plasma corticosterone or corticosterone changes due to an acute stressor. These data are important because they suggest that it is unlikely that the increases in exploratory behavior shown by young and old prenatal choline supplemented rats compared to control rats are secondary to diet-induced changes in activity, or in responsiveness of the HPA axis. Further work examining rats of other ages, other measures of stress (e.g., measuring adrenocorticotropic hormone after acute or prolonged stress) and activity (e.g., home cage activity) will be necessary to rule out this potential mechanism
3.3. Prenatal choline supplementation enhances markers of hippocampal plasticity in aged rats
We have previously reported that prenatal choline supplementation markedly increases hippocampal neurogenesis in 8-month old female rats (Glenn et al., 2007). Prenatal choline supplementation also increases the concentration of several hippocampal growth factors in both young and middle aged male (BDNF, IGF-1 and NGF in 70-day olds, Wong-Goodrich et al., 2008a; IGF-1 in 1-year olds, Wong-Goodrich et al., 2008), and female rats (NGF in 20- and 90- day olds, Sandstrom et al., 2004; BDNF in 8–month olds, Glenn et al., 2007). Note that in each of these studies only one sex was examined and usually only one age, and in each case rats had different experiences prior to the growth factor assays, which may have influenced hippocampal function.
One goal of the present study was to determine whether increases in these features of hippocampal plasticity would still be evident in aged male and female rats whose mothers received supplemental choline only during ED 12–17. We found that hippocampal cell proliferation was elevated in both male and female by about 20–25%, although numbers of proliferating cells were still quite low in our aged, prenatally choline supplemented rats. While this increase in new cells in the dentate gyrus is not large, other studies have reported that the effects of 30 min/day of exercise on a treadmill on hippocampal cell proliferation in Sprague Dawley male rats are quite pronounced in 2 month old rats, but decrease to only a 20–25 % increase over control by 14 months of age (Kim et al., 2004). Thus, we believe the effect of prenatal choline supplementation on hippocampal cell proliferation, is in the range of what is seen with acute exercise in rats. In this study, we did not distinguish between cell proliferation or survival and gave all rats 10 daily injections of BrdU to increase the number of labeled cells and permit a more accurate estimate of cell numbers using the optical fractionators and the principles on unbiased stereology. We also did not attempt to identify whether labeled cells were new neurons or glia. A high proportion of the cells that are generated in the subgranular zone and migrate to the granule cell layer commit to a neuronal phenotype and in 8-month old female rats we have demonstrated both increased hippocampal cell proliferation and neurogenesis in prenatally choline supplemented rats (Glenn et al., 2007). However, past research has shown that some experiences, like environmental enrichment, increase the numbers of newly divided cells that become neurons without affecting overall new cell numbers (Kempermann et al., 1998). Thus, while we think it likely that there were more new neurons underlying the effects reported, future work will need to examine whether prenatal choline supplementation increased only the baseline level of precursor cell proliferation, or actually led to a net increase in granule cell numbers.
We hypothesized that increases in basal cell proliferation might be due to changes in the production of hippocampal stem/progenitor cell proliferation factors as has been suggested by several other laboratories (Shetty et al., 2005; Kuhn et al., 1996; Hallbergson et al., 2003). Examination of a number of target growth factors revealed a significant increase in hippocampal VEGF concentration in 25 month old prenatally choline supplemented female rats compared to aged control females and a possible increase in NT-3, which did not reach significance. No differences in NGF, BDNF, or IGF-1 levels were found in females and we found no long-term changes in these growth factors in males due to prenatal choline availability. While these data suggest that preservation of several growth factors may be a contributing factor to our enhanced cell proliferation in aged prenatally choline supplemented rats, it is unlikely to be the only explanation. Others have suggested that age-related decline in hippocampal cell proliferation may be due to increased cell death in the hippocampal granule cell layer (Gould and Tanapat, 1997), or increasing levels of stress hormones and the neurotransmitter serotonin (Gould et al., 1998; Gould, 1999), or a decline in hippocampal vasculature (Palmer et al., 2000; Monje et al., 2002). An alternate hypothesis is that some other growth factor that we did not measure may be preserved in prenatally choline supplemented rat hippocampus. For example, Napoli and colleagues (Napoli et al, 2008) have shown an increase in IGF2 protein and mRNA levels in the hippocampus of young prenatally choline supplemented rats compared to control rats.
Nevertheless, our finding of increased VEGF protein in the hippocampus of 25-month-old female rats whose mothers were supplemented with choline prenatally is intriguing. VEGF is expressed in cerebral microvessels and the source of VEGF is likely from both endothelial cells and pericytes (Hoehn et al., 2002; Rosenstein and Krum, 2004), although it has been recently reported that VEGF is also expressed in quiescent progenitor cells (QNPs) and in astrocyes, with low but detectable expression in dentate granule cells (Segi-Nishida et al., 2008). VEGF stimulates adult neurogenesis and improves cognitive function in adult rats (Cao et al., 2004; Greenberg & Jin, 2004) but its expression declines greatly by 12 months of age in the rat and does not decline further in old age (Shetty et al., 2005). Several recent reports reveal that VEGF can act directly on neurons to produce neuroprotective effects. Examples include reduced hypoxic death of cultured cortical neurons (Jin et al., 2000b), and protection of cultured hippocampal neurons from glutamate neurotoxicity (Matsuzaki et al., 2001). Perhaps higher levels of VEGF in prenatal choline supplemented rats leads to a form of neuroprotection from aging.
3.4 Exploratory behavior, hippocampal plasticity, and cognitive function
One goal of the current study was to examine the potential behavioral and neural mechanisms that might underlie our findings of preserved cognitive function in old age in rats whose mothers had consumed a choline-supplemented diet during embryonic days 12–17 (Meck et al., 2008; Meck and Williams, 2003). We hypothesized prenatal choline supplementation might alter behaviors that contribute to risk or resilience in cognitive aging. We provide evidence that prenatally choline supplemented rats are not more active and do not appear to show alterations in stress reactivity, thus it is not likely that preservation of cognitive function occurs because rats have had more lifelong exercise or lower levels of plasma corticosterone, both factors that contribute to improved cognition in old age (see Cameron and McKay, 1999; Cotman and Berchtold, 2002). Our findings showing that prenatally choline supplemented female rats are more exploratory, or perhaps less fearful when they are young and prenatally choline supplemented males show preserved exploration when they are old, suggest that prenatal choline supplementation may alter the way that rats interact with their environment. This idea is supported by other findings from our laboratory. For example, Cheng and colleagues (Cheng et al., 2008) have recently reported that prenatally choline supplemented rats are better able to regulate behavioral inhibition when they are trained to withhold lever pressing for a minimum amount of time following the previous lever press, in order to earn reinforcement. Together these data suggest that future studies should examine the extent to which these beneficial effects of prenatal choline supplementation might generalize to other aspects of emotional behavior.
We also hypothesized that prenatal choline supplementation enables rats to maintain a “neural reserve” as they age, that might allow them to show preserved cognitive function. We provide some evidence that this may be true. Prenatally choline supplemented males and females showed increased hippocampal cell proliferation at 25 months of age, compared to control rats and in females this was accompanied by an increase in VEGF. Because some aspects of choline metabolism are sexually dimorphic and estrogen sensitive (Fisher et al. 2007), it is perhaps not surprising that we found sex differences in the emergence of choline’s effects in males and females. Together these data suggest that prenatal choline supplementation sets in motion a series of neural and behavioral changes that include increased exploration and increased hippocampal cell proliferation leading to preserved cognitive function in the aged brain.
4 Experimental Procedure
4.1. Experimental Overview
The present investigation employed a longitudinal design to examine rats’ anxiety and exploratory behaviors prior to the onset of puberty and again in old age as a function of prenatal control-treatment or choline-supplementation (see below). Behavioral measures (described in detail below) included reactions to and exploration of a novel open field test and novel objects and were collected initially on PDs 35 and 36 and again after rats were 24 months of age. Also at the first time point, the corticosterone (CORT) response to acute restraint stress was examined in all rats. The first time point was selected to permit behavioral and physiological measures of reactions to novelty and stress that reflected solely the organizational impact of hormones and diet independent of the activational effects of hormones that would occur after the onset of puberty. Female rats in our laboratory typically show vaginal opening around PD 35 but do not show their first estrous cycle until PD 41 or 42. Therefore we classified this period as prepubertal, and well before the onset of the first estrous cycle.
Two weeks after all behavioral measures were collected at the 24-month time point, rats were given 10 daily injections of the cell division marker, bromodeoxyuridine (BrdU; 100 mg/kg i.p.; Sigma) and were killed 24 hours after the last injection. Brains were rapidly extracted and one hemisphere from each was postfixed in 4% paraformaldehyde and retained for BrdU immunohistochemistry (see below). The hippocampus was dissected from the other hemisphere (counterbalanced across and between conditions), treated with x, and stored at −80°C until assayed for growth factor protein levels (see below).
4.2. Animals and Diet Manipulation
All rats in this study were housed in individually ventilated, polycarbonate cages (Allentown, Inc., Allentown, NJ). Colony conditions were monitored and recorded daily and kept at 21°C and between 30–40% humidity. Rats were kept on a 12:12 light/dark cycle with lights on at 08:00. Water was always freely available through an automatic feature of the caging system. All experimental procedures adhered to and were approved by the Duke University Institutional Care and Use Committee.
Timed pregnant Sprague Dawley rats (n=20; CD strain; Charles Rivers Breeders, Kingston NY) arrived in the colony on embryonic day (ED) 9. Dams were given ad lib access to a synthetic diet (AIN76A; Dyets, Inc., Bethlehem, PA) that served as the control diet throughout the study and contained 1.1 g/kg choline chloride substituted for choline bitartrate. On the evening of ED10 the food a subset of dams (n=10) was switched to a choline supplemented diet (AIN76A; 5 g/kg choline chloride). The supplemented diet was available ad lib to these dams through the morning of ED18, at which time they were returned to the control diet. On the day after birth, postnatal day (PD) 1, litters were removed from control and supplemented dams, marked for identification of diet condition, and mixed litters of 10 pups (male; female; control; and supplemented) were returned to foster, control-fed mothers. Litters were weaned on PD24 into same-sex pairs. Male (n=8) and female (n=8) rats from control-fed mothers (CON) and male (n=8) and female (n=8) from supplemented mothers (SUP) were selected to serve as subjects in this study. Of these rats, one female CON, one female SUP, two male CON, and one male SUP died before behavioral data could be collected from the aged time point. The behavioral data from these animals collected at the first time point was excluded from the overall analyses. In addition, one female SUP and one male CON rat developed pituitary tumors (confirmed at death) before neural measures could be collected. Their behavioral data were kept in the analysis but there are no neural data reported for these rats.
4.3. Behavioral Measures
All behavioral tests were conducted in the same way and under similar conditions at the two time points. Rats were experimentally naïve prior to the first set of tests but served as subjects in another study from approximately for about 9 months beginning at 120 days of age in which they were food restricted to about 90% of their free-feeding weight, and trained daily for about 30 min in an operant task. All rats were treated identically during this period. At 18 months of age rats were returned to free feeding and were not disturbed except for regular cage cleaning until behavioral training at 24 months of age.
4.3.1. Open field exploration
The open field was a 100 × 100 cm arena constructed of opaque Plexiglas with 40 cm high walls and a clear plexiglass lid. It was located in a large, dimly lit room. Rats were placed in the field facing a corner, one at a time, for 5 min. The rat was recorded by an overhead ceiling-mounted digital camera attached to a PC compatible laptop computer.
The field was divided into 16 equal-sized squares for analysis. Behavioral measures that were extracted from the digital video recordings included: percentage of squares entered, number of crossings into the 12 squares that made up the perimeter, latency to enter one of the 4 squares that made up the center area, number of crossings into the 4 center squares, total time spent in the center area, and the average duration of visits to the center area.
4.3.2. Novel object exploration
On the day following the open field test, each rat was returned to the same arena for another 5-minute assessment. For this test, four novel objects were located approximately 15 cm diagonally from each corner. This placement was designed to allow us to examine the inclination of rats to explore the objects from the perimeter versus the center of the field.
The objects were distinct from each other: they ranged in size from approximately 10–15 cm high and wide, were constructed of ceramic, metal, and plastic, and were differently reflective along the spectrum of light presumed detectable by albino rats. They were selected from a pool of objects pre-tested for a moderate level of interest by rats; that is, not avoided or excessively investigated. They were also prescreened and carefully selected to avoid materials that elicit chewing behaviors or excessive oral palpitation. These criteria served to ensure that we would not have floor or ceiling effects in object exploration and that we could adequately and sufficiently distinguish between exploratory behaviors independent of oral manipulation of the objects. In this way, we could more accurately judge interest in exploring objects by rats and search for differences amongst our experimental conditions.
As with the first day in the open field, each rat was digitally recorded. Behavioral measures of object exploration included: latency to explore an object; latency to explore each of the four objects; total time spent exploring objects, number of visits to an object, and the average duration of object visits. We also distinguished between whether rats were exploring objects from the perimeter or center of the field and extracted total time for each, as well as number and duration of visits from the center. The same open field exploration measures described above were extracted for this test as well.
4.4. CORT response to acute stress in young rats
On PD 37 and 38 male and female rats, respectively, underwent 30 min of restraint stress and the response of the hypothalamic-pituitary-adrenal axis was assessed by measuring plasma CORT levels from blood samples taken periodically through the test. The test was conducted between 10:00 and 14:00 on each day. Rats were brought into a quiet, dimly lit one at a time and placed in a clear Plexiglas immobilization tube that was specially constructed to easily accommodate pre–pubescent male and female rats but was designed to ensure that rats could not turn along either the vertical or horizontal axes. Approximately 200 µl of blood was collected from the tail at each of four collection times. The first blood sample was taken within two minutes of placing rats in the restrainer and reflected basal levels of CORT. The second sample was taken just prior to the end of the 30 minutes of restraint, before removing the rat, and allowed us to examine the change in CORT from basal levels in response to the stressor. For the next two hours, rats were placed in individual holding cages in a separate room. Two more samples were taken, 60 and 120 minutes after the onset of the stressor (30 and 90 min after the end of the stressor) and reflected the return of CORT to basal levels.
Blood samples were stored on ice until centrifuged and supernatant was collected and stored at −80°C until assayed. Levels of CORT in samples were assessed using radioimmunoassay (RIA rat kit for CORT; Diagnostic Products) and are shown in pg/ml.
4.5 Neural measures in old rats
4.5.1. BrdU immunohistochemistry
Approximately two weeks after the collection of behavioral measures, aged rats were started on a 10-day regime of daily BrdU injections (Sigma; 100 mg/kg, i.p.; based on research by Kempermann et al., 1997). Twenty-four hours after the last injection rats were anesthesized with a ketamine/xylazine cocktail (80 and 10 mg/kg, respectively, i.p.) and decapitated. Brains were rapidly extracted and the hippocampus dissected out of one hemisphere for Elisa. The other half of each brain was immersion-fixed in 4% paraformaldehyde for 4 days and stored in 0.1% sodium azide thereafter.
Fixed half brains were sectioned on a vibratome. Every 5th 60–µm section was retained for BrdU immunohistochemistry. Procedures for BrdU staining were based on Kuhn et al. (1996) and are described in Glenn et al. (2007). In brief, free-floating sections was rinsed with Tris-buffered saline (TBS, pH 7.3) followed by 30 min in 0.6% hydrogen peroxide in TBS at room temperature to reduce nonspecific staining. After rinsing again in TBS, tissue was treated for 2 hr in 50% formamide and 2 × SSC (0.3 m NaCl, 0.03 m sodium citrate) at 65 °C, rinsed in 2 × SSC for 10 min, incubated in 2 m HCl for 30 min at 37 °C, and rinsed in 0.1 m boric acid (pH 8.5) for 15 min. Sections were rinsed in TBS, incubated in 0.1% Triton X-100 (TTX; Sigma) and 3% normal horse serum (Vector Laboratories, Burlingame, CA) in TBS for 30 min at room temperature, and then incubated with the primary antibody (monoclonal mouse anti-BrdU, 1 : 400; Boehringer Mannheim, Indianapolis, IN) for 48 hr at 4 °C. Following this, the tissue was rinsed with TBS and incubated with the secondary antibody (biotinylated horse antimouse, 1 : 200; Vector Laboratories) for 2 hr at room temperature. The tissue was then rinsed in TBS, incubated in an avidin-biotin complex (ABC, Vector Laboratories) for 1 hr at room temperature, rinsed again in TBS, and treated for peroxidase detection with diaminobenzidine (Vector Laboratories, nickel intensified) for 10 min. Stained sections were mounted on gelatin–coated slides, dehydrated, and coverslipped.
4.5.2. Unbiased stereology to quantify numbers of BrdU–labeled cells
The numbers of newly proliferated cells labeled with the BrdU marker were estimated in the dentate gyrus using modified procedures based on the principles of unbiased stereology and the optical fractionator (Mouton, 2002; West, 1993). The modifications were necessary to overcome the low level of BrdU labeling that is characteristic of very old brains (Kempermann, Kuhn, and Gage, 1998). Unbiased practices were maintained by counting cells that were only evident in an inner 20 um block of tissue and stained cells that were only in focus in the top and bottom 2 µm were excluded from counts. Our sampling procedure also ensured that every cell in each section examined was counted without possibility of being recounted. Using an optical fractionator, all labeled cells in 6–10 sections through the rostral-caudal extent of the dentate gyrus were counted and the value multiplied by 5, to account for every 5th section being analyzed, and 2 to account for the hemisphere that was not examined.
4.5.3. Elisa for growth factor protein content
Whole tissue extracts of hippocampus were prepared by adding lysis buffer (50 mm Tris pH 7.5, 150 mm NaCl, 1% Nonidet NP-0, 10% glycerol, 2 mm 4-(2-aminoethyl)-benzenesulphonyl fluoride, 1 µg/mL leupeptin, 2 µg/mL aprotinin, 2 µg/mL pepstatin) to tissue, followed by gentle sonication, incubation on ice for 15 min, and a brief centrifugation to clear. The ChemiKine™sandwich ELISA kits (Chemicon Int, Inc.) were used to assay the levels of the following growth factors in hippocampal lysates: brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and vascular-epithelial growth factor (VEGF), neurotrophin-3 (NT-3), and insulin-like growth factor-1 (IGF-1). Lysates were diluted 1 : 5 with sample or standard diluent (Chemicon). Protein levels were measured according to manufacturer's instructions and pg/mL values were obtained as raw data.
4.6. Statistical Analyses
The mean and standard error (SEM) for all dependent variables were calculated for each group of female, male, CON, and SUP rats. These are displayed in figures for all behavioral data and CORT data. The neural measures of growth factor protein levels and numbers of BrdU+ cells were transformed to percent control values; the female CON mean was used to calculate percent control values for all females and the male CON mean was used to calculate percent control values for all males. Mixed analyses of variance (ANOVA) were conducted using the between factors of Sex (male and female) and Diet (CON and SUP) and the repeated measure of Age (1 and 24 months of age) or Time (0, 30, 60, and 120 min from stressor onset). Between ANOVAs were conducted using the Sex and Diet factors. Due to the small sample size in the study (n=5–8) we planned a priori to examine males and females separately (independent or dependent samples student t-tests) or post hoc Tukey tests.
Acknowledgements
The authors would like to thank Cynthia Kuhn for her help with the corticosterone measures and radioimmunoassay. This research was supported by National Institute on Aging grants F32 AG025052 to MJG and P01 AG009525 to CLW and JKB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altum M, Bergman E, Edstrom E, Johnson H, Ulfhake B. Behavioral impairments of the aging rat. Physiol. Behav. 2007;92:911–923. doi: 10.1016/j.physbeh.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Ballini C, Colivicchi MA, Della Corte L, Giovannini MG, Pepeu G. Investigation on acetylcholine, aspartate, glutamate and GABA extracellular levels from ventral hippocampus during repeated exploratory activity in the rat. Neurochem. Res. 2003;28:565–573. doi: 10.1023/a:1022881625378. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Granholm A-CE, Nelson ME, Moore AB. Patterns of neurotrophin protein levels in male and female Fischer 344 rats from adulthood to senescence: How young is “young” and how old is “old”? Expt. Aging Res. 2008;34:13–26. doi: 10.1080/03610730701761908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA, Lippman HR, Chen JJ. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol. Behav. 1975;14:601–608. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RDG. Restoring production of hippocampal neurons in old age. Nature Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cao L, XJiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genetics. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary adrenal regulation in the female rat. J. Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Cermak JM, Holler T, Jackson DA, Blusztajn JK. Prenatal availability of choline modifies development of the hippocampal cholinergic system. FASEB J. 1998;12:349–357. doi: 10.1096/fasebj.12.3.349. [DOI] [PubMed] [Google Scholar]
- Cheng R-K, MacDonald CJ, Wiliams CL, Meck WH. Prenatal choline supplementation alters the timing, emotion, and memory performance (TEMP) of adult male and female rats as indexed by differential reinforcement of low-rate schedule behavior. Learn. Mem. 2008;15:153–162. doi: 10.1101/lm.729408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex differences in resting pituitary-adrenal function in the rat. Am. J. Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Delacour J. A central activation role for the hippocampus: a viewpoint. Neurosci. Res. Commun. 1995;16:1–10. [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LM, daCosta KA, Kwock L, Stewart PW, Lu TS, Stabler SP, Allen RH, Zeisel SH. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am. J. Clin. Nutr. 2007;85:1275–1285. doi: 10.1093/ajcn/85.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frielingsdorf H, Simpson DR, Thal LJ, Pizzo DP. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol. Dis. 2007;26:47–55. doi: 10.1016/j.nbd.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neurosci. 2001;106:43–53. doi: 10.1016/s0306-4522(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur. J. Neurosci. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;11:S46–S51. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neurosci. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Gray A. The neuropsychology of anxiety. Oxford: Oxford University Press; 1982. [Google Scholar]
- Greenberg DA, Jin K. Experiencing VEGF. Nature Genet. 2004;36:792–793. doi: 10.1038/ng0804-792. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Milner TA, Leranth C. Sex steroids and the dentate gyrus. Prog. Brain Res. 2007;163:399–415. doi: 10.1016/S0079-6123(07)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbergson AF, Gnatenco C, Peterson DA. Neurogenesis and brain injury: managing a renewable resource for repair. J. Clin. Invest. 2003;112:1128–1133. doi: 10.1172/JCI20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp. Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav. Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Hoehn BD, Harik SI, Hudetz AG. VEGF mRNA expressed in microvessels of neonatal and adult rat cerebral cortex. Mol. Brain Res. 2002;101:103–108. doi: 10.1016/s0169-328x(02)00175-4. [DOI] [PubMed] [Google Scholar]
- Hohmann CF, Berger-Sweeney J. Cholinergic regulation of cortical development and plasticity. New twists to an old story. Perspect. Dev. Neurobiol. 1998;5:401–425. [PubMed] [Google Scholar]
- Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor rescues HN33 neural cells from death induced by serum withdrawal. J. Mol. Neurosci. 2000;14:197–203. doi: 10.1385/JMN:14:3:197. [DOI] [PubMed] [Google Scholar]
- Jones JP, Meck WH, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Dev. Brain Res. 1999;118:159–167. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Semba R, Takeuchi IK, Kato K. Age-related changes in levels of brain-derived neurotrophic factor in selected brain regions of rats, normal mice and senescence-accelerated mice: a comparison to those of nerve growth factor and neurotrophin-3. Neurosci. Res. 1998;31:227–234. doi: 10.1016/s0168-0102(98)00040-6. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kim Y-P, Kim H, Shin M-S, Chang H-K, jang M-H, Shin M-C, Lee H-H, Yoon J-H, Jeong I-G, Kim C-J. Age-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Neurosci. Let. 2004;355:152–154. doi: 10.1016/j.neulet.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Kitay JI, Coyne MD, Swygert NH, Gaines KE. Effects of gonadal hormones and ACTH on the nature and rates of secretion of adrenalcortical steroids by the rat. Endocrinology. 1971;89:565–570. doi: 10.1210/endo-89-2-565. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-Iameliorates the age-related decline in hippocampal neurogenesis. Neurosci. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lores-Arnaiz S, Bustamante J, Arismendi M, Vilas S, Paglia N, Basso N, Capani F, Coirini H, Costa JJ, Arnaiz MR. Extensive enriched environments protect old rats from the aging dependent impairment of spatial cognition, synaptic plasticity and nitric oxide production. Behav. Brain Res. 2006;169:294–302. doi: 10.1016/j.bbr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Longitudinal increases in cortisol during human aging predicts hippocampal atrophy and memory deficits. Nat. Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J. 2001;15:1218–1220. [PubMed] [Google Scholar]
- McEwen BS. Stress and the aging hippocampus. Front. Neuroendocrinol. 1999;20:49–70. doi: 10.1006/frne.1998.0173. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Pre-and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev. Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav. Neurosci. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. NeuroReport. 1997;8:3045–3051. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front. Integrative Neurosci. 2008 doi: 10.3389/neuro.07.007.2007. 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott TJ, Kowall NW, Lopez-Coviella I, Blusztajn JK. Prenatal choline deficiency increases choline transporter expression in the septum and hippocampus during postnatal development and in adulthood in rats. Brain Res. 2007;1151:1–11. doi: 10.1016/j.brainres.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and practices of unbiased stereology. Baltimore, MD: The John Hopkins University Press; 2002. [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol. Aging. 2003;24:273–284. doi: 10.1016/s0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Napoli I, Blusztajn JK, Mellott TJ. Prenatal choline availability increases the expression of IGF2 and its receptor in the hippocampus and frontal cortex of rats. Brain Res. 2008 doi: 10.1016/j.brainres.2008.08.046. (2008, this issue). [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Paris JM, Lorens SA, Van de Kar LD, Urban JH, Richardson-Morton KD, Bethea CL. A comparison of acute stress paradigms: hormonal responses and hypothalamic serotonin. Physiol. Behav. 1987;39:33–43. doi: 10.1016/0031-9384(87)90341-6. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Williams CL, Meck WH, Swartzwelder HS. Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J. Neurophysiol. 1998;79:1790–1796. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur. J. Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Renner MJ, Bennett AJ, White JC. Age and sex as factors influencing spontaneous exploration and object investigation by preadult rats (Rattus norvegicus) J. Comp. Psych. 1992a;106:217–227. doi: 10.1037/0735-7036.106.3.217. [DOI] [PubMed] [Google Scholar]
- Renner MJ, Dodson DL, Leduc PA. Scopolamine suppresses both locomotion and object contact in a free-exploration situation. Pharmacol. Biochem. Behav. 1992b;41:625–636. doi: 10.1016/0091-3057(92)90384-r. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Krum JM. New roles for VEGF in nervous tissue- beyond blood vessels. Exp. Neurol. 2004;187:246–253. doi: 10.1016/j.expneurol.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Loy R, Williams CL. Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Res. 2002;947:9–16. doi: 10.1016/s0006-8993(02)02900-1. [DOI] [PubMed] [Google Scholar]
- Schenk F, Brandner C. Indirect effects of peri- and postnatal choline treatment on place-learning abilities in rat. Psychobiol. 1995;23:302–313. [Google Scholar]
- Segi-Nishida E, Warner-Schmidt JL, Duman RS. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc. Nat. Acad. Sci. U.S.A. 2008;105:11352–11357. doi: 10.1073/pnas.0710858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R, Lu BN. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn. Mem. 2006;13:307–315. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tees RC, Mohammadi E. The effects of neonatal choline dietary supplementation on adult spatial and configural learning and memory in rats. Dev. Psychobiol. 1999;35:226–240. [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol. Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH, Heyer D, Loy R. Choline-induced development of visuospatial memory in male and female rats: an analysis of nerve growth factor-sensitive basal forebrain neurons. Brain Res. 1998;794:225–238. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Mellot TJ, Glenn MJ, Blusztajn JK, Williams CL. Prenatal choline supplementation attenuates neuropathological response to status epilepticus in the adult rat hippocampus. Neurobiol. Dis. 2008a;30:255–269. doi: 10.1016/j.nbd.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Glenn MJ, Mellot TJ, Blusztajn JK, Meck WH, Williams CL. Spatial memory and hippocampal plasticity are differentially sensitive to the availability of choline in adulthood as a function of choline supply in utero. Brain Res. 2008b doi: 10.1016/j.brainres.2008.08.074. (2008, this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Nutritional importance of choline for brain development. J. Am.Coll. Nutr. 2004;23:621S–626S. doi: 10.1080/07315724.2004.10719433. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. The fetal origins of memory: the role of dietary choline in optimal brain development. J. Pediatr. 2006;149:S131–S136. doi: 10.1016/j.jpeds.2006.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]