Abstract
Background:
In dialysis patients, activated vitamin D therapy is associated with reduced mortality. Observational data suggests that low 25-hydroxyl vitamin D levels are associated with diabetes mellitus, hypertension and cancers. However, whether low serum 25-hydroxyl vitamin D (25(OH)D) levels are associated with mortality in the general population is unknown.
Methods:
We tested the association of low 25(OH)D levels with all-cause, cancer and cardiovascular disease (CVD) mortality in 13,331 nationally representative adults ≥20 years old from the Third National Health and Examination Survey (NHANES III) Linked Mortality Files. Participant vitamin D levels were collected in 1988-1994 and individuals were passively followed for mortality through 2000.
Results:
In cross-sectional multivariable analyses, increasing age, female sex, non-white race/ethnicity, diabetes, current smoking, and higher BMI were all independently associated with higher odds of 25(OH)D deficiency (lowest quartile of 25(OH)D <17.8 ng/ml), while greater physical activity, vitamin D supplementation and non-winter season were inversely associated. During a median 8.7 years of follow-up, there were 1806 deaths including 777 from CVD. In multivariable models (adjusted for baseline demographics, season, traditional and novel CVD risk factors), compared to the highest quartile, 25(OH)D levels <17.8 ng/ml were associated with a 26% increased rate of all-cause mortality (1.26, 95% CI 1.08-1.46) and a population attributable risk of 3.1%. The adjusted models of CVD and cancer mortality revealed a higher risk which was not statistically significant.
Conclusions:
The lowest quartile of 25(OH)D (<17.8 ng/ml) is independently associated with all-cause mortality in the general population.
Introduction
Several studies have suggested that 25(OH)D deficiency is an unrecognized contributor to the development of cardiovascular disease (CVD), cancer and mortality. 1,25-dihydroxyvitamin D affects the renin-angiotensin system,1 cardiac myocyte hypertrophy2 and has anti-inflammatory effects,3 all of which may influence CVD risk.4 In addition, 1,25-dihydroxyvitamin D has anti-proliferative activity, which may influence cancer risk5. Activated vitamin D therapy is associated with lower mortality in those with end-stage renal disease (ESRD)6-10. In addition, low 25(OH)D levels have been associated with multiple CVD risk factors in the NHANES III population11 as well as separately with hypertension12, congestive heart failure13, cancer14 and diabetes mellitus15, 16. Low 25(OH)D levels in incident hemodialysis patients have recently been shown to be associated with all-cause mortality17. A meta-analysis of 18 randomized clinical trials of vitamin D supplementation in mostly older individuals found that randomization to vitamin D was associated with lower all-cause mortality18. Despite these suggested associations, we found no published studies evaluating the relationship between 25(OH)D levels and mortality risk in the general population.
The optimal level of 25(OH)D has been suggested to be ≥30 ng/ml (75 nmol/L)19, 20, a level associated with maximal suppression of parathyroid hormone and reduced fracture rates and postulated to be associated with better health outcomes. Approximately 41% of men and 53% of women in the United States, however, have levels of 25(OH)D below 28 ng/ml (70 nmol/L)21.
We hypothesized that low serum 25(OH)D is a risk factor for cancer, CVD and all-cause mortality among adults in the United States. The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality dataset provides an excellent opportunity to test the association of 25(OH)D deficiency with mortality in a large, multi-racial sample of the general population.
Methods
Study Participants
NHANES III is a nationwide probability sample of non-institutionalized civilian persons conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention. Details regarding informed consent and statistical methods are outlined elsewhere22. We restricted our analyses to adults ≥20 years of age who had a physical examination and laboratory testing at baseline (October 1988-October 1994) and for whom vital status information was known at follow-up (through December 31, 2000). Non-Hispanic blacks, Mexican-Americans and the elderly were over-sampled in NHANES III to allow for more precise estimates in these groups23. In order to ensure comparable conditions at each NHANES survey site, northern states were surveyed during the summer and southern states were surveyed during the winter. Participants were excluded if they did not have complete data on the study variables of interest. The remaining 13,331 participants represent approximately 175 million people in the United States. The institutional review boards at the Johns Hopkins Bloomberg School of Public Health and the Albert Einstein College of Medicine determined this analysis to be exempt.
Study Variables
Interview questions, physical examination and laboratory values, such as C-reactive protein (CRP), glucose, albumin, creatinine, and lipids levels, were assessed in all study participants at baseline during 1988-1994 and processed per standard protocol22. Laboratory draws occurred during either morning, afternoon or evening sessions. Participants were asked to fast 12 hours before the morning examination or 6 hours for the afternoon or evening examination. In order to minimize hemolysis of blood samples, which will affect serum calcium but not serum 25(OH)D levels, all phlebotomists were certified and completed training in standardized laboratory procedures annually. Serum 25(OH)D was measured using the Diasorin radioimmunoassay (RIA) kit (Diasorin, Stillwater, MN) on frozen serum (<−20°C) between February 1994 and December 1995 (Total CV from quality control samples 13-19%). The RIA kit was calibrated using HPLC-purified 25(OH)D every 6 months. Race/ethnicity was self-identified. Hypertension was defined as systolic blood pressure (BP) ≥140 mm Hg, diastolic BP ≥90 mm Hg, and/or use of antihypertensive medications. A participant was considered to have diabetes mellitus if he or she reported ever being told by a doctor that he or she had diabetes or “sugar diabetes” at a time other than during pregnancy, was taking insulin or a “diabetes pill” at the time of the questionnaire, or had a fasting blood glucose >126 mg/dL or a non-fasting blood glucose >200 mg/dL. Smoking was classified as never, current or former smoker. Low socioeconomic status (SES) was defined as ≤200% of the poverty index. The use of cholesterol medication was based on the response to the question, “To lower your blood cholesterol, are you now following this advice to take prescribed medicine?”
Creatinine levels were calibrated to the Cleveland Clinic laboratory by subtracting 0.23 mg/dl from NHANES III values, and estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine using the abbreviated Modification of Diet in Renal Disease (MDRD) formula24, 25. We assigned an eGFR value of 200 for all individuals with an eGFR >200 ml/min per 1.73m2 (n=94) because these were thought to be biologically implausible. The main analyses were restricted to those participants with an eGFR >15 ml/min/1.73m2. Urinary albumin-creatinine ratio (ACR) was based on a spot urine sample. Both ACR and CRP were log-transformed to achieve approximate normality for statistical analyses.
Physical activity level was based on the participant‘s metabolic expenditures in the past month and categorized as low physical activity intensity (≤3.5), moderate (3.6-14.9) or high intensity (≥15)26. No information was available about whether the physical activities were performed indoors or outdoors. Vitamin D supplementation use was coded as positive for patients who reported taking a supplement or multivitamin containing >0 IU of vitamin D. Those taking repletion doses of vitamin D (50,000 IU) were excluded from the analysis (n=3).
Linkage and Causes of Death
Mortality outcomes were collected on NHANES III participants through December 31, 2000 using probabilistic matching, with up to 12 identifying data elements, to National Death Index (NDI) records and were made available in June 2005. A selected sample of death certificates was reviewed manually to validate the process. The underlying cause of death was coded according to the 9th revision of the International Statistical Classification of Diseases, Injuries and Causes of Death (ICD-9) for deaths occurring between 1988 and 1998, and according to the 10th revision (ICD-10) for deaths occurring in 1999 and 2000. All deaths from 1988 through 1998 coded under ICD-9 guidelines were recoded into comparable groups based on the ICD-10 underlying cause of death27, 28. For this analysis, CVD mortality included deaths coded as due to hypertensive disease (I10-I13), ischemic heart disease (I20-I25), arrhythmia (I44-I49), heart failure (I50), cerebrovascular disease (I60-I69) or atherosclerosis or other diseases of the arteries (I70-I78). Cancer mortality included deaths coded as all malignant neoplasm deaths (C00-C95) including malignant neoplasms of digestive organs (C15-C26), respiratory and intrathoracic organs (C30-39), and genital organs (C50-63). A sub-analysis of cancers previously linked to low 25(OH)D levels included colorectal cancer (C18-21), breast cancer (C50) and prostate cancer (C61). Infectious deaths included deaths from infectious and parasitic diseases (A00-B99), meningitis (G00, G03), acute rheumatic fever (I00-I02), endocarditis (I33), acute upper and lower respiratory infections including influenza and pneumonia (J00-J06, J10-J22) and urinary tract infections (N39). External causes of mortality included deaths from accidents, falls, suicide, homicide, and complications of medical and surgical care (V01-Y89).
Statistical analyses
Because of the complex sampling design of NHANES III, weighted analyses using the survey command in Stata 9.0 (Stata Corp., College Station, TX) were used. The distributions of participant characteristics were first examined by level of serum 25(OH)D unweighted quartiles (see Table 1 for quartile cut-offs). Multivariable logistic regression analyses were used to determine independent predictors of 25(OH)D deficiency defined as being in the lowest quartile (25(OH)D levels <17.8 ng/ml). A sensitivity analysis was performed using multivariable linear regression with log-transformed 25(OH)D levels as the dependent variable. Power calculations using PS power and sample size program29 revealed a detectable mortality rate ratio (MRR) of greater than 1.17 or less than 0.84 for all-cause mortality (1.25 or 0.78 for CVD mortality) assuming 80% power and an α of 0.05.
Table 1.
Baseline characteristics of 13,331 participants of NHANES III by 25(OH)D quartiles. Results presented as means or percents ± standard error.
| 25-hydroxyvitamin D levels (ng/ml)* | |||||
|---|---|---|---|---|---|
| >32.1 | 24.4-32.1 | 17.8-24.4 | <17.8 | P-value | |
| Characteristic | (n=3359) | (n=3242) | (n=3344) | (n=3386) | |
| Age (years) | 41.9 ± 0.5 | 45.2 ± 0.6 | 46.7 ± 0.6 | 45.5 ± 0.6 | <0.001 |
| Women (%) | 45.0 ± 1.2 | 50.5 ± 1.0 | 56.1 ± 1.4 | 66.3 ± 1.4 | <0.001 |
| Race (%) | |||||
| Non-Hispanic white | 91.4 ± 0.9 | 82.6 ± 1.2 | 69.3 ± 1.8 | 48.1 ± 2.5 | <0.001 |
| Non-Hispanic black | 2.1 ± 0.3 | 5.4 ± 0.5 | 13.3 ± 0.9 | 32.8 ± 1.9 | <0.001 |
| Mexican-American | 2.6 ± 0.3 | 4.8 ± 0.5 | 6.7 ± 0.6 | 7.2 ± 0.7 | <0.001 |
| Other | 3.9 ± 0.9 | 7.1 ± 0.9 | 10.7 ± 1.3 | 11.8 ± 1.3 | <0.001 |
| Diabetic (%) | 3.8 ± 0.5 | 6.6 ± 0.5 | 8.2 ± 0.8 | 10.9 ± 0.8 | <0.001 |
| History of CVD (%) | 6.0 ± 0.5 | 7.9 ± 0.8 | 9.5 ± 0.9 | 8.2 ± 0.9 | 0.002 |
| Hypertension (%) | 19.3 ± 1.1 | 23.8 ± 1.2 | 27.8 ± 1.2 | 28.9 ± 1.3 | <0.001 |
| BMI (kg/m2) | 25.3 ± 0.1 | 26.4 ± 0.2 | 27.6 ± 0.2 | 28.1 ± 0.2 | <0.001 |
| Total cholesterol (mg/dl) | 202.2 ± 1.0 | 202.8 ± 1.3 | 207.5 ± 1.3 | 203.5 ± 1.2 | 0.04 |
| HDL cholesterol (mg/dl) | 51.2 ± 0.5 | 49.8 ± 0.5 | 50.4 ± 0.5 | 51.8 ± 0.4 | 0.73 |
| Cholesterol medication use (%) |
2.4 ± 0.3 | 2.7 ± 0.4 | 3.3 ± 0.4 | 3.7 ± 0.7 | 0.02 |
| Systolic BP (mm Hg) | 120.1 ± 0.6 | 122.3 ± 0.5 | 124.0 ± 0.5 | 124.4 ± 0.6 | <0.001 |
| Diastolic BP (mm Hg) | 73.6 ± 0.2 | 74.2 ± 0.3 | 74.7 ± 0.3 | 75.0 ± 0.3 | <0.001 |
| Smoking (%) | |||||
| Former | 28.3 ± 1.2 | 26.8 ± 1.1 | 25.3 ± 1.2 | 21.5 ± 1.3 | 0.001 |
| Current | 29.0 ± 1.4 | 26.0 ± 1.4 | 26.9 ± 1.3 | 32.6 ± 1.3 | 0.26 |
| Season (%) | |||||
| Winter (Jan.-March) | 10.6 ± 2.1 | 15.3 ± 2.3 | 20.6 ± 3.6 | 27.3 ± 4.7 | <0.001 |
| Spring (April-June) | 22.0 ± 4.1 | 28.8 ± 4.6 | 28.8 ± 4.3 | 26.3 ± 4.3 | 0.04 |
| Summer (July-Sept.) | 43.4 ± 5.5 | 33.1 ± 5.6 | 26.0 ± 4.2 | 19.2 ± 3.5 | <0.001 |
| Fall (Oct.-Dec.) | 24.0 ± 4.7 | 22.8 ± 4.5 | 24.7 ± 4.0 | 27.2 ± 4.1 | 0.34 |
| eGFR <60 ml/min/1.73m2 (%) |
3.3 ± 0.4 | 4.1 ± 0.4 | 4.8 ± 0.5 | 4.6 ± 0.6 | <0.001 |
| Albumin excretion ≥30 mg/g creatinine (%) |
6.1 ± 0.4 | 7.4 ± 0.7 | 9.9 ± 0.6 | 12.6 ± 1.1 | <0.001 |
| C-reactive protein >0.21 mg/dl (%) |
24.0 ± 1.5 | 26.6 ± 1.6 | 30.7 ± 1.6 | 37.0 ± 1.7 | <0.001 |
| Serum albumin (g/dl) | 4.23 ± 0.02 | 4.21 ± 0.03 | 4.14 ± 0.02 | 4.08 ± 0.02 | <0.001 |
| Low SES (%) | 29.8 ± 1.5 | 29.4 ± 1.4 | 35.7 ± 2.0 | 41.9 ± 1.6 | <0.001 |
| Vitamin D use (%) | 35.2 ± 1.3 | 30.1 ± 1.2 | 25.2 ± 1.2 | 16.3 ± 1.3 | <0.001 |
| Physical Activity | |||||
| Moderate (%) | 55.6 ± 1.3 | 56.4 ± 1.1 | 53.7 ± 2.0 | 48.1 ± 1.4 | 0.002 |
| High (%) | 28.7 ± 1.5 | 20.9 ± 1.0 | 16.6 ± 1.1 | 11.7 ± 1.3 | <0.001 |
To convert to SI units, multiply 25(OH)D value by 2.496.
Poisson regression models were used to examine the independent associations between 25(OH)D levels and cancer, CVD, other causes and all-cause mortality. For the analyses of cause-specific mortality, participants were censored at the time of death from other causes. We explored the continuous, potentially nonlinear, relationship of mortality risk associated with 25(OH)D levels using fully adjusted restricted cubic spline models.
Because serum 25(OH)D levels vary by season30 all multivariable analyses were adjusted for season of examination. Inclusion in the final model was based on the variable of interest being associated with both 25(OH)D levels and mortality (p<0.20) and on a priori determination of confounders of the association between 25(OH)D levels and mortality. Covariates included in the final model were age, sex, race, season, hypertension, history of prior CVD, diabetes mellitus, smoking, BMI, HDL cholesterol, total cholesterol, the use of cholesterol-lowering medications, eGFR categories, serum albumin, log urinary albumin-creatinine ratio, log CRP, physical activity level, vitamin D supplementation and low SES. No pairs of covariates with correlations greater than or equal to 0.7 were included in the models to avoid issues of collinearity. Interactions were tested by adding a product term for 25(OH)D quartile and each of the following covariates: age, sex, race, history of CVD, diabetes status, hypertension status, obesity status, physical activity level, smoking and baseline eGFR <60 ml/min/1.73m2. Because non-Hispanic blacks have lower serum 25(OH)D levels compared to non-Hispanic whites, the highest quartile of 25(OH)D, the comparison group, only had 371 non-Hispanic blacks, compared to 2,164 in the lowest quartile. Because this small comparison group may spuriously influence results, we elected to analyze non-Hispanic black specific quartiles for the sub-group analysis. The cut-off values are: Quartile 1: >24 ng/ml, Quartile 2: ≤24 -18 ng/ml, Quartile 3: ≤18 – 13.5 ng/ml, and Quartile 4: ≤13.5 ng/ml.
In addition, to test the robustness of the association we performed sensitivity analyses adjusting for serum calcium and phosphate levels which are affected by vitamin D status and are associated with mortality in patients with ESRD31 and in the general population32. Because diabetes mellitus and/or hypertension may be in the causal pathway between low 25(OH)D levels and mortality, we developed models with and without diabetes mellitus and hypertension. Because of potential non-linear associations between continuous variables such as age and BMI and mortality, we also performed a sensitivity analysis where all variables were added to the model as categorical variables (quartiles of the continuous variables). We also further tested the non-linear association found in the spline by using the following cut-offs for 25(OH)D levels: <20, 20-29, 30-39, 40-49 and >50 ng/ml with the 30-39 as the reference group. We calculated the attributable risk percent by dividing the elevation from baseline rate (1.0) by the MRR for the lowest quartile of 25(OH)D. We calculated the population attributable risk percent by multiplying the attributable risk percent by the percent of the population who were in the lowest 25(OH)D quartile. For all analyses, a p-value <0.05 for 2-tailed tests were considered statistically significant. P-values were not adjusted for multiple comparisons.
Results
Factors associated with low 25(OH)D levels
Older participants, women and non-Hispanic blacks had lower 25(OH)D levels (Tables 1 and 2). Mean systolic and diastolic BP, mean BMI, and percentage of diabetics, those with elevated ACR and elevated CRP increased and serum albumin decreased across decreasing quartiles of 25(OH)D (highest quartile to lowest). There were more participants with low SES and fewer participants taking vitamin D supplementation and participating in high and moderate levels of physical activity in the lowest quartile of 25(OH)D (<17.8 ng/ml).
Table 2.
Baseline characteristics of 13,331 participants of NHANES III by 25(OH)D quartiles for multi-category variables. Results presented as means or percents ± standard error.
| 25-hydroxyvitamin D levels (ng/ml)* | ||||
|---|---|---|---|---|
| >32.1 | 24.4-32.1 | 17.8-24.4 | <17.8 | |
| Characteristic | (n=3359) | (n=3242) | (n=3344) | (n=3386) |
| Race (%) | ||||
| Non-Hispanic white | 43.5 ±1.4 | 28.5 ± 0.9 | 18.5 ± 0.8 | 9.5 ± 0.6 |
| Non-Hispanic black | 7.8 ± 0.8 | 14.4 ± 1.0 | 27.5 ± 0.9 | 50.3 ± 2.1 |
| Mexican-American | 20.3 ± 1.3 | 27.1 ± 1.1 | 29.2 ± 1.2 | 23.4 ± 1.6 |
| Other | 19.4 ± 2.7 | 25.9 ± 1.9 | 30.1 ± 2.4 | 24.6 ±2.3 |
| Smoking (%) | ||||
| Never | 34.8 ± 1.5 | 27.8 ± 0.9 | 21.9 ± 0.9 | 15.6 ± 0.9 |
| Former | 39.9 ± 1.9 | 27.4 ± 1.2 | 20.1 ± 1.2 | 12.6 ± 0.9 |
| Current | 37.9 ± 1.4 | 24.6 ± 1.2 | 19.7 ± 1.0 | 17.8 ± 1.1 |
| Season (%) | ||||
| Winter (Jan.-March) | 23.7 ± 1.5 | 24.8 ± 2.0 | 26.0 ± 2.0 | 25.5 ± 1.9 |
| Spring (April-June) | 31.5 ± 1.9 | 29.8 ± 1.1 | 23.1 ± 0.8 | 15.6 ± 1.2 |
| Summer (July-Sept.) | 48.3 ± 1.8 | 26.6 ± 1.4 | 16.2 ± 0.6 | 8.9 ± 0.7 |
| Fall (Oct.-Dec.) | 36.5 ± 1.7 | 25.1 ± 1.4 | 21.1 ± 8.8 | 17.3 ± 1.4 |
| Physical Activity | ||||
| Low (%) | 23.9 ± 1.1 | 25.1 ± 1.1 | 25.5 ± 1.0 | 25.5 ±1.3 |
| Moderate (%) | 37.9 ± 1.3 | 27.8 ± 0.9 | 20.6 ± 0.9 | 13.7 ± 0.8 |
| High (%) | 49.5 ± 2.2 | 26.1 ± 1.0 | 16.1 ± 1.2 | 8.4 ± 0.8 |
In multivariable models, increasing age, female sex, non-white race/ethnicity (particularly non-Hispanic black race/ethnicity), diabetes, current smoking, and increasing BMI were all independently associated with an increased odds of being 25(OH)D deficient (i.e., lowest quartile) (Table 3). Physical activity, vitamin D supplementation and non-winter season were associated with decreased odds of deficiency. In unadjusted analysis, low SES was associated with a higher risk of deficiency (OR 1.60 (1.40-1.81)) which after adjustment became a lower risk of deficiency 0.73 (0.60-0.89). The primary confounders of this association were race and physical activity; both associated with a >15% change in the point estimate. When using log-transformed 25(OH)D levels in a linear regression all of the associations shown in Table 3 remained the same, including the reversal of the low SES point estimate with adjustment.
Table 3.
Independent predictors of 25(OH)D deficiency (25(OH)D levels <17.8 ng/ml)* among 13,331 adults >20 years of age (multivariable model adjusted for all factors listed in Table).
| Characteristics** | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Age, per 10 yrs | 1.10 | 1.04-1.17 | 0.001 |
| Female sex | 2.26 | 1.90-2.69 | <0.001 |
| Non-Hispanic black race/ethnicity | 10.17 | 8.13-12.72 | <0.001 |
| Mexican-American race/ethnicity | 2.45 | 1.96-3.06 | <0.001 |
| “Other” race/ethnicity | 3.03 | 2.18-4.20 | <0.001 |
| Diabetes | 1.46 | 1.16-1.84 | 0.002 |
| History of Prior CVD | 0.87 | 0.64-1.18 | 0.36 |
| Hypertension | 0.96 | 0.77-1.21 | 0.74 |
| BMI, per 1 kg/m2 | 1.04 | 1.02-1.06 | <0.001 |
| Total cholesterol, per 10 mg/dL | 0.98 | 0.97-1.00 | 0.01 |
| HDL cholesterol, per 10 mg/dL | 1.02 | 0.97-1.06 | 0.44 |
| Use of cholesterol lowering medications | 1.39 | 0.85-2.29 | 0.19 |
| Former Smoking | 1.09 | 0.91-1.31 | 0.33 |
| Current Smoking | 1.59 | 1.35-1.87 | <0.001 |
| Spring (April-June) | 0.67 | 0.49-0.92 | 0.01 |
| Summer (July – Sept) | 0.31 | 0.23-0.43 | <0.001 |
| Fall (Oct-Dec) | 0.63 | 0.45-0.86 | 0.005 |
| eGFR < 60 ml/min/1.73 m2 | 1.05 | 0.75-1.47 | 0.77 |
| Albuminuria >30 mg/g | 1.26 | 0.96-1.64 | 0.09 |
| CRP >0.21 mg/dL | 0.96 | 0.78-1.20 | 0.73 |
| Serum albumin, per 1 g/dl | 0.93 | 0.72-1.21 | 0.58 |
| Low SES | 0.73 | 0.60-0.89 | 0.002 |
| Vitamin D supplementation | 0.45 | 0.36-0.56 | <0.001 |
| Moderate Physical Activity | 0.65 | 0.53-0.80 | <0.001 |
| High Physical Activity | 0.44 | 0.32-0.61 | <0.001 |
To convert to SI units, multiply 25(OH)D value by 2.496.
Reference categories are: for race: non-Hispanic white race/ethnicity; for smoking: non-smokers; for eGFR: >60 ml/min/1.73 m2; for physical activity: low physical activity; for season: winter season (January-March).
Associations between 25(OH)D levels and all-cause mortality
During a median 8.7 years of follow-up (intraquartile range (IQR) 7.1-10.2), there were 1806 deaths, of which 777 (43%) were ascribed to CVD, 424 (23%) were ascribed to cancers, 105 (6%) were ascribed to infectious etiologies and 92 (5%) were ascribed to external causes of death. Of those who died from CVD, 590 (76%) died from atherosclerotic CVD, 145 (19%) from cerebrovascular disease, and 42 (5%) from congestive heart failure. Those who died had a mean age of 66.4 years, were 46% female, 58% were hypertensive, 19% diabetic and 32% had prior cardiovascular disease.
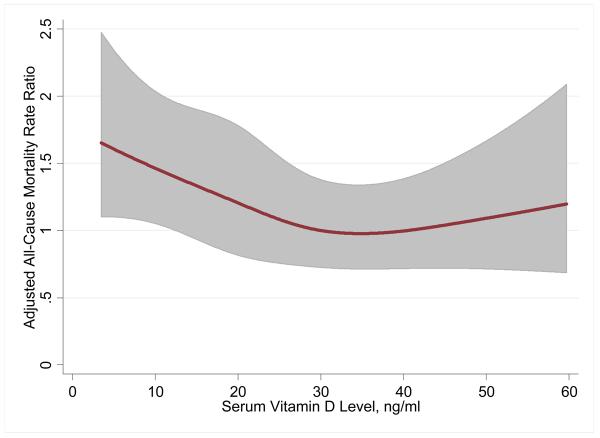
In unadjusted analysis, having a 25(OH)D level in the lowest quartile was associated with a 78% increased risk of all-cause mortality (Table 4). The continuous, fully adjusted, association between vitamin D levels and all-cause mortality is shown graphically in Figure 1. After further adjusting for known CVD risk factors including BMI and renal function, low SES, and markers of healthy lifestyle including physical activity and use of vitamin D supplementation, there was still a 26% higher rate of all-cause mortality for the lowest quartile of 25(OH)D (MRR 1.26, 95% CI 1.08-1.46) compared to the highest quartile. This adjusted IRR translates to an attributable risk percent of 20.6% and a population attributable risk percent of 3.1% associated with the lowest quartile of 25(OH)D.
Table 4.
Rate Ratios of All-Cause and CVD Mortality by 25(OH)D† Quartiles in 13,331 participants of NHANES III.
| All-Cause Mortality Rate Ratio |
>32.1 ng/ml | 24.4-32.1 ng/ml | 17.8-24.4 ng/ml | <17.8 ng/ml |
|---|---|---|---|---|
| Unadjusted | 1.0 (Ref) | 1.14 (0.94-1.39) | 1.49 (1.24-1.78) | 1.78 (1.44-2.21) |
| Limited* | 1.0 (Ref) | 0.92 (0.78-1.08) | 1.11 (0.95-1.31) | 1.52 (1.31-1.77) |
| Full** | 1.0 (Ref) | 0.93 (0.79-1.10) | 1.06 (0.89-1.24) | 1.26 (1.08-1.46) |
| Full without diabetes mellitus and hypertension |
1.0 (Ref) | 0.94 (0.80-1.12) | 1.06 (0.90-1.26) | 1.28 (1.11-1.48) |
|
CVD Mortality Rate Ratio |
>32.1 ng/ml | 24.4-32.1 ng/ml | 17.8-24.4 ng/ml | <17.8 ng/ml |
| Unadjusted | 1.0 (Ref) | 1.07 (0.82-1.41) | 1.31 (1.01-1.71) | 1.70 (1.22-2.37) |
| Limited* | 1.0 (Ref) | 0.85 (0.67-1.09) | 0.98 (0.76-1.27) | 1.53 (1.12-2.08) |
| Full** | 1.0 (Ref) | 0.83 (0.65-1.07) | 0.88 (0.69-1.14) | 1.20 (0.87-1.64) |
| Full without diabetes mellitus and hypertension |
1.0 (Ref) | 0.85 (0.66-1.09) | 0.89 (0.69-1.15) | 1.22 (0.90-1.65) |
|
Cancer Mortality Rate Ratio |
>32.1 ng/ml | 24.4-32.1 ng/ml | 17.8-24.4 ng/ml | <17.8 ng/ml |
| Unadjusted | 1.0 (Ref) | 0.97 (0.65-1.45) | 1.52 (1.10-2.10) | 1.31 (0.96-1.81) |
| Limited* | 1.0 (Ref) | 0.78 (0.53-1.16) | 1.11 (0.82-1.51) | 1.05 (0.74-1.47) |
| Full*** | 1.0 (Ref) | 0.80 (0.54-1.19) | 1.08 (0.80-1.46) | 0.91 (0.63-1.31) |
|
Infectious Disease Mortality Rate Ratio |
>32.1 ng/ml | 24.4-32.1 ng/ml | 17.8-24.4 ng/ml | <17.8 ng/ml |
| Unadjusted | 1.0 (Ref) | 1.28 (0.65-2.53) | 1.22 (0.67-2.21) | 1.46 (0.73-2.91) |
| Limited* | 1.0 (Ref) | 1.06 (0.53-2.12) | 0.90 (0.46-1.76) | 1.10 (0.47-2.56) |
| Full** | 1.0 (Ref) | 1.01 (0.53-1.93) | 0.87 (0.43-1.74) | 0.84 (0.38-1.86) |
|
External Causes of Death Rate Ratio |
>32.1 ng/ml | 24.4-32.1 ng/ml | 17.8-24.4 ng/ml | <17.8 ng/ml |
| Unadjusted | 1.0 (Ref) | 0.88 (0.25-3.08) | 1.59 (0.58-4.37) | 1.17 (0.44-3.07) |
| Limited* | 1.0 (Ref) | 0.85 (0.26-2.83) | 1.47 (0.54-3.99) | 1.05 (0.45-2.48) |
| Full*** | 1.0 (Ref) | 0.92 (0.27-3.11) | 1.68 (0.63-4.47) | 1.27 (0.56-2.87) |
To convert to SI units, multiply 25(OH)D value by 2.496.
Limited Model adjusted for age, sex, race, and season
Fully Adjusted Model includes age, sex, race, season, hypertension, history of prior CVD, diabetes, smoking, HDL cholesterol, total cholesterol, use of cholesterol medications, eGFR categories, serum albumin, log(albumin-creatinine ratio), log(CRP), BMI, physical activity level, vitamin D supplementation and low SES.
Fully Adjusted Cancer and External Causes of Death Models include age, sex, race, season, cigarette use, BMI, log (CRP), serum albumin, physical activity level, vitamin D supplementation and low SES.
Figure 1.
Restricted cubic spline showing the fully adjusted associations between serum 25(OH)D levels and all-cause mortality in 13,331 participants of NHANES III. Knots are at 10.9, 20.5, 28.9 and 45.9 ng/ml. To convert to SI units (nmol/L), multiply 25(OH)D value by 2.496.
Associations between 25(OH)D levels and cause-specific mortality
For CVD mortality, the unadjusted analysis revealed that having a 25(OH)D level in the lowest quartile was associated with 70% higher risk of mortality. This association did not remain statistically significant in the fully adjusted model, though the magnitude of the point estimate was similar to that for all-cause mortality (MRR 1.20, 95% CI 0.87-1.64) (Table 3). There was a non-significant association between lowest quartile of 25(OH)D and cancer-specific mortality in an unadjusted model (1.31 (0.96-1.81)), which disappeared after adjustment for age, sex, race and season (1.05 (0.74-1.47)). When testing only cancers previously associated with 25(OH)D levels, colorectal, breast and prostate (n=116), the lowest quartile had an adjusted MRR of 1.38 (0.49-3.93) and the 17.8-24.4 ng/ml quartile had an MRR of 2.05 (1.14-3.68).
Sensitivity Analyses
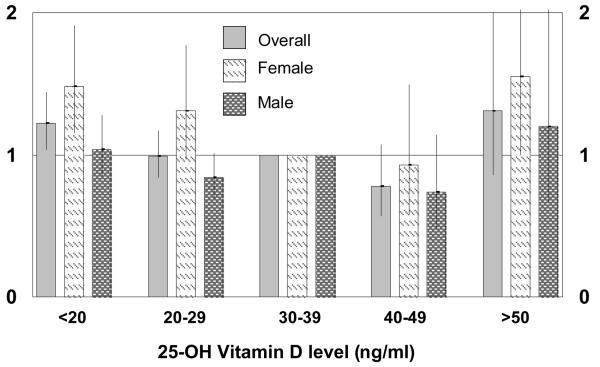
Results did not change significantly when serum calcium and phosphate levels were entered in the fully-adjusted model (MRR for lowest quartile of 25(OH)D = 1.27 (1.10-1.47) for all-cause mortality; 1.21 (0.89-1.65) for CVD mortality). Likewise, the associations remained relatively unchanged in models assessing all-cause mortality not including diabetes mellitus and hypertension (Table 4), and separately without diabetes (MRR=1.28 (1.11-1.48)) and without hypertension (MRR=1.26 (1.08-1.45)). When continuous variables such as age were added to the model as categorical variables the association between the lowest quartile of 25(OH)D and mortality strengthened (MRR=1.34 (1.15-1.57). Results also did not differ when analyzed by different 25(OH)D cut-offs. As seen in Figure 2, in females, having both low levels (<20 ng/ml) and high levels (>50 ng/ml) was associated with an increased rate of mortality.
Figure 2.
Associations between 25(OH)D levels and all-cause mortality in 13,331 participants of NHANES III overall and by gender.
Subgroup analyses
The lowest quartile of 25(OH)D was more strongly associated with mortality among participants without a history of CVD (MRR=1.54; 1.23-1.94) than in those with a history of CVD (MRR=0.90; 0.66-1.22, p-interaction=0.006) (Table 5). Stronger associations, of borderline statistical significance, were found for participants without hypertension (p-interaction=0.09), participants without diabetes (p-interaction=0.09) and for women (p-interaction=0.06). No interaction was observed for age, race, obesity, physical activity level, smoking or estimated GFR.
Table 5.
The adjusted* associations of 25(OH)D levels in 13,331 participants of NHANES III and all-cause mortality in specific participant subgroups.
| 25-hydroxyvitamin D levels (ng/ml) † | |||||
|---|---|---|---|---|---|
| Sub-group (n=n at risk/deaths) |
>32.1 | 24.4-32.1 | 17.8-24.4 | <17.8 | p- interaction |
| Age >65 (n=2867/1243) |
1.0 (Ref) | 0.97 (0.79-1.19) |
0.99 (0.82-1.20) |
1.26 (1.03-1.54) |
0.14 |
| Age <65 (n=10464/563) |
1.0 (Ref) | 0.81 (0.58-1.14) |
1.13 (0.81-1.56) |
1.28 (0.93-1.76) |
|
| Men (n=6277/1047) |
1.0 (Ref) | 0.82 (0.64-1.05) |
0.94 (0.75-1.19) |
1.04 (0.83-1.30) |
0.06 |
| Women (n=7054/759) |
1.0 (Ref) | 1.16 (0.87-1.55) |
1.27 (0.97-1.66) |
1.51 (1.15-1.98) |
|
| Non-Hispanic white (n=5696/1056) |
1.0 (Ref) | 0.92 (0.78-1.08) |
1.06 (0.88-1.27) |
1.22 (1.03-1.45) |
|
| Non-Hispanic black‡ (n=3597/401) |
1.0 (Ref) | 0.80 (0.59-1.10) |
1.03 (0.77-1.36) |
1.05 (0.77-1.44) |
0.74 |
| Mexican-American (n=3511/318) |
1.0 (Ref) | 1.28 (0.72-2.25) |
1.40 (0.85-2.30) |
1.46 (0.89-2.42) |
0.87 |
| Other (n=527/31) |
1.0 (Ref) | 0.65 (0.08-4.92) |
0.78 (0.11-5.70) |
2.50 (0.59-10.60) |
0.27 |
| CVD present (N=1396/632) |
1.0 (Ref) | 0.67 (0.49-0.92) |
0.75 (0.53-1.04) |
0.90 (0.66-1.22) |
0.006 |
| CVD absent (n=11935/1174) |
1.0 (Ref) | 1.10 (0.84-1.43) |
1.22 (0.96-1.57) |
1.54 (1.23-1.94) |
|
| Hypertension present (n=4064/1114) |
1.0 (Ref) | 0.87 (0.72-1.05) |
1.00 (0.80-1.25) |
1.12 (0.89-1.41) |
0.09 |
| Hypertension absent (n=9267/692) |
1.0 (Ref) | 0.96 (0.66-1.38) |
1.13 (0.86-1.50) |
1.42 (1.00-2.01) |
|
| Diabetes present (n=1295/408) |
1.0 (Ref) | 0.77 (0.53-1.11) |
1.17 (0.85-1.62) |
1.10 (0.77-1.58) |
0.09 |
| Diabetes absent (n=12036/1398) |
1.0 (Ref) | 0.97 (0.79-1.18) |
1.03 (0.84-1.27) |
1.36 (1.14-1.61) |
|
| BMI <30 kg/m2 (n=9963/1434) |
1.0 (Ref) | 0.95 (0.79-1.13) |
1.01 (0.83-1.23) |
1.22 (1.01-1.48) |
0.68 |
| BMI ≥30 kg/m2 (n=3368/372) |
1.0 (Ref) | 0.81 (0.56-1.17) |
1.09 (0.74-1.62) |
1.38 (0.92-1.08) |
|
| eGFR <60 ml/min/1.73m2 (n=743/417) |
1.0 (Ref) | 1.13 (0.85-1.50) |
1.08 (0.78-1.49) |
1.28 (0.96-1.70) |
0.35 |
| eGFR >60 ml/min/1.73m2 (n=12588/1389) |
1.0 (Ref) | 0.87 (0.71-1.07) |
1.03 (0.85-1.25) |
1.20 (1.00-1.44) |
|
| Low Physical Activity Level (n=4359/819) |
1.0 (Ref) | 0.96 (0.71-1.29) |
1.13 (0.91-1.40) |
1.35 (1.03-1.76) |
0.49 |
| Moderate Physical Activity Level (n=6772/896) |
1.0 (Ref) | 0.92 (0.75-1.12) |
1.04 (0.81-1.33) |
1.25 (0.97-1.61) |
|
| High Physical Activity Level (n=2,200/91) |
1.0 (Ref) | 1.26 (0.68-2.33) |
1.21 (0.66-2.23) |
0.95 (0.29-3.12) |
|
| Non-smokers (n=6,542/697) |
1.0 (Ref) | 0.98 (0.76-1.25) |
1.15 (0.83-1.57) |
1.37 (0.98-1.93) |
|
| Former smokers (n=3,322/681) |
1.0 (Ref) | 0.97 (0.74-1.28) |
0.99 (0.76-1.29) |
1.18 (0.85-1.63) |
0.58 |
| Current smokers (n=3,467/428) |
1.0 (Ref) | 0.83 (0.56-1.23) |
1.09 (0.79-1.51) |
1.16 (0.82-1.63) |
|
Models adjusted for age, sex, race, season, hypertension, history of prior CVD, diabetes, smoking, HDL cholesterol, total cholesterol, use of cholesterol medications, eGFR categories, serum albumin, log(albumin-creatinine ratio), log(CRP), BMI, physical activity level, vitamin D supplementation and low SES except for the variable in the subgroup.
To convert to conventional units, divide 25(OH)D value by 2.496.
Using African-American specific quartiles: Quartile 1: >24 ng/ml, Quartile 2: ≤24 −18 ng/ml, Quartile 3: ≤18 − 13.5 ng/ml, and Quartile 4: ≤13.5 ng/ml.
Discussion
We found in the general US population that 25(OH)D deficiency (the lowest quartile <17.8 ng/ml) was associated with a 26% higher risk of all-cause mortality, independent of baseline demographics, traditional and non-traditional CVD risk factors, and measures of a healthy lifestyle. The estimated association with increased risk of CVD mortality was similar, though of weaker statistical significance. We did not find an association with cancer mortality or other causes of death. This is the first study to our knowledge to explore the association between 25(OH)D levels and mortality in the general population.
Several lines of evidence suggest that vitamin D deficiency may be a risk factor for cardiovascular, cancer and all-cause mortality. Ecological studies reveal that CVD events are higher in the winter when vitamin D levels are lower33 and cancer survival is better if the cancer is diagnosed in the summer when vitamin D levels are higher34. In addition, observational data shows that the use of activated vitamin D in patients with ESRD is associated with decreased mortality6-10. Vitamin D receptor knock-out mice experience cardiac myocyte hypertrophy.2 Activated vitamin D has anti-proliferative properties5. In mice, vitamin D is an inhibitor of the renin-angiotensin system1. Other inhibitors of the renin-angiotensin system, such as ACE inhibitors, reduce mortality and morbidity in multiple disease states35.
Low 25(OH)D levels are also associated with hypertension, diabetes mellitus, insulin resistance and an elevated BMI, all of which are risk factors for CVD and all-cause mortality. Low 25(OH)D levels12, but not vitamin D intake36, are associated with incident hypertension. There are two small clinical trials that suggest that vitamin D supplementation37, 38 reduced systolic BP. The association of 25(OH)D deficiency with obesity39, 40, glucose intolerance15, 16, 41, and the metabolic syndrome42 is another potential mechanism for increased CVD risk. We found that diabetes mellitus and increased BMI were independently associated with increased odds of being 25(OH)D deficient. We adjusted for hypertension, diabetes mellitus and BMI in our analyses and still found a significant association with all-cause mortality. Analyses with and without hypertension and diabetes mellitus in the model did not reveal a difference in the risk estimates associated with low 25(OH)D levels. However, this analysis did not account for incident diabetes and hypertension developing after the NHANES survey period.
The association of low 25(OH)D levels with mortality was strongest in those without CVD, without hypertension and without diabetes mellitus, arguing against low vitamin D levels being just a marker of poor general health. If diabetes mellitus and hypertension are in the causal pathway between low 25(OH)D levels and mortality, i.e. low 25(OH)D leads to hypertension which leads to higher mortality, it follows that the effect is more pronounced in those without pre-existing diabetes mellitus, hypertension and CVD. The fact that the associations were stronger in those without CVD at baseline suggests that if a causal relationship exists, 25(OH)D deficiency may play a role before CVD is established.
It is unclear why the association between 25(OH)D levels and mortality was more pronounced amongst women. It may be that there is a hormone interaction between estrogens and 25(OH)D. Women tend to develop atherosclerosis later in life compared to men suggesting that, similarly to the no CVD subgroup, perhaps low 25(OH)D levels play a role before the development of atherosclerosis. This area deserves further research and may help reveal the mechanisms behind the associations seen, if in fact they are causal.
A recently published randomized clinical trial of calcium and 400 IU/day vitamin D supplementation in generally healthy post-menopausal women did not show a lower risk of cardiovascular events in those randomized to vitamin D and calcium43. However, a meta-analysis of 18 randomized clinical trials showed that participants randomized to vitamin D supplementation experienced fewer deaths compared to those randomized to placebo18. In that analysis, as in ours, they were not able to establish the specific cause of death responsible for the lower mortality.
Several authors have commented that the optimal levels of 25(OH)D should be greater than 30 ng/ml19, 20. In our observational study we found that there was a lower risk of mortality at levels 30-49 ng/ml but that at levels >50 ng/ml there was again a higher risk of mortality in women. This is similar to findings about anti-oxidant vitamins and vitamin E, which show that too much may be harmful44, 45.
Several associations between vitamin D deficiency and clinical factors need further explanation. In adjusted analysis, low SES was associated with a lower risk of being 25(OH)D deficient. Most of the confounding was due to the very strong association between non-Hispanic black race and low 25-OH D status. The mechanism for the protective effect of low SES is unknown. However, one may conjecture that participants with low SES may spend more time outdoors and therefore have more sun exposure.
Our study is limited in that it is an observational study and therefore causality cannot be inferred. We adjusted for all known traditional CVD risk factors and non-traditional risk factors, including markers of a healthy lifestyle, such as the use of vitamin D supplementation. Residual confounding, however, may still exist. Specifically, the excess mortality observed in the lowest quartile may be a reflection of the participant's overall poor condition, or an unmeasured confounder, which we could not fully adjust for in our analysis. We were not able to distinguish the specific causes of mortality which accounted for the elevated all-cause mortality risk associated with 25(OH)D deficiency in our population, perhaps due to limitations of power or to the use of potentially imprecise death certificate data. Northern states were only sampled in the summer in NHANES III and therefore the full extent of 25(OH)D deficiency in the population is probably under-estimated. This probably attenuates the association seen in our results. Lastly, one must view the subgroup analyses with caution due to issues of multiple comparisons.
In conclusion, the lowest quartile of 25(OH)D (<17.8 ng/ml) is associated with a higher risk of all-cause mortality in the general US population. Further observational studies are needed to confirm these findings and establish the mechanisms underlying these observations. If confirmed, randomized clinical trials will be needed to determine whether vitamin D supplementation therapy at higher doses could have any potential benefit in reducing future mortality risk in those with 25(OH)D deficiency.
Acknowledgements
The National Center for Health Statistics (NCHS) is the source of the data used in this analysis. All analyses, interpretations and conclusions are made by the authors and do not represent views of the NCHS. We are indebted to Christopher Rogers, PhD, of the NCHS for his aid with the analysis. MLM and this analysis were supported by grant F32DK069017 and K23-DK078774 and EDM was supported by grant T32-HL007024 from the National Institutes of Health. EDM is also supported by the PJ Schafer Cardiovascular Research Fund. WP is supported, in part, by the Paul Beeson Physician Faculty Scholars in Aging Program. EDM has received consulting fees from Abbott Pharmaceuticals. The funding organizations had no role in the design and conduct of the study, collection, management, analysis and interpretation of data or the preparation, review or approval of the manuscript. Drs. Melamed and Michos had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
MLM and this analysis were supported by grant F32-DK069017 and K23-DK078774 and EDM was supported by grant T32-HL007024 from the National Institutes of Health. EDM is also supported by the PJ Schafer Cardiovascular Research Fund. WP is supported, in part, by the Paul Beeson Physician Faculty Scholars in Aging Program. EDM has received consulting fees from Abbott Pharmaceuticals.
REFERENCES
- 1.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002 Jul;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005 Jan;288(1):E125–132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 3.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002 Apr;8(4):174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 4.Levin A, Li YC. Vitamin D and its analogues: do they protect against cardiovascular disease in patients with kidney disease? Kidney Int. 2005 Nov;68(5):1973–1981. doi: 10.1111/j.1523-1755.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee P, Chatterjee M. Antiproliferative role of vitamin D and its analogs--a brief overview. Mol Cell Biochem. 2003 Nov;253(12):247–254. doi: 10.1023/a:1026072118217. [DOI] [PubMed] [Google Scholar]
- 6.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005 Apr;16(4):1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 7.Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006 Jul;70(2):351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 8.Tentori F, Hunt WC, Stidley CA, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006 Nov;70(10):1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006 Aug;70(4):771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 10.Shoji T, Shinohara K, Kimoto E, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004 Jan;19(1):179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 11.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin d in the United States: data from the third national health and nutrition examination survey. Arch Intern Med. 2007 Jun 11;167(11):1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 12.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-Hydroxyvitamin D Levels and Risk of Incident Hypertension. Hypertension. 2007 Mar 19; doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 13.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003 Jan 1;41(1):105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006 Apr 5;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 15.Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006 Mar;29(3):650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 16.Chonchol M, Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int. 2007 Jan;71(2):134–139. doi: 10.1038/sj.ki.5002002. [DOI] [PubMed] [Google Scholar]
- 17.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007 Aug 8; doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 18.Autier P, Gandini S. Vitamin D Supplementation and Total Mortality: A Meta-analysis of Randomized Controlled Trials. Arch Intern Med. 2007 Sep 10;167(16):1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 19.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998 Mar 19;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006 Jul;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 21.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005 Autumn;15(4 Suppl 5):S5–97. 101. [PubMed] [Google Scholar]
- 22.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994 Jul;(32):1–407. [PubMed] [Google Scholar]
- 23.Ezzati TM, Massey JT, Waksberg J, Chu A, Maurer KR. Sample design: Third National Health and Nutrition Examination Survey. Vital Health Stat 2. 1992 Sep;(113):1–35. [PubMed] [Google Scholar]
- 24.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002 May;39(5):920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003 Jul 15;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 26.Zhu S, St-Onge MP, Heshka S, Heymsfield SB. Lifestyle behaviors associated with lower risk of having the metabolic syndrome. Metabolism. 2004 Nov;53(11):1503–1511. doi: 10.1016/j.metabol.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 27.International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) Washington, D.C.: 1991. [Google Scholar]
- 28.International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. 1992;1 Vol Revision. [PubMed] [Google Scholar]
- 29.Dupont W, Plummer WD. PS power and sample size program available for free on the Internet. Controlled Clin Trials. 1997;18:274. [Google Scholar]
- 30.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002 May;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 31.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004 Aug;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 32.Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007 May 14;167(9):879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 33.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005 Oct;94(4):483–492. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]
- 34.Lim HS, Roychoudhuri R, Peto J, Schwartz G, Baade P, Moller H. Cancer survival is dependent on season of diagnosis and sunlight exposure. Int J Cancer. 2006 Oct 1;119(7):1530–1536. doi: 10.1002/ijc.22052. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000 Jan 20;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 36.Forman JP, Bischoff-Ferrari HA, Willett WC, Stampfer MJ, Curhan GC. Vitamin D intake and risk of incident hypertension: results from three large prospective cohort studies. Hypertension. 2005 Oct;46(4):676–682. doi: 10.1161/01.HYP.0000182662.82666.37. [DOI] [PubMed] [Google Scholar]
- 37.Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998 Aug 29;352(9129):709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 38.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001 Apr;86(4):1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 39.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000 Sep;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 40.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003 Jan;88(1):157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 41.Hypponen E, Power C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabetes Care. 2006 Oct;29(10):2244–2246. doi: 10.2337/dc06-0946. [DOI] [PubMed] [Google Scholar]
- 42.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005 May;28(5):1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 43.Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007 Feb 20;115(7):846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 44.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005 Jan 4;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 45.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007 Feb 28;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]