Introduction
Development and homeostasis of sensory neurons requires interaction of a broad array of growth factors that are produced by sensory neuron targets, immune cells and sensory neurons themselves. Over the last decade, two families of growth factors have been shown to be particularly important: the neurotrophin family, which includes NGF, NT-3, BDNF and NT-4, and the glial cell-line derived neurotrophic family that includes GDNF, artemin, neurturin and persephin. Nociceptors, sensory neurons that detect painful or potentially damaging stimuli, have been shown to be dependent on target-derived NGF for survival during development 1, 2. Studies from our laboratories and others have also found that although members of the GDNF neurotrophic factor family have a modest impact on developmental survival, they can affect the number and functional phenotype of postnatal nociceptors 3-5. Mice that overexpress GDNF have more IB4-positive afferents that exhibit lower mechanical thresholds 5. Mice that overexpress artemin also have increased numbers of sensory neurons and show increased responsiveness to noxious heat stimuli and decreased heat thresholds 4. In addition to these developmental effects, new observations presented below demonstrate that short-term, acute exposure to GDNF-family ligands (GFLs) can affect sensitivity of adult nociceptive neurons in vitro and in vivo. These observations have led us to propose the general hypothesis that neurotrophins are required for sensory neuron embryogenesis, but from late development onward to postnatal and adult ages, neurotrophins and GFLs co-modulate and maintain sensory neuron phenotype.
It is well established that sensory neuron survival during development is dependent on growth factors, many of which are produced by or proximal to targets of sensory endings 6-10. In 1997, Molliver et al. 11 demonstrated that for at least one population of sensory afferents, two growth factors, NGF and GDNF, cooperate to produce fully mature adult sensory neurons. This population of small neurons bind the isolectin B4 (IB4; also referred to as “non-peptidergic neurons”) and project to lamina II of the dorsal horn. Early in development these neurons express trkA and are NGF responsive; postnatally, these sensory neurons downregulate trkA, express the oncogene/receptor tyrosine kinase, ret, and depend on GDNF for trophic support. Ret signaling is mediated by a receptor complex of ret and the GFRα 1−4 co-receptor family (a family of GPI-linked receptors); these co-receptors bind GDNF, neurturin, artemin or persephin respectively (Figure 1). Studies in knockout mice lacking GDNF revealed a neurotrophic role for GDNF during development; deletion of GDNF causes a 23% reduction in the number of DRG neurons in neonatal mice 12 (adult data is not available because the mice die at birth), and overexpression of GDNF in the skin increased the number of DRG neurons 27% 5. In contrast, artemin knockout mice are reported to have no change in the total number of sensory neurons or in the expression of the artemin-specific receptor GFRα3 13. These data suggest a neuromodulatory rather than a neurotrophic role for artemin. Mice lacking neurturin have significantly fewer GFRα2 (the co-receptor for neurturin)-expressing neurons14. However, it should be noted that mice lacking GFRα2 have no significant loss of either myelinated or unmyelinated neurons, although there is a decrease in the axonal diameter of both populations 15. These data suggest that suggesting the decrease in GFRα2-expressing cell number reflects a decrease in receptor expression and not cell loss. A recent study 16 found that >70% of the non-peptidergic (i.e. IB4-positive) nerve endings were absent in the skin of neurturin knockout mice, but CGRP-positive (i.e. peptidergic) fibers were unaffected. Surprisingly, the neurturin knockout mice exhibited normal detection of noxious heat, but nocifensive behaviors elicited with formalin were significantly decreased, suggesting reduced pain perception. However, these mice showed significantly increased sensitivity to noxious cold in a tail flick test. This phenotype is consisted paradoxical hyperalgesia in human CRPS type 1 patients in which the magnitude of hyperalgesia was correlated with the extent of loss of peripheral innervation 17, 18 (but see 19). These studies are consistent with a model in which neurturin is required for trophic support of cutaneous non-peptidergic C-fiber nociceptors, but compensatory mechanisms following the loss of these fibers can result in both decreased sensation and increased pain sensitivity.
Figure 1. Neurotrophins (NGF, NT-3, BNDF, NT-4) and GFLs (artemin, neurturin, GDNF) bind to specific membrane receptors.
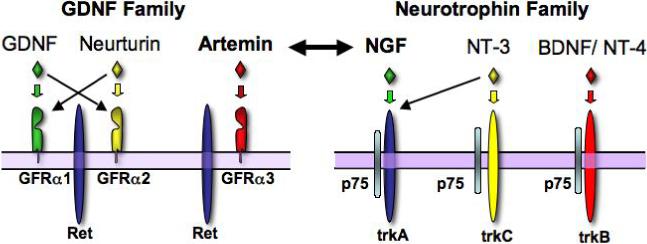
Both families of neurotrophic factors employ a two-receptor complex to produce specific growth factor effects. For the neurotrophin family, p75 can bind all four ligands, but maximal activity is obtained only the ligand binds both p75 and its specific trk receptor. Similarly, GFLs bind both a specific GPI-linked GFRα receptor and ret. But unlike the neurotrophin receptor complex, the GFRs are not thought to initiate any signaling independent of ret (whereas p75 can signal via the ceramide pathway).
NGF regulates sensitivity of adult sensory neurons via TRPV1
A prominent role for NGF in the regulation of plasticity in mature sensory neurons has been well-described. For example, in vivo injection of NGF produces thermal hypersensitivity within 30 minutes and mechanical sensitivity within hours in both rodents and humans 20, 21. The mechanism for NGF-induced heat sensitivity may involve the transient receptor potential vanilloid type 1 (TRPV1) receptor. TRPV1 is a nonselective cation channel gated by noxious heat, protons and vanilloid compounds such as capsaicin 22, 23 and is required for inflammation-induced heat hyperalgesia 24, 25. Under normal conditions, repeated activation results in progressive desensitization of TRPV1 (tachyphylaxis; Figure 3a). NGF potentiates TRPV1 function, antagonizing or blocking tachyphylaxis and is upregulated in the periphery following inflammation, leading to the hypothesis that NGF plays a major role in acute inflammatory hyperalgesia 26-29.
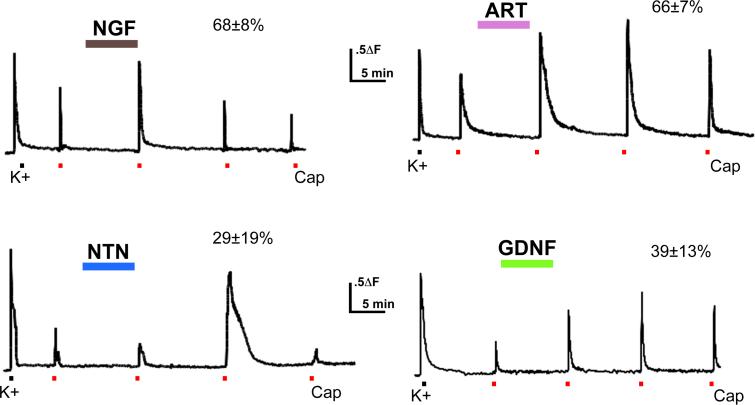
Figure 2. Acute potentiation of TRPV1 by growth factors.
Ca++ influx following 1 mM capsaicin treatment before and after 7 minute perfusion of nerve growth factor (NGF), Glial-derived neurotrophic factor (GDNF), artemin (ART), or neurturin (NTN) (all at 100ng/ml) was examined in isolated DRG sensory neurons. Each coverslip received a single growth factor perfusion. Growth factor treatment increased capsaicin responses (both peak and area measurements) in many cells; percent of capsaicin responders potentiated is given above each record. Red bar indicates capsaicin application.
Recent studies of transgenic mice overexpressing artemin in the skin suggest that GDNF family members may also modulate thermal sensation (4; see accompanying paper in this volume (Wang et al.)). Mice overexpressing artemin in the skin have increased thermal sensitivity and decreased thermal thresholds in C-fiber nociceptors as compared to wild type mice.
Distribution of GDNF family ligand receptors (GFRs)
As discussed above, the actions of growth factors in the GDNF family are mediated by ret in combination with one of the GFRs family members. The GFRs, required for specific binding of each of the three growth factors, appear to be differently distributed in mouse and rat. In rat, roughly equal percentages of neurons expressing each GFR were identified by in situ hybridization in back-labeled sciatic afferents (32−42% for each receptor 30). In similar experiments in mouse, significant differences in expression of each GFR are reported (lumbar DRG neurons: GFRα1 17%, GFRα2 = 22%, GFRα3 = 34%). However, these percentages may differ from those reported in rat because cell counts were performed on whole lumbar ganglia 31. Neurons may also express multiple GFRs, in rat 30% of cells are proposed to express both GFRα1 and GFRα2 32; no data is available on GFRα3 overlap. However, no overlap was detected between GFRα1 and GFRα2 31; these authors did detect overlap in expression of GFRα1 and GFRα3 as well as GFRα2 and GFRα3. Immunocytochemical studies in adult mouse conflict with these in situ results: GFRα3 was detected in peptidergic, trkA-positive nociceptors distinct from either neurturin- (GFRα2-positive) or GDNF (GFRα1-positive)-responsive neurons 33. Based on these results, little overlap between GFRα3 and GFRα1 or GFRα2 is expected at the functional level.
TRPV1-positive neurons express receptors for neurturin or artemin
Widespread expression of TrkA in TRPV1-expressing neurons 33, 34 supports a model in which modulation of TRPV1 by NGF underlies the thermal hyperalgesia induced by NGF injection. However, extensive co-localization TRPV1 and GFRα3 immunoreactivity in both rat and mouse DRG neurons has also been described 33. We have confirmed the extent of overlap between GFRα3 and TRPV1 (Figure 2) 35. Although GFRα2 and TRPV1 are not extensively co-localized, a subset of neurons (∼22% of TRPV1-positive cells) were clearly positive for both TRPV1 and GFRα2 (double-labeled cells in Figure 2; arrows). It should also be noted that the majority (>65%) of TRPV1-positive cells co-expressed GFRα3 (Figure 2). Thus, assuming that there is little overlap between artemin and neurturin responsive afferents, the immunohistochemical data suggests that virtually all TRPV1-expressing neurons will respond to one of these two members of the GDNF family. In addition, TRPV1 is modulated by NGF in >50% of sensory neurons, indicating that trkA is co-expressed with GFRs in some sensory neurons.
Figure 2. GFRα2 and GFRα3 co-localize with TRPV1.
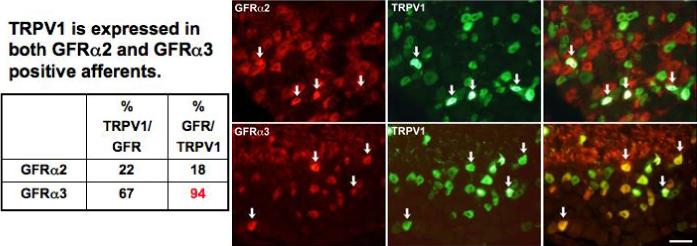
Images show immunohistochemical staining for GFRα2 or GFRα3 (red) with TRPV1 (green) in sections of mouse L4 DRG. A. TRPV1 labeled small and medium-sized neurons with a range of staining intensity, as widely described in the literature. Staining for GFRα2 was intense in axons and neuronal cell bodies of small and medium size. Only a minority of TRPV1-positive neurons were also GFRα2-positive. Arrows in merged panel indicate double-labeled neurons. B. GFRα3 stained cell bodies and axons of a subset of small neurons. In contrast to GFRα2 staining, most GFRα3-positive neurons also expressed TRPV1 (see arrows in merged panels). Scale bar = 50mm. Quantification of staining is provided in table at left of the figure.
GFLs are more efficacious at potentiating TRPV1 responses than NGF
The observation that the receptors for GDNF family members are expressed in TRPV1-positive cells suggested that these growth factors might modulate capsaicin responses, as seen with NGF. This has been investigated using calcium imaging of acutely dissociated (< 24 hr) mouse sensory neurons grown in the absence of additional growth factors 36. Ca++ responses to 1μM capsaicin were recorded in isolated sensory neurons using fura-2 imaging before and after a 10 minute exposure to NGF, artemin, neurturin or GDNF (each at 100ng/ml) 35. 1μM capsaicin administered at 10 minute intervals elicited responses that showed significant tachyphylaxis due to desensitization of the TRPV1 channel (Figure 3A; see also 27). NGF given during this 10 minute interval maintained and often potentiated TRPV1 response magnitude. (Potentiation was defined as a response to subsequent capsaicin applications greater in magnitude (peak or area) than that elicited by the first capsaicin application.) All four growth factors significantly potentiated capsaicin-evoked Ca++ transients in some neurons (Figure 3). NGF and artemin each potentiate a majority (>65%) of capsaicin responses, while neurturin- and GDNF- responsive cells are seen less frequently (29% and 39%, respectively).
The magnitude of capsaicin response potentiation also varies with growth factor 35. Using the area under the curve as a measure of the extent of potentiation, the mean maximal potentiation induced by NGF was a 2.03 ± 0.23 -fold increase over the initial capsaicin response. The magnitude of potentiation elicited by artemin (2.93 ± 0.41) was significantly greater than that elicited by NGF. Neurturin and GDNF potentiated capsaicin responses with a similar efficacy to NGF, increasing the capsaicin response magnitude by 1.89 ± 0.52-fold and 1.65 ± 0.7-fold, respectively. These mean fold increases in TRPV1 response magnitude are population averages; for each growth factor, changes in TRPV1 function were calculated in all cells, including cells that did not respond to the given growth factor and exhibited tachyphylaxis. Therefore, neurturin and GDNF appear to have less effect on average than artemin because these growth factors affect significantly fewer capsaicin-responsive cells. However, when the analysis is restricted to those cells in which capsaicin responses were potentiated by growth factor application, artemin, neurturin and GDNF were all found to potentiate capsaicin responses significantly more than NGF (fold increases: NGF 2.49 ± 0.27; artemin 4.21 ± 0.55; neurturin 4.19 ± 1.3; GDNF 4.13 ± 0.93).
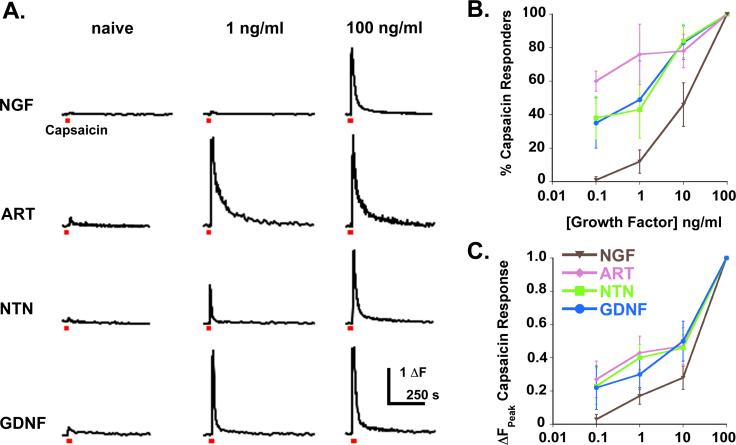
Further evidence of GFL efficacy in potentiating TRPV1 is provided by dose-response curves for the four growth factors (Figure 4) 35. At 0.1 ng/ml, NGF does not potentiate capsaicin responses in any cells, however artemin, neurturin and GDNF all potentiate many cells at this concentration. Even at 10ng/ml, GDNF family members are more broadly effective than NGF, potentiating most responsive cells (∼80% in each case) whereas NGF affects only 46% of responsive neurons. These data demonstrate that artemin, neurturin and GDNF are ∼10−100x more potent than NGF in potentiating capsaicin responses in sensory neurons.
Figure 4. GDNF-family members are more potent than NGF in TRPV1 potentiation.
Capsaicin-evoked Ca++ influx was recorded before and 10 minutes after perfusion of growth factors on isolated DRG neurons. Each growth factor concentration (0.1, 1, 10, 100ng/ml) was tested on individual coverslips, cells were allowed to recover for 30 minutes and maximal doses of each growth factor were given to identify all potential responders in the field and to determine maximal potentiation magnitude for each responsive cell. Representative traces are shown in A. Note that the magnitude of potentiation increases with increasing growth factor concentration (A and C), as does the number of cells potentiated (B). Although all four factors potentiated capsaicin-evoked responses, all GDNF family members were substantially more potent than NGF.
The majority of TRPV1-positive cells respond to both NGF and artemin
Because over 60% of all capsaicin-sensitive cells exhibit potentiation to either artemin or NGF, it seemed likely that some neurons would respond to both growth factors 35. By pairing each of the GDNF family members with NGF we found that most cells (63%) cells potentiated by NGF were also potentiated by artemin. NGF/GDNF or NGF/neurturin overlap was rarely detected (10% and 5%, respectively), whereas there was almost total overlap in GDNF- and neurturin-responsive subsets of cells (80% of neurturin-responsive cells were also GDNF-sensitive). These data define at least 2 subsets of TRPV1-positive sensory neurons: a major population responsive to NGF and artemin, presumably co-expressing TrkA and GFRα3, and minor population responsive to neurturin and GDNF, presumably expressing GFRα1 and/or GFRα2.
Alterations in expression of NGF and GFL during inflammation
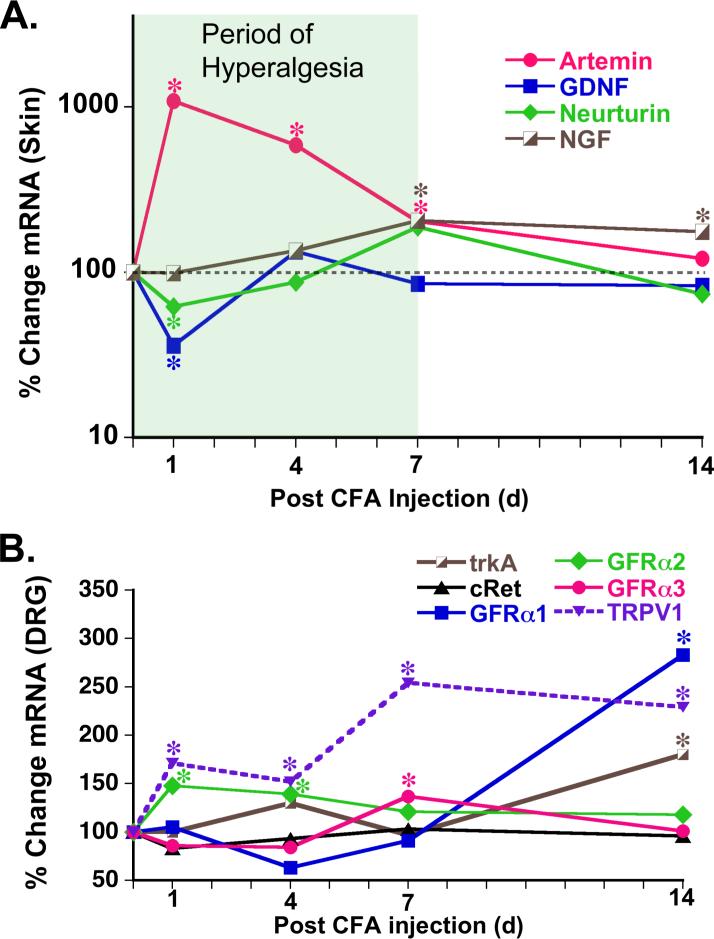
NGF is proposed to play a central role in inflammation-induced hyperalgesia because it is up-regulated following injury and that this change lasts for the duration of hyperalgesia. As GFLs are even more effective than NGF at potentiating the response of putative nociceptors to capsaicin, the expression levels of artemin, neurturin and GDNF mRNA in footpad skin were examined during complete Freunds adjuvant (CFA) -induced inflammation 35. CFA injection causes thermal hyperalgesia with a consistent time course that begins within 1 day and lasts for 7−10 days 37, 38. During this time period, mRNA for all four growth factors are dynamically regulated. NGF mRNA increases slowly over the first 7 days to 2-fold over naive levels (Figure 5). In contrast, artemin increases 10-fold in the first 24 hr and remained elevated for 7 days. GDNF and neurturin expression decreases after CFA: GDNF between days 1 and 4, and neurturin at day 1 and during the second week of inflammation. However, the increase in artemin is more robust within the first 24 hours of hyperalgesia development. In contrast, the increase in NGF mRNA is not significant (at least in our hands) until day 4 post inflammation.
Figure 5. Growth factors are up-regulated in the skin during inflammation.
Time course and magnitude of changes in growth factor mRNA levels in hindpaw skin during inflammation evoked by injection of CFA and measured by 32P-RT-PCR (A). Shaded box indicates the period of thermal hyperalgesia recorded in a previous experiment 38. Artemin is most dramatically upregulated (10-fold) at day 1 following CFA injection; this immediately precedes the time period of greatest thermal hyperalgesia in CFA-induced inflammation (1−4 days). NGF was also upregulated, but at later time points (4, 7, 15 days) and to a lesser degree (2-fold) than artemin. B. Expression levels of growth factor receptors and TRPV1 were measured using real-time PCR in L3,4,5 DRG during inflammation evoked by CFA injection. GFRα2, the neurturin receptor, is upregulated during the first week of inflammation; GFRα3, the artemin receptor, is upregulated at day 7, during the period of intense thermal hyperalgesia. Expression of TrkA, the NGF receptor, and GFRα1 are not changed until day 14. *p < .05 vs. naive
GFL directly induce thermal hypersensitivity in vivo
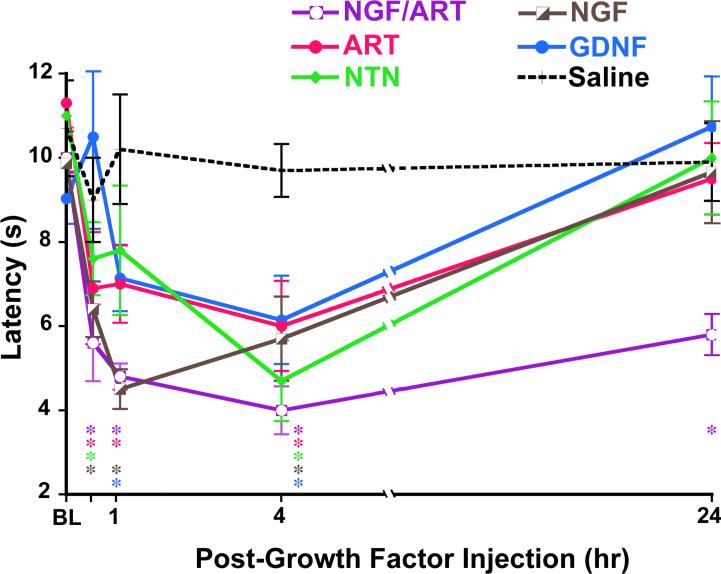
A number of cytokines and growth factors are known to be increased by inflammation. These compounds can be directly injected into the mouse hindpaw thermal or mechanical hypersensitivity monitored to probe the direct contribution of individual factors to hyperalgesia. This has been done previously for NGF that has been shown to increase thermal sensitivity 39. Injection of artemin, neurturin and GDNF (0.2mg/20ml) are also capable of increasing thermal sensitivity in vivo (Figure 9) 35. The time course of thermal hypersensitivity induced by different growth factors were distinct and all effects resolved in 24 hrs. At 30 minutes, 1 hr and 4 hr post-growth factor injection, NGF, neurturin and artemin all induced significant (p < .01) thermal hyperalgesia compared to naive and contralateral thermal thresholds. GDNF effects took longer to develop: GDNF-induced thermal hyperalgesia was not evident until 1 hr post-injection. Surprisingly, combined injection of NGF and artemin, both of which are upregulated in inflamed skin (see Figure 6), resulted in significant (p < .005) thermal hyperalgesia lasting 7 days.
Figure 6. Growth factors acutely potentiate heat responses in vivo.
Latency to thermal nociceptive response was measured in mice using the Hargreaves apparatus before and after injection of 0.2mg/20ml growth factor (or saline control) into the glabrous skin of the hindpaw. All growth factors tested (NGF, artemin, neurturin, GDNF) caused significant thermal hyperalgesia during the first 4 hours; these effects of single growth factor injection resolved by 24 hours post-injection. Co-injection of NGF and artemin (0.2mgNGF/0.2mg artemin/20mlPBS) resulted in persistent thermal hyperalgesia lasting 6 days. These data suggest that GDNF family members, as well as NGF, directly potentiate nociceptive thermal signaling in vivo. Additionally, the effects of growth factors on thermal signaling are additive in vivo. *p < .05 vs. saline control injection
Implications for future studies
That GFLs produce hyperalgesia was not predicted by at least 3 other studies. In the first, artemin was actually shown to reverse hyperalgesia produced by spinal nerve ligation in rat 40. In these studies, injection of artemin in the region of the nerve injury (over the hip) was effective when given before or after spinal nerve ligation. Gardell et al. (2003) reported no change in thermal or mechanical sensitivity after their artemin injection. However, they did not test thermal hyperalgesia at the site of artemin injection, as in the experiments discussed above. Perhaps the acute affects of artemin we observed represent local action of artemin on nociceptors in the glabrous skin that was directly tested in the Hargreaves apparatus. Also, Gardell and colleagues used artemin concentrations that were 300 times greater than that used in this study 40; this difference raises the possibility that high artemin concentrations have other effects which mask the hyperalgesic action. Finally, these investigators only tested their animals 24 hrs after artemin injection, at a time that our studies showed the hyperalgesia produced by artemin had been resolved. However, it should also be noted that a similar rat study found that artemin injection (either i.p.(5μm/kg) or i.t.(10μg/day)) in rats did not block hyperalgesia produced by spinal nerve ligation (no mention was made as to whether hyperalgesia developed in these animals) 41. A separate study by McMahon and colleagues compared intrathecal injections of GDNF to NGF. Both growth factors could increase the expression of CGRP, but only NGF produced hyperalgesia 42. Again, the difference between this study and those described by Malin and coworkers may be due to the site of growth factor injection. Our conclusion from these conflicting results is that site of injection of these growth factors is critical in terms of their behavioral effects. We are confident that peripheral administration of these GFLs can produce hyperalgesia as almost identical results have been obtained by independent laboratories (R. Torres, Regeneron Inc. personal communications).
It might seem that results by Malin et al., 35 diminish the importance of NGF as the crucial growth factor regulating inflammatory hyperalgesia. However, our interpretation is that that these studies do not minimize the importance of NGF as much as they refocus our attention on a unique population of nociceptors that not only respond to NGF, but also respond to artemin and express TRPV1. These nociceptors respond to two growth factors that are upregulated during inflammation and express a multifunctional receptor (TRPV1) that appears to be required for inflammatory thermal hyperalgesia; similar requirements for mechanical hypersensitivity are not clear. These neurons innervate virtually every tissue in the body, but are especially abundant among visceral and muscle afferents (Malin, unpublished). Thus, one potentially fruitful area for the development of new pain therapeutics would be the identification of compounds that specifically target these neurons (e.g. via GFRα3) or via combination therapy that decreases circulating levels of NGF and artemin (e.g. via anti-NGF/artemin antibodies).
References
- 1.Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci. 1994;14(3 Pt 2):1422–32. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76(6):1001–11. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 3.Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci. 2006;26(11):2981–90. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, et al. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26(33):8578–87. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, et al. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22(10):4057–65. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies AM. Studies of neurotrophin biology in the developing trigeminal system. J Anat. 1997;191(Pt 4):483–91. doi: 10.1046/j.1469-7580.1997.19140483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farinas I, Cano-Jaimez M, Bellmunt E, Soriano M. Regulation of neurogenesis by neurotrophins in developing spinal sensory ganglia. Brain Res Bull. 2002;57(6):809–16. doi: 10.1016/s0361-9230(01)00767-5. [DOI] [PubMed] [Google Scholar]
- 8.Kirstein M, Farinas I. Sensing life: regulation of sensory neuron survival by neurotrophins. Cell Mol Life Sci. 2002;59(11):1787–802. doi: 10.1007/PL00012506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors, and pain. Microsc Res Tech. 1999;45(4−5):252–61. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<252::AID-JEMT9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Saarma M, Sariola H. Other neurotrophic factors: glial cell line-derived neurotrophic factor (GDNF). Microsc Res Tech. 1999;45(4−5):292–302. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<292::AID-JEMT13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, et al. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19(4):849–61. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 12.Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382(6586):76–9. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 13.Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, et al. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35(2):267–82. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- 14.Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, et al. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22(2):253–63. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- 15.Stucky CL, Rossi J, Airaksinen MS, Lewin GR. GFR alpha2/neurturin signalling regulates noxious heat transduction in isolectin B4-binding mouse sensory neurons. J Physiol. 2002;545(Pt 1):43–50. doi: 10.1113/jphysiol.2002.027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindfors PH, Voikar V, Rossi J, Airaksinen MS. Deficient nonpeptidergic epidermis innervation and reduced inflammatory pain in glial cell line-derived neurotrophic factor family receptor alpha2 knock-out mice. J Neurosci. 2006;26(7):1953–60. doi: 10.1523/JNEUROSCI.4065-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albrecht PJ, Hines S, Eisenberg E, Pud D, Finlay DR, Connolly MK, et al. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120(3):244–66. doi: 10.1016/j.pain.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Oaklander AL, Rissmiller JG, Gelman LB, Zheng L, Chang Y, Gott R. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy). Pain. 2006;120(3):235–43. doi: 10.1016/j.pain.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 19.Janig W, Baron R. Is CRPS I a neuropathic pain syndrome? Pain. 2006;120(3):227–9. doi: 10.1016/j.pain.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci. 1994;6(12):1903–12. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 21.Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, et al. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology. 1997;48(2):501–5. doi: 10.1212/wnl.48.2.501. [DOI] [PubMed] [Google Scholar]
- 22.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 23.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 24.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 25.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–7. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 26.Shu XQ, Mendell LM. Neurotrophins and hyperalgesia. Proc Natl Acad Sci U S A. 1999;96(14):7693–6. doi: 10.1073/pnas.96.14.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274(3):159–62. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- 28.Shu X, Mendell LM. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol. 2001;86(6):2931–8. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- 29.Zhu W, Galoyan SM, Petruska JC, Oxford GS, Mendell LM. A developmental switch in acute sensitization of small dorsal root ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J Neurophysiol. 2004 doi: 10.1152/jn.00356.2004. [DOI] [PubMed] [Google Scholar]
- 30.Bennett DL, Boucher TJ, Armanini MP, Poulsen KT, Michael GJ, Priestley JV, et al. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci. 2000;20(1):427–37. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baudet C, Mikaels A, Westphal H, Johansen J, Johansen TE, Ernfors P. Positive and negative interactions of GDNF, NTN and ART in developing sensory neuron subpopulations, and their collaboration with neurotrophins. Development. 2000;127(20):4335–44. doi: 10.1242/dev.127.20.4335. [DOI] [PubMed] [Google Scholar]
- 32.Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18(8):3059–72. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13(11):2177–82. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- 34.Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19(5):1844–54. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, et al. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26(33):8588–99. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2(1):152–60. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- 37.Fairbanks CA, Schreiber KL, Brewer KL, Yu CG, Stone LS, Kitto KF, et al. Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proc Natl Acad Sci U S A. 2000;97(19):10584–9. doi: 10.1073/pnas.97.19.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwick M, Molliver DC, Lindsay J, Fairbanks CA, Sengoku T, Albers KM, et al. Transgenic mice possessing increased numbers of nociceptors do not exhibit increased behavioral sensitivity in models of inflammatory and neuropathic pain. Pain. 2003;106(3):491–500. doi: 10.1016/j.pain.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Amann R, Schuligoi R, Herzeg G, Donnerer J. Intraplantar injection of nerve growth factor into the rat hind paw: local edema and effects on thermal nociceptive threshold. Pain. 1996;64(2):323–9. doi: 10.1016/0304-3959(95)00120-4. [DOI] [PubMed] [Google Scholar]
- 40.Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, et al. Multiple actions of systemic artemin in experimental neuropathy. Nat Med. 2003;9(11):1383–9. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- 41.Bolon B, Jing S, Asuncion F, Scully S, Pisegna M, Van GY, et al. The candidate neuroprotective agent artemin induces autonomic neural dysplasia without preventing peripheral nerve dysfunction. Toxicol Pathol. 2004;32(3):275–94. doi: 10.1080/01926230490431475. [DOI] [PubMed] [Google Scholar]
- 42.Ramer MS, Bradbury EJ, Michael GJ, Lever IJ, McMahon SB. Glial cell line-derived neurotrophic factor increases calcitonin gene-related peptide immunoreactivity in sensory and motoneurons in vivo. Eur J Neurosci. 2003;18(10):2713–21. doi: 10.1111/j.1460-9568.2003.03012.x. [DOI] [PubMed] [Google Scholar]