Abstract
Objectives
The objectives were to determine the time course for ovarian failure in rats caused by 4-vinylcyclohexene diepoxide (VCD) and develop a model for ovarian cancer in which ovarian neoplasms were chemically induced in an animal that was follicle depleted, but retained residual ovarian tissue.
Methods
Initially, female Fisher 344 rats were treated with VCD (to induce ovarian failure) or vehicle control (sesame oil). Three or six months after treatment ovaries were collected and processed for histological evaluation for confirmation of ovarian failure.
A further set of female rats was assigned to four groups exposed to combinations of vehicle control, VCD and/or DMBA (directly applied to the ovary) in a novel model for examining early stages of ovarian neoplasia.
Results
Three and six months following VCD dosing there was a significant reduction of ovarian weight and follicle number. Treatment with DMBA subsequent to VCD resulted in tumors in 42% of animals at three months and 57% at five months. All neoplasms were classified Sertoli-Leydig cell tumors (SLCT). No tumors occurred in animals treated with vehicle or DMBA alone.
Conclusions
These studies demonstrate that the VCD-treated rat can be used as a model for peri- and post-menopause. DMBA induction of ovarian neoplasms was greater in those rats treated with VCD. Whether this increase was due to tumor initiation by VCD or was the result of ovarian failure cannot be distinguished from these results. This represents the only animal model to date for sex cord stromal tumors.
Introduction
Ovarian cancer is the most lethal of gynecologic malignancies [1]. The incidence of ovarian cancer increases by about ten-fold in women during the peri- to post-menopausal period, when compared to younger women [2]. This increase is attributed, in part, to three major factors associated with ovarian senescence, depletion of oocytes, loss of ovarian steroid production, and increased circulating gonadotropic hormones resulting from loss of negative feedback from ovarian hormones on the pituitary [2]. Thus, development of an animal model for ovarian cancer in which ovarian failure has been induced and the animal is follicle depleted, but retains residual ovarian tissue would provide a model with improved physiological relevance compared with a cycling animal.
Previous studies in rats and mice have shown that the occupational chemical, 4-vinylcyclohexene diepoxide (VCD) specifically targets and destroys primordial and primary follicles in rats and mice while leaving large pre-antral (secondary) and antral follicles unaffected [3, 4]. Mechanistic studies have determined that this selective follicle loss is due to enhancement of the natural process of atresia (apoptosis) [4–6]. Therefore, VCD has been used in mice to accelerate ovarian failure and generate an animal model for peri- and post-menopause [7]. Extensive investigation has determined that, whereas, VCD destroys small pre-antral follicles, it does not produce effects on larger follicles or any other tissues [3, 8], thus ovarian failure results only after secondary and antral follicles have become depleted via ovulation or atresia. Therefore, compared with ovariectomized animals more commonly used for modeling menopause, the VCD-induced ovarian failure model is more relevant to the study of post-menopause because the animal retains residual ovarian tissue. Furthermore, unlike the ovariectomized animal, in the VCD-treated animal, onset of ovarian failure is gradual, providing a model for the peri-menopausal transition [9]. Thus, adaptation of the VCD-treated animal to a relevant model for ovarian cancer would represent an important advancement and provide a model useful for developing diagnostic, therapeutic and preventative strategies.
In modeling ovarian cancer, recent approaches have induced ovarian tumors in rodents using carcinogens. One particularly promising approach for inducing epithelial ovarian cancer in rats has utilized direct application of 7,12-dimethylbenz[a]anthracene (DMBA) to the ovary [10–17].
The present study was designed to determine the time-course for impending VCD-induced ovarian failure in rats and apply the DMBA approach in those animals for the development of ovarian neoplasms. This will provide a more physiologically relevant animal model for ovarian cancer in peri/post-menopausal women when compared with that in cycling rats. The hypothesis being tested is that ovarian neoplasms can be induced more readily in animals that have undergone chemical-induced ovarian failure.
Materials and Methods
Animals
Female Fisher-344 rats (age d21) were purchased from Harlan, and housed and used in accordance with NIH guidelines and the policies of the University of Arizona Institutional Animal Care and Use Committee. Temperature, humidity, and photoperiod were constant (12 hr light, 12 hr dark at 22C). Animals were allowed access to food and water Ad Libitum. Rats were allowed to acclimate seven days before the experiment began.
Reagents
4-vinylcyclohexene diepoxide (VCD), 7,12-Dimethyl- benz[a]anthracene (DMBA) and sesame oil were purchased from Sigma Chemical Company (St. Louis MO). Tribromoethanol and 2-methyl-2-butanol were from Aldrich Chemical Company (St. Louis MO). Anti- inhibin-α antibody clone R1 and cytokeratin 7 were from Dako Inc. (Carpenteria CA). MAK-6 cocktail of antibodies (cytokeratins 8, 14, 15, 16, 18, and 19) was made at the University of Arizona.
Experiments 1 and 2
Dosing
Day 28 old rats were injected (i. p.) daily (25d) with sesame oil vehicle (2.5 ml/kg) or VCD (160 mg/kg, 1.14 mmol/kg). The day 25 time of dosing was chosen based on the results of a preliminary study comparing ovotoxicity in F344 rats following 25 or 30 days of repeated daily dosing with VCD (160 mg/kg; unpublished). Following 30 days of dosing, rats began to show adverse effects such as weight loss. There was no difference (p>0.05) in primordial/primary follicle loss between the two days. Thus, for the present study, 25 days of dosing was chosen.
Experiment 1
Three or six months after the end of VCD dosing, rats (n=4) were killed by CO2 inhalation and ovaries were collected, weighed and processed for histological evaluation.
Experiment 2
Four months after the end of VCD dosing, using a surgical approach, rats (n=9-14/treatment-timepoint) received a single injection of sesame oil (vehicle) or DMBA (100 μg DMBA in 10 μl) under the bursa of the right ovary. The left ovary was not injected. Anesthesia was induced by injection (i.p.) with 2% Avertin (stock solution:25 gm tribromoethanol in 15.5 ml 2-butanol; 2 ml stock in 100 ml sterile saline). After anesthesia (1.28 ml/100 gm body weight) right flank region skin was cleaned with Providone-iodine and 70% ethanol. The ovary was accessed by incising through the skin and muscle layer, located within the fat pad near the kidney, exteriorized, injected under the bursa, and returned intact to the body cavity. The muscle layer was closed with 3-0 braided silk suture and the skin closed with wound clips.
Experimental Design
Rats were assigned to one of four experimental groups. Group 1 (n=22) received sesame oil by i.p. injection (2.5 ml/kg/day; 25d) and a single ovarian injection of sesame oil (10 μl) four months after the end of dosing (Con/Con). Group 2 (n=18) received VCD (160 mg/kg/day i.p.; 25d) and a single ovarian injection of oil as in group 1 (VCD/Con). Group 3 (n=17) received oil by i.p. injection as in group 1 (25d) and a single ovarian injection of DMBA (100 μg in 10 μl) four months after the end of oil (Con/DMBA). Group 4 (n=26) received VCD as in group 2 (25d) and a single ovarian injection of DMBA as in group 3 (VCD/DMBA). Ovaries were collected three (n=8-12/treatment) or five (n=9-14/treatment) months post-surgery (seven or nine months, respectively, after the end of VCD dosing).
Histology
Ovaries were fixed in Bouin’s solution (2–4 h), transferred to 70% ethanol, dehydrated, embedded in paraffin blocks, sectioned (5 μm), and every 20th section was mounted, and stained with hematoxylin and eosin (H & E). In addition, unstained sections (2–4 sections/slide) were collected from the central portion of each ovary and mounted as serial sections on 5–10 slides for staining for immunohistochemistry.
Follicle Counting
Follicles were counted in ovaries stained with H & E.. Numbers of oocyte-containing follicles (showing a distinct oocyte nucleus) were classified and counted in every 20th section as previously described [18]. Briefly, primordial follicles contained a single layer of squamous granulosa cells and primary follicles a single layer of cuboidal granulosa cells. Secondary follicles contained multiple layers of granulosa cells and antral follicles contained at least two layers of granulosa cells and a fluid filled antral space.
Immunohistochemistry
Immunohistochemistry was performed using an ES immunostainer (Ventana) for 24 minutes. Two cytokerarin immunostains were used selectively; cytokeratin-7 antibody specific for ovarian surface epithelial cells and MAK-6 antibody cocktail. Antibody application was followed by a biotin-conjugated goat anti-mouse antibody, and avidin-D conjugated horse radish peroxidase and then 3,3′-diaminobenzidine tetrahydrochloride with copper enhancement as color substrate and hematoxylin as counterstain. After clearing in graded alcohols and xylene, slides were cover-slipped. Dark brown structures indicated positive immunostaining.
Tumor Analyses
Histological grade was assigned according to structural patterns of human Sertoli-Leydig cell tumors (SLCT) [19]: Grade 1 (well differentiated) predominantly tubular with no nuclear atypia or mitotic activity, and a prominent Leydig cell component; Grade 2 (intermediate differentiation): misshapen tubules with moderate nuclear atypia, 5–10 mitoses/10hpf, less frequent Leydig cell clusters, and occasionally hyperchromatic spindle cell stroma; Grade 3 (poorly differentiated): non-lobular with a predominant sarcomatoid composition; slit-like spaces or compact clusters of irregular shape were occasionally present; and mitoses were often >20/10hpf, and Leydig cells were scarce.
Statistical Analysis
Follicle numbers were determined in ovaries from individual animals, averaged, and the mean (±S.E.) in control versus treated animals in each group was calculated. Differences between groups were analyzed by two-way analysis of variance (ANOVA) with significance set at p < 0.05. Post-hoc tests (Fisher’s Protected Least Significant Difference) were used where appropriate.
Results
VCD-induced follicle depletion was performed in F344 female rats to determine an optimal time of impending ovarian failure in which DMBA could directly be applied to the ovary (Table 1, Figure 1). Three months following the end of the 25 days of VCD dosing there was a reduction (p<0.05) in follicles of all sizes, relative to age-matched (vehicle-treated) controls, whereas, by six months, no primordial or primary follicles remained and only a few secondary and antral follicles were observed (Table 1). Morphological appearance of VCD-treated ovaries demonstrated a lack of those functional structures that were observed in cycling controls at both time points (Fig. 1). Although no oocytes were observed in ovaries from VCD-treated animals, rings of residual granulosa cells were seen (figure 1B). Additionally, ovarian weight normalized to body weight (gm/gm X 100) in VCD-treated rats was reduced (p<0.05) at three months (cyclic controls, 0.036 ± .002; VCD, 0.022 ± 0.001) and six months (cycling controls, 0.029 ± 0.001; VCD, 0.011 ± 0.001 gm).
Table 1.
| 3 Months | 6 Months | |||
|---|---|---|---|---|
| Follicle Class | Controlc | VCD | Controlc | VCD |
| Primordial | 112.00±10.9 | 0.25±0.3* | 64.00±6.35 | 0.00* |
| Primary | 37.25±3.3 | 0.50±0.3* | 22.75±5.65 | 0.00* |
| Secondary | 9.50±1.8 | 0.25±0.3* | 9.25±1.11 | 0.50±0.5* |
| Antral | 8.25±0.9 | 1.5±0.3* | 6.50±1.85 | 1.5±1.19* |
daily dosing-vehicle control or VCD (160mg/kg-25 days)
n=4
age matched cycling controls
p<0.05 different from control
Figure 1.
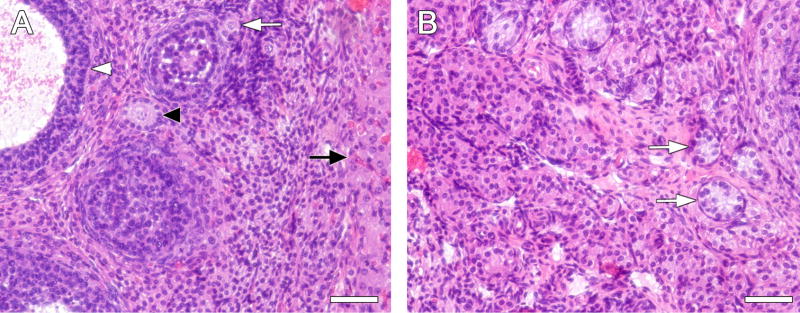
Effect of VCD dosing on ovarian follicles stained with hematoxylin and eosin. Female F344 rats were dosed daily (25d) with vehicle control (A) or VCD (B). Ovaries were collected 3 mo. following the end of VCD dosing, and prepared for histological evaluation as described in materials and methods. A) White arrow, primordial follicle; black arrowhead, primary follicle; white arrowhead antral follicle; black arrow, corpus luteum. B) White arrows, degenerative pre-antral follicles. Magnification = 200X. Scale bars (A and B) = 50 μm
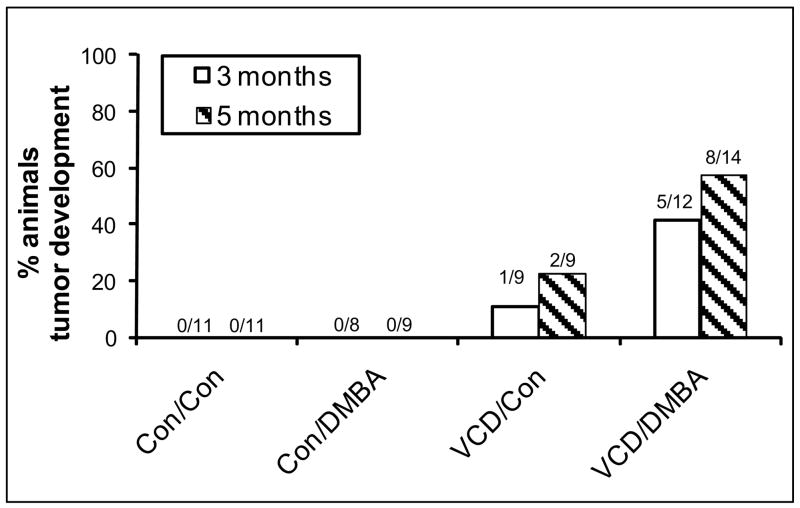
An evaluation of the ability of DMBA (directly applied to the right ovary) to cause tumor development in cycling control relative to VCD-treated rats was made(Figure 2). Ovaries were collected for histological evaluation at two time points following DMBA exposure, three months and five months. No tumors were recorded at either time point in cycling control (Con/Con) or DMBA-only treated (Con/DMBA) animals. Tumor development was observed in one of nine (11%) VCD-treated rats at three months, and in two of nine (22%) VCD-treated rats at five months (one bilaterally). In rats treated with VCD, then DMBA, tumors were seen in five of 12 animals (42%) at three months, and eight of 14 animals (57%) at five months. Table 2 lists the morphological characteristics of these tumors. A series of micrographs representative of their appearances is shown in Figure 3. All neoplasms were sex cord-stromal types with Sertoli and Leydig cell components (figure 3C–F). Characteristic for Sertoli-Leydig cell tumor classification was the presence of stromal lutenized cells, designated Leydig cells. Nests of Leydig cells had abundant cytoplasm with eosinophilic granules and uniform normochromatic nuclei with prominent nucleoli. Fewer Leydig cells were present in less differentiated tumors. No Reinke crystals were noted in Leydig cells; no heterologous tissue differentiation occurred involving the epithelial component. Most tumors were solid, a few were cystic. All neoplasms were confined to the ovary, except for one that had extension into the peri-ovarian fat. Neoplastic growth occurred in degenerative ovaries with loss of normal architectural features such as a loss of a peripheral rim of empty, small follicular remnants. Frequently neoplasms had nests of acinar/tubular epithelial-like structures reminiscent of follicle remnants without luminal lining. Similar neoplasms occurred following treatment with VCD/DMBA as well as with VCD alone. Epithelial variations other than sex cord occurred infrequently. The ovarian surface epithelium usually remained flat and inconspicuous, but occasionally was mildly proliferative (slight stratification without nuclear abnormality). In cycling control ovaries, extensive corpus luteum protrusion produced invagination-like clefts of the surface epithelium, but invaginations were lost as ovaries degenerated and lost nodularity. There was no indication of glandular inclusions on the ovarian surface but glandular inclusions were identified around the oviduct insertion where they showed columnar ciliated epithelium and in the hilum of the ovary, perhaps a remnant of rete ovarii of mesonephric origin. These non-neoplastic glands with distinct smooth luminal lining had non-neoplastic cuboidal/columnar cells.
Figure 2.
Incidence of tumor development. Female F344 rats were dosed daily (25d) with vehicle control (Con) or VCD. Four months following the final dose of VCD, surgery was performed for injection of vehicle control (Con) or DMBA under the bursa of the right ovary. Three or five months following exposure to DMBA ovaries were collected and prepared for histological evaluation or immunohistochemistry as described in materials and methods. Bars represent per cent of animals that developed tumors in each group. Numbers over the bars are the animal number with tumors versus total animals in that group. Open bars, three months; hatched bars, five months.
Table 2.
Morphological characteristics of ovarian tumors in individual animals
| Treatment | Grade | Size | Lesion |
|---|---|---|---|
| VCD/Con 3 months. | 3 | 1.5 mm | solid |
| VCD/Con 3 months. | 3 | 1.5 mm | solid |
| VCD/Con 3 months. Bilateral | 2 and 2 | 1.0 and 0.7 mm | solid |
| VCD/DMBA 3 months. | 3 | 2.0 mm | solid |
| VCD/DMBA 3 months. | 3 | 3.0 mm | solid |
| VCD/DMBA 3 months. | Too early | 2.0 mm | early |
| VCD/DMBA 3 months. | Too early | <1.0 mm | early |
| VCD/DMBA 3 months. | 3 | 0.5 mm | cystic |
| VCD/DMBA 5 months. | 3 | 2.0 mm (multifocal) | solid |
| VCD/DMBA 5 months. | 3 | 7.0 mm | cystic |
| VCD/DMBA 5 months. | 3 | 4.0 mm | solid/cystic |
| VCD/DMBA 5 months. | 3 | 5.0 mm | solid |
| VCD/DMBA 5 months. | 3 | 7.0 mm | solid |
| VCD/DMBA 5 months. | 1 and 2 | 4.0 mm | cystic |
| VCD/DMBA 5 months. | 3 | 5.0 mm | solid/cystic |
| VCD/DMBA 5 months. | 3 | 2.0 mm | solid |
| VCD/DMBA 5 months. | 2 | 2.0 mm | solid |
Figure 3.
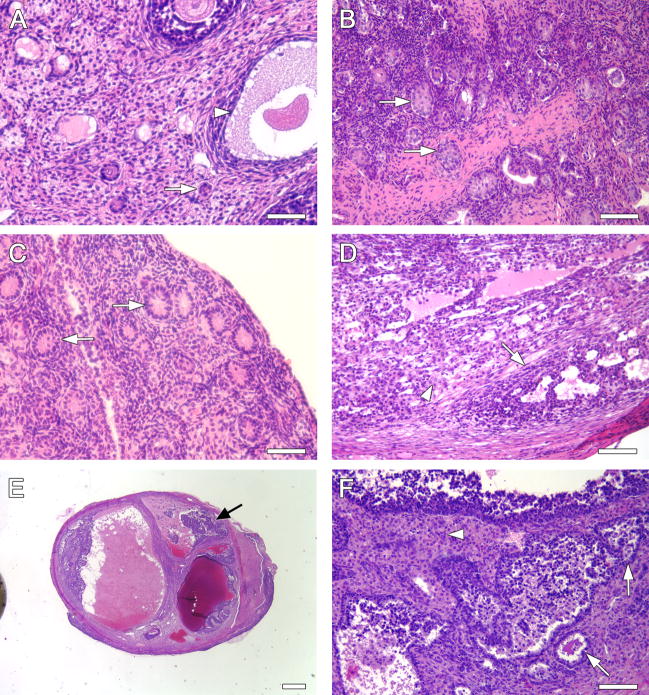
Representative ovaries stained with hematoxylin and eosin. A) Con/Con, 3mo. Arrow; primordial follicle, arrowhead, antral follicle. B) VCD/Con, 3 mo. Arrows, degenerative pre-antral follicles. C) Con/DMBA, 5 mo. Arrows, degenerative pre-antral follicles. D) VCD/DMBA, 3 mo. Intermediate differentiated Sertoli-Leydig cell tumor. Arrow, Sertoli cells with irregular tubule formation; arrowhead, nests of Leydig cells. E) VCD/DMBA, 5 mo. Ovary with cystic Sertoli-Leydig cell tumor (black arrow). F) same tumor as in E at higher magnification. Intermediate differentiated tumor with irregular spaces formed by Sertoli cells (arrows) and aggregates of Leydig cells (arrowhead). Magnification A–D, F = 200X; E = 20X. Scale bars (A–D, F) = 50 μm. E = 500 μm
To assist in classification of tumor origin, immunohistochemistry (IHC) for α-inhibin was performed as a marker for sex cord stromal tumor (Figure 4). Cytokeratin-7 was used as a marker for ovarian surface epithelium. In all tumors, staining was positive for α-inhibin and negative for cytokeratin MAK-6.
Figure 4.
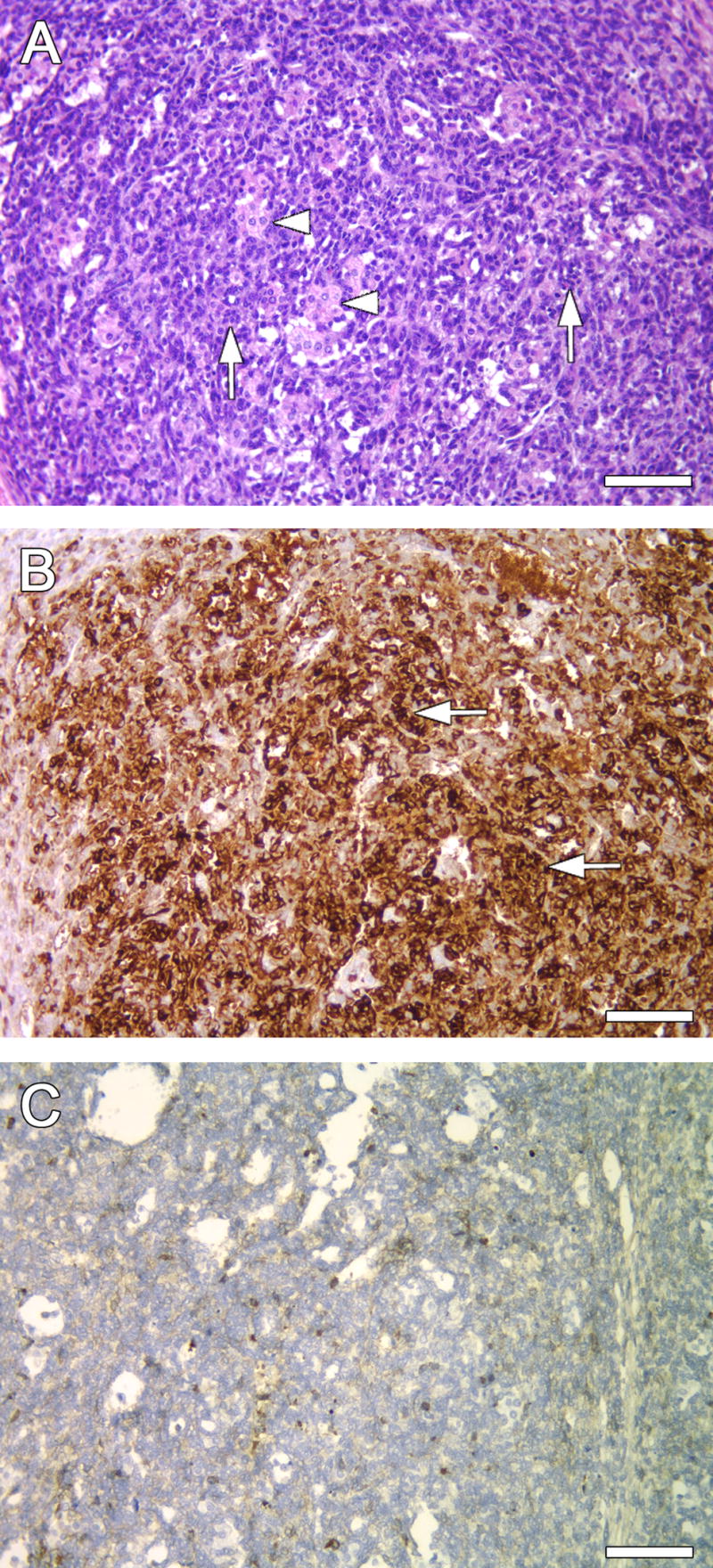
Immuno-staining for cell markers in representative VCD/DMBA (3 month) ovary. A) Poorly differentiated tumor with diffuse, sarcomatoid Sertoli component (arrows) and nests of Leydig cells (arrowheads). B) Same ovary as in A, Sertoli cells staining strongly positive for α-inhibin (arrows). C) Same ovary as in A, negative staining for cytokeratin. Magnification 200X. Scale bars (A–C) = 50 μm.
Discussion
In the mouse model for VCD-induced ovarian failure, repeated daily dosing of mice with VCD (15d) resulted in onset of ovarian failure at 59 days following the onset of dosing (1.5 months after dosing had stopped) [7]. Mice are known to be more susceptible to ovotoxic effects of VCD than rats [20]. Thus, in rats, a longer period of dosing (25 days) was required and a longer time to onset of ovarian failure resulted (> six months after dosing had stopped). The three to six month range of time following VCD dosing showed similar degrees of impending ovarian failure, although complete ovarian failure had not occurred. Therefore, this range of time would represent the late peri-menopausal to post-menopausal transition in women [21]. Four months after VCD dosing was chosen as the optimal time for application of DMBA.
Three and five months after DMBA exposure (seven and nine months after VCD dosing, respectively), rats were evaluated for development of neoplasms. These time points following exposure to DMBA are earlier than reported tumors in previous studies (10–15). Because no previous studies had used DMBA in rats that had undergone ovarian senescence, earlier time points were used to determine whether tumor development would be accelerated following ovarian failure. Presumably, if longer time points had been used, tumor development in DMBA/cycling animals would also have been observed. Interestingly, in a previous study, DMBA in cycling animals [10] did not produce tumors until six months (22% animals developing tumors). Therefore, the development of neoplasms (57% of animals) at five months in DMBA-treated animals that had undergone ovarian failure (VCD-treated), demonstrates an increased susceptibility to DMBA. Whether the increase in DMBA-induced tumors is due to VCD acting as a tumor initiator for DMBA (promoter) or relates to increased sensitivity to DMBA resulting from ovarian failure cannot be determined from this study. Ovarian neoplasm development was seen in three animals (16%) treated with VCD alone. Whether this was due to direct VCD effects or the result of ovarian failure must be determined by further studies.
Previous studies utilized DMBA to generate ovarian adenocarcinomas in rats [10–15], by using direct exposure of ovaries to the carcinogen either by introduction of a DMBA-saturated suture under the ovarian surface [10–13] or by injection of DMBA directly into the ovary [14, 15] in cycling animals. Tumor development and/or progression ranged from 22% of animals at 6–12 months [10] to 77% of animals at one year [13]. Cellular origin of the tumors was somewhat variable, however, most of the studies reported at least some of the tumors originating from the surface epithelium, based on morphological appearance or immunostaining for cytokeratin, a marker of ovarian surface epithelium [10,13]. Tumor progression was enhanced by subsequent exposure of animals to 17β-estradiol [11], or repetitive gonadotropin hormone stimulation [10], whereas, exposure to various endocrine disrupting chemicals retarded carcinogenesis [15]. Interestingly, one study removed an ovary prior to exposure of the rat to DMBA with the specific purpose of concentrating ovulation upon the remaining ovary and hastening a senescent hormonal milieu [11] because of the acknowledged benefit of developing a postmenopausal model of the increased incidence of cancer in this population [2].
The DMBA carcinogenic effect in rats has previously been classified as adenocarcinoma rather than sex cord-stromal in nature. Nishida et al.[12] noted neoplasms in 19 of 40 rats (47.5%). Seventeen were classified as adenocarcinoma, and two as fibrosarcoma. In 2002 Tanaka et al. [15] reported seven of 20 rats (35%) with adenocarcinoma 51 weeks following injection of the ovary with DMBA. In 2004 Tanaka et al. [14], reported 9/20 rats (45%) treated with DMBA developed adenocarcinoma. Crist et al. [13] reported 23/30 (77%) ovarian neoplasms; nine adenocarcinomas, eight granulosa-thecal tumors, five sarcomas and one undifferentiated carcinoma but morphologic illustrations do not exclude sex cord types for some cases. In the present study, tumors were classified as sex cord-stromal of the Sertoli-Leydig cell type due to the presence of both epithelial and stromal luteinized cells [19] as well as by the presence of strong α-inhibin staining in all tumors.
None of the previous studies included rats with VCD-induced ovarian failure nor did they describe SLCT’s as were observed in this study and they described a longer exposure time to DMBA of 6–13 months compared to the 3–5 months exposure in our study [10, 13, 15]. Since none of the prior studies included α-inhibin immunostaining, carcinogenic effects of DMBA may have included tumors similar to the type observed in this study. Alpha-inhibin immunostaining is critical for separating adenocarcinomas (negative for α-inhibin) from sex cord-stromal tumors (expressing α-inhibin) because histologic evaluation, particularly of high grade tumors, does not readily differentiate adenocarcinoma from sex cord stromal tumors [22, 23]. Inhibin, especially α-inhibin, has been found to be the most useful immunomarker to date for sex cord stromal tumors.
Cytokeratin-7, a specific marker for surface epithelial cells, but negative in sex cord tumors was not used in the other studies [10, 13], instead Pan-cytokeratin was used as the cytokeratin antigen, which may show overlapping patterns of expression with other ovarian tumors. Sertoli cell tumors can express epithelial markers such as cytokeratin AE1/AE3 and CK8/18) in 38% to 100% of cases [24]. Therefore, in the absence of staining for inhibin, it is difficult to differentiate adenocarcinoma from SLCT’s and other sex cord stromal tumors.
The effect of a variety of carcinogens on the rodent ovary is similar in that follicular destruction precedes follicular cell proliferation and neoplasia [16,17]. Thus, tumor origin may be related more to perturbation of follicular tissue than disruption of the surface epithelium. In the present study, minor effects on the surface epithelium were occasionally noted (focal hyperplasia, invaginations and microcysts, fibrosis and fibrous adhesions), but they were not related to the observed tumors.
In human ovaries, Sertoli-Leydig tumors comprise <0.5% of neoplasms [19]. They are confined to the ovary in 97% of cases. Only one animal in the present study demonstrated extension to peri-ovarian adipose tissue at five months. This lack of metastasis supports our conclusion that the tumors produced were consistent with SLCT’s and not adenocarcinomas, as 97% of SLCT’s are confined to the ovary. This is in contrast to epithelial ovarian cancers which readily metastasize early, resulting in late stage diagnosis in the majority of the epithelial cancers in women.
In summary, these studies demonstrate that the rat can be used as a VCD model for peri-and post-menopause, although onset to ovarian failure is substantially longer than that seen in mice. DMBA induction of ovarian neoplasms was only observed in those rats with VCD-induced ovarian failure. This approach provided an earlier onset of tumor development compared with previous studies using cycling rats. Therefore, this model may be useful for developing early screening methods or biomarkers for early detection of menopause-associated ovarian cancer in particular SLCT’s in women.
Acknowledgments
These studies were supported by R01s AG021948 (PBH) and CA119200 (JKB) and center grant ES06694. We wish to thank Andrea Grantham for preparation of tissues for histological evaluation, Doug Cromey for help with the micrographs, and Nivedita Sen for help with the figures.
Footnotes
Conflict of Interest Statement:
The authors declare that there are no conflicts of interest.
References
- 1.Stakleff KD, Von Gruenigen VE. Rodent models for ovarian cancer research. Int J Gynecol Cancer. 2003;13:405–412. doi: 10.1046/j.1525-1438.2003.13317.x. [DOI] [PubMed] [Google Scholar]
- 2.Vanderhyden BC. Loss of ovarian function and the risk of ovarian cancer. Cell Tiss Res. 2005;322:117–124. doi: 10.1007/s00441-005-1100-1. [DOI] [PubMed] [Google Scholar]
- 3.Kao S, Sipes I, Hoyer PB. Early effects of ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod Toxicol. 1999:67–75. doi: 10.1016/s0890-6238(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 4.Springer L, McAsey M, Flaws JA, Tilly J, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmicol. 1996;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- 5.Hu X, Christian PJ, Thompson KE, Sipes IG, Hoyer PB. Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol Reprod. 2001;65:87–93. doi: 10.1095/biolreprod65.1.87. [DOI] [PubMed] [Google Scholar]
- 6.Hu X, Christian PJ, Flaws JA, Sipes IG, Hoyer PB. Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2002;67:718–724. doi: 10.1095/biolreprod.102.004259. [DOI] [PubMed] [Google Scholar]
- 7.Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-depleted mouse ovary produces androgen. Biol Reprod. 2004;71:130–138. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 8.Wright LE, Christian PJ, Rivera Z, Van Alstine WG, Funk JL, Bouxsein ML, Hoyer PB. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Mineral Res. 2008;23:1296–1303. doi: 10.1359/jbmr.080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohff JC, Christian PJ, Marion SL, Hoyer PB. Effect of duration of dosing on onset of ovarian failure in a chemical-induced mouse model of perimenopause. Menopause. 2006;13:482–488. doi: 10.1097/01.gme.0000191883.59799.2e. [DOI] [PubMed] [Google Scholar]
- 10.Stewart SL, Querec TD, Ochman AR, Gruver BN, Bao R, Babb JS, Wong TS, Koutroukides T, Pinnola AD, Klien-Szanto A, Hamilton TC, Patriotis C. Characterization of a carcinogenesis rat model of ovarian preneoplasia and neplasia. Cancer Res. 2004;64:8177–8183. doi: 10.1158/0008-5472.CAN-04-1702. [DOI] [PubMed] [Google Scholar]
- 11.Ting AY, Kimler BF, Fabian CJ, Petroff BK. Characterization of a preclinical model of simultaneous breast and ovarian cancer progression. Carcinogenesis. 2007;28:130–135. doi: 10.1093/carcin/bgl140. [DOI] [PubMed] [Google Scholar]
- 12.Nishida T, Sugiyama TO, Kataoka A, Ushijima K, Yakushiji M. Histologic characterization of rat ovarian carcinoma induced by intraovarian insertion of a 7,12-dimethylbenz[a]anthracene-coated suture. Cancer. 1998;83:965–970. doi: 10.1002/(sici)1097-0142(19980901)83:5<965::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Crist KA, Zahng Z, You M, Gunning WT, Conran PB, Steele VE, Lubet RA. Characterization of rat ovarian adenocarcinomas developed in response to direct instillation of 7,12-dimethylbenz[a]anthracene (DMBA) coated suture. Carcinogenesis. 2005;26:951–957. doi: 10.1093/carcin/bgi039. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Kohno H, Suzuki R, Sugie S. Lack of modifying effects of an estrogenic compound atrazine on 7,12-dimethylbenz[a]anthracene-induced ovarian carcinogenesis in rats. Cancer Lett. 2004;210:129–137. doi: 10.1016/j.canlet.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Kohno J, Tanino M, Yanaida Y. Inhibitory effects of estrogenic compounds, 4-nonylphenol and genistein, on 7,12-dimethylbenz[a]anthracene-induced ovarian carcinogenesis in rats. Ecotoxicol and Environ Safety. 2002;52:38–45. doi: 10.1006/eesa.2002.2159. [DOI] [PubMed] [Google Scholar]
- 16.Kuwahara I. Experimental induction of ovarian tumors in mice treated with single administration of 7, 12-dimethylbenz[a]anthracene, and it’s histopathological observation. GANN. 1967;58:253–266. [PubMed] [Google Scholar]
- 17.Krarup T. Oocyte destruction and ovarian tumorigenesis after direct application of a chemical carcinogen (9:10-dimethyl-1:2-benzanthrene) to the mouse ovary. Int J Cancer. 1969;4:61–75. doi: 10.1002/ijc.2910040109. [DOI] [PubMed] [Google Scholar]
- 18.Flaws J, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide-induced ovotoxicity in rats. Reprod toxicol. 1994;8:509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 19.Young RH, Scully RE. Sex cord-stromal, steroid cell, and other ovarian tumors. In: Kurman R, editor. Blaustein’s Pathology of the Female Genital Tract. 5. New York: Springer-Verlag; 2002. pp. 905–966. [Google Scholar]
- 20.Smith BJ, Mattison DR, Sipes IG. The role of epoxidation in 4-vinylcyclohexene-induced ovarian toxicity. Toxicol Appl Pharmicol. 1990;105:372–381. doi: 10.1016/0041-008x(90)90141-g. [DOI] [PubMed] [Google Scholar]
- 21.Haas JR, Christian PJ, Hoyer PB. The effects of VCD-induced impending ovarian failure on fertility in C57Bl/6 female mice. Comp Med. 2007;57:443–449. [PubMed] [Google Scholar]
- 22.McCluggage WG. Recent advances in immunohistochemistry in the diagnosis of ovarian neoplasms. J Clin Pathol. 2000;53:327–334. doi: 10.1136/jcp.53.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deavers MT, Malpica A, Liu J, Broaddus R, Silva E. Ovarian sex cord-stromal tumors: an immunohistochemical study including a comparison of calretinin and inhibin. Mod Pathol. 2003;16:584–590. doi: 10.1097/01.MP.0000073133.79591.A1. [DOI] [PubMed] [Google Scholar]
- 24.Zhao C, Bratthauer GL, Barner R, Vang R. Comparative analysis of alternative and traditional immunohistochemical markers for the distinction of ovarian Sertoli cell tumor from endometrioid tumors and carcinoid tumor. Am J Surg Pathol. 2007;31:255–266. doi: 10.1097/01.pas.0000213355.72638.f4. [DOI] [PubMed] [Google Scholar]