Abstract
OBJECTIVE
We sought to determine if Chlamydia pneumoniae impairs invasive trophoblast function and is associated with preeclampsia.
STUDY DESIGN
We conducted cell viability and invasion assays using primary extravillous trophoblast cells isolated from first trimester placentas. We performed a case-control study to identify C. pneumoniae in trophoblast cells dissected by laser-capture microscopy from placentas in women with severe preeclampsia and controls who delivered at term.
RESULTS
Trophoblast cell viability and invasion through extracellular matrices were decreased after infection with C. pneumoniae (both P<0.05). C. pneumoniae DNA was detected in trophoblast cells in 15/48 cases but only 3/30 controls (OR=4.1, P=0.02). Positive and negative controls yielded expected results.
CONCLUSION
Chlamydia pneumoniae infection can reduce trophoblast invasion into the uterine wall and is associated with preeclampsia. Further investigation of the mechanisms by which C. pneumoniae induces trophoblast dysfunction, and the identification of therapies to prevent adverse outcomes attributed to trophoblast dysfunction, are warranted.
Keywords: adverse pregnancy outcome, Chlamydia pneumoniae, placental dysfunction, preeclampsia, trophoblast
Introduction
Preeclampsia is a hypertensive disorder that is exclusive to human pregnancy. Although the etiology is unknown, common pathologic features include decreased endovascular trophoblast migration and atherosclerotic changes in the uterine vasculature.1–3 Placental migration into the maternal uterine wall and blood vessels is mediated by extravillous (invasive) trophoblast cells. Failed invasion by extravillous trophoblasts leads to placental dysfunction and adverse obstetric outcomes associated with placental dysfunction, such as preeclampsia. Preeclampsia shares many risk factors and pathophysiological features with atherosclerotic vascular disease.
In the past two decades, researchers have reported an association between Chlamydia pneumoniae and vascular disease.4 Chlamydia pneumoniae is a common gram-negative, intracellular bacterium that is able to infect most of the cell types involved in atherogenesis, including endothelium, smooth muscle cells, and macrophages.5, 6 Seropositivity for antibodies against C. pneumoniae (IgG class) is correlated with coronary artery disease.7 Chlamydia pneumoniae is found more frequently in atherosclerotic vascular lesions than in vascular tissue from healthy controls.8 Furthermore, patients who are seropositive for C. pneumoniae antibodies have a significantly greater arterial intimal medial thickness than seronegative patients,9, 10
Dissemination of C. pneumoniae into the peripheral blood during pregnancy may expose the placenta to infection. Although C. pneumoniae DNA has been detected previously in placental tissue,11 and circulating maternal antibodies against C. pneumoniae have been found in patients with preeclampsia,12–14 the direct effects of the bacterium in trophoblast cells have not been investigated yet.
We hypothesize that placental infection with C. pneumoniae is not a rare event and may be associated with an increased risk of obstetric complications resulting from placental dysfunction, such as preeclampsia. Consequently, we sought to determine (i) if infection of extravillous trophoblast cells by C. pneumoniae reduces cell viability and/or reduces cell invasion, and (ii) if placental infection with C. pneumoniae is associated with preeclampsia.
Materials and Methods
Infection of extravillous trophoblast cells with Chlamydia pneumoniae
We used immortalized first trimester trophoblast cells (HTR-8/SVneo) and primary extravillous trophoblast cells in our experiments. The HTR-8/SVneo cells were generously provided by C. H. Graham (Queen’s University, Ontario, Canada). These cells, originally obtained from explant cultures of human first-trimester placenta,15 exhibit phenotypic characteristics of extravillous trophoblasts, including invasive properties through extracellular matrices.16 The cells were cultured at 37°C with Dulbecco modified Eagle medium, DMEM (Gibco BRL, Grand Island, NY), supplemented with 10 percent fetal bovine serum (FBS). All media contained gentamicin (100 μg/ml) and amphotericin B (2.5 μg/ml).
Primary extravillous trophoblast cells were isolated and propagated from first trimester placental tissues (8–13 wk gestation) as originally described by Graham et al and modified in our laboratory.17, 18 Briefly, finely minced chorionic villi were cultured at 37° C in DMEM containing 10 percent FBS. Extravillous trophoblast cells, which outgrew from attached villous fragments, were separated from villous trophoblast cells on the 10th to 12th day of culture. The isolated extravillous trophoblast cells were then seeded and propagated in the same cell culture medium. The extravillous trophoblast cells used in these experiments were characterized by immunostaining of cytokeratin filaments 8 and 18.19 Trophoblast cells were infected with cell-free C. pneumoniae TWAR strain TW 183 (ATCC VR2282). 20, 21 The infectivity, as determined by inclusion forming units (IFU) of bacterial preparation, was determined in HEp2 cells (a human epithelial cell line that is a known host for C. pneumoniae, ATCC CCL-23). Aliquots of 0.1 – 1.0 IFU/cell were used to infect duplicate cultures of HTR-8/SVneo cells overnight, after which culture medium was replaced with fresh DMEM not containing C. pneumoniae. Viability and invasion experiments were performed 24 and 48 hours after infection.
Alternatively, HTR-8/SVneo cells were incubated with infected C. pneumoniae-infected peripheral blood mononuclear cells (PBMCs). Chlamydia pneumonia isolates were propagated in PBMCs, which were isolated from healthy volunteers and processed as described elsewhere.22, 23 PBMCs were stimulated with phytohaemagglutinin and infected with 0.1 – 1.0 IFU/cell of C. pneumoniae by centrifugation for 1 hour, 500 g, at 37° C and then added to trophoblast cells overnight.24 Culture medium was replaced, and viability and invasion experiments were performed 24 and 48 hours after co-infection.
Assessment of Chlamydia pneumoniae infection in extravillous trophoblast cells
In order to confirm that C. pneumoniae could infect extravillous trophoblasts, infected cells were harvested for DNA extraction using Qiagen DNA mini kits (Qiagen, Valencia, CA). Two ng of extracted DNA were subjected to nested touchdown PCR to detect a portion of the major outer membrane protein (MOMP)-coding gene.25 The MOMP gene product is a key component in the structure of C. pneumoniae, because it maintains the structural integrity of the infectious elementary body form. PCR was performed to amplify a 333 base-pair (bp) fragment of the MOMP gene using outer primers CP1 and CP2 (Table 1). The initial PCR products were diluted 10 times in water and 5 μl of amplified product were used for a second PCR using the inner primers CPC and CPD to amplify a 207-bp fragment within the MOMP gene that is specific for C. pneumoniae. Amplification with primers specific for β-globin (S-GH20 and SPCO04) was used to confirm the presence of intact cellular DNA.26 Reactions were completed with Ready-to-Go PCR beads (Amersham Biosciences, Piscataway, NJ). In a final volume of 25 μl, each reaction contained 1.5 units of Taq DNA polymerase, 10mM tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, 200 μM of each dNTP (dATP, dGTP, dTTP, and dCTP) and 10 μl of each oligonucleotide (0.4 μM of CP1 and CP2 primers for the first step, and 1 μM of CPC and CPD primers for the second reaction). PCR amplification was performed in a thermocycler, GeneAmp PCR System 9700 (Amersham Biosciences) as shown in Table 1. Each set of PCR amplifications included positive controls (DNA extracted from HEp2 cells infected with C. pneumoniae, ATCC CCL-23) and negative controls (non-infected HTR-8/SVneo cells). A 10 μl aliquot of both the first and second reaction volume was subjected to electrophoresis in a 2 percent agarose gel containing ethidium bromide. Ultraviolet transillumination was used to identify the 333 bp and 207 bp C. pneumoniae MOMP band and a 268 bp β-globin band.
Table 1.
Oligonucleotides used for PCR analyses
| Target gene | Sequence 5′-3′ | PCR product (bp) | Cycles | Annealing temperature | References |
|---|---|---|---|---|---|
| MOMP1 | First step oligonucleotides CP1 TTA CAA GCC TTG CCT GTA GG CP2 GCG ATC CCA AAT GTT TAA GGC |
333 | 40 | Step down 65 - 55 | Tong and Sillis (23) |
| Second step oligonucleotides CPC TTA TTA ATT GAT GGT ACA ATA CPD ATC TAC GGC AGT AGT ATA GTT |
207 | 30 | 50 | ||
| β-globin | SGH20 GAA GAG CCA AGG ACA GGT AC PCO04 CAA CTT CAT CCA CGT TCA CC |
40 | 55 | Saiki et al (24) |
MOMP, major outer membrane protein
Functional assays
Cells and culture medium were collected to perform cell viability and invasion assays 24 and 48 hours after infection with cell-free C. pneumoniae or C. pneumoniae-infected PBMCs. Negative controls for these experiments consisted of non-infected primary extravillous trophoblast cells, non-infected HTR-8/SVneo cells, and cells co-cultured with non-infected PBMCs. All experiments were conducted in triplicate in two separate sets.
Cell viability based on lactate dehydrogenase (LDH) release was measured using Cyto Tox 96 Non-Radioactive Cytotoxicity Assay kit according to the manufacturer’s protocol (Promega, Madison, WI).27 Briefly, after collecting cell culture medium, infected cells and cells used as negative controls were lysed, and equal volumes (50 μl) of medium and lysis buffer (containing LDH released from lysed cells) were transferred to separate 96-well plates. After the addition of a reconstituted substrate mix provided with the kit, absorbance based on the reaction between LDH and substrate was recorded using a microplate reader at 490 nm. Results were obtained after subtracting background values; the absorbance of lysis buffer/absorbance of lysis buffer + cell culture medium ratio was calculated to determine the percentage of cells that remained viable at each time point.28
Invasion of infected trophoblast cells through an extracellular matrix (ECM) Matrigel was determined using Cell Invasion Assay Kits (Chemicon International, Temecula, CA) according to the manufacturer’s protocol. In these experiments, 1 × 106 cells/ml of infected and negative control cells were placed in invasion chambers at different time points after infection. After 48 hours, cells that invaded through the ECM Matrigel were stained with Cell Stain provided by the manufacturer and treated with 10 percent acetic acid. A volume of 150 μl of the dye/solute mixture was transferred to 96-well plates and invasion was measured by colorimetric absorbance at OD 560 nm. Levels of invasion were determined by comparing average OD values of infected cells to those of non-infected cells at day 0.28
Case-control study design
In order to associate C. pneumoniae infection of the placenta with preeclampsia, we conducted a case-control study in which we collected placentas from 78 subjects. Cases were defined as women who developed severe preeclampsia requiring delivery before 37 weeks’ gestation. Controls included women who delivered at term with no obstetrical or medical complications. Medical records were reviewed by a subspecialty certified perinatologist (SP) before subjects were included in the study. This study was approved by the Office of Regulatory Affairs at the University of Pennsylvania (protocol 700943), and informed consent was obtained from all subjects. Criteria for severe preeclampsia outlined by the American College of Obstetricians and Gynecologists included blood pressure ≥160/100 mmHg on two occasions sustained for ≥6 hours and proteinuria (≥5 grams in a 24-hour urine specimen or ≥3+ on two random urine samples).29 We excluded women with risk factors for developing preeclampsia, including multiple gestations, diabetes mellitus, systemic lupus erythematosus, other renal diseases, and chronic hypertension. Pregnant women with fetal malformations and sexually transmitted diseases during the index pregnancy also were excluded from the cohort.
Histological sections from the basal plate region of placentas from cases and controls were placed on membrane slides for microdissection by laser-capture microscopy (Molecular machines and Industries, Knoxville, TN). We isolated extravillous trophoblast cells from invasive trophoblast columns and villous trophoblast cells from the periphery of chorionic villi by using the PixCell II Laser Capture Microdissection System (Arcturus Engineering, Mountain View, CA). DNA was extracted from the laser-captured trophoblast cells using Qiagen DNA mini kits following the manufacturer’s instructions. PCR to detect a portion of the C. pneumoniae MOMP-coding gene was conducted as described above. Laser dissection and PCR assays were performed blinded to the samples from cases and controls.
Statistical analysis
Mean OD values and standard errors were calculated and compared between infected and control cells using analysis of variance (ANOVA). Mean, median values and standard deviations were utilized for comparing demographics and outcomes data between cases and controls. Chi-square tests or Fisher’s exact tests for dichotomous variables and t-tests for continuous variables were utilized to compare rates of C. pneumoniae DNA detection between cases and controls. A P-value of 0.05 was indicative of statistical significance.
Results
We successfully infected 1 × 105 HTR-8/SVneo cells and primary extravillous trophoblast cells with 1.0 IFU cell-free C. pneumoniae or 1.0 IFU C. pneumoniae-infected PBMCs. MOMP-coding gene sequences were detected by PCR at 24 and 48 hours after infection. Because trophoblast cells were successfully infected with cell-free C. pneumoniae and 1.0 IFU C. pneumoniae-infected PBMCs, we performed our functional assays using trophoblast cells infected with cell-free C. pneumoniae.
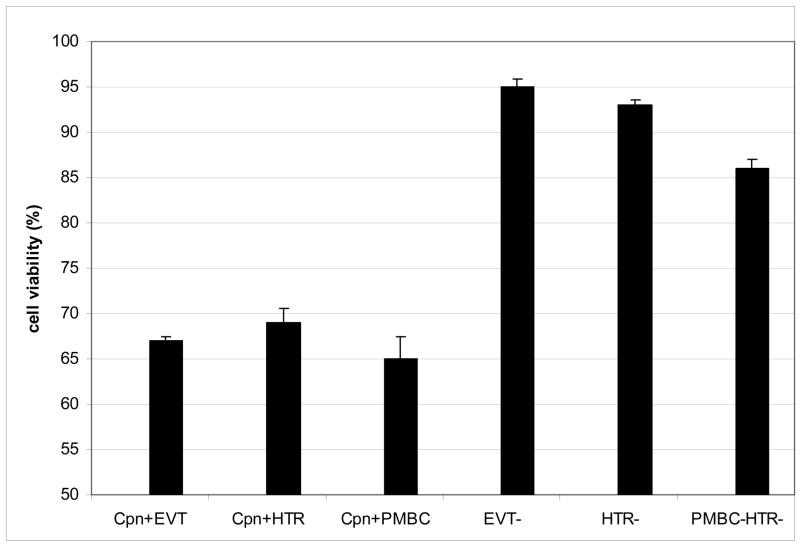
At 24 hours post-infection, the viability of infected primary extravillous trophoblast cells, infected HTR-8/SVneo cells, and PBMC co-infected HTR-8/SVneo cells was significantly reduced compared with non-infected extravillous trophoblast cells, non-infected HTR-8/SVneo cells, and cells co-cultured with C. pneumoniae-free PBMCs (68 percent viable, 70, 65, 96, 93, and 87 percent, respectively). At 48 hours post-infection, viability ranged from 65 to 69 percent among infected trophoblast cells and from 86 to 95 percent among negative controls (P<0.01, Figure 1A).
Figure 1. Functional assays demonstrating adverse effects of C. pneumoniae infection of extravillous trophoblast cells at 48 hours post-infection. All experiments were conducted in triplicate in two separate sets.
Figure 1A: The viability of C. pneumoniae infection of primary extravillous trophoblast cells (Cpn+EVT), HTR-8/SVneo cells (Cpn+HTR), and PBMC co-infected HTR-8/SVneo cells (Cpn+PBMC) was compared with negative controls, including primary extravillous trophoblast cells (EVT−), HTR-8/SVneo cells (HTR−) and PBMC-co-cultured HTR-8/SVneo cells (PBMC-HTR) that were not infected. Cell viability was measured by LDH release assays. Mean results are expressed as a percentage along the Y-axis. (*)
(*) P<0.01 by ANOVA
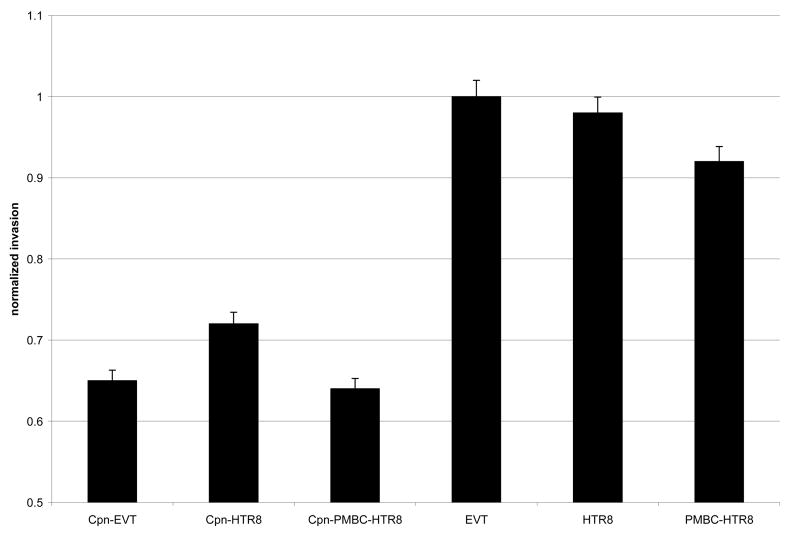
Figure 1B: Invasion of infected trophoblast cells through an ECM was measured using Cell Invasion Assay Kits (Chemicon International) and was compared with negative controls. Levels of invasion were normalized to 1.0 in non-infected extravillous trophoblast cells and are mean values are indicated on the Y-axis. Invasion was determined by spectrophotometric measurement of labeled cells that invaded through the ECM. (*)
(*) P<0.05 by ANOVA
Invasion of infected trophoblast cells through the ECM significantly decreased in C. pneumoniae-infected cells. Invasion rates were normalized to 1.0 in non-infected primary extravillous trophoblast cells, and invasion rates were reduced to 0.6 to 0.8 after infection with Chlamydia pneumoniae (P<0.05, Figure 1B).
Demographic characteristics from our case–control study are shown in Table 2. Based on our study design, the gestational age at delivery and birthweight were lower in cases than in controls: 32 weeks (26–36 weeks) for severe preeclampsia cases versus 39 weeks (37–42 weeks) for controls, P< 0.0001; 1,588 ± 469 gm for severe preeclampsia versus 3,344 ± 527 gm for controls, P< 0.0001. Severe preeclampsia cases were more likely to be nulliparous (68 percent) compared with controls (23 percent; P< 0.0001). The same difference was observed when comparing the presence of intrauterine growth restriction among severe preeclampsia cases (20 percent) versus controls (0; P=0.01).
Table 2.
Demographics and outcomes data for case-control study
| Severe PE, n=48 | Controls, n=30 | P-value1 | |
|---|---|---|---|
| Age (year ± SD) | 25.9 ± 7.2 | 26.3 ± 6.6 | NS |
| Nulliparity (percent) | 68.7 | 23.0 | <0.0001 |
| GA del (wks, range) | 32 (26–36) | 39 (37–42) | <0.0001 |
| BW (gm) | 1588 ± 469 | 3344 ± 527 | <0.0001 |
| IUGR (percent) | 20 | 0 | 0.01 |
PE, preeclampsia;
GA del, gestational age at delivery;
BW, birthweight;
IUGR, intrauterine growth restriction (birthweight less than the 10th percentile for gestational age).
P-values determined by t-test for continuos variables and by Fisher’s exact test for dichotomous variables.
Among all subjects in the case-control study, C. pneumoniae DNA was identified in trophoblast cells in 18/78 (23 percent) placentas. Chlamydia pneumoniae DNA was detected significantly more frequently in trophoblast cells from cases (15/48, 31 percent) than controls (3/30, 10 percent; OR=4.1, P=0.02 Table 3). Positive and negative controls yielded expected results. In a secondary analysis, C. pneumoniae DNA was identified in the extravillous region of 11/78 (14 percent) placentas. The presence of C. pneumoniae DNA in extravillous regions of placental samples from cases of severe preeclampsia was greater than the detection rate in controls (9/48, 19 percent, versus 2/30, 7 percent), but this difference did not achieve statistical significance (OR=3.23, P=0.1) (Table 3).
Table 3.
Chlamydia pneumoniae detection in trophoblast cells from preeclampsia cases and controls.
| Severe PE, n=48 | Controls, n=30 | P-value * | |
|---|---|---|---|
| VT1 or EVT (+) | 15 | 3 | |
| VT and EVT (−) | 33 | 27 | 0.02 |
|
| |||
| EVT2 (+) | 9 | 2 | |
| EVT (−) | 39 | 28 | 0.1 |
VT = villous trophoblast cells
EVT = extravillous trophoblast cells;
P-values determined by Fisher’s exact tests comparing case groups to controls
Comments
We demonstrated that C. pneumoniae is able to infect human invasive extravillous trophoblast cells, and that this infection reduces cellular viability and impairs cell invasive properties through an ECM. We also demonstrated an association between the presence of C. pneumoniae infection of the placenta and severe preeclampsia before term.
A strong point of our study is that we were able to correlate the results of our case-control study with in vitro assays that demonstrated the ability of C. pneumoniae-infected PBMCs and cell-free C. pneumoniae to infect primary and immortalized extravillous trophoblast cells. The functional studies we conducted showed that infected extravillous trophoblast cells had decreased viability (P<0.01) and reduced invasive properties (P<0.05) compared to non infected cells. The PCR method we used had been validated previously for sensitivity (level of detection of ≤1 IFU) and specificity (against other Chlamydial species) compared to other diagnostic methods as cell culture and immunofluorescence.30 The bacterium strain used in our experiments, TW 183, had been used successfully by other investigators to conduct functional assays in infected epithelial cells.31 Another strength of our study is that DNA extraction from extravillous and villous regions of the placentas from cases and controls by laser capture dissection techniques allowed us to identify which cells were infected with C. pneumoniae (Figures 2A,2B). Our study had some limitations. First, the results of in vitro experiments must be interpreted with caution when applying these results to in vivo conditions. The C. pneumoniae strain utilized in our study had not been used to infect placental tissue before. The pathological effects that we observed following C. pneumoniae infection may have resulted in part from cell detachment from the culture plates. However, the use of appropriate controls permitted us to conclude that the pathological effects we observed occurred as a consequence of C. pneumoniae infection. Finally, the women comprising our case-control study were almost exclusively inner city pregnant women and largely African-American, so our observations will require validation in other cohorts before they can be generalized.
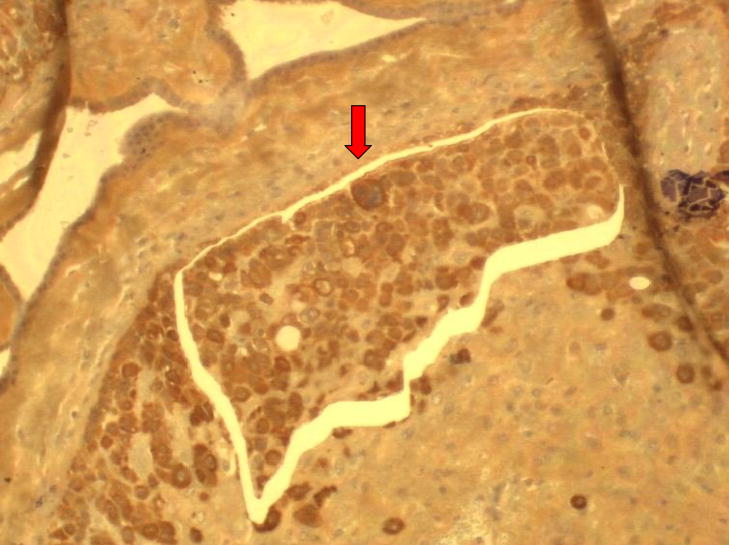
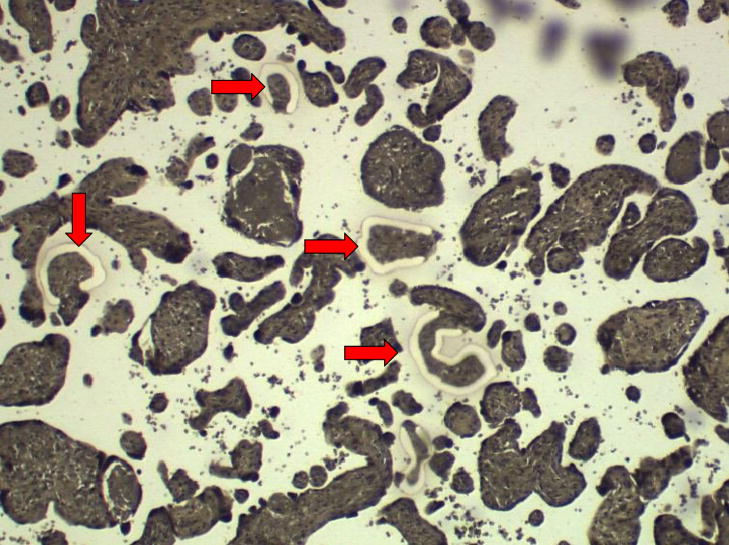
Figure 2. Laser capture dissection of trophoblast cells.
Figure 2A: Photomicrograph of placental villi from the placental basal plate region (magnification: 20X).
Arrow indicates region where cells were captured. Photomicrograph provided by our laboratory.
Figure 2B: Photomicrograph of extravillous trophoblast region from the basal plate of placenta (magnification: 100X).
Arrow indicates region where cells were captured. Photomicrograph provided by our laboratory.
Our interest in C. pneumoniae as a possible cause of preeclampsia emerged from the association of C. pneumoniae with atherosclerosis.5, 8, 9 Recently, serological studies have attempted to correlate C. pneumoniae infection with preeclampsia with conflicting results. 12–14, 32, 33 In a recent publication, Conde-Agudelo et al., reviewed the correlation of different maternal infection conditions and the risk of preeclampsia. They concluded that there was no significant difference in the seroprevalence of antibodies against Chlamydia pneumoniae in women with preeclampsia and normotensive ones.34 However, serology cannot prove or disprove a causal association. A more reliable surrogate is the detection of C. pneumoniae in placentas from cases with adverse pregnancy outcomes. Baboonian et al., previously detected C. pneumoniae DNA in placental tissue, although they failed to find a difference in the presence of bacterial DNA in placentas from women with fetal growth restricted fetuses (25/32, 78 percent) compared to normal controls (19/27, 70 percent; P=0.44).11 The results of our case-control study suggest that C. pneumoniae infection of the placenta is related to poor obstetric outcomes, such as severe preeclampsia before term (Table 3). We detected infection in villous and extravillous trophoblast cells but did not study the impact of C. pneumoniae infection of villous trophoblast viability and function. Not all subjects with C. pneumoniae DNA detection in our cohort developed severe preeclampsia (3/30 controls; Table 3). We speculate that in some cases this may be due to the individual host response to C. pneumoniae rather than to the infection.35 Alternatively, same as in atherosclerosis, some forms of the bacterium may remain dormant inducing no functional effects on infected cells until certain triggers prompt the manifestation of the disease.36
Chlamydia pneumoniae interact with target cells via blood dissemination; however direct extravascular tissue invasion has been previously described.22, 31 The transmission of C. pneumoniae to extravillous trophoblasts and villous trophoblasts may occur via ascending infection from a previously colonized genital tract.
We hypothesize that C. pneumoniae may play a role in the pathogenesis of preeclampsia by two different mechanisms: cellular and humoral. Failed trophoblast invasion, a common feature in preeclampsia,1 may be induced by placental infection. Our lab has shown previously that infection of invasive extravillous trophoblasts by viruses reduces their ability to invade through an ECM.28, 37, 38 Based on the results of our in vitro experiments, we propose that circulating C. pneumoniae during pregnancy may infect invasive trophoblast cells at the maternal-fetal interface inducing pathologic changes that interfere with placental invasion into the uterine wall, resulting in reduced placental perfusion, placental dysfunction, and adverse obstetric outcomes. Meanwhile, humoral responses induced by C. pneumoniae could be similar to those described in atherosclerosis. Elevation of inflammatory markers and endothelial dysfunction precede vascular injury.39 Enhanced intravascular inflammation is a common scenario in preeclampsia.40 The degree of vascular lesion in atherosclerosis correlates with levels of heat shock protein and C-reactive protein (CRP) induced by C. pneumoniae.10 Elevated levels or CRP have been associated with high titers of anti C. pneumoniae IgG antibodies in women with preeclampsia who delivered before term.32
Collectively, our data indicate that C. pneumoniae is able to infect invasive trophoblast cells and induce pathological sequelae that are associated with placental dysfunction and preeclampsia. Our findings provide a scientific basis for the investigation of the role of C. pneumoniae in pregnancy complications related to placental dysfunction. Future research should be directed toward studying mechanisms by which C. pneumoniae infection reduces viability and invasion of trophoblast cells, such as apoptosis. In addition, the role of humoral markers of C. pneumonia infection, such as CRP and heat shock proteins, need to be corroborated in placental infection by C. pneumoniae. We also speculate that C. pneumoniae, an intracellular bacterium, may interact with cytoplasmic regulatory proteins such as NOD-like receptors leading to inflammatory and apoptotic responses. Our observations need to be confirmed with larger numbers and in different populations. If C. pneumoniae is found to play a role in the etiology of preeclampsia, this will have significant implications in the investigation for treatment of affected women.
Acknowledgments
This research was supported by the NIH grant HD42100 (SP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kong TY, DeWolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and by small-for-gestational age infants. BJOG. 1986;93:1049–59. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 2.Frusca T, Morassi L, Pecorelli S, Grigolato P, Gastaldi A. Histological features of uteroplacental vessels in normal and hypertensive patients in relation to birthweight. BJOG. 1989;96:835–9. doi: 10.1111/j.1471-0528.1989.tb03324.x. [DOI] [PubMed] [Google Scholar]
- 3.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe preeclamptic pregnancies. BJOG. 1994;101:669–74. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 4.Saikku P. Seroepidemiology in Chlamydia pneumoniae-atherosclerosis association. European Heart J. 2002;23:263–4. doi: 10.1053/euhj.2001.2913. [DOI] [PubMed] [Google Scholar]
- 5.Grayston JT, Kuo CC, Wang SP, Altman J. A new Chlamydia psitacci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986;315(3):161–8. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- 6.Gaydos CA, Summergill JT, Sahney NN, Ramírez JA, Quinn TC. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–20. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong Y-K, Gallagher PJ, Ward ME. Chlamydia pneumoniae and atherosclerosis. Heart. 1999;81:232–8. doi: 10.1136/hrt.81.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saikku P, Leinonen M, Matilla K, et al. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;2:983–6. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 9.Sander D, Winbeck K, Klingelhöfer J, Etgen T, Conrad B. Enhanced progression of early carotid atherosclerosis is related to Chlamydia pneumoniae (Taiwan acute respiratory) seropositivity. Circulation. 2001;103:1390–5. doi: 10.1161/01.cir.103.10.1390. [DOI] [PubMed] [Google Scholar]
- 10.Sander D, Winbeck K, Klingelhofer J, Etgen T, Conrad B. Reduced progression of early carotid atherosclerosis after antibiotic treatment and Chlamydia pneumoniae seropositivity. Circulation. 2002;106:2428–33. doi: 10.1161/01.cir.0000036748.26775.8d. [DOI] [PubMed] [Google Scholar]
- 11.Babbonian C, Smith DA, Shapland D, et al. Placental infection with Chlamydia pneumoniae and intrauterine growth restriction. Cardiovasc Res. 2003;60:165–9. doi: 10.1016/s0008-6363(03)00321-3. [DOI] [PubMed] [Google Scholar]
- 12.Heine RP, Ness RB, Roberts JM. Seroprevalence of antibodies to Chlamydia pneumoniae in women with preeclampsia. Obstet Gynecol. 2003;101:221–6. doi: 10.1016/s0029-7844(02)02591-7. [DOI] [PubMed] [Google Scholar]
- 13.von Dadelszen P, Magee LA, Krajden M, et al. Levels of antibodies against cytomegalovirus and Chlamydophila pneumoniae are increased in early onset preeclampsia. BJOG. 2003;110:725–30. [PubMed] [Google Scholar]
- 14.Goulis DG, Chappell L, Gibbs RG, Williams D, Dave JR, Taylor P. Association of raised titres of antibodies to Chlamydia pneumoniae with a history of preeclampsia. BJOG. 2005;112:299–305. doi: 10.1111/j.1471-0528.2004.00423.x. [DOI] [PubMed] [Google Scholar]
- 15.Graham CH, Hawley TS, Hawley RG, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–11. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 16.Appleton SD, Lash GE, Marcks GS, et al. Effect of glucose and oxygen deprivation on hem oxygenase expression in human chorionic villi explants and immortalized trophoblast cells. Am J Physiol Regul Integr Com Physiol. 2003;285:R1453–60. doi: 10.1152/ajpregu.00234.2003. [DOI] [PubMed] [Google Scholar]
- 17.Graham CH, Lysiak JJ, McCrae KR, Lala PK. Localization of transforming growth factor-beta at the human fetal-maternal interface: role in trophoblast growth and differentiation. Biol Reprod. 1992;46:561–572. doi: 10.1095/biolreprod46.4.561. [DOI] [PubMed] [Google Scholar]
- 18.Koi H, Zhang J, Makrigiannakis A, et al. Syncytiotrophoblast is a barrier to maternal-fetal transmission of Herpes simplex virus. Biol Reprod. 2002;67:1572–9. doi: 10.1095/biolreprod.102.004325. [DOI] [PubMed] [Google Scholar]
- 19.Neudeck H, Oei SL, Stiemer B, Hopp H, Graf R. Binding of antibodies against high and low molecular weight cytokeratin proteins in the human placenta with special reference to infarcts, proliferation and differentiation processes. Histochem J. 1997;29:419–30. doi: 10.1023/a:1026499203743. [DOI] [PubMed] [Google Scholar]
- 20.Grayston JT. Immunization against Trachoma. Pan American Health Organization Scientific Publication. 1965;147:549. [Google Scholar]
- 21.Grayston JT, Wang SP, Kuo CC, Campbell LA. Current knowledge on Chlamydia pneumoniae strain TWAR, an important cause of pneumonia and other acute respiratory diseases. Eur J Clin Microbiol Infec Dis. 1989;8:191–202. doi: 10.1007/BF01965260. [DOI] [PubMed] [Google Scholar]
- 22.Boman J, Söderberg S, Forsberg J, et al. High prevalence of Chlamydia pneumoniae DNA in peripheral blood mononuclear cells in patients with cardiovascular disease and in middle-aged blood donors. J Infect Dis. 1998;178:274–7. doi: 10.1086/517452. [DOI] [PubMed] [Google Scholar]
- 23.Parry S, Zhang J, Koi H, Arechavaleta-Velazco F, Elovitz M. Transcytosis of Human immunodeficiency virus 1 across the placenta is enhanced by treatment with tumor necrosis factor alpha. J Gen Virol. 2006;87:2269–78. doi: 10.1099/vir.0.81071-0. [DOI] [PubMed] [Google Scholar]
- 24.Edfeldt K, Agerbrth B, Rottenberg ME, et al. Involvement of the antimicrobial peptide LL-37 in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2006:1151–7. doi: 10.1161/01.ATV.0000223901.08459.57. [DOI] [PubMed] [Google Scholar]
- 25.Tong CYW, Sillis M. Detection of Chlamydia pneumoniae and Chlamydia psitacci in sputum samples by PCR. J Clin Pathol. 1993;46:313–7. doi: 10.1136/jcp.46.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saiki RK, Bugawan TL, Horn GT, Mullis KB, Erlich HA. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986;324:163–6. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- 27.Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;115:61–9. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 28.Gómez LM, Ma Y, Ho C, McGrath, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod. 2008;23:709–15. doi: 10.1093/humrep/dem404. [DOI] [PubMed] [Google Scholar]
- 29.American College of Obstetricians and Gynecologists. Practice Bulletin N° 33. Diagnosis and Management of Preeclampsia and Eclampsia Obstet Gynecol. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 30.Dowell SF, Peeling RW, Boman J, et al. Standardizing Chlamydia pneumoniae assays: Recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada) Clin Infect Dis. 2001;33:492–502. doi: 10.1086/322632. [DOI] [PubMed] [Google Scholar]
- 31.Rajalingam K, Al-Younes H, Müller A, Meyer TF, Szczepek A, Rudel T. Epithelial cells infected with Chlamydophila pneumoniae (Chlamydia pneumoniae) are resistant to apoptosis. Infect Immun. 2001;69(12):7880–8. doi: 10.1128/IAI.69.12.7880-7888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karinen L, Leinonen M, Bloigu A, et al. Maternal serum Chlamydia pneumoniae antibodies and CRP levels in women with preeclampsia and gestational hypertension. Hypertens Pregnancy. 2008;27(2):143–58. doi: 10.1080/10641950701885188. [DOI] [PubMed] [Google Scholar]
- 33.Raynor BD, Bonney EA, Jang KT, Coto W, Garcia MS. Preeclampsia and Chlamydia pneumoniae: is there a link? Hypertens Pregnancy. 2004;23(2):129–34. doi: 10.1081/PRG-120028284. [DOI] [PubMed] [Google Scholar]
- 34.Conde-Agudelo A, Villar J, Lindheimer M. Maternal infection and risk of preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2008;198:7–22. doi: 10.1016/j.ajog.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 35.Neumann FJ. Chlamydia pneumoniae-atherosclerosis link. A sound concept in search for clinical relevance. Circul. 2002;106:2414–6. doi: 10.1161/01.cir.0000040403.57597.48. [DOI] [PubMed] [Google Scholar]
- 36.Grayston JT. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J Infec Dis. 200;181:S402–10. doi: 10.1086/315596. [DOI] [PubMed] [Google Scholar]
- 37.Arechavaleta-Velasco F, Ma Y, Zhang J, McGrath CM, Parry S. Adeno-associated virus-2 (AAV-2) causes trophoblast dysfunction, and placental AAV-2 infection is associated with preeclampsia. Am J Pathol. 2006;168:1951–9. doi: 10.2353/ajpath.2006.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou D, Ma Y, Zhang J, McGrath C, Parry S. Cytomegalovirus infection of trophoblast cells elicits an inflammatory response: a possible mechanism of placental dysfunction. Am J Obstet Gynecol. 2006;194:535–41. doi: 10.1016/j.ajog.2005.07.073. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Hennekes CH, Buring JE, Rifai N. C reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 40.Redman CWG, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]