Abstract
Introduction
Osteonecrosis of the jaw (ONJ) has been reported in patients treated with bisphosphonates. The incidence and risk factors associated with this disorder have not been clearly defined.
Materials and Methods
We conducted a retrospective analysis of 4019 patients treated with intravenous bisphosphonates between 1996 and 2004. Our goals were to estimate the frequency, understand the clinical presentation, and identify risk factors associated with ONJ development.
Results
Sixteen of 1338 patients with breast cancer (1.2%) and 13 of 548 patients with multiple myeloma (2.4%) developed ONJ. The median dose and duration of treatment with pamidronate or zoledronic acid were significantly higher in patients with ONJ (p < 0.0001). Multivariate Cox proportional hazards regression analysis identified treatment with zoledronic acid (hazards ratio [HR], 15.01; 95% CI: 2.41–93.48; p = 0.0037), treatment with pamidronate followed by zoledronic acid (HR, 4.00; 95% CI: 0.86–18.70; p = 0.078), and dental extractions (HR, 53.19; 95% CI: 18.20–155.46; p < 0.0001) as significant risks for ONJ in breast cancer. In multiple myeloma, dental extractions (HR, 9.78; 95% CI: 3.07–31.14; p = 0.0001) and osteoporosis (HR, 6.11; 95% CI: 1.56–23.98; p = 0.0095) were significant risk factors while controlling for bisphosphonate therapy. Thirteen of 29 patients were followed for a median of 17.1 mo (range, 7–67 mo); lesions healed in 3 patients during this period.
Conclusions
ONJ is an uncommon but long-lasting disorder that occurs mainly in breast cancer and multiple myeloma patients treated with intravenous bisphosphonates. High cumulative doses of bisphosphonates, poor oral health, and dental extractions may be significant risk factors for ONJ development. ONJ resolved in 23% of patients with conservative therapy.
Key words: bisphosphonates, osteonecrosis of the jaw, breast cancer, multiple myeloma, bone metastasis, osteoporosis
INTRODUCTION
Until recently, osteonecrosis of the jaw (ONJ) was an entity seen primarily in patients with head and neck cancers treated with radiation therapy. This entity, also termed osteoradionecrosis, occurs in ∼3% of patients with head and neck cancers.(1,2) Its severity, prolonged morbidity, association with poor dental health, and the lack of effective therapy led to the application of routine dental evaluation and treatment before radiation therapy. The past 4 yr have seen a re-emergence of this condition in patients not treated with head and neck irradiation.(3–17) The common thread in these reports was the use of intensive intravenous bisphosphonate and oncologic therapy, which led the investigators to propose an association between bisphosphonate use and ONJ.
The newest generation of bisphosphonates belongs to the class of amino-substituted bisphosphonates. These pharmacologic agents have a high binding affinity for hydroxyapatite and inhibit bone resorption by causing osteoclast apoptosis.(18) Bisphosphonates are effective for treatment of metastatic bone disease and disorders associated with increased bone turnover such as osteoporosis, hypercalcemia of malignancy, and Paget's disease.(18,19) Both pamidronate and zoledronic acid have been extensively studied. Their efficacy in patients with metastatic bone diseases has been confirmed in several prospective randomized trials.(20–24) In these patients, bisphosphonate treatment has resulted in reduction and/or delay of onset of skeletal-related events such as fractures, cord compression, or hypercalcemia. Because of efficacy and good clinical tolerability, they have been widely and routinely used in these patients. Side effects, with appropriate monitoring, are mild and include an acute and self-limited febrile inflammatory response, hypocalcemia, and kidney dysfunction.(25,26) However, the recent reports of ONJ have prompted concern.
This study was initiated to determine the frequency, clinical presentation, and risk factors associated with ONJ in patients treated with intravenous bisphosphonates at the University of Texas M.D. Anderson Cancer Center.
MATERIALS AND METHODS
Study design and cohort
We conducted a retrospective analysis of 4019 patients treated with pamidronate and/or zoledronic acid between September 1996 and February 2004. The chart review was conducted from June 2004 until June 2005.
Permission to conduct the study was granted by the institutional review board at the University of Texas M.D. Anderson Cancer Center, and the need for written informed consent was waived.
The primary objectives were to estimate the frequency of ONJ in patients treated with intravenous bisphosphonates; to better understand the clinical presentation of ONJ; and to identify risk factors associated with the development of osteonecrosis in these patients.
Definition and diagnosis of ONJ
The criterion used to define ONJ was exposed nonhealing bone with or without pain of at least 3-mo duration.
All cases, identified in the chart review as having an oral disorder, including osteomyelitis, delayed healing after dental extraction, periodontal disease, or extensive dental decay, were reviewed and discussed in a weekly group meeting with Dr Béla B Toth, Dental Oncologist/Oral Pathologist, at our institution. Cases that met the definition of ONJ and had not been treated with radiation therapy to the head and neck area were classified as ONJ. Cases that met the definition but had received radiation treatment to the head and neck were classified as osteoradionecrosis of the jaw. Cases that had one medical note indicating jawbone exposure but were not seen at the dental clinic or were not available for evaluation because of death or loss to follow-up were classified as suspicious for ONJ.
Data elements
For each chart reviewed, we collected the following information: demographics, indication for bisphosphonate treatment, cancer diagnosis, treatments received, histological diagnosis, tumor staging, other medical conditions with respective treatments, details regarding bisphosphonate therapy (type, dose, duration), and dental history.
Additional information collected on patients defined as having ONJ included symptoms associated with osteonecrosis, date of diagnosis, location of osteonecrosis, any associated event (dental extraction, periodontitis, bone exostosis, dentures, trauma, or infection), treatments received, and outcome.
Assessment of clinical course of ONJ
Thirteen patients from the 29 included in this cohort were followed in the Dental Clinic for periods of >6 mo after the identification of osteonecrosis up until June 2006. For each of these patients, information on the status of the osteonecrosis lesion was obtained. In addition, we noted the duration of treatment with bisphosphonate after diagnosis of ONJ.
Management of ONJ
All patients with ONJ were evaluated at least once by the dental service at our institution. The patients were managed using conservative techniques with avoidance of large-scale debridement and/or surgical excision of necrotic bone. The treatment incorporated judicious use of antibiotics for organisms not considered to be normal oral flora and attention to oral hygiene (flossing, brushing, oral rinses with SaliCept [Carrington Laboratories, Irving, TX, USA], baking soda, or Periogard [Colgate Oral Pharmaceuticals, NY, USA]). Debridement or sequestrectomy, when performed, was limited to protruding bone. A biopsy was performed in 15 patients to exclude metastatic cancer and each showed necrotic bone.
Role of the sponsor
Novartis Pharmaceuticals provided partial funding support but did not participate in the collection or the final analysis of data. The statistical analysis was performed by the Division of Quantitative Sciences at our institution. The lead authors wrote the manuscript, which was reviewed by all authors. A draft was provided to Novartis for comment, but all decisions regarding content and interpretation were made by the authors.
Statistical analysis
From 4019 charts reviewed, 25 were excluded from statistical analysis because of incomplete data. Characteristics of the remaining 3994 patients were summarized by using standard descriptive statistics for continuous variables or tabulations for categorical variables. The association of ONJ with demographic, health, and treatment variables were examined using Fisher's exact tests or Wilcoxon rank-sum tests, as appropriate. Total dose of pamidronate and zoledronic acid was determined by combined information from chart review and the pharmacy database with comparison between groups using Wilcoxon rank-sum or Kruskal-Wallis tests, as appropriate.
Using univariate and multivariate logistic regression methods, the probability of ONJ diagnosis (presence or absence) was modeled, and ORs and corresponding 95% CIs were computed. Independent variables, other than type of bisphosphonate therapy (pamidronate alone, zoledronic acid alone, or pamidronate followed by zoledronic acid), were chosen based on known or suspected risk factors and included age, smoking status, alcohol use, estrogen receptor status, progesterone receptor status, presence of osteoporosis, prior dental extractions, chronic renal failure, and treatment factors (use of anthracycline, tamoxifen, aromatase inhibitors, trastuzumab, melphalan, glucocorticoids, taxanes, or thalidomide). Initial models included each independent variable alone. Variables that were associated with ONJ occurrence at p ≤ 0.10 were entered simultaneously for the full multivariate model. The full multivariate model was reduced using a backward selection procedure. Similarly, univariate and multivariate Cox proportional hazards regression models were used to study the prognostic effects of the above factors on time to ONJ. Type of bisphosphonate therapy was retained in all multivariate models regardless of statistical significance.
Time to ONJ was computed using Kaplan-Meier methods. In addition, cumulative incidence of ONJ was estimated. Patients with no evidence of ONJ were considered censored, and time to osteonecrosis was defined as the number of years from the initiation of bisphosphonate therapy to the last follow-up date. For patients diagnosed with ONJ, time to osteonecrosis was calculated as the number of years from the initiation of bisphosphonate therapy to the date of osteonecrosis diagnosis. Separate analyses were conducted for all patients or the group as a whole, for the subgroup of patients diagnosed with breast cancer, and for those diagnosed with multiple myeloma. All reported p values are two sided at a significance level of 5%. Analyses were performed with SAS for Windows (release 9.0; SAS Institute, Cary, NC, USA) and S-PLUS (release 2000; Insightful, Seattle, WA, USA).
RESULTS
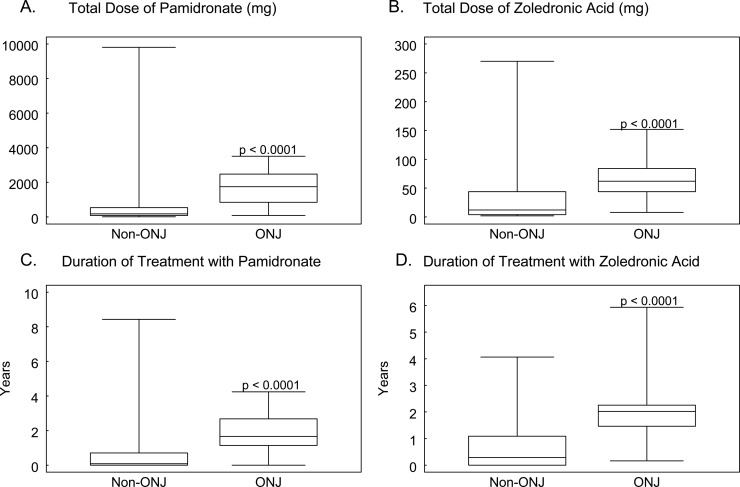
We identified 29 cases of ONJ, 7 cases of osteoradionecrosis of the jaw, and 3 suspected cases of ONJ in the analyzed cohort of 3994 patients. The baseline characteristics of these 3994 patients are presented in Table 1. The indications for intravenous bisphosphonate treatment included bone metastases, hypercalcemia, multiple myeloma, osteoporosis, and Paget's disease. There were no significant differences in age (p = 0.60) or sex (p = 0.18) of patients with or without ONJ. However, patients with ONJ had a longer median duration of malignant disease (5.75 [range, 0.91–24.37yr] versus 3.11 yr [range, 0–46.04 yr]; p = 0.0001) and longer median duration of bone metastases (5.22 [range, 0.80–13.24 yr] versus 1.53 yr [range, 0–18.92 yr]; p < 0.001) than did patients without osteonecrosis. Of the 3994 patients, 2288 patients were treated with pamidronate, 1180 with zoledronic acid, and 526 with pamidronate followed by zoledronic acid. Patients with ONJ received significantly higher doses of pamidronate (median dose, 1755 versus 180 mg) or zoledronic acid (median dose, 62 versus 12 mg) and were treated with bisphosphonates for a longer period of time (Fig. 1; Table 1). In addition, treatment with zoledronic acid was more frequently observed in patients who developed ONJ (76%) compared with patients who did not develop ONJ (42%; 22 of 29 patients versus 1684 of 3965; p = 0.0004; Table 1).
Table 1.
Patient Characteristics (3994 Patients)
| Characteristics | Non-ONJ (N = 3965) | ONJ (N = 29) | p |
| Median age in (yr; minimum-maximum) | 60 (3–101) | 62 (43–82) | 0.60* |
| Sex [male/female (n)] | 1637/2328 | 8/21 | 0.18* |
| Cancer diagnosis (n) | |||
| Breast cancer | 1457† | 16 | |
| Multiple myeloma | 541‡ | 13 | |
| Renal cell carcinoma | 327 | 0 | |
| Lung cancer | 380 | 0 | |
| Prostate cancer | 185 | 0 | |
| Others | 1075 | 0 | |
| Indication for bisphosphonate therapy (n)§ | |||
| Bone metastases | 2392 | 16 | |
| Multiple myeloma | 535 | 13 | |
| Hypercalcemia | 1023 | 0 | |
| Osteoporosis | 271 | 0 | |
| Paget's disease | 11 | 0 | |
| Median years duration of disease (minimum-maximum) | 3.11 (0–46.04) | 5.75 (0.91–24.37) | 0.0001* |
| Median duration of follow-up (minimum-maximum) | 1.77 (0–36.45) | 5.39 (0.81–12.75) | <0.0001* |
| Bisphosphonate (n) | |||
| Pamidronate | 2281 | 7 | <0.0001¶ |
| Zoledronic acid | 1171 | 9 | |
| Pamidronate + zoledronic acid | 513 | 13 | |
| Median dose (mg) of bisphosphonate (minimum-maximum) | |||
| Pamidronate | 180 (13–9810) | 1755 (90–3510) | <0.0001* |
| Zoledronic acid | 12 (2–270) | 62 (8–152) | <0.0001* |
| Median duration of bisphosphonate treatment in years (minimum-maximum) | |||
| Pamidronate | 0.08 (0.003–8.43) | 1.67 (0.003–4.24) | <0.0001* |
| Zoledronic acid | 0.29 (0.003–4.07) | 2.02 (0.17–5.94) | <0.0001* |
| Dental extractions (yes/no) | 136/3829 | 16/13 | <0.0001¶ |
| Pamidronate use (yes/no) | 2794/1171 | 20/9 | 0.8400¶ |
| Zoledronic acid use (yes/no) | 1684/2281 | 22/7 | 0.0004¶ |
Duration of follow-up was defined as the time interval between first visit to last visit at our institution.
* p values were calculated with the use of the Wilcoxon rank-sum test.
† The total number of breast cancer patients without ONJ was 1457 (1322 treated with bisphosphonates for bone metastases and 135 treated for osteoporosis).
‡ The total number of patients with myeloma without ONJ was 541 (535 treated with bisphosphonates for myeloma bone disease and 6 treated for osteoporosis).
§ A few patients had more than one indication for bisphosphonate therapy.
¶ p values were calculated with the use of the Fisher's exact test to examine the association of dental extraction, pamidronate use and zoledronic acid use with development of ONJ.
FIG. 1.
Dose and duration of treatment with pamidronate and zoledronic acid in patients with and without ONJ. Duration and total dose of pamidronate and zoledronic acid treatments were compared between patient groups using a Wilcoxon rank-sum test. The comparison was made between patients with ONJ and non-ONJ patients. (A) Total dose of pamidronate. (B) Total dose of zoledronic acid. Patients with ONJ received significantly higher doses of pamidronate and zoledronic acid than patients without ONJ (p < 0.0001). (C) Duration of treatment with pamidronate. (D) Duration of treatment with zoledronic acid. Patients with ONJ had a longer duration of treatment (p < 0.0001).
Clinical presentation and course of ONJ
ONJ was identified in 16 of the 1338 patients with breast cancer (1.2%) and 13 of the 548 patients with myeloma (2.4%). No cases of ONJ were identified in patients treated with intravenous bisphosphonates for osteoporosis, hypercalcemia of malignancy, or Paget's disease.
The mandible was more frequently involved (70%) than the maxilla (30%). Approximately two thirds of the patients presented with bone exposure or a failure of closure of an open wound without associated pain. No patient developed a severe manifestation of osteonecrosis such as a cutaneous fistula, and none required extensive surgery.
Our analysis identified factors, previously associated with development of ONJ, in 28 of the 29 patients: dental extraction in 16 patients, periodontal disease in 14; bone exostosis (mandibular or maxillary tori) in 10; intubation-induced trauma in 1; trauma from manipulation of dental implants in 1; and denture-induced trauma in 3 (Table 2).
Table 2.
Characteristics of Patients with ONJ
| Cancer diagnosis | Age (yr)/sex | Time to ONJ (mo) | Type of bisphosphonate (cumulative dose, mg) | Oral findings and interventions |
| 1. Breast cancer | 60/F | 17 | Zoledronic acid (48) | Dental extraction |
| 2. Breast cancer | 70/F | 41 | Pamidronate (1800) | Periodontal disease, dental extraction |
| Zoledronic acid (44) | ||||
| 3. Breast cancer | 42/F | 7 | Zoledronic acid (28) | Dental extraction, bone exostosis |
| 4. Breast cancer* | 45/F | 24 | Pamidronate (1530) | Trauma, bone exostosis |
| Zoledronic acid (44) | ||||
| 5. Breast cancer | 61/F | 17 | Pamidronate (90) | Periodontal disease, dental extraction |
| Zoledronic acid (128) | ||||
| 6. Breast cancer | 69/F | 51 | Pamidronate (1710) | Dental extraction |
| Zoledronic acid (108) | ||||
| 7. Breast cancer | 46/F | 38 | Pamidronate (810) | Periodontal disease, dental abscess |
| Zoledronic acid (84) | ||||
| 8. Breast cancer | 63/F | 54 | Pamidronate (2790) | Periodontal disease, dental extraction |
| Zoledronic acid (76) | ||||
| 9. Breast cancer* | 78/F | 19 | Zoledronic acid (72) | Periodontal disease, dental extraction |
| 10. Breast cancer | 63/F | 25 | Zoledronic acid (32) | Periodontal disease, dental extraction, trauma |
| 11. Breast cancer | 60/F | 66 | Pamidronate (2700) | Periodontal disease, dental extraction |
| Zoledronic acid (132) | ||||
| 12. Breast cancer | 62/F | 39 | Pamidronate (1710) | Bone exostosis |
| Zoledronic acid (72) | ||||
| 13. Breast cancer* | 47/F | 39 | Pamidronate (3510) | Periodontal disease |
| 14. Breast cancer* | 64/F | 23 | Pamidronate (1980) | Dental Infection |
| 15. Breast cancer* | 63/F | 68 | Pamidronate (900) | Dental extraction |
| Zoledronic acid (64) | ||||
| 16. Breast cancer* | 60/F | 4 | Zoledronic Acid (8) | Bone exostosis† |
| 17. Myeloma | 79/F | 27 | Pamidronate (1260) | Dental extraction, bone exostosis |
| Zoledronic acid (32) | ||||
| 18. Myeloma | 67/M | 56 | Pamidronate (2520) | Periodontal disease, bone exostosis |
| Zoledronic acid (60) | ||||
| 19. Myeloma | 61/M | 4 | Pamidronate (360) | Bone exostosis, periodontal disease, dental extraction |
| 20. Myeloma | 71/F | 12 | Zoledronic acid (44) | Trauma |
| 21. Myeloma | 51/F | 19 | Zoledronic acid (56) | None |
| 22. Myeloma | 53/F | 28 | Pamidronate (1800) | Bone exostosis |
| 23. Myeloma | 63/M | 7 | Zoledronic acid (28) | Dental extraction, periodontal disease, periodontal surgery |
| 24. Myeloma | 42/M | 66 | Pamidronate (2520) | Bone exostosis |
| Zoledronic acid (144) | ||||
| 25. Myeloma* | 50/M | 20 | Pamidronate (1800) | Bone exostosis |
| 26. Myeloma | 64/M | 33 | Pamidronate (90) | Periodontal disease, dental extraction |
| Alendronate | ||||
| 27. Myeloma | 49/M | 39 | Zoledronic acid (152) | Dental extraction, dental decay |
| 28. Myeloma | 64/F | 47 | Pamidronate (2430) | Periodontal disease, trauma |
| Zoledronic acid (72) | ||||
| 29. Myeloma* | 66/M | 8 | Pamidronate (720) | Periodontal disease, dental extraction, trauma |
* Patients deceased.
† Patient with extensive metastatic breast cancer diagnosed in February 2003. Started on zoledronic acid March 2003. Unknown oral status before presentation to dental clinic with exposed bone in the left mandible in July 2003. Deceased in February 2004.
All patients with ONJ were seen at least once at the dental clinic. Thirteen of these patients were prospectively followed in the dental clinic for a period of >6 mo (median, 17.1 mo; range, 7–67 mo). Analysis of these 13 patients showed that all received a dose of zoledronic acid within 3 mo of the initial diagnosis, and 10 of the 13 patients had a prior history of pamidronate use. Zoledronic acid was discontinued in eight patients, replaced by weekly alendronate (70 mg) in one patient, decreased in frequency in two patients, and continued without changes in two patients. None of the subgroup of patients with breast cancer had healed, despite follow-up periods of 12–67 mo (median, 21.1 mo). Three of the seven patients with multiple myeloma healed completely. One healed 10.6 mo after discontinuation of intravenous zoledronic acid, a second patient healed 9.5 mo after intravenous zoledronic acid was replaced by oral alendronate (70 mg weekly), and a third patient healed 3 mo after a reduction in dosing frequency of zoledronic acid (reduced from 4 mg monthly to 4 mg every 3 mo).
Subgroup analysis of ONJ in breast cancer or multiple myeloma
In both breast cancer (p = 0.08) and multiple myeloma (p = 0.02) groups, patients with ONJ had longer durations of malignancy (Table 3). In addition, breast cancer patients with osteonecrosis had a significantly greater duration of bone metastasis compared with breast cancer patients without osteonecrosis. Among breast cancer patients and myeloma patients, those with ONJ received substantially greater cumulative doses of pamidronate (3- to 6-fold) or zoledronic acid (2- to 2.4-fold) and were treated for longer periods of time (2.8- to 5-fold). All of these differences were highly significant. This dataset also shows that patients with breast cancer and ONJ had a higher percentage of estrogen receptor positivity than did breast cancer patients without osteonecrosis (94% versus 67%; p = 0.028).
Table 3.
Characteristics of Patients With Breast Cancer and Multiple Myeloma
| Characteristics |
Breast cancer (N = 1338)
|
Multiple myeloma (N = 548)
|
||||
| Non-ONJ (N = 1322) | ONJ (N = 16) | p* | Non-ONJ (N = 535) | ONJ (N = 13) | p* | |
| Median age in years (minimum-maximum) | 57 (25–93) | 62 (44–79) | 0.3496 | 60 (28–88) | 64 (43–82) | 0.4796 |
| Median duration of bone metastases in years (minimum-maximum) | 2.02 (0–18.81) | 4.84 (0.8–11.03) | 0.0010 | NA | NA | NA |
| Median duration of disease in years (minimum-maximum) | 5.73 (0.03–37.48) | 7.27 (0.91–24.37) | 0.0801 | 3.18 (0–19.45) | 5.17 (1.73–13) | 0.0242 |
| Estrogen receptor status (yes/no) | 886/436 | 15/1 | 0.0284 | NA | NA | NA |
| Median does (mg) of bisphosphonate (minimum-maximum) | ||||||
| Pamidronate | 540 (45–9810) | 1710 (90–3510) | 0.0137 | 270 (30–9300) | 1800 (90–2520) | 0.0045 |
| Zoledronic acid | 32 (4–270) | 68 (8–132) | 0.0336 | 24 (4–172) | 58 (28–152) | 0.0158 |
| Median duration of treatment in years | ||||||
| Pamidronate | 0.59 (0.003–8.43) | 1.68 (0.90–4.24) | 0.0005 | 0.30 (0.003–6.70) | 1.55 (0.003–3.13) | 0.0104 |
| Zoledronic acid | 0.73 (0.003–4.07) | 2.04 (0.17–5.94) | 0.0004 | 0.67 (0.003–3.59) | 1.85 (0.74–2.92) | 0.0034 |
* p values were calculated with the use of the Fisher's exact tests or Wilcoxon rank-sum tests.
NA, not applicable.
Relationship between osteonecrosis and bisphosphonate type
Among patients who developed ONJ, median time to ONJ in breast cancer was 2.49 yr (range, 1.73–3.24 yr) for pamidronate; 1.38 yr (range, 0.3–1.60 yr) for zoledronic acid; and 3.33 yr (range 1.28–5.51 yr) for those treated sequentially with the two drugs (p = 0.0181). The median time to development of ONJ in multiple myeloma was 1.59 yr (range, 0.25–2.72 yr) for pamidronate; 1.26 yr for zoledronic acid (range, 0.57–3.10 yr); and 4.18 yr for those treated sequentially (range, 2.18–5.35 yr; p = 0.0685).
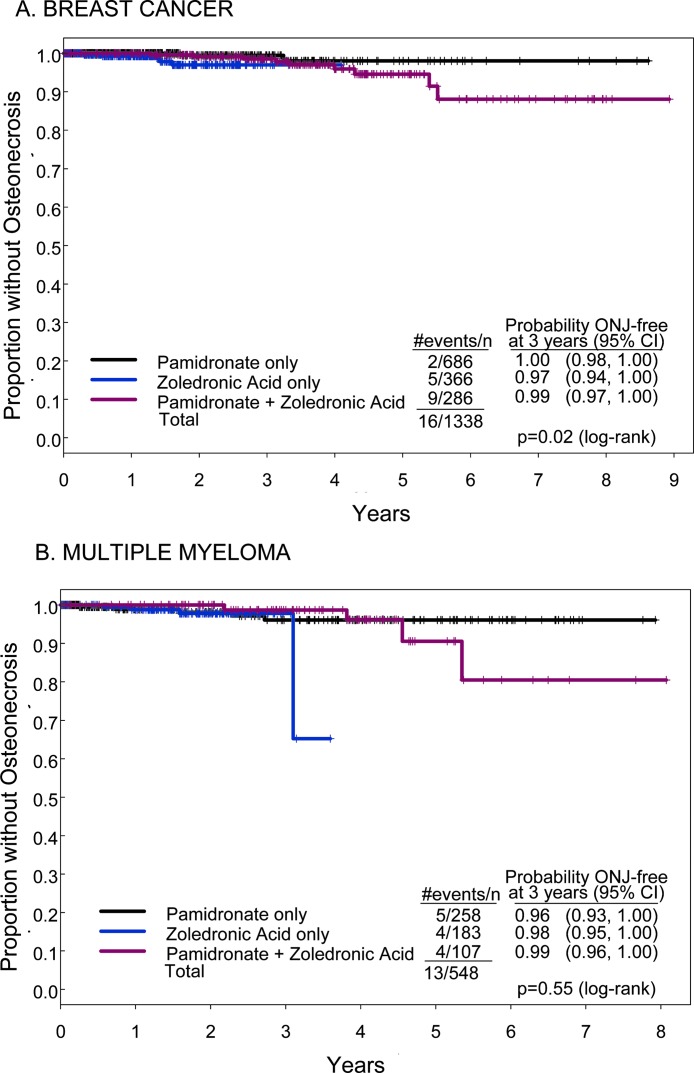
To further explore the relationships in these three groups (pamidronate alone, zoledronic acid alone, or pamidronate followed by zoledronic acid), we analyzed the time to onset of ONJ using two different techniques: Kaplan-Meier methods (Fig. 2) and cumulative incidence (Fig. 3). There was no significant difference in the probability of being free of ONJ between pamidronate, zoledronic acid, or pamidronate followed by zoledronic acid therapy in the breast cancer or myeloma cohorts at 3 yr of therapy (Fig. 2). However, by 5 yr of therapy, this analysis showed a time-dependent decrease in the percentage of patients free of osteonecrosis in the pamidronate group followed by the zoledronic acid group for both breast cancer and myeloma (Fig. 2). Whereas the probability of being free of ONJ was high at 3 yr for breast cancer patients treated with pamidronate followed by zoledronic acid (98.6%; 95% CI: 96.9–100%), it fell to 94.6% (95% CI: 90.5–98.9%) at 5 yr. A similar and even more striking pattern was observed for patients with myeloma treated with pamidronate followed by zoledronic acid: 98.7% (95% CI: 96.2–100%) were osteonecrosis-free at 3 yr and 90.6% (95% CI: 79.4–100%) were osteonecrosis-free at 5 yr. There are insufficient data available for treatment with zoledronic acid alone to comment on the long-term hazard of development of ONJ.
FIG. 2.
Kaplan-Meier estimates of the proportion of patients free of ONJ over time according to type of bisphosphonate received. The Kaplan-Meier method was used to estimate the number of patients free of ONJ at 3 and 5 yr of bisphosphonate treatment. (A) Kaplan-Meier curve in patients with breast cancer. (B) Same analysis in patients with multiple myeloma. Black line, patients treated with pamidronate; blue line, patients treated with zoledronic acid; red line, patients treated with both pamidronate and zoledronic acid. In patients with breast cancer, the probability of being ONJ-free at 3 yr was 99.5% (95% CI: 98.4–100%) in the pamidronate only group, 97% (95% CI: 94.3–99.8%) in the zoledronic acid group, and 98.6% (95% CI: 96.9–100%) in patients treated with pamidronate followed by zoledronic acid. The comparable values for 5 yr were 98% (95% CI: 95.1–100%), inadequate data for zoledronic acid, and 94.6% (95% CI: 90.5–98.9%). In patients with multiple myeloma, the probability of being ONJ-free at 3 yr was 96% (95% CI: 92.7–99.6%) in the pamidronate group, 97.8% (95% CI: 95.4–100%) in the zoledronic acid group, and 98.7% (95% CI: 96.2–100%) in patients treated with both. The comparable data for 5 yr is 96.1% (95% CI: 92.7–99.6%), inadequate for zoledronic acid, and 90.6% (95% CI: 79.4–100%). Time to ONJ differed significantly between the bisphosphonates treatment groups.
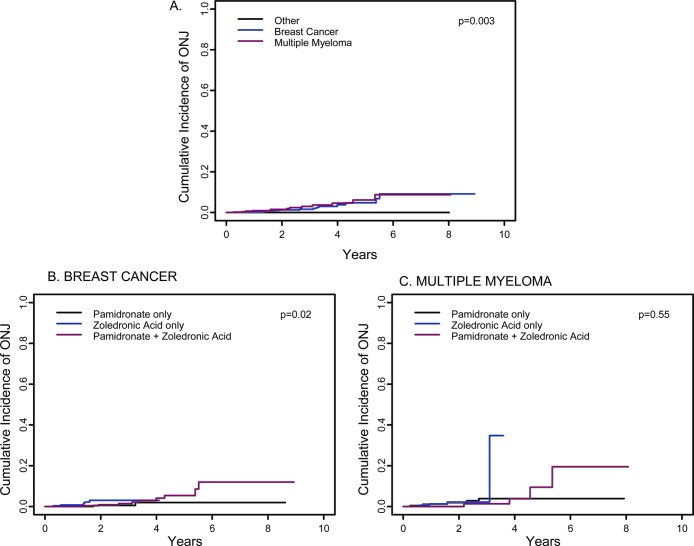
FIG. 3.
Estimate of cumulative incidence of ONJ. (A) Cumulative incidence of ONJ in 3994 patients with breast cancer (blue line), multiple myeloma (red line), or other disorders (black line). The estimated cumulative incidence of ONJ at 3 yr was 1.6% (95% CI: 0.005–0.027) in breast cancer, 3% (95% CI: 0.009–0.050) in multiple myeloma, and 0% in other disorders (p = 0.003). The comparable data at 5 yr are 4.8% (95% CI: 1.7–7.9%); 6.2% (95% CI: 1.9–10.4%); and 0%. (B and C) Cumulative incidence of ONJ at 3 yr in breast cancer and multiple myeloma patients analyzed by type of bisphosphonate used. In the breast cancer group at 3 yr (B), the estimated cumulative incidence of ONJ is 0.5% (95% CI: 0.000–0.016) with pamidronate, 3.0% (95% CI: 0.000–0.058) with zoledronic acid, and 1.4% (95% CI: 0.000–0.031) with pamidronate followed by zoledronic acid (p = 0.02). The comparable data for the breast cancer group at 5 yr are 2% (95% CI: 0–4.9%), inadequate numbers for zoledronic acid, and 5.4% (95% CI: 1.1–9.7%). The differences are significant at 5 yr. In the multiple myeloma group, the estimated cumulative incidence of ONJ or maxilla at 3 yr is 3.9% (95% CI: 0.004–0.074) with pamidronate, 2.2% (95% CI: 0.000–0.047) with zoledronic acid, and 1.3% (95% CI: 0.000–0.039) with pamidronate followed by zoledronic acid (p = 0.55). The comparable data for the multiple myeloma group at 5 yr are 3.9% (95% CI: 0.4–7.4%), inadequate for zoledronic acid, and 9.4% (95% CI: 0–21.7%).
When this same dataset was analyzed by estimating cumulative incidence of ONJ (Fig. 3), a nearly identical pattern emerged. The cumulative incidence of ONJ in breast cancer or myeloma treated for >5 yr was 4.8% (95% CI: 1.7–7.9%) or 6.2% (95% CI: 1.9–10.4%), respectively.
A subgroup analysis of bisphosphonate use in breast cancer (Fig. 3B) or myeloma (Fig. 3C) showed a similar pattern. Patients who were treated with pamidronate followed by zoledronic acid for >5 yr had a substantially greater cumulative incidence of ONJ among breast cancer (5.4%, 95% CI: 1.1–9.7%) and myeloma patients (9.4%, 95% CI: 0–21.7%). Again there were insufficient data to analyze the effects of zoledronic acid alone.
Risk factors associated with ONJ
We examined a number of factors including known risk factors for avascular necrosis or ONJ, as well as other factors related to the primary oncologic diagnosis or its treatment. By comparing the 29 cases of ONJ to those without osteonecrosis, we identified dental extractions (p < 0.0001) and treatment with zoledronic acid (p = 0.0004) as significant factors associated with development of ONJ (Fisher's exact test; Table 1). Further analysis of risk factors was performed within the subgroups of patients with breast cancer and multiple myeloma.
Risk factors in breast cancer
To better understand potential risk factors for development of ONJ in the context of breast cancer, we performed univariate and multivariate logistic regression analyses. Factors significant at p < 0.10 in univariate analysis and considered for multivariate modeling included estrogen receptor status, dental extractions, use of aromatase inhibitors, and type of bisphosphonate therapy. The reduced model showed significant associations between the diagnosis of ONJ and estrogen receptor positive status (OR, 15.44; 95% CI: 1.59–149.78; p = 0.0183), dental extractions (OR, 159.48; 95% CI: 42.39–599.94; p < 0.0001), and treatment with pamidronate followed by zoledronic acid compared with pamidronate alone (OR, 17.26; 95% CI: 2.88–103.55; p = 0.0018). In patients treated with zoledronic acid alone, the risk of osteonecrosis was also higher than that of patients treated with pamidronate alone, but the difference in risk did not reach statistical significance (OR, 5.58; 95% CI: 0.90–34.42; p = 0.0641).
Treatment with anthracyclines, tamoxifen, taxanes, trastuzumab, and aromatase inhibitors were not significantly associated with a greater risk to develop ONJ.
Using the multivariate Cox proportional hazards regression analysis, we found that the type of bisphosphonate therapy and dental extractions were significantly associated with time to ONJ. The risk of ONJ was greater in patients treated with zoledronic acid alone (hazards ratio [HR], 15.01; 95% CI: 2.41–93.48; p = 0.0037) or in patients treated with pamidronate followed by zoledronic acid (HR, 4.00; 95% CI: 0.86–18.70; p = 0.078) than in patients treated with pamidronate alone. Having a dental extraction was also a significant risk factor (HR, 53.19; 95% CI: 18.20–155.46; p < 0.0001).
The Cox proportional hazards regression analysis did not indicate that estrogen receptor positive status was a significant independent risk factor for ONJ (HR, 4.66; 95% CI: 0.61–35.35; p = 0.14). The association of estrogen receptor positive tumors with increased risk of ONJ can be explained by the longer duration of disease and resultant greater doses of bisphosphonate used to treat metastatic bone disease. There were 901 patients with estrogen receptor–positive tumors and 437 patients with estrogen receptor–negative tumors. Patients with estrogen receptor–positive tumors had longer duration of disease (6.05 [range, 0.03–37.48 yr] versus 5.01 yr [range, 0.12–36.8 yr]; p = 0.0037), longer duration of bone metastases (2.22 [range, 0.00–18.81 yr] versus 1.64 yr [range, 0.00–17.74 yr]; p < 0.0001), and received significantly higher cumulative doses of pamidronate (630 [range, 45–9810 mg] versus 450 mg [range, 60–7110 mg]; p = 0.0009) or zoledronic acid (40 [range, 4–216 mg] versus 20 mg [range, 4–270 mg]; p < 0.0001).
Risk factors in multiple myeloma
In a multivariate logistic regression model that controlled for type of bisphosphonate therapy, history of osteoporosis (OR, 10.67; 95% CI: 2.41–47.15; p = 0.0018) and dental extractions (OR, 13.22; 95% CI: 3.70–47.29; p < 0.0001) were significant risk factors for development of ONJ. Other parameters analyzed that were not found to be significantly associated with ONJ included patient age, renal insufficiency, alcohol use, smoking, and treatment with anthracyclines, melphalan, glucocorticoids, or thalidomide.
The multivariate Cox proportional hazards regression analysis modeling time to ONJ confirmed that a history of osteoporosis (HR, 6.11; 95% CI: 1.56–23.98; p = 0.0095) and dental extraction (HR, 9.78; 95% CI: 3.07–31.14; p = 0.0001) were significant risks for development of ONJ while controlling for type of bisphosphonate therapy. In myeloma patients, there was no statistically significant difference between the development of ONJ in patients treated with pamidronate, zoledronic acid, or both. The osteonecrosis cases in myeloma were uniformly distributed between the three groups of bisphosphonates: four treated with pamidronate alone; four treated with zoledronic acid alone; and four treated with pamidronate and zoledronic acid. One patient received one infusion of pamidronate (90 mg) followed by alendronate 40 mg daily (5 mo) and 80 mg daily (17 mo).
Glucocorticoids and ONJ
Because glucocorticoids have been implicated in the development of avascular necrosis of other sites, we evaluated the doses of prednisone and dexamethasone received by all patients. A total of 2840 patients in the non-ONJ group received dexamethasone (median dose, 252 mg; range, 1–21,380 mg) and 432 patients received prednisone (median dose, 380 mg; range, 5–37,800 mg). In the ONJ group, 21 patients were treated with dexamethasone (median, 565 mg; range, 16–3560 mg) and 2 were treated with prednisone (median, 67.5 mg; range, 55–80 mg). Although the median dose of dexamethasone in the ONJ group was 2-fold greater than in the group without ONJ, this difference did not reach statistical significance (p = 0.22).
Lack of ONJ in other malignancies
To explore why ONJ is predominantly seen in patients with breast cancer and multiple myeloma, we compared cumulative dose and duration of bisphosphonate therapy in breast cancer or multiple myeloma patients versus patients with metastatic bone disease from other cancers. Patients with breast cancer or myeloma received significantly greater cumulative doses of pamidronate or zoledronic acid and for a longer period of time than did patients with bone metastases from other cancers. The median dose of pamidronate was 540 mg (range, 45–9810 mg) in patients with breast cancer, 270 mg (range, 30–9300 mg) in patients with myeloma, and 90 mg (range, 30–4140 mg) in patients with other cancers (p < 0.0001). The median dose of zoledronic acid was 32 mg (range, 4–270 mg) in breast cancer, 28 mg (range, 4–172 mg) in myeloma, and 8 mg (range, 2–156 mg) in other cancers (p < 0.0001). Duration of treatment with zoledronic acid or pamidronate was also longer in both breast cancer and myeloma patients versus patients with other cancers (p < 0.0001).
DISCUSSION
Bisphosphonates were first introduced into clinical use in the 1970s. Continued study of bisphosphonate structure and function led to the development of more potent bisphosphonates able to inhibit bone resorption more efficiently with minimal or no detrimental effects in bone mineralization.(18,19) These agents (pamidronate, alendronate, risedronate, ibandronate, and zoledronic acid) constitute the bulk of currently used bisphosphonates and are characterized by the presence of a nitrogen molecule within the chemical structure. These compounds have been used successfully to treat conditions in which bone resorption is a major component of the disease process such as Paget's disease of bone, osteoporosis, hypercalcemia of malignancy, and metastatic bone disease. The use of intravenous bisphosphonates in patients with multiple myeloma, breast cancer, and other solid tumors metastatic to the bones has led to a remarkable improvement of quality of life by reducing skeletal-related events.(20–24)
In 2003, the first reports of ONJ in patients treated with bisphosphonates appeared. ONJ, previously associated with radiation therapy to the head and neck area, was identified in patients receiving bisphosphonate therapy without radiation exposure.(3–6) These reports brought this condition to the attention of the medical community but lacked information regarding incidence and risk factors. Several subsequent reports have described a frequency of ONJ that ranges from 0.6% to 6.2% for bisphosphonate-treated patients with breast cancer and 1.7% to 11% for those with myeloma.(7–17) It is important to note that no one (including this series) has shown a direct cause–effect relationship between bisphosphonates and ONJ in a controlled study; however, the infrequency of recognition of this disorder in nonirradiated patients with cancer before 2003 provides a compelling case for such an association.
We conducted an extensive analysis of patients treated with intravenous bisphosphonate at our institution over a period of 8 yr. This study differs from other published reports because it evaluates the frequency and risk factors associated with ONJ in a large cohort of patients that is not restricted to the diagnosis of multiple myeloma or breast cancer. In patients who received bisphosphonate therapy as part of their treatment, 0.72% (1.2% in breast cancer; 2.4% in multiple myeloma) developed ONJ. This figure is lower than some of the reports but is similar to the experience in another major cancer center, where ∼1% of patients with breast cancer treated with intravenous bisphosphonates developed ONJ(10) and similar to two recently published studies that identified a frequency of 1.7% and 1.9% of ONJ in multiple myeloma patients.(13,15) We examined the potential explanations for our generally lower frequency of ONJ. One potential explanation is a failure to ascertain all cases. We think this is unlikely because patients receiving their oncologic care at our institution are most commonly referred for dental evaluation internally when they develop an unusual dental problem. The purpose is to exclude the possibility of metastatic cancer or an infectious process that could act as a septic focus. Such a visit would have been recorded in the medical record and ascertained. However, cases of ONJ in patients who transferred their care to another institution would not be ascertained. Another explanation for a low incidence of ONJ in our institution is that routine dental care in advance of oncologic therapy reduces the overall incidence of ONJ. Based on our review of the medical records, we estimate that ∼30% of women with breast cancer and 50% of multiple myeloma patients received pretherapy dental evaluation and care at the Dental Clinic.
Review of the studies reported to date suggests that the frequency of ONJ may be different according to geographical regions.(7–17) Most of the published studies are from the United States, Italy, Poland, and Greece. The frequency of ONJ in multiple myeloma patients seems to be lower in the United States, Poland, and Italy (1.2–3.5%) and higher in Greece (7.4–11%).(8,9,12,13,15–17) In breast cancer patients, the frequency varies from 0.6%(10) to the 1.2% observed in this study in the United States, 2.9% in Greece,(8) and 6.2% in a recent study from Italy.(14) These differences raise interesting questions regarding potential influence of genetic backgrounds in the development of ONJ and/or reflect differences in oral health from these different countries.
In this study, there was no difference in age or sex of patients who developed ONJ. However, it is evident that development of ONJ was associated with longer median duration of malignant disease, longer median duration of bone metastases, which relates to longer treatment duration, and greater cumulative doses of pamidronate and/or zoledronic acid. In agreement with these findings, ONJ was identified largely in patients with breast cancer and myeloma. None of our 2108 patients with prostate, renal, lung, or other cancers developed ONJ. In addition, none of the patients treated with intravenous bisphosphonates for indications other than myeloma or metastatic bone disease, including osteoporosis, hypercalcemia, and Paget's disease, developed ONJ. The absence of ONJ in these groups is likely related to the lower cumulative dose of bisphosphonates and duration of therapy in these groups, although our data do not exclude the possibility of other explanations. Finally, we identified no relationship between any specific chemotherapy agent or protocol and ONJ.
Another important question is whether the potency of the bisphosphonate has an impact on the development of ONJ. There has been a progressive increase in the potency of bisphosphonates over the past three decades. Studies show a rank order of bone resorption potency as follows: zoledronic acid > ibandronate > risedronate > alendronate > pamidronate > etidronate.(27) The most potent are several orders of magnitude more than the least. Our results and reports from others(7–9,11) suggest an increase in the recognition of this disorder coincident with the approval of zoledronic acid, the most potent bisphosphonate. Indeed, the multivariate analysis of our dataset showed that the use of zoledronic acid is an independent risk factor in women with breast cancer. In addition, among breast cancer patients who developed ONJ, the time between initiating bisphosphonate therapy to development of ONJ was shorter in patients treated with zoledronic acid alone compared with patients treated with pamidronate or pamidronate followed by zoledronic acid. Our dataset is not robust enough to conclude that zoledronic acid use is the major factor, because the majority of the patients who developed ONJ received sequential pamidronate and zoledronic acid therapy. We suspect that development of ONJ is related to a complex combination of dose, duration, and potency of bisphosphonate therapy.
Precipitating factors
Our study confirmed and highlighted observations made by others regarding the relationship between preexisting dental health and development of ONJ. In 28 of 29 patients with this disorder, we were able to identify precipitating events that included dental extractions (55%), dental implants (2.9%), periodontal disease (41%), trauma related to intubation or poorly fitting dentures (17%), and bone exostosis at the site of osteonecrosis (34%). These findings further reinforce the recommendation for prebisphosphonate dental evaluation as a strategy for prevention of ONJ.(28–31) In addition, caution should be exercised in performing dental extraction or dental implants in patients receiving intravenous bisphosphonate treatment as part of a cancer therapy regimen.
Our dataset provides no guidance regarding the safety of dental extractions or implants after discontinuation of intravenous bisphosphonate treatment because all cases occurred during active treatment. Our strategy has shifted to a focus on prebisphosphonate dental evaluation and treatment. As is evident from our dataset, several patients were continued on intravenous bisphosphonate at the same or a reduced frequency even after the identification of osteonecrosis and at least 2 of the 13 patients healed despite continuation of bisphosphonates.
Examination of other factors
Our dataset elucidated several other features that have not been rigorously examined. The first is the lack of an association between glucocorticoid use and dosage and ONJ as assessed by univariate or multivariate analyses. Second, there is a relationship between estrogen receptor status in breast cancer and the development of ONJ. Further examination by Cox proportional hazards regression analysis showed that this effect was related to the longer survival period of patients with estrogen receptor–positive tumors, who received larger cumulative doses of bisphosphonate and a longer duration of therapy. Third, in our cohort of long-term follow-up patients, it is clear that the clinical course of ONJ is variable. The limited size and the complexity of the dataset make a formal analysis impossible. There are, however, a few relevant observations. First, for most patients, ONJ is a long-lasting disorder. Observation over a 3-yr period showed healing in <25% of patients. Second, in this cohort, discontinuation of bisphosphonate therapy had no impact in healing. Two of the three patients (all with myeloma) who healed continued on bisphosphonate therapy, and none of the breast cancer patients have healed despite the fact that four of seven have discontinued bisphosphonate treatment.
We recognize that some may recommend the avoidance of bisphosphonates in patients with bone metastasis or myeloma. It is important to recall, however, that bisphosphonates were introduced to prevent skeletal events, which have much greater morbidity/mortality than ONJ. There is general consensus in the oncologic community that skeletal-related events, substantial problems in the prebisphosphonate era, have diminished substantially since the introduction of bisphosphonates, leading to improved quality and possibly duration of life. These effects are so profound that it is unlikely bisphosphonate use will be discontinued until there are suitable alternatives.
It is also important to recognize that these results are not applicable to the use of oral bisphosphonates for the treatment of osteoporosis. Whereas there have been reported cases of ONJ associated with oral bisphosphonate use (including the one case associated with high-dose oral bisphosphonate in this report), it is clear from the paucity of these reports that such events are significantly less common than in cancer patients treated with intravenous bisphosphonates.(5,6) Our observation that duration of therapy seems to be a factor in the development of ONJ may have relevance to the long-term use of oral bisphosphonates to prevent or treat osteoporosis. Whereas there are long-term safety data available for several oral bisphosphonates,(32,33) including bone biopsies showing normal remodeling, there remains concern that long-term treatment and accumulation of bisphosphonate in bone could lead to detrimental effects.(34) Ongoing surveillance is clearly warranted.
In conclusion, ONJ is an uncommon but significant complication that occurs in patients treated with bisphosphonates. In this study, the development of ONJ was associated with longer treatment and higher cumulative doses of intravenous bisphosphonate therapy and with dental extractions and treatment with zoledronic acid in breast cancer patients. Because bisphosphonates have had a critical role in management of metastatic bone disease, their continued use is justified. However, the medical community should focus on prevention of ONJ by detection and management of dental disease before treatment with bisphosphonates and routine surveillance during therapy. The retrospective nature of the study and the statistical analysis of a small number of cases of ONJ are limitations of our study. We suggest that prospective studies should be performed to confirm these results, and in addition, more careful studies to define the minimum dose and duration of therapy with bisphosphonates necessary to prevent skeletal complications of malignancy are also needed.
ACKNOWLEDGMENTS
We thank Loy Deloney for administrative support, Mary Jean Klein for protocol assistance, and Bernice Pines for secretarial assistance. This study was supported in part by NIH/NCI 2P30 CA016672 30, the Nellie B. Connally Breast Cancer Research Program, and an unrestricted grant from Novartis Pharmaceuticals.
Footnotes
Dr Gagel has served as a speaker and received research support from Novartis Pharmaceuticals. Drs Hortobagyi and Hoff received research support from Novartis Pharmaceuticals. All other authors state that they have no conflicts of interest.
REFERENCES
- 1.Reuther T, Schuster T, Mende U, Kubler A. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients: A report of a thirty year retrospective review. Int J Oral Maxillofac Surg. 2003;32:289–295. doi: 10.1054/ijom.2002.0332. [DOI] [PubMed] [Google Scholar]
- 2.Wahl MJ. Osteoradionecrosis Prevention Myths. Int J Radiat Oncol Biol Phys. 2006;64:661–669. doi: 10.1016/j.ijrobp.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Carter GD, Goss AN. Bisphosphonates and avascular necrosis of the jaws. Aust Dent J. 2003;48:268. [PubMed] [Google Scholar]
- 4.Migliorati CA. Bisphosphonates and oral cavity avascular bone necrosis. J Clin Oncol. 2003;21:4253–4254. doi: 10.1200/JCO.2003.99.132. [DOI] [PubMed] [Google Scholar]
- 5.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 6.Ruggiero SL, Mehotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the Jaws associated with the use of bisphosphonates: A review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353:99–100. doi: 10.1056/NEJM200507073530120. [DOI] [PubMed] [Google Scholar]
- 8.Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukos V, Gika D, Anagnostopoulos A, Papadimitriou C, Terpos E, Dimopoulos MA. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factors. J Clin Oncol. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 9.Badros A, Weikel D, Salama A, Goloubeva O, Schneider A, Rapoport A, Fenton R, Gahres N, Sausville E, Ord R, Meiller T. Osteonecrosis of the jaw in multiple myeloma patients: Clinical features and risk factors. J Clin Oncol. 2006;24:945–952. doi: 10.1200/JCO.2005.04.2465. [DOI] [PubMed] [Google Scholar]
- 10.Van Poznak CH, Estilo CL, Sauter NP, Hudis C, Tunick S, Huryn JM, Halpern J. Osteonecrosis of the jaw in patients with metastatic breast cancer. Breast Cancer Res Treat. 2004;88(Suppl 1):S131. [Google Scholar]
- 11.Woo S-B, Hellstein JW, Kalmar JR. Systematic review: Bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 12.Tosi P, Zamagni E, Cangini D, Tachetti P, DiRaimondo F, Catalano L, DÁrco A, Ronconi S, Cellini C, Offidani M, Perrone G, Ceccolinin M, Brioli A, Tura S, Baccarani M, Cavo M. Osteonecrosis of the jaws in newly diagnosed multiple myeloma patients treated with zoledronic acid and thalidomide-dexamethasone. Blood. 2006;108:3951–3952. doi: 10.1182/blood-2006-07-033571. [DOI] [PubMed] [Google Scholar]
- 13.Kraj M, Ryszard P, Stanislaw M, Owczarska K. The incidence of jaw osteonecrosis in multiple myeloma patients treated with bisphosphonates. Acta Pol Pharm. 2006;63:450–452. [PubMed] [Google Scholar]
- 14.Sanna G, Zampino MG, Pelosi G, Nole F, Goldhirsh A. Jaw avascular bone necrosis associated with long-term use of bisphosphonates. Ann Oncol. 2005;16:1207–1208. doi: 10.1093/annonc/mdi206. [DOI] [PubMed] [Google Scholar]
- 15.Pozzi S, Marcheselli R, Sacchi S, Baldini L, Angrili F, Pennese E, Quarta G, Stelitano C, Caparotti G, Luminari S, Musto P, Natale D, Broglia C, Cuoghi A, Dini D, Di Tonno P, Leonardi G, Pianezze G, Pitini V, Polimeno G, Ponchio L, Masini L, Musso M, Spriano M, Pollastri G. Bisphosphonate-associated osteonecrosis of the jaw: A review of 35 cases and an evaluation of its frequency in multiple myeloma patients. Leuk Lymphoma. 2007;48:56–64. doi: 10.1080/10428190600977690. [DOI] [PubMed] [Google Scholar]
- 16.Zervas K, Verrou E, Teleioudis Z, Vahtsevanos K, Banti A, Mihou D, Krikelis D, Terpos E. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: A single-centre experience in 303 patients. Br J Haematol. 2006;134:620–623. doi: 10.1111/j.1365-2141.2006.06230.x. [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulos MA, Kastritis E, Anagnostopoulos A, Melakopoulos I, Gika D, Moulopoulos LA, Bamia C, Terpos E, Tsionos K, Bamias A. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: Evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91:968–971. [PubMed] [Google Scholar]
- 18.Rodan GA, Fleisch HA. Bisphosphonates: Mechanism of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleisch H. Bisphosphonates: Mechanism of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 20.Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, Wheeler H, Simeone JF, Seaman J, Knight RD. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases: Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 21.Theriault RL, Lipton A, Hortobagyi GN, Leff R, Gluck S, Stewart JF, Costello S, Kennedy I, Simeone J, Seaman JJ, Knight RD, Mellars K, Heffernan M, Reitsma DJ. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: A randomized, placebo-controlled trial-Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol. 1999;17:846–854. doi: 10.1200/JCO.1999.17.3.846. [DOI] [PubMed] [Google Scholar]
- 22.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein M, Coleman RE, Reitsma DJ, Seaman JJ, Chen BL, Ambros Y. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer and osteolytic lesions of multiple myeloma: A phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 23.Rosen LS, Gordon D, Tchekmedyan S, Yanagihara R, Hirsh V, Krakowski M, Pawlicki M, de Souza P, Zheng M, Urbanowitz G, Reitsma D, Seaman JJ. Zoledronic acid versus placebo in the treatment of skeletal metastases I patients with lung cancer and other solid tumors: A phase III, double-blind, randomized trial-the Zoledronic Acid Lung cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 24.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B, Zoledronic Acid Prostate Cancer Study Group A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 25.Maalouf NM, Heller HJ, Odvina CV, Kim PJ, Sakhaee K. Bisphosphonate-induced hypocalcemia: Report of 3 cases and review of literature. Endocr Pract. 2006;12:48–53. doi: 10.4158/EP.12.1.48. [DOI] [PubMed] [Google Scholar]
- 26.Guarneri V, Donati S, Nicolini M, Giovanelli S, D'Amico R, Conte PF. Renal Safety and efficacy of i.v. bisphosphonates in patients with skeletal metastases treated for up to 10 years. Oncologist. 2005;10:842–848. doi: 10.1634/theoncologist.10-10-842. [DOI] [PubMed] [Google Scholar]
- 27.Green JR, Muller K, Jaeggi KA. Preclinical pharmacology of CGP 42'446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res. 1994;9:745–751. doi: 10.1002/jbmr.5650090521. [DOI] [PubMed] [Google Scholar]
- 28.Bilezikian JP. Osteonecrosis of the jaw—do bisphosphonates pose a risk. N Engl J Med. 2006;355:2278–2281. doi: 10.1056/NEJMp068157. [DOI] [PubMed] [Google Scholar]
- 29.Ruggiero S, Gralow J, Marx RE, Hoff AO, Schubert MM, Huryn JM, Toth B, Damato K, Valero V. Practical guidelines for the prevention, diagnosis and treatment of osteronecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2:7–14. doi: 10.1200/jop.2006.2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Body JJ, Coleman R, Clezardin P, Ripamonti C, Rizzoli R, Aapro M. International Society of geriatric oncology (SIOG) clinical practice recommendations for the use of bisphosphonates in elderly patients. Eur J Cancer. 2007;43:852–858. doi: 10.1016/j.ejca.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Weitzman R, Sauter N, Eriksen EF, Tarassoff PG, Lacerna LV, Dias R, Altmeyer A, Csermak-Renner K, McGrath L, Lantwicki L, Hohneker JA. Critical review: Updated recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in cancer patients–May 2006. Crit Rev Oncol Hematol. 2007;62:148–152. doi: 10.1016/j.critrevonc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Bone HG, Hosking D, Devogeloer JD, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA, Alendronate Phase III Osteoporosis Treatment Study Group Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 33.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, FLEX Research Group Effects of continuing or stopping alendronate after 5 years of treatment: The Fracture Intervention Trial Long-term Extension (FLEX): A randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 34.Odvina CV, Zerweck JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: A potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]