Abstract
Introduction
Hip fracture is the most devastating osteoporotic fracture type with significant morbidity and mortality. Several studies in humans identified chromosomal regions linked to hip size and bone mass. Animal models, particularly the inbred rat, serve as complementary approaches for studying the genetic influence on hip fragility. The purpose of this study is to identify sex-independent and sex-specific quantitative trait loci (QTLs) for femoral neck density, structure, and strength in inbred Copenhagen 2331 (COP) and Dark Agouti (DA) rats.
Materials and Methods
A total of 828 (405 males and 423 females) F2 progeny derived from the inbred COP and DA strains of rats were phenotyped for femoral neck volumetric BMD (vBMD), cross-sectional area, polar moment of inertia (Ip), neck width, ultimate force, and energy to break. A whole genome screen was performed using 93 microsatellite markers with an average intermarker distance of 20 cM. Recombination-based marker maps were generated using MAPMAKER/EXP from the COP × DA F2 data and compared with published Rat Genome Database (RGD) maps. These maps were used for genome-wide linkage analyses to detect sex-independent and sex-specific QTLs.
Results
Significant evidence of linkage (p < 0.01) for sex-independent QTLs were detected for (1) femoral neck vBMD on chromosomes (Chrs) 1, 6, 10, and 12, (2) femoral neck structure on Chrs 5, 7, 10, and 18, and (3) biomechanical properties on Chrs 1 and 4. Male-specific QTLs were discovered on Chrs 2, 9, and 18 for total vBMD, on Chr 17 for trabecular vBMD, on Chr 9 for total bone area, and on Chr 15 for ultimate force. A female-specific QTL was discovered on Chr 2 for ultimate force. The effect size of the individual QTL varied between 1% and 4%.
Conclusions
We detected evidence that sex-independent and sex-specific QTLs contribute to hip fragility in the inbred rat. Several QTLs regions identified in this study are homologous to human chromosomal regions previously linked to QTLs contributing to femoral neck and related phenotypes.
Key words: quantitative trait loci, sex-independent, sex-specific, BMD, hip fracture
INTRODUCTION
Osteoporosis is a common polygenic disorder with reduced BMD and increased susceptibility to fracture at multiple skeletal sites.(1) Hip fracture is one of the most devastating osteoporotic fracture types causing significant morbidity and mortality(2) and has a greater incidence in women then men. BMD, structure, and strength are the primary skeletal determinants of hip fracture risk.(3–6) These phenotypes are under substantial genetic control,(7–9) and there is evidence that the heritability of bone structure at the hip is higher in women than men.(8)
In humans, several studies have shown linkage for hip BMD, axis length, and bone size to multiple chromosomal regions.(10–30) Some recent studies also provide evidence of sex-specific bone mass regulation at different skeletal sites including the hip in humans.(21,24,30) Unfortunately, none of these studies has yet identified a gene or genes underlying the variation in these hip phenotypes. To expedite the gene discovery process, it would be most effective to use extensive skeletal phenotyping along with gene expression and proteomic analyses to prioritize potential candidate genes. Unfortunately, such studies are extremely difficult, if not impossible, in humans but they can be undertaken in animal models.
Previously, we showed that skeletal mass, structure, and strength varies among inbred strains of rats.(31) We also showed that, despite similar body size, substantial variation exists in bone geometry and biomechanical properties between adult Copenhagen 2331 (COP) and Dark Agouti (DA) rats.(32) Femoral neck work to failure in COP rats was >3-fold greater than DA rats because of thicker femoral neck and shorter femur axis length in COP rats. Thus, COP rats seem to have alleles that substantially affect the hip structure, and these inbred rats may provide a unique resource to identify the genetics underlying the phenotypic variation at the femoral neck.
We generated a large number of second filial (F2) progeny derived from a cross of COP and DA inbred rats having significant differences in their hip structure. We obtained multiple hip phenotypes, many of which could only be obtained in a rodent model. The purpose of this study was to identify the quantitative trait loci (QTLs) contributing to the variation in hip structure. In addition, by testing male and female F2, we were able to also test for sex-specific genetic regulation of hip bone mass and strength.
MATERIALS AND METHODS
Animal breeding
Reciprocal mating of COP rats with DA rats was performed to first create a hybrid F1 population, and the F1 rats were intercrossed to create a total of 828 (405 males and 423 females) second filial (F2) offspring. Once an F2 population was created, the rats were allowed to grow to 6 mo (26 wk) of age before they were killed. Rat identities were recorded on data chips implanted subcutaneously and were verified using a scanner from Biomedic Data System (Seaford, DE, USA). The rats were housed at Indiana University's Laboratory Animal Resource Center (LARC), two rats per cage, and provided standard rat chow (NIH-31 Mouse/Rat diet 7017; Harlan Teklad, Madison, WI, USA) and water ad libitum. After death, rat left and right femora attached with muscle were dissected out, and the right femora were immediately stored at −20°C for biomechanical testing. The left femora were stripped of the muscle and soft tissues and transferred to 70% ethyl alcohol and stored at 4°C for densitometry analyses. The excised spleens were immediately stored in liquid nitrogen before transferring them to −80°C. The procedures performed throughout the experiment followed the guidelines of the Indiana University Animal Care and Use Committee (IACUC).
pQCT
The proximal ends of the left femora were placed in plastic tubes filled with 70% ethyl alcohol and centered in the gantry of a Norland Stratec XCT Research SA+pQCT (Stratec Electronics, Pforzheim, Germany). Single slice measurements of 0.26 mm thickness and a voxel size of 0.07 mm were taken perpendicularly through the femoral neck. Five consecutive scans perpendicular to the neck axis were obtained 0.25 mm apart from each other starting at the base of the femoral head and ending at the greater trochanter. For each slice, the X-ray source was rotated through 180° of projection. Volumetric BMD (vBMD; mg/cm3), cross-sectional area (CSA; mm2), and polar moment of inertia (Ip; mm4) of the femoral neck were measured from the average values of all five pQCT slices using the XCT Research SA Plus, software version 5.40. A density threshold of 900 was used to identify the boundary between trabecular bone and cortical bone, and the lower threshold of 300 was used to distinguish trabecular bone from soft tissue. The accuracy and repeatability of the pQCT measurements was confirmed using a method described previously.(33)
Femoral neck width measurement
The femoral neck width of each rat was measured in the anterior–posterior direction using digital calipers accurate to 0.01 mm, with a precision of +0.005 mm (Mitutoyo, Aurora, IL, USA). The shortest transverse distance of the femoral neck was considered the width of the femoral neck.
Biomechanical testing
The frozen right femora were brought to room temperature slowly in a saline bath and sectioned in the femur shaft at 25% of their total length from the distal end. The proximal ends of the femora were mounted vertically in a special chuck that clamped the femoral shaft to the lower platen of the material testing machine (Alliance RT/5; MTS Systems, Eden Prairie, MN, USA). The bones were held in place by a small (1 N) preload, and load was applied directly downward at a cross-head speed of 20 mm/min onto the femoral head at room temperature in monotonic axial compression until fracture. Force and displacement measurements were collected every 0.05 s. From the force versus displacement curves, ultimate force (Fu; N) and energy to break (U; mJ) were calculated in TestWorks software, version 4.06. Fu reflects the strength of the bone or maximum load that the bone can support before failing; U reflects the amount of energy the specimen can absorb before fracture.
DNA isolation and microsatellite marker genotyping
Genomic DNA was isolated from the individual rat spleen using the Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. Genotyping for each animal was accomplished using PCR with microsatellite markers (Research Genetics, Birmingham, AL, USA) previously shown to be polymorphic for COP and DA rats. A total of 93 markers, with an average intermarker spacing of 20 cM, were genotyped in 828 F2 progeny to provide coverage of the entire genome (chromosomes [Chrs] 1–20, X). The PCR primer sequences were designed for multiplexing using fluorescent dyes. The Qiagen Multiplex PCR Kit was used for all multiplex PCR reactions (Qiagen, Valencia, CA, USA). Allele sizes of the fluorescently labeled PCR products were determined on an ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using the program, Genotyper, version 3.6.
Heritability measurement
Heritability of the phenotypic measures was estimated as unity minus the ratio of the pooled variance in the parental animals (COP and DA) to the phenotypic variance in the F2 progeny. These variance estimates provided indices of the environmental and genetic variances, respectively, for each trait.
Quantitative linkage analysis
Quality assessment of the data was performed. Genotyping data for each marker were tested to ensure the expected 1:2:1 mendelian ratio. Recombination-based marker maps were generated using MAPMARKER/EXP with genotype data from all 828 F2 animals.(34) Marker order and distances were compared with those previously published at the Rat Genome Database (RGD) website (http://rgd.mcw.edu/; June 2007). The following femoral neck phenotypes were prioritized for analysis: femoral neck total vBMD, cortical vBMD, trabecular vBMD, neck width, polar Ip, total area, cortical area, trabecular area, ultimate force, energy to break, stiffness, and elongation. The distribution of each phenotype was reviewed, and all outliers were verified or removed from further analysis. In addition, phenotypic distributions were also examined to detect sex differences. When sex differences were found (e.g., body weight), analyses were performed in the male and female F2 separately, and combined analyses of the male and female F2 were not performed. For the vast majority of phenotypes, different distributions were not observed for the two sexes, and analyses were performed in the male and female F2 rats and the combined sample.
The recombination-based marker map generated using the data from the 828 F2 animals was used to perform multipoint linkage analyses using the program R/qtl.(35) Permutation tests were performed to obtain appropriate genome-wide significance levels for the linkage results.(36) Because the phenotypes are correlated, the set of phenotypes for each rat was kept together and randomly reassigned (permuted) to another rat in the sample. This phenotypic reassignment was performed for each of the 828 F2 animals to generate a permutated sample. This process was repeated to generate 5000 permutated datasets, and genome-wide linkage analysis was performed in each of the 5000 permutated datasets.(37) All phenotypes measured for each rat were included in the permutation approach. Thus, genome-wide significance thresholds appropriate for each phenotype were obtained. The maximum LOD scores for linkage for each phenotype was recorded in each permutated dataset. In this manner, the LOD significance thresholds for the 95th (suggestive) and 99th (significant) percentile of the maximum genome-wide LOD scores across all phenotypes were found to be 3.5 and 4.3, respectively. ANOVA was performed using the most significant marker in each QTL region to further characterize significant genotypic group differences and obtain effect size estimates. For measures resulting in detection of multiple QTLs, these markers were also included in an overall ANOVA model to estimate their combined contribution to phenotypic variation.
Sex specificity of each QTL detected in our primary analysis was evaluated according to the method described by Solberg et al.,(38) which compares two linkage models that differ only in a QTL-by-sex interaction effect. The full model (model 5) contains terms for QTL, sex, and QTL-by-sex interaction effect, whereas the reduced model (model 4) contains terms for QTL and sex only. At the position of each QTL achieving genome-wide significance in the primary screen (LOD > 3.5), a traditional likelihood ratio test (1-df χ2) was performed using the full and reduced models. This represents a test for sex specificity of the QTLs for the phenotype and chromosomal position considered.
RESULTS
Phenotypic heritability
Heritability of femoral neck total vBMD, cortical vBMD, trabecular vBMD, total area, cortical area, ultimate force, and energy to break was 0.74, 0.32, 0.79, 0.32, 0.32, 0.46, and 0.65, respectively.
Genetic loci for body weight
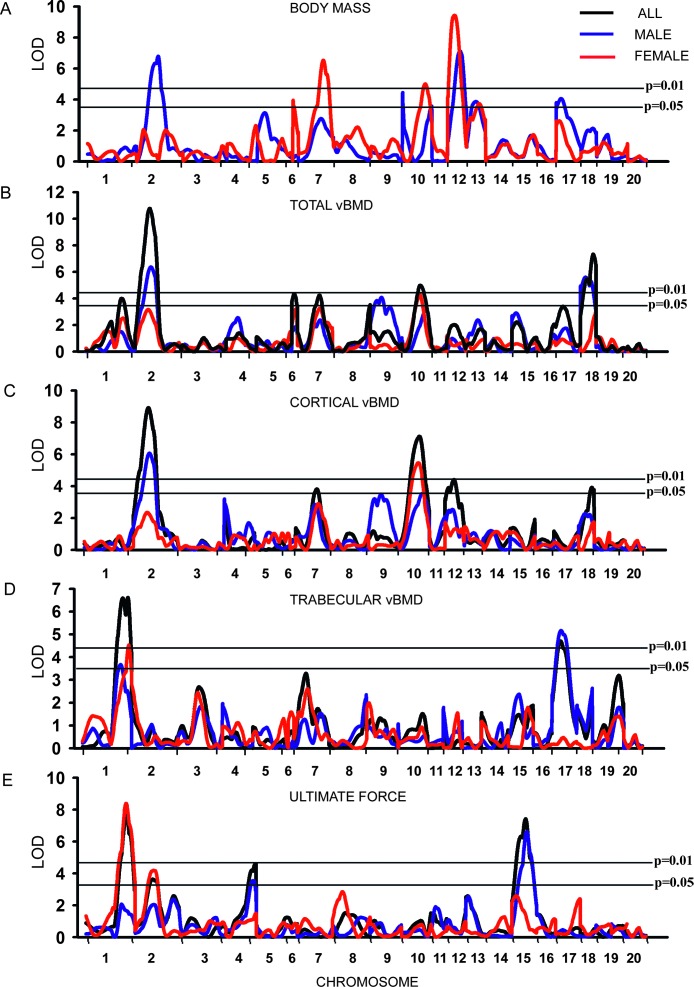
Significant evidence of linkage (p < 0.01) of body weight was observed on Chrs 2 (LOD = 6.8) and 12 (LOD = 7.1) in male and Chrs 7 (LOD = 6.5), 10 (LOD = 5.0), and 12 (LOD = 9.4) in female rats (Fig. 1A). In addition, suggestive linkage (p < 0.05) for body mass was observed on Chrs 10, 13, and 17 in male and on Chrs 6 and 13 in female rats (Fig. 1A; Table 1). All bone phenotypes reported below were adjusted to correct for variation in body weight.
FIG. 1.
Genome-wide linkage analysis for body mass (A), total vBMD (B), cortical vBMD (C), trabecular vBMD (D), and ultimate force (E) for rat Chrs 1–20 (excluding X and Y chromosomes). Multipoint LOD scores plotted on the y axis vs. the relative location along each chromosome on the x axis. The horizontal lines indicate the threshold value for genome-wide significance at p = 0.05 (bottom line) and p = 0.01 (top line). The blue linkage line indicates male, the red linkage line indicates female, and the black linkage line indicates the whole F2 sample.
Table 1.
Summary of Genome Screen LOD Score for Density, Biomechanics, and Structure of Femoral Neck*
|
Density
|
Biomechanics
|
Structure
|
||||||||||
|
LOD (position in cM)†
|
LOD (position in cM)†
|
LOD (position in cM)†
|
||||||||||
| Chromosome | Phenotype | All | Male | Female | Phenotype | All | Male | Female | Phenotype | All | Male | Female |
| 1 | Total vBMD | 4.0 (102) | Ultimate force | 7.6 (114) | 8.3 (114) | Neck width | 4.0 (113) | 3.5 (113) | ||||
| Trabecular vBMD | 6.6 (127) | 3.6 (108) | 4.5 (130) | |||||||||
| 2 | Total vBMD | 10.7 (31) | 6.3 (33)‡ | Ultimate force | 3.6 (36) | 4.1 (38)‡ | ||||||
| Cortical vBMD | 8.9 (32) | 6.0 (33) | Energy to break | 3.6 (88) | ||||||||
| 4 | Ultimate force | 4.5 (96) | 3.5 (87) | |||||||||
| 5 | Cortical area | 4.4 (3) | ||||||||||
| 6 | Total vBMD | 4.3 (10) | ||||||||||
| 7 | Total vBMD | 4.2 (53) | Total area | 5.4 (52) | ||||||||
| Cortical vBMD | 3.8 (51) | Ip | 5.1 (53) | |||||||||
| 8 | Total vBMD | 3.5 (82) | ||||||||||
| 9 | Total vBMD | 4.0 (27)‡ | ||||||||||
| 10 | Total vBMD | 4.9 (44) | 4.3 (43) | Total area | 3.5 (13)‡ | |||||||
| Cortical vBMD | 7.1 (45) | 3.5 (38) | 5.4 (44) | Ip | 4.5 (43) | |||||||
| 12 | Cortical vBMD | 4.4 (30) | Total area | 5.2 (43) | 3.6 (44) | |||||||
| 15 | Ultimate force | 7.4 (33) | 6.6 (36)‡ | |||||||||
| Energy to break | 6.6 (35) | 4.1 (36) | ||||||||||
| 17 | Trabecular vBMD | 4.7 (18) | 5.1 (19)‡ | Cortical area | 4.5 (21) | |||||||
| 18 | Total vBMD | 7.3 (31) | 5.6 (14)‡ | |||||||||
| Cortical vBMD | 3.9 (32) | |||||||||||
* All LOD scores above the significance thresholds for the 95th percentile (LOD = 3.5) are reported.
† Position in centimorgans is based on the RGD map.
‡ Represents male sex-specific QTLs.
Genetic loci for femoral neck vBMD
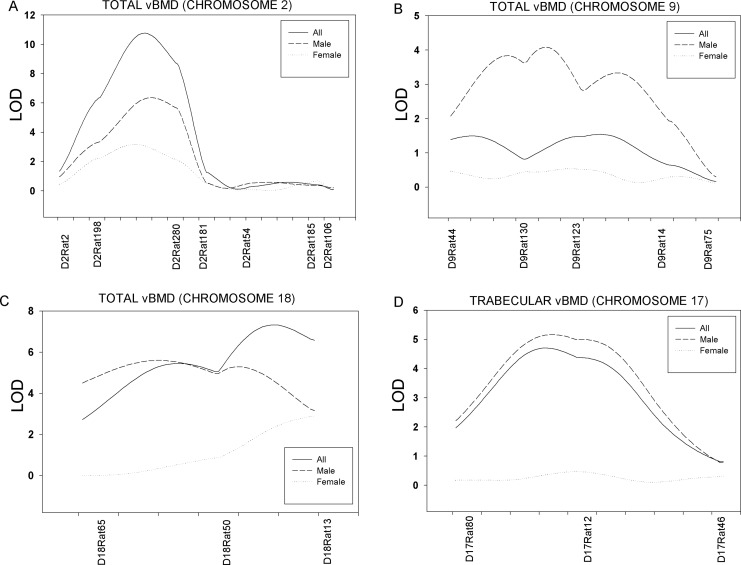
Evidence of significant linkage (p < 0.01) of femoral neck total vBMD was observed on Chrs 2, 6, 10, and 18 in the full sample of 828 F2 animals (LOD score of 10.7, 4.3, 4.9, and 7.3, respectively; Fig. 1B; Table 1). Significant linkage for total vBMD was observed on Chrs 2 (LOD = 6.3) and 18 (LOD = 5.6) in male and Chr 10 (LOD = 4.3) in female rats. Suggestive linkage (p < 0.05) for total vBMD was observed on Chrs 1, 7, and 8 in the full F2 sample. Linkage of male-specific total vBMD QTLs was detected on Chrs 2 (sex-specific p = 0.04), 9 (p = 0.00002), and 18 (p = 0.002), and of trabecular vBMD on Chr 17 (p = 0.002). Figure 2 shows the sex-specific linkage for total vBMD on Chrs 2 (A), 9 (B), and 18 (C) and trabecular vBMD on Chr 17 (D).
FIG. 2.
Sex-specific linkage for femoral neck total vBMD on Chr 2 (A), Chr 9 (B), and Chr 18 (C) and trabecular vBMD on Chr 17 (D).
The compartmentalized analysis of neck density showed that cortical and trabecular density at the femoral neck was linked to several QTLs. Evidence of significant linkage (p < 0.01) of femoral neck cortical vBMD was observed on Chrs 2 (LOD = 8.9), 10 (LOD = 7.1), and 12 (LOD = 4.4) in the full sample (Fig. 1C; Table 1). Suggestive linkage (p < 0.05) for cortical vBMD was observed on Chrs 7 and 18 in the full sample and Chr 10 in male rats. Significant linkage for cortical vBMD was observed on Chr 2 (LOD = 6.0) in male and Chr 10 (LOD = 5.4) in female rats. Two major femoral neck trabecular vBMD QTLs (p < 0.01) were detected on Chrs 1 (LOD = 6.6) and 17 (LOD = 4.7) in the full sample of F2 rats (Fig. 1D; Table 1). Significant linkage (p < 0.01) was detected on Chr 17 (LOD = 5.1) in male and on Chr 1 (LOD = 4.5) in female rats. Suggestive linkage (p < 0.05) for trabecular vBMD was observed on Chr 1 in male F2.
Several cortical vBMD QTLs were found to be significantly affecting the QTLs for total density. The cortical density QTLs on Chr 2 in male and on Chr 10 in female were found on the same region of Chrs 2 and 10, where total density QTLs is located. On the other hand, we found no significant (p < 0.01) density QTLs affecting both sexes in any chromosomes. However, cortical density QTL on Chr 10 in female found to be suggestively linked to male cortical density. Similarly trabecular density QTL on Chr 1 in female was found to be suggestively linked to male trabecular density.
Genetic loci for femoral neck structure
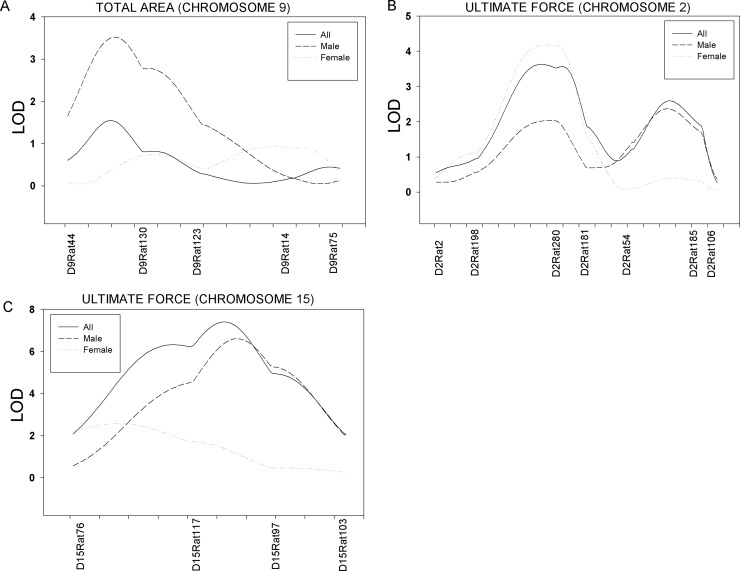
Significant linkage (p < 0.01) of femur neck cross-sectional total area was observed on Chrs 7 and 10 in the combined sample with LOD score of 5.4 and 5.2, respectively (Table 1). Suggestive linkage (p < 0.05) of total area was detected on Chr 10 in female rats. Significant linkage (p < 0.01) for cortical area was found on Chrs 5 (LOD = 4.4) and 17 (LOD = 4.5) in the full sample. Significant linkage (p < 0.01) of polar moment of inertia (Ip) was observed on Chrs 7 and 10 with a LOD score of 5.1 and 4.5, respectively. In addition, suggestive linkage (p < 0.05) for neck width was observed on Chr 1 both the combined and female samples. Also, a male-specific QTL for total area was found on Chr 9 (sex-specific p = 0.0001). Figure 3A shows the sex-specific linkage for total bone area on Chr 9.
FIG. 3.
Sex-specific linkage for femoral neck total area on Chr 9 (A), ultimate force on Chr 2 (B), and ultimate force on Chr 15 (C).
Genetic loci for femoral neck strength
Significant linkage (p < 0.01) of femur neck ultimate force was observed on Chrs 1, 4, and 15 in the full sample with LOD scores of 7.6, 4.5, and 7.4, respectively (Fig. 1E; Table 1). A locus significantly linked (p < 0.01) to ultimate force was found on Chr 1 (LOD = 8.3) in female rats. Also, a significant male-specific QTL for ultimate force was found on Chr 15 (sex-specific p = 0.0006), and a female-specific ultimate force was detected on Chr 2 (p = 0.003). Figure 3 shows the sex-specific linkage for ultimate force on Chrs 2 (B) and 15 (C). Suggestive linkage (p < 0.05) of ultimate force was detected on Chr 2 in the full sample, on Chr 4 in male rats, and on Chr 2 in female rats. Significant linkage (p < 0.01) was observed for energy to break on Chr 15 (LOD = 6.6) in the combined sample (Table 1). In addition, suggestive linkage (p < 0.05) was observed for energy to break on Chrs 2 and 15 in male rats.
DISCUSSION
We identified several sex-independent and sex-specific chromosomal regions linked to femoral neck density, structure, and strength in inbred COP and DA F2 rats. Analysis of the full sample of F2 rats showed significant sex-independent linkage on Chrs 1, 6, 10, and 12 for density, Chrs 5, 7, 10, and 18 for structure, and Chrs 1 and 4 for strength. Subsequent analyses of the full sample by sex detected male-specific QTLs on Chrs 2, 9, and 18 for total vBMD, Chr 17 for trabecular vBMD, Chr 9 for total area, and Chr 15 for ultimate force. A female-specific QTL was found on Chr 2 for ultimate force. In addition, we observed allele-specific effects on several chromosomal regions. The c/c alleles contributed beneficially on Chrs 2, 10, 17, and 18 for neck density, Chrs 7 and 17 for structure, and Chrs 4 and 15 for biomechanics (Table 2). On the other hand, the c/c alleles had detrimental effects on Chrs 1, 6, 7, and 12 for neck density, Chr 10 for structure, and Chr 1 for biomechanics.
Table 2.
Genotypic Mean Values for Femoral Neck Bone Mass, Structure, and Strength QTLs in all F2 Samples*
|
Genotype
|
Effect of COP alleles | r2 | ANOVA p value | |||||
| Phenotype | Chromosome | Marker | c/c | c/d | d/d | |||
| Total vBMD (mg/mm3) | 2 | D2Rat280 | 1067.9 ± 3.7 | 1052.1 ± 2.7 | 1033.3 ± 3.8 | A | 0.03 | <0.0001 |
| 6 | D6Rat46 | 1039.2 ± 4.0 | 1051.5 ± 2.7 | 1062.2 ± 3.7 | A | 0.01 | 0.0002 | |
| 7 | D7Rat23 | 1037.3 ± 3.9 | 1055.8 ± 2.6 | 1056.0 ± 4.1 | R | 0.02 | 0.0003 | |
| 10 | D10Rat162 | 1062.7 ± 3.9 | 1054.0 ± 2.7 | 1035.6 ± 4.0 | A | 0.02 | <0.0001 | |
| 18 | D18Rat13 | 1064.8 ± 3.6 | 1051.6 ± 2.8 | 1035.1 ± 3.8 | A | 0.02 | <0.0001 | |
| Cortical vBMD (mg/mm3) | 2 | D2Rat280 | 1259.8 ± 2.7 | 1247.4 ± 2.0 | 1236.4 ± 2.8 | A | 0.03 | <0.0001 |
| 10 | D10Rat162 | 1257.2 ± 2.8 | 1250.4 ± 2.0 | 1234.4 ± 2.9 | A | 0.04 | <0.0001 | |
| 12 | D12Rat91 | 1240.4 ± 2.7 | 1247.0 ± 2.0 | 1256.9 ± 2.9 | R | 0.01 | 0.0002 | |
| Trabecular vBMD (mg/mm3) | 1 | D1Rat169 | 735.1 ± 1.8 | 742.7 ± 1.2 | 748.3 ± 1.7 | A | 0.02 | <0.0001 |
| 17 | D17Rat12 | 746.8 ± 1.7 | 743.2 ± 1.2 | 735.3 ± 1.8 | A | 0.02 | <0.0001 | |
| Total area (mm2) | 7 | D7Rat23 | 5.5 ± 0.1 | 5.4 ± 0.1 | 5.2 ± 0.1 | A | 0.02 | <0.0001 |
| 10 | D10Rat162 | 5.3 ± 0.1 | 5.3 ± 0.1 | 5.6 ± 0.1 | R | 0.02 | <0.0001 | |
| Cortical area (mm2) | 17 | D17Rat12 | 3.5 ± 0.1 | 3.4 ± 0.1 | 3.3 ± 0.1 | A | 0.01 | <0.0001 |
| Ip (mm4) | 7 | D7Rat23 | 5.4 ± 0.1 | 5.1 ± 0.1 | 4.7 ± 0.1 | A | 0.02 | <0.0001 |
| 10 | D10Rat162 | 4.9 ± 0.1 | 4.9 ± 0.1 | 5.5 ± 0.1 | R | 0.02 | <0.0001 | |
| Ultimate force (N) | 1 | D1Rat169 | 102.6 ± 1.0 | 104.3 ± 0.6 | 109.9 ± 0.9 | R | 0.03 | <0.0001 |
| 4 | D4Rat72 | 108.6 ± 0.9 | 105.0 ± 0.7 | 102.6 ± 0.9 | A | 0.02 | <0.0001 | |
| 15 | D15Rat117 | 109.2 ± 1.0 | 105.9 ± 0.7 | 101.6 ± 1.0 | A | 0.02 | <0.0001 | |
| Energy to break (mJ) | 15 | D15Rat97 | 29.7 ± 0.9 | 26.2 ± 0.6 | 22.8 ± 0.9 | A | 0.03 | <0.0001 |
* Values are mean ± SE in all F2 samples.
A, additive; R, recessive; c/c, homozygous for COP alleles; c/d, heterozygous; d/d, homozygous for DA alleles.
Significant QTLs affecting only density without influencing structure or strength were found on Chr 18 (Table 1). Although the QTL linked to total vBMD in male on Chr 18 is separated 17 cM from the QTL linked in all F2 rats for the same phenotype, they seem to be a single QTL. Remarkably, syntenic regions corresponding to these QTLs regions were also linked to femur neck BMD on 5q32 and 18p11 in humans (Table 3).(13,29) A locus on Chr 5 was found affecting only neck structure without affecting density or strength. On the other hand, QTLs influencing neck strength were discovered on Chrs 4 and 15 with no linkage to either density or structures. The syntenic regions for rat Chr 15 QTLs were linked to hip and neck density and structure on 10q22, 13q21, and 14q23 in humans.(18–20)
Table 3.
Rat QTLs That Overlap With Homologous Human QTLs and Related Phenotypes*
| Chromosome | Rat femoral neck phenotypes | Sex | Flanking markers | Human synteny and phenotype |
| Chr 1 | Trabecular vBMD | All samples | D1Rat169–D1Rat82 | 9q21, Neck BMD(21) |
| Female only | D1Rat169–D1Rat82 | 10q26, Hip BMD(15) | ||
| Ultimate force | All samples | D1Rat169–D1Rat82 | 11q12–13, Neck BMD(13,22) | |
| Female only | D1Rat169–D1Rat82 | |||
| Chr 2 | Total vBMD | All samples | D2Rat198–D2Rat280 | 3q24–26, Pelvic axis length(23) |
| Male only | D2Rat198–D2Rat280 | 5p12–15, Trochanter size(11) | ||
| Cortical vBMD | All samples | D2Rat198–D2Rat280 | 5q11, Neck axis length(14) | |
| Male only | D2Rat198–D2Rat280 | 4q23–26, Neck BMD and axis length(14,24) | ||
| Chr 4 | Ultimate force | All samples | D4Rat104–D4Rat72 | |
| Chr 5 | Cortical area | All samples | D5Rat187–D5Rat3 | 1p35–36, Neck and hip BMD(16,17,25,29) |
| Chr 6 | Total vBMD | All samples | D6Rat46–D6Rat42 | |
| Chr 7 | Total vBMD | All samples | D7Rat23–D7Rat78 | 8q22–24, Hip bone size and Ward's BMD(26,28) |
| Total area | All samples | D7Rat23–D7Rat78 | ||
| Ip | All samples | D7Rat23–D7Rat78 | ||
| Chr 8 | Energy to break | Female only | D8Rat111–D8Rat15 | 3q21–24, Pelvic axis length(23) |
| 11q22, Neck BMD(30) | ||||
| 15q22–25, Neck and hip BMD, neck cross-sectional area(18) | ||||
| Chr 10 | Total vBMD | All samples | D10Rat162–D10Rat124 | 5q31–35, Neck BMD and axis length(13) |
| Female only | D10Rat162–D10Rat124 | 17p11–13, Neck and hip BMD(10) | ||
| Cortical vBMD | All samples | D10Rat162–D10Rat124 | ||
| Female only | D10Rat162–D10Rat124 | |||
| Ip | All samples | D10Rat162–D10Rat124 | ||
| Total area | All samples | D10Rat162–D10Rat124 | ||
| Chr 12 | Cortical vBMD | All samples | D12Rat91–D12Rat30 | 7p22–23, Neck BMD(27) |
| Chr 15 | Ultimate force | All samples | D15Rat117–D15Rat97 | 10q22, Neck cross-sectional area(20) |
| Male only | D15Rat117–D15Rat97 | 13q21, Hip BMD(19) | ||
| Energy to break | All samples | D15Rat117–D15Rat97 | 14q23, Neck BMD(18) | |
| Chr 17 | Trabecular vBMD | All samples | D17Rat80–D17Rat12 | 9q21–22, Neck BMD(21) |
| Male only | D17Rat80–D17Rat12 | |||
| Cortical area | All samples | D17Rat80–D17Rat12 | ||
| Chr 18 | Total vBMD | All samples | D18Rat50–D18Rat13 | 5q32, Neck BMD(13) |
| Male only | D18Rat65–D18Rat50 | 18p11, Neck BMD(29) |
* All rat QTLs with LOD scores above the significance thresholods for the 99th percentile (LOD = 4.3) are reported.
Sex-independent pleiotropic QTLs affecting both density and structure were found on Chrs 1, 7, and 10. The trabecular density QTL found on Chr 1 was also linked to neck width (Table 1). The corresponding human syntenic regions on 9q21 and 10q26 were previously linked to neck and hip BMD (Table 3).(15,21) The density QTLs discovered on Chr 7 in the full sample were also linked to neck structure phenotypes. A syntenic region in humans on 8q22–24 for this Chr 7 QTL in rat was linked to hip bone size and Ward's BMD.(26,28) Similarly, the QTLs linked to total and cortical vBMD on Chr 10 in the full sample and females were also linked to neck structure phenotypes. The corresponding human syntenic region on 5q31–35 and 17p11–13 were linked to neck BMD and axis length.(10,13)
Sex-independent pleiotropic QTLs affecting both density and strength were found on Chr 1. Trabecular density QTL on Chr 1 was also linked to ultimate force (Table 1). The syntenic chromosomal regions for these QTLs on Chr 1 in rats were linked to neck and hip BMD on 9q21, 10q26, and 11q12–13 in human (Table 3).(13,15,21,22) The only pleiotropic QTL that affected density, structure, and strength was found on Chr 1 (Table 1).
Sex-specific QTLs analyses showed that male-specific density QTL was found on Chrs 2, 9, and 18 for total vBMD (Fig. 2; Table 1). A male-specific trabecular vBMD was found on Chr 17. A male-specific neck structure QTL was found on Chr 9 for total area, and a male-specific strength QTL was detected on Chr 15 for ultimate force (Fig. 3). On the other hand, a female-specific QTL for ultimate force was found on Chr 2. This same QTL on Chr 2 also significantly linked to both total and cortical vBMD. The QTL linked to energy to break on Chr 2 is at a different location from the QTL for density or strength on the same chromosome, suggesting that this QTL influences bone strength independently. The corresponding human syntenic region for this Chr 2 QTL in rat was extensively linked to neck BMD and structure on 3q24–26, 4q23–26, 5p12–15, and 5q11.(11,14,23,24) The syntenic regions for male-specific ultimate force QTL on Chr 15 in rat coincides with the neck BMD on 14q23,(18) hip BMD on 13q21,(19) and neck cross-sectional area on 10q22(20) in humans (Table 3).
In this study, we analyzed each femoral neck trait independently as we did for our previous studies. However, the underlying correlation among the phenotypes can potentially be used to improve the ability to identify QTLs contributing to multiple traits. Therefore, we measured the correlation among the phenotypes when these traits map to a common QTL region. At the 1% genome-wide significance level, total vBMD and cortical vBMD are both linked to the same QTL regions on Chrs 2 and 10. The correlation coefficient between these two phenotypes is 0.77, supporting the localization of a QTL that affects common variability in both phenotypes. On the other hand, trabecular vBMD and ultimate force are both linked to the same QTL region on Chr 1. The correlation coefficient between these two phenotypes is −0.13. Thus, it would possible that within the QTL region there could be separate genes contributing to these traits, or there could be a common gene that has been identified that would not have been detected based on the correlation structure of these phenotypes.
The statistically significant individual QTLs detected in the combined male and female sample for femoral neck phenotypes in our study were of modest effect, with ANOVA r 2 estimates between 1% and 4% (Table 2). We also evaluated the combined effect of the significant QTLs for each phenotype with multiple findings and found that all the QTLs that we report remained significant (p < 0.01) when considered simultaneously. The total variance explained in each phenotype was 11.4% for total vBMD, 8.6% for cortical vBMD, 4.9% for trabecular vBMD, 5.4% for total area, and 9.4% for ultimate force. These estimates are all approximately equal to the sum of the individual QTL effects, indicating that they act independently to influence the femoral neck phenotypes measured.
In a previous study, we identified several genetic loci that affect femoral neck BMD and a single locus on rat Chr 4 that strongly affects femoral neck structure and strength in inbred female Fischer 344 and Lewis rats.(33) However, in this study, we found only a couple of QTLs influencing ultimate force on Chr 4 in COP and DA rats, suggesting that COP and DA rats harbor different genetic variants that influence the femoral neck. Additionally, we found several chromosomal locations in both of Fischer 344 × Lewis and COP × DA inbred models overlapped for femoral neck phenotypes. A QTL for cortical vBMD in Fischer 344 and LEW rats coincides with the QTLs for total vBMD, cortical vBMD, and ultimate force in COP and DA rats on Chr 2. Similarly, a QTL on Chr 4 for cortical vBMD in the F344 × LEW study coincides with the QTL for ultimate force in the COP × DA study. A QTL for total vBMD, trabecular vBMD, and trabecular area on Chr 15 in the F344 and LEW F2 sample coincides with the QTL for ultimate force and energy to break in COP and DA F2 rats. In addition, QTLs for total and cortical area on Chr 18 in F344 and LEW rats coincide with the QTLs for total and cortical vBMD in COP and DA rats.
In summary, we detected several sex-independent and sex-specific QTLs that contribute to hip fragility in inbred COP and DA rats. Several rat QTLs found in this study are syntenic to multiple human QTLs previously linked to different femoral neck phenotypes in both men and women. Besides these syntenic QTLs observed in this study, some new QTLs were also discovered for different femoral neck phenotypes. These QTLs harbor hundreds of candidate genes contributing genetic determinants of density, structure, and strength at the hip. Further studies involving fine mapping of individual QTLs through the development of congenic lines and cDNA microarray and proteomic analyses will be necessary to identify the key genes contributing to the risk of hip fracture.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health through the following grants: R01AR047822 (CHT) and P01AG018397 (CHT, DLK, TF, MJE).
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.NIH Consensus Development Conference. Diagnosis, prophylaxis and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Looker AC, Orwoll ES, Johnston CC, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 3.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 4.Peacock M, Turner CH, Liu G, Manatunga AK, Timmerman L, Johnston CC., Jr Better discrimination of hip fracture using bone density, geometry and architecture. Osteoporos Int. 1995;5:167–173. doi: 10.1007/BF02106096. [DOI] [PubMed] [Google Scholar]
- 5.Faulkner KG, Cumming SR, Black D, Palermo L, Gluer CC, Genant HK. Simple measurement of femoral geometry predicts hip fracture: The study of osteoporotic fractures. J Bone Miner Res. 1993;8:1211–1217. doi: 10.1002/jbmr.5650081008. [DOI] [PubMed] [Google Scholar]
- 6.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 7.Peacock M, Turner CH, Econs MJ, Foroud T. Genetics of osteoporosis. Endocr Rev. 2002;23:303–326. doi: 10.1210/edrv.23.3.0464. [DOI] [PubMed] [Google Scholar]
- 8.Arden NK, Baker J, Hogg C, Bann K, Spector TD. The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: A study of postmenopausal twins. J Bone Miner Res. 1996;11:530–534. doi: 10.1002/jbmr.5650110414. [DOI] [PubMed] [Google Scholar]
- 9.Garnero P, Arden NK, Griffiths G, Delmas PD, Spector TD. Genetic influence on bone turnover in postmenopausal twins. J Clin Endocrinol Metab. 1996;81:140–146. doi: 10.1210/jcem.81.1.8550741. [DOI] [PubMed] [Google Scholar]
- 10.Huang QY, Xu FH, Shen H, Zhao LJ, Deng HY, Liu YJ, Dvomyk V, Conway T, Davies KM, Li JL, Liu YZ, Recker RR, Deng HW. A second-stage genome scan for QTLs influencing BMD variation. Calcif Tissue Int. 2004;75:138–143. doi: 10.1007/s00223-004-0088-y. [DOI] [PubMed] [Google Scholar]
- 11.Huang QY, Xu FH, Shen H, Zhao LJ, Deng HY, Conway T, Liu YJ, Liu YZ, Li JL, Li MX, Davies KM, Recker RR, Deng HW. Genome scan for QTLs underlying bone size variation at 10 refined skeletal sites: Genetic heterogeneity and the significance of phenotype refinement. Pysiol Genomics. 2004;17:326–331. doi: 10.1152/physiolgenomics.00161.2002. [DOI] [PubMed] [Google Scholar]
- 12.Koller DL, White KE, Liu G, Hui SL, Conneally PM, Johnston CC, Econs MJ, Foroud T, Peacock M. Linkage of structure at the proximal femur to chromosomes 3, 7, 8, and 19. J Bone Miner Res. 2003;18:1057–1065. doi: 10.1359/jbmr.2003.18.6.1057. [DOI] [PubMed] [Google Scholar]
- 13.Koller DL, Econs MJ, Morin PA, Christian JC, Hui SL, Parry P, Curran ME, Rodriguez LA, Conneally PM, Joslyn G, Peacock M, Johnston CC, Foroud T. Genome screen for QTLs contributing to normal variation in bone mineral density and osteoporosis. J Clin Endocrinol Metab. 2000;85:3116–3120. doi: 10.1210/jcem.85.9.6778. [DOI] [PubMed] [Google Scholar]
- 14.Koller DL, Liu G, Econ MJ, Hui SL, Morin PA, Joslyn G, Rodriguez LA, Conneally PM, Christian JC, Johnston CC. Genome screen for quantitative trait loci underlying normal variation in femoral structure. J Bone Miner Res. 2001;16:985–991. doi: 10.1359/jbmr.2001.16.6.985. [DOI] [PubMed] [Google Scholar]
- 15.Deng HW, Xu FH, Huang QY, Shen H, Deng H, Conway T, Liu YJ, Liu YZ, Li JL, Zhang HT. A whole-genome linkage scan suggests several genomic regions potentially containing quantitative loci for osteoporosis. J Clin Endocrinol Metab. 2002;87:5151–5159. doi: 10.1210/jc.2002-020474. [DOI] [PubMed] [Google Scholar]
- 16.Devoto M, Shimoya K, Caminis J, Ott J, Tnenhouse A, Whyte MP, Sereda L, Hall S, Considine E, Williams CJ. First-stage autosomal genome screen in extended pedigrees suggests genes predisposing to low bone mineral density on chromosomes 1p, 2p and 4q. Eur J Hum Genet. 1998;6:151–157. doi: 10.1038/sj.ejhg.5200169. [DOI] [PubMed] [Google Scholar]
- 17.Devoto M, Specchia C, Li HH, Caminis J, Tenenhouse A, Rodriguez H, Spotila LD. Variance component linkage analysis indicates a QTL for femoral neck bone mineral density on chromosome 1p36. Hum Mol Genet. 2001;10:2447–2452. doi: 10.1093/hmg/10.21.2447. [DOI] [PubMed] [Google Scholar]
- 18.Peacock M, Koller DL, Hui S, Johnson CC, Conneally PM, Foroud T, Econs MJ. Peak bone mineral density at the hip is linked to chromosomes 14q and 15q. Osteoporos Int. 2004;15:489–496. doi: 10.1007/s00198-003-1560-7. [DOI] [PubMed] [Google Scholar]
- 19.Kammerer CM, Schneider JL, Cole SA, Hixson JE, Samollow PB, O'Connell JR, Perez R, Dyer TD, Almasy L, Blangero J, Bauer RL, Mitchell BD. Quantitative trait loci on chromosomes 2p, 4p and 13q influence bone mineral density of the forearm and hip in Mexican Americans. J Bone Miner Res. 2003;18:2245–2252. doi: 10.1359/jbmr.2003.18.12.2245. [DOI] [PubMed] [Google Scholar]
- 20.Ralston SH, Galwey N, MacKay I, Albagha O, Cardon L, Compston JE, Cooper C, Duncan E, Keen R, Langdahl B, McLellan A, O'Riordan J, Pols HA, Reid DM, Uitterlinden AG, Wass J, Bennett ST. Loci for regulation of bone mineral density in men and women identified by genome wide linkage scan: The FAMOS study. Hum Mol Genet. 2005;14:943–951. doi: 10.1093/hmg/ddi088. [DOI] [PubMed] [Google Scholar]
- 21.Ioannidis JPA, Ng MY, Sham PC, Zintzaras E, Lewis CM, Deng HW, Econs MJ, Karasik D, Devoto M, Kammerer CM, Spector T, Andrew T, Cupples LA, Duncan EL, Foroud T, Kiel DP, Koller D, Langdahl B, Mitchell BD, Peacock M, Recker R, Shen H, Sol-Church K, Spotilla LD, Uitterlinden AG, Wilson SG, Kung AW, Ralston SH. Meta-analysis of genome-wide scans provides evidence for sex- and site-specific regulation of bone mass. J Bone Miner Res. 2007;22:173–183. doi: 10.1359/jbmr.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koller DL, Rodriguez LA, Christian JC, Slemenda CW, Econs MJ, Hui SL, Morin P, Conneally PM, Joslyn G, Curran ME, Peacock M, Johnston CC, Foroud T. Linkage of a QTL contributing to normal variation in bone mineral density to chromosome 11q12-13. J Bone Miner Res. 1998;13:1903–1908. doi: 10.1359/jbmr.1998.13.12.1903. [DOI] [PubMed] [Google Scholar]
- 23.Xiong DH, Shen H, Xiao P, Guo YF, Long JR, Zhao LJ, Liu YZ, Deng HY, Li JL, Recker RR, Deng HW. Genome-wide scan identified QTLs underlying femoral neck cross-sectional geometry that are novel studied risk factors of osteoporosis. J Bone Miner Res. 2006;21:424–437. doi: 10.1359/JBMR.051202. [DOI] [PubMed] [Google Scholar]
- 24.Peacock M, Koller DL, Lai D, Hui S, Foroud T, Econs MJ. Sex-specific quantitative trait loci contribute to normal variation in bone structure at the proximal femur in men. Bone. 2005;37:467–473. doi: 10.1016/j.bone.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson SG, Reed PW, Bansal A, Chiano M, Lindersson M, Langdown M, Prince RL, Thompson D, Thompson E, Bailey M, Kleyn PW, Sambrook P, Shi MM, Spector TD. Comarison of genome screens for two independent cohorts provide replication of suggestive linkage of bone mineral density to 3p21 and 1p36. Am J Hum Genet. 2003;72:144–155. doi: 10.1086/345819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen H, Long JR, Xiong DH, Guo YF, Xiao P, Liu YZ, Zhao LJ, Liu YJ, Deng HY, Li JL, Recker RR, Deng HW. A genomewide scan for quantitative trait loci underlying area bone size variation in 451 Caucasian families. J Med Genet. 2006;43:872–879. doi: 10.1136/jmg.2006.041251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu YH, Xu X, Terwedow HA, Niu T, Hong X, Wu D, Wang L, Brain JD, Bouxsein ML, Cummings SR, Rosen CJ, Xu X. Large-scale genome-wide linkage analysis for loci linked to BMD at different skeletal sites in extreme selected sibships. J Bone Miner Res. 2007;22:184–194. doi: 10.1359/jbmr.061015. [DOI] [PubMed] [Google Scholar]
- 28.Karasik D, Myers RH, Cupples LA, Hannan MT, Gagnon DR, Herbert A, Kiel DP. Genome screen for quantitative trait loci contributing to normal variation in bone mineral density: The Framingham study. J Bone Miner Res. 2002;17:1718–1727. doi: 10.1359/jbmr.2002.17.9.1718. [DOI] [PubMed] [Google Scholar]
- 29.Streeten EA, McBride DJ, Pollin TI, Ryan K, Shapiro J, Ott S, Mitchell BD, Shuldiner AR, O'Connell JR. Quantitative trait loci for BMD identified by autosome-wide linkage scan to chromosomes 7q and 21 in men from the Amish family osteoporosis study. J Bone Miner Res. 2006;21:1433–1442. doi: 10.1359/jbmr.060602. [DOI] [PubMed] [Google Scholar]
- 30.Huang QY, Ng MYM, Cheung CL, Chan V, Sham PC, Kung AWC. Identification of two sex-specific quantitative trait loci in chromosome 11q for hip bone mineral density in Chinese. Hum Hered. 2006;61:237–243. doi: 10.1159/000095216. [DOI] [PubMed] [Google Scholar]
- 31.Turner CH, Roeder RK, Wieczorek A, Foroud T, Liu G, Peacock M. Variability in skeletal mass, structure, and biomechanical properties among inbred strains of rats. J Bone Miner Res. 2001;16:1532–1539. doi: 10.1359/jbmr.2001.16.8.1532. [DOI] [PubMed] [Google Scholar]
- 32.Sun Q, Turner CH. Two inbred rat strains that differ substantially in hip fragility. Calcif Tissue Int. 2003;72:498–504. doi: 10.1007/s00223-002-1040-7. [DOI] [PubMed] [Google Scholar]
- 33.Alam I, Sun Q, Liu L, Koller DL, Fishburn T, Carr LG, Econs MJ, Foroud T, Turner CH. Identification of a quantitative trait locus on rat chromosome 4 that is strongly linked to femoral neck structure and strength. Bone. 2006;39:93–99. doi: 10.1016/j.bone.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L. MAPMARKER: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- 35.Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- 36.Doerge RW, Churchill GA. Permutation tests for multiple loci affecting a quantitative character. Genetics. 1996;142:285–294. doi: 10.1093/genetics/142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basten CJ, Weir BS, Zeng ZB. Zmap-a QTL cartographer. In: Smith C, Gavora JS, Chesnais BBJ, Fairfull W, Gibson JP, Kennedy BW, Burnside EB, editors. Computing Strategies and Software; 5th World Congress on Genetics Applied to Livestock Production; Canada: Ontario; 1994. pp. 65–66. [Google Scholar]
- 38.Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, Li R, Turek FW, Churchill GA, Takahashi JS, Redei EE. Sex- and lineage-specific inheritance of depression-like behavior in the rat. Mamm Genome. 2004;15:648–662. doi: 10.1007/s00335-004-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]