Abstract
Low-level lead (Pb) exposure is associated with behavioral and cognitive dysfunction but it is not clear how Pb produces these behavioral changes. Pb has been shown to alter auditory temporal processing in both humans and animals. Auditory temporal processing occurs in the superior olivary complex (SOC) in the brainstem where it is an important component in sound detection in noisy environments and in selective auditory attention. The SOC receives a serotonergic innervation from the dorsal raphe, and serotonin has been implicated in auditory temporal processing within the brainstem and inferior colliculus. Because Pb exposure modulates auditory temporal processing, the serotonergic system is a potential target for Pb. The current study was undertaken to determine whether developmental Pb exposure preferentially changes the serotonergic system within the SOC. Pb-treated mice were exposed to no Pb, very low Pb (0.01 mM), or low Pb (0.1 mM) throughout gestation and through 21 days postnatally. Brainstem sections from control and Pb-exposed mice were immunostained for the vesicular monoamine transporter 2 (VMAT2), serotonin, and dopamine beta hydroxylase (DβH, a marker for norepinephrine) in order to elucidate the effect of Pb on monoaminergic input into the SOC. Sections were also immunolabeled with antibodies to VGLUT1, VGAT and VAChT to determine whether Pb exposure alters the glutaminergic, gaba-ergic, or cholinergic systems. Pb exposure caused a significant decrease in VMAT2, 5HT, and DβH expression while VGLUT1, VGAT and VAChT showed no change. These results provide evidence that Pb exposure during development alters normal monoaminergic expression in the auditory brainstem.
Keywords: VMAT2, serotonin, auditory, dopamine beta hydroxylase, dorsal raphe
Introduction
Lead (Pb) has long been recognized as a toxic agent that has a significant impact on human health (Mannino et al., 2003; Mannino et al., 2005; Sanborn et al., 2002; Tong et al., 2000; Toscano and Guilarte, 2005). Neurotoxic effects of low doses of Pb have been shown to result in behavioral and cognitive deficits and in 1991, The Centers for Disease Control and Prevention set the acceptable blood lead level at 10 ug/dl. However, an increasing body of evidence has demonstrated that Pb blood lead levels below 10 ug/dL produce many behavioral deficits including lowered IQ, attention deficit hyperactivity disorder, and dyslexia (Braun et al., 2006; Canfield et al., 2003a; Canfield et al., 2003b; Chen et al., 2007; Chiodo et al., 2004; Gilbert and Weiss, 2006; Glotzer et al., 1995; Kamel et al., 2003; Lanphear et al., 2000).
It is not clear how Pb produces these behavioral changes but both low-level lead exposure and learning disabilities have been associated with altered auditory temporal processing in both humans and animals (Finkelstein et al., 1998; Gray, 1999; Lurie et al., 2006). Temporal processing is used to decode complex sounds and to detect a signal within a noise background. It is thought that neurons of the superior olivary complex (SOC) in the brainstem play a role in sound detection in noisy environments and in selective auditory attention (Mulders and Robertson, 2005). The SOC receives a catecholaminergic and a serotonergic innervation from the locus coeruleus and the dorsal raphe respectively (Mulders and Robertson, 2005; Thompson and Hurley, 2004).
While the physiological role of the noradrenergic input to olivocochlear neurons has yet to be defined (Mulders and Robertson, 2005), serotonin (5-HT) has been shown to alter auditory temporal processing in the mammalian auditory brainstem (Hall and Hurley, 2007; Hurley, 2007; Hurley and Pollak, 2005a; Hurley et al., 2002). Olivocochlear neurons project to the inferior colliculus, and serotonin alters both the magnitude and the latency of neuronal responses to auditory stimuli within the IC (Hurley, 2007). Serotonin has been shown to modulate neuronal spike count, first-spike latency, temporal precision, and the interspike interval, all of which may alter temporal processing (Hurley and Pollak, 2005b). Because Pb exposure modulates auditory temporal processing, the serotonergic system is a potential target for Pb within auditory brainstem nuclei.
The current study was undertaken to determine whether developmental Pb exposure changes the expression of serotonin within the superior olivary complex (SOC). Brainstem sections from control and Pb-exposed mice were immunostained for the vesicular monoamine transporter 2 (VMAT2), serotonin, and dopamine beta hydroxylase (DβH), a marker for norepinephrine) in order to elucidate the effect of Pb on monoaminergic input into the SOC. Control and Pb-exposed brainstem sections were also immunolabeled for the vesicular glutamate transporter 1 (VGLUT1), the vesicular acetylcholine transporter (VAChT), and the vesicular GABA transporter (VGAT) in order to determine the effect of Pb on glutaminergic, gaba-ergic, and cholinergic neurotransmitter systems. Glutamate is considered to be the primary neurotransmitter for ascending auditory information (reviewed in Trussell, 2002) but GABA, acetycholine, and glycine are also important neurotransmitters within the auditory brainstem (Maison et al., 2003; Sato et al., 2000; Trussell, 2002).
We found that developmental Pb exposure reduces immunoreactivity for VMAT2, serotonin, and DβH within the Lateral Superior Olive (LSO) but has no effect on VGLUT 1 and 2, VAChT, or VGAT. This affect appears to be specific to the LSO. The motor trigeminal nucleus (located near the SOC in the brainstem) did not show a decrease in 5HT, nor did western blot analysis reveal any decreased monoamergic expression within the entire brainstem. These findings demonstrate that developmental Pb exposure has a significant effect on the monoaminergic innervation to the LSO.
Materials and Methods
Animals
Breeding pairs of CBA mice were obtained from The Jackson Laboratory (Bar Harbor, Maine). All mice used in these studies were maintained in micro isolator units in the University of Montana specific pathogen free animal facility. The animal care facility housed all mice on a 12 h light/dark cycle in a temperature-controlled environment. Cages, bedding, and food were sterilized by autoclaving and mice were handled with aseptic gloves. Mice were allowed food and water ad libitum. All animal use procedures were in accordance with NIH and the University of Montana IACUC guidelines using approved animal protocols.
Lead Exposure
Breeding pairs were randomly assigned to three groups and given unlimited access to water containing 0 mM (control), 0.01 mM (very low), or 0.1 mM (low) Pb acetate. Breeding pairs were given Pb in their drinking water at the time of pairing and offspring were exposed to Pb throughout gestation and through the dam’s milk until postnatal day 21–24 (P21). P21 was chosen as an endpoint for these studies because at this time the auditory system is mature and the mice are considered to be adult.
Blood Lead Levels
Blood was collected from anesthetized mice by retro-orbital puncture. Blood Pb levels were measured by the Montana Health Department in Helena, MT. The no Pb group included values of <1.0 μg/dL which were included in the data set as equal to 1.0 μg/dL.
Western Blot Analysis
Animals were sacrificed at P21 by cervical dislocation and the brainstem removed (n=16 brains per treatment group). The auditory region of the mouse brainstem was isolated using a 1mm mouse brain matrix. A 2mm thick section was cut and then flash frozen on a microscope slide using liquid nitrogen and dissected into three separate regions: the cochlear nucleus (CN); the ventral brainstem region (VBS), and the dorsal brainstem region (DBS) as described in Jones et al, 2008, followed by storage at −80 C. The VBS region contains containing the superior olivary complex (SOC; the lateral superior olive (LSO), medial superior olive (MSO), and the medial nucleus of the trapezoid body (MNTB)).
Pooled samples containing four brains each from the VBS fraction only were analyzed. Samples were homogenized in lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate and 1 mM Na3VO4. (Cell Signaling Technology, Beverly, MA) for a total of n=4 separate homogenates per treatment group. Additions were made giving final concentrations of 0.5% Na-deoxycholate, 0.5% sodium dodecyl sulfate, 1μM okadaic acid, 1 mM phenylmethylsulfonyl fluoride, 0.1 mg/ml benzamidine, 8 μg/ml calpain inhibitors I and II and 1 μg/ml each leupeptin, pepstatin A and aprotinin. Tissue was homogenized on ice in .6 ml of ice-cold lysis buffer, incubated on ice for 30 minutes followed by 30 seconds of sonication and centrifugation at 50,000 rpm for 20 minutes at 4°C. The supernatant was assayed for protein concentration using the Bio-Rad Protein Assay (Bio-Rad #500-0001, Hercules CA) and aliquots were stored at −80°C for use in Western analysis. Protein separation was done by SDS-PAGE using NuPAGE® 4–12% Bis-Tris polyacrylamide gels (Invitrogen, Carlsbad, CA). Aliquated samples were mixed with distilled deionized water and NuPAGE® LDS sample buffer followed by 10 minutes at 70°C on a standard VWR® heatblock (West Chester, PA). Gels were loaded with 20 μg of denatured protein per well along with 10 μl MagicMark™ XP Western Protein Standard (Invitrogen, Carlsbad, CA) and 5 ul of Kaleidoscope™ Western Protein Standard (Bio-Rad). Gels were run in NuPAGE® MOPS SDS Running Buffer (Invitrogen) with 500 μl of NuPAGE® Antioxidant (Invitrogen) for 55 min. Membranes were pretreated in methanol for 15 seconds, washed in distilled water for three minutes and soaked in transfer buffer for 5 minutes prior to transfer. Gels were transferred for 1 hr on ice at 100 V in a cold room using a Bio-Rad Power Pac 200 power supply (Bio-Rad). Post-transfer membranes were blocked in 5% dried nonfat milk, 0.1% tween and TBS for 1.5 hours at room temperature. Membranes were washed in TBST 3 times for 10 minutes and incubated with primary antibody in blocking buffer overnight at 4°C (see Table 1 for information on primary antibodies). Membranes were washed in TBST for 5 minutes and the appropriate peroxidase secondary antibody (Vector Labs, Burlingame CA) was applied at 1:2000 for 1 hr at room temp. Membranes were washed three times for 10 minutes in TBST and then visualized using an electrochemiluminescence western blotting detection reagents (Amersham Biosciences, Piscataway NJ). Exposures were taken in a Fuji film Intelligent Darkbox using a Fujifilm LAS-3000 camera (Fujifilm, Valhalla NY). All blots were normalized to GADPH.
TABLE 1.
List of Primary Antibodies
| Target Protein | Antigen | Species | Dilution | Supplier and Cat. No. |
|---|---|---|---|---|
| Anti-GAPDH | GAPDH from rabbit muscle | Mouse monoclonal | 1:2000 (WB) | Chemicon MAB374 |
| Anti-DβH | Full length protein from cow adrenal medulla | Rabbit polyclonal | 1:800 (IF) | Abcam AB43868 |
| Anti-5HT | Rabbit serotonin coupled to bovine serum albumin with paraformaldehyde | Rabbit polyclonal | 1:10,000 (IF) | ImmunStar Inc. Cat#:20080 |
| Anti-VAChT | Strep-Tag fusion protein containing C-terminal residues 475–530 of VAChT from rat | Rabbit polyclonal | 1:500 (WB) 1:2000 (IF) |
Synaptic systems, 139103 |
| Anti-VGLUT1 | Strep-Tag fusion protein containing amino acid residues 456–560 of VGLUT1/BNP1 from rat | Rabbit polyclonal | 1:2000 (WB) 1:1000 (IF) |
Synaptic Systems, 135303 |
| Anti-VMAT2 | AA 496–515 from rat VMAT2 | Rabbit polyclonal | 1:500 (WB) 1:2000 (IF) |
Synaptic Systems, 138302 |
| Anti-VGAT | Synthetic peptide representing AA 75–87 of rat VGAT | Rabbit polyclonal | 1:2000 (WB) 1:200 (IF) |
Synaptic Systems, 131002 |
| Anti-Synaptophysin | Vesicular fraction of bovine brain | Mouse monoclonal | 1:200 (IHC) | Millipore MAB5258 |
IF, immunofluorescence; IHC, immunohistochemistroy; WB, Western blot
Immunohistochemistry
At P21, mice from all three treatment groups (n=5 per group) were deeply anesthetized and perfused transcardially with 4% Na-periodate-lysine-paraformaldehyde fixative (PLP, final concentrations 0.01M sodium periodate, 0.075M lysine, 2.1% paraformaldehyde, 0.037M phosphate). Brains were removed and post-fixed for 2 hours at 4°C in PLP, rinsed 3 times for 10 minutes each in PBS (pH 7.4) and transferred to a 30% sucrose solution in PBS overnight at 4°C. Brains were bisected between the forebrain and brainstem and tissue was embedded cut-side down into 1.5 cm square embedding cups filled with optimal cutting temperature (O.C.T.) compound. Brains were then frozen in liquid nitrogen and stored at −20°C. Ten micron tissue sections were cut on a Thermo Shandon Cryotome Cryostat (Thermo Shandon, Pittsburgh PA) and a one in three series of sections was collected for each brain.
Tissue from control and Pb exposed animals were run together to minimize variability. Sections were rinsed in PBS, permeabilized for 30 minutes with 0.5% Triton X-100 in PBS and then immunostained as previously described (Lurie and Durham, 2000; Wishcamper et al., 2001). Briefly, tissue sections were blocked for 20 minutes with either 4% Normal Goat or Rabbit Serum in PAB (1% sodium azide, 0.5% bovine serum albumin in PBS) and incubated with primary antibody for 24 hours in a humid chamber at 4° C (see Table 1 for details on primary antibodies). Control sections received no primary antibody. The tissue was rinsed and then stained with the appropriate secondary antibody for 1 hr. at room temperature (Alexa Fluor 488--1:400, or 488 Avidin biotin complex-1:500). Sections were then rinsed and the slides coversliped with FluorSave™ (Calbiochem®, San Diego CA) and stored at 4° C. Sections were then viewed and analyzed on a BioRad Radiance 2000 confocal microscope.
For light microscopy and DAB immunohistochemistry, an additional set of control and Pb exposed brains were paraffin embedded as previous described (Lurie and Durham, 2000; Wishcamper et al., 2001) and a one in six series was collected and mounted onto slides. Tissue was then immunostained for synaptophysin using the standard peroxidase anti-peroxidase procedure using the Vector ABC kit with appropriate secondary antibodies (Vector Laboratories Burlingame, CA). Visualization was carried out using 3-3′ diaminobenzidine (DAB, Sigma, St. Louis MO) in 0.1M Tris buffer with 0.001M imidazole and 0.1% hydrogen peroxide as the chromagen. Sections were then rinsed in water, dehydrated, and coverslipped using DPX mounting media (BDH Limited, Poole U.K.).
Antibody Characterization
The GAPDH antibody recognizes a single band at 36 kD in our Western blots of mouse brainstem tissue similar to that seen by Wang et al, 2005.
The DβH antiserum recognizes a band of 68 kD (Zhou et al, 2004) and demonstrates a virtually identical staining pattern in our tissue as other DβH polyclonal antibodies (Eugene Tech International) used to label the rat nucleus tractus solitarii (Nosjean et al, 2002), the zebrafinch song control nuclei (Mello et al, 1998) and the cat SOC (DiaSorin; Behrens et al, 2002).
The 5-HT antibody immunostains our mouse brainstem tissue in a virtually identical staining pattern as other studies using this antiserum to label the mouse superior olive and inferior colliculus (Hurley et al, 2002; Thompson, 2006; 2008) In addition, preadsorption with BSA does not affect immunostaining, but preadsorption with the 5HT/BSA conjugate protein (10 micrograms/ml) eliminates all immunoreactivity.
The vesicular acetylcholine transporter (VAChT) antiserum recognizes a strong band in Western blots at ~50kD, and weaker bands at 67kD and 80kD in our mouse brainstem preps. This antibody recognizes both glycosylated and unglycosylated VAChT. The staining pattern we observe in the murine brainstem is virtually identical to that seen with this antibody in the rat LSO (Yao and Godfrey, 1998) and guinea pig forebrain (Hartig et al, 2007).
The vesicular glutamate transporter 2 (VGLUT 2) antiserum recognizes a band at 60kD in Western blots of our mouse brainstem preps similar to that reported by Takamori et al, 2000a. We also observe a virtually identical staining pattern to that seen in the rat LSO (Billups, 2005).
The antibody to the vesicular monoamine transporter (VMAT) recognizes a strong band at ~55kD on Western blots of our brainstem preps. Some additional bands are also seen that are thought to represent n-glycosylation of VMAT2 (Yao and Hersh, 2007) and we also observe a similar pattern of WB staining as reported for other polyclonal VMAT antibodies (Millipore). Our immunostaining in the murine brainstem is virtually identical to the staining pattern seen in the rat median eminence (Weihe et al, 1996).
The vesicular GABA transporter (VGAT) antiserum recognizes a strong band at ~50kD in Western blots of our mouse brainstem preps similar to the bands at 50 and 57 kD observed by others with this antiserum (Takamori et al, 2000b). This antiserum stains a virtually identical pattern in our murine brainstem tissue to that observed in rat hippocampal neurons (Baer et al, 2007; Prange et al, 2004).
The antibody to synaptophysin recognizes a band of 38 kD (Eastwood and Harrison, 2001). Our murine brainstem tissue immunolabels with a virtually identical staining pattern to that seen in the ferret superior olive (Alvarado et al, 2004), and the rat superior olive [immunostained with a different mouse monoclonal antibody against synaptophysin (Sigma; Caminos et al 2007)].
Tissue Analysis
All fluorescent slides were viewed at 60x magnification using a BioRad Radiance 2000 Laser Scanning System connected to a Dell PC. Images were collected and then converted from color tiff files to black and white, 12 bit tiff files. Two to five sections per animal were analyzed from the middle of the nucleus of interest. A fixed pattern of area of interest boxes (three-five per section) were overlaid onto the tissue section and the integrated optical density of the immunostaining was measured using MediaCybernetics Image-Pro software (Bethesda MD). Integrated optical density measurements were used for quantification of immunostaining because it analyzes both the area of immunostained tissue that met threshold as well as the intensity of the immunostaining. In summary, a threshold of immunostaining in no Pb animals was set for each antibody such that all immunoreactivity met threshold. This set a control threshold that was unique for each antibody and was used as a comparison to the Pb treatment groups. It is important to note that Pb did not decrease the intensity of our fluorescent signal for all antibodies, rather there was a decrease in the amount of label per area of tissue. Immunostaining within three-five random areas (430 square microns) within LSO, MNTB, and the motor trigeminal nucleus in control and Pb-exposed mice was then quantified and averaged. Statistical differences in immunostaining between control and Pb exposed animals were analyzed using Synergy Software’s KaleidaGraph software (Reading PA).
Light microscopic brainstem sections (synaptophysin immunoreactivity) were viewed at 40x magnification with a Nikon E-800 attached to a CRI Nuance multi spectral imaging system (CRI, Inc., Woburn PA). Images were analyzed with NIH Image 1.62 in order to quantify the density of DAB staining. Images containing the entire LSO and MNTB were captured on the screen and the entire LSO and MNTB within each section was analyzed. In control sections of LSO and MNTB, a threshold was set such that the reaction product for synpatophysin reached this threshold. The number of pixels that reached threshold was then calculated by the computer, and the mean pixel count was calculated to determine a single value for all control and Pb exposed brains examined. Measurements were performed on two to three sections per animal.
Neuronal cell counts
Estimates of dorsal raphe neuron numbers in control and Pb treated groups were obtained using a one-in-six series of 10 μm sections collected throughout the entire dorsal raphe and immunolabeled for 5-HT. Slides were viewed at 40x magnification with a Nikon E-800 microscope. Sections were selected and matched for anatomical location and then slides were blinded for analysis. Estimates of dorsal raphe neuron number were obtained from 4–6 consecutive sections located in the middle of the dorsal raphe nucleus. Neurons were counted if they displayed a clearly defined nuclear envelope and a well-defined cytoplasm. Estimates of neuron counts were then corrected using the Abercrombie Factor. Neurons in 4 to 6 sections of the dorsal raphe nucleus per animal were counted. In this way an estimate sample of neuronal number is obtained for each animal.
Photography
Digital images were captured using the BioRad confocal microscope or using a Nikon E-800 microscope attached to the Nuance Spectral Imaging system. The captured images were processed in Adobe Photoshop version 7.0 and contrast, brightness, and sharpness were slightly adjusted to ensure consistency among images.
Statistical Analysis
Data are expressed as mean ± SEM and were analyzed using one-way analysis of variance with Dunnet’s and/or Tukey’s post-hoc analyses where appropriate; p<0.05 was considered significant. Neuron counts were analyzed using a Wilcoxin-Mann-Whitney rank sum test for comparison.
Results
Blood Lead Levels
The current study uses three different doses of Pb in the drinking water, the no Pb control, very low Pb (0.01mM), and low Pb (0.1mM). The blood lead levels (mean ± SEM) for these mice are as follows: No Pb control (1.36 ± 0.014 μg/dL), very low Pb (8.0 ± 0.45), and low Pb (42.26 ± 1.97 μg/dL). None of our doses of Pb produced changes in size or body weight (data not shown) and the animals appeared unaffected by Pb, indicating that the doses used in this study can be considered a sub-toxic dose.
Pb exposure results in decreased expression of VMAT-2, serotonin, and DBH
In order to determine whether developmental Pb exposure results in alterations in monoaminergic neurotransmitters, control and Pb-exposed brainstem sections were immunolabeled for the monoaminergic vesicular transporter, VMAT 2. In control animals, VMAT 2 immunoreactivity is abundant in LSO (Figure 1A) but very little immunoreactivity is found in the medial nucleus of the trapezoid body (MNTB; data not shown). This is in agreement with previous studies that have reported the presence of many 5-HT immunoreactive fibers in the murine LSO, and relatively sparse numbers of 5-HT immunopositive fibers in MNTB (Thompson and Hurley, 2004).
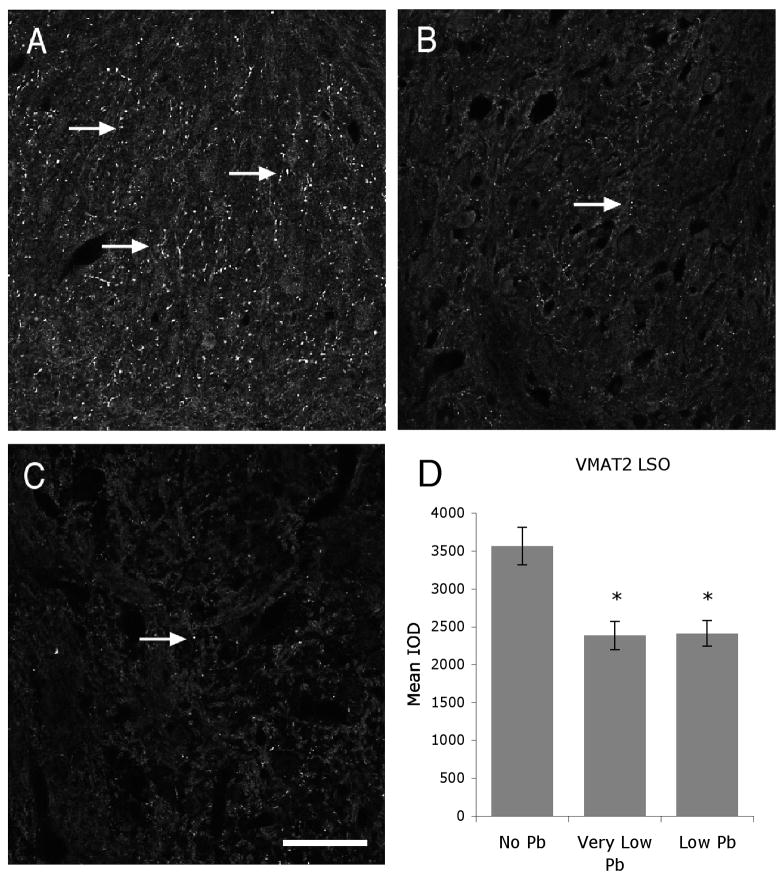
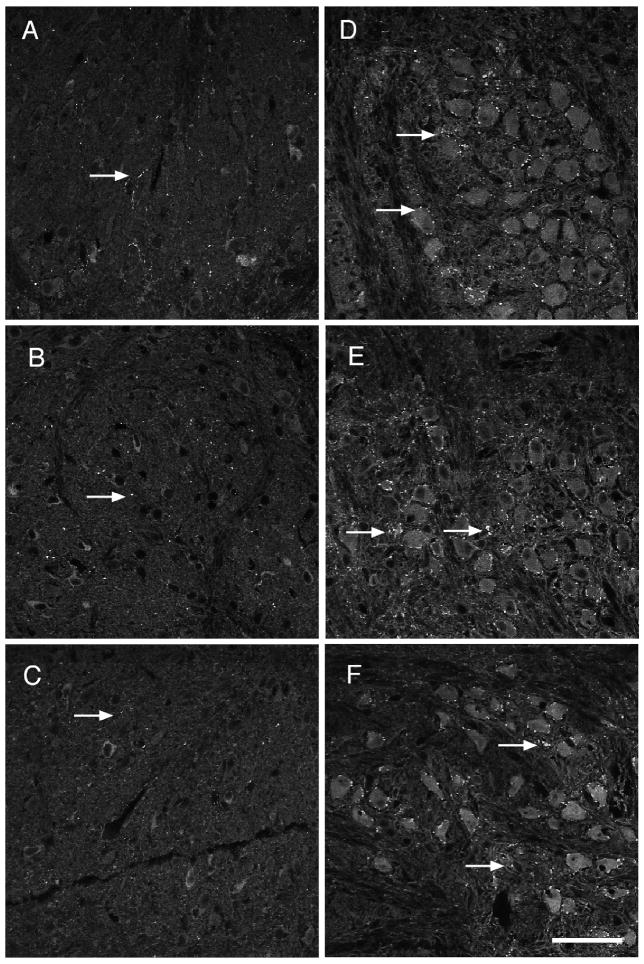
Figure 1.
Pb exposure decreases VMAT2 expresssion within the LSO. A–C) Representative micrographs of immunofluorescent staining for VMAT2 in the LSO in no (A), very low (B), and low (C) Pb mice reveal a decrease in immunoreactivity with Pb treatment (Arrows). Quantification of staining for VMAT2 in the LSO reveals that this decrease is statistically significant (D). Arrows point to immunostaining. Graphs illustrate mean ± the standard error of the mean (SEM). (n=5 per group) *P < 0.05, One-way Anova with Tukey’s all pairs comparison. Bar = 50 μm for panels A–C.
Developmental Pb exposure results in a significant decrease in VMAT 2 immunoreactivity in both the very low and low Pb mice. Figure 1 illustrates the dramatic decrease in VMAT 2 immunoreactivity within the LSO in Pb-treated mice compared to controls. Quantification of the immunostaining reveals a 33% decrease in the VMAT 2 staining.
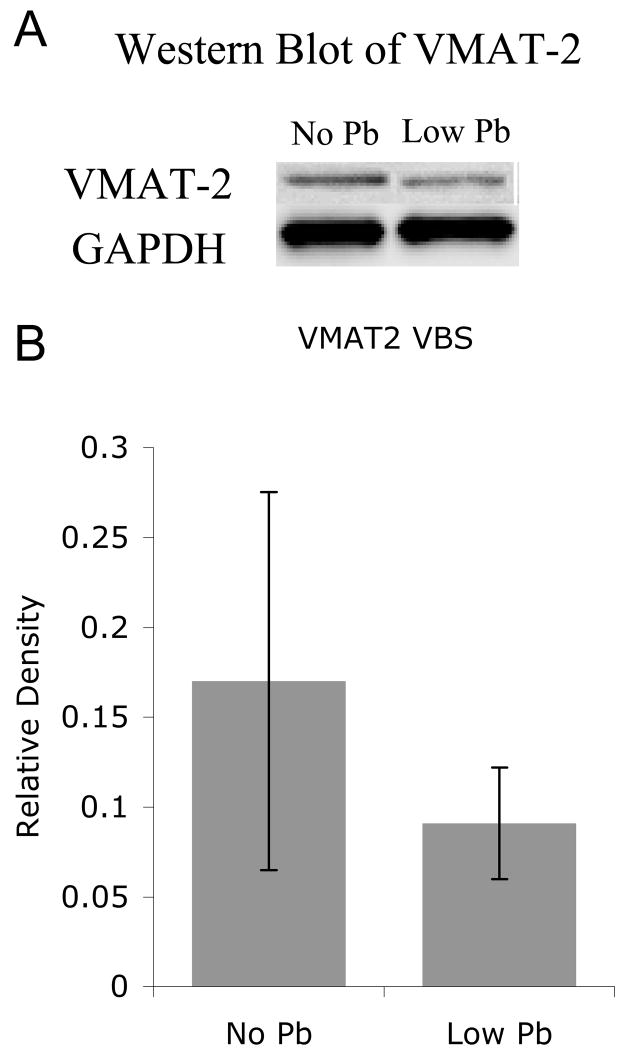
However, it is important to note that this decrease is not a general result of Pb exposure. Western analysis reveals no significant decrease in VMAT 2 protein following Pb exposure within the entire brainstem (Figure 2).
Figure 2.
Pb exposure does not change VMAT2 expression levels in the entire ventral brainstem. A) Western analysis demonstrates no significant change in VMAT2. B) Quantification of the western blots reveals no significant change in VMAT 2 with Pb exposure. Representative blots are shown above the quantification (n=4 separate homogenates; 4 brainstem/homogenate). The graphs illustrate mean ± the standard error of the mean (SEM).
Pb exposure does not alter the expression of other neurotransmitter transporters
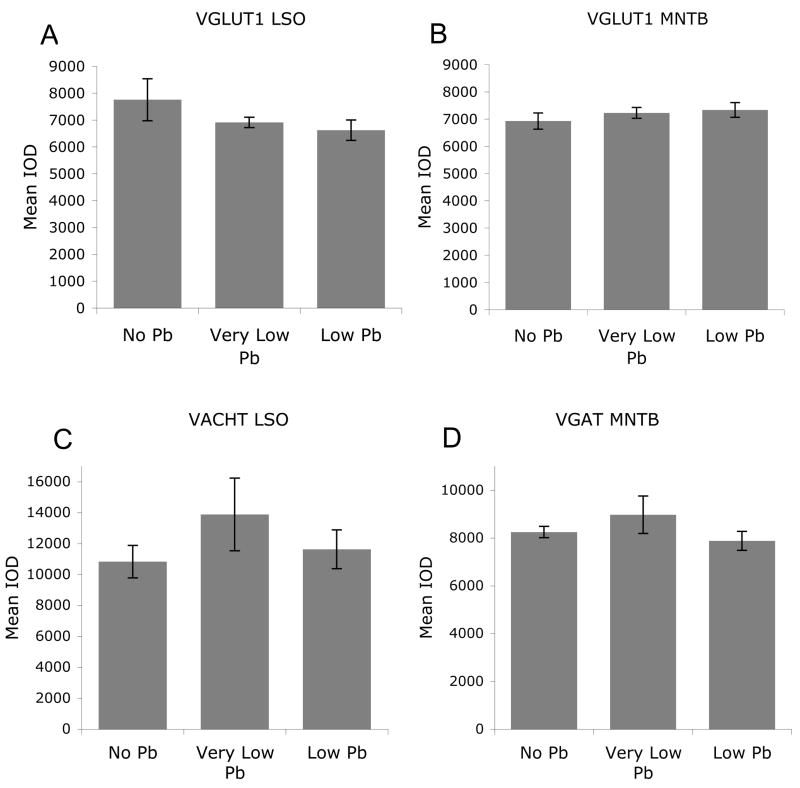
In order to determine if Pb specifically affects the monoamine system, the expression of other neurotransmitter transporters such as VGLUT 1, VGAT, and VAChT was examined in brainstem sections immunolabeled with antibodies to the appropriate transporter protein. Glutamate is the major excitatory neurotransmitter that is used by the auditory system and we found robust immunostaining for VGLUT 1 in both the LSO and MNTB in control mice (Figure 3). This immunostaining is not changed by Pb exposure (Figures 3 and 5), indicating that Pb does not significantly alter glutamate transporter expression within the auditory brainstem. Similarly, immunostaining for VGAT and VAChT is not changed with Pb exposure, suggesting that both gaba-ergic and cholinergic expression levels remain unaffected by Pb (Figures 4 and 5). Western analysis does not show any Pb-induced changes in VGLUT 1, VGAT, and VAChT levels in the entire brainstem (data not shown).
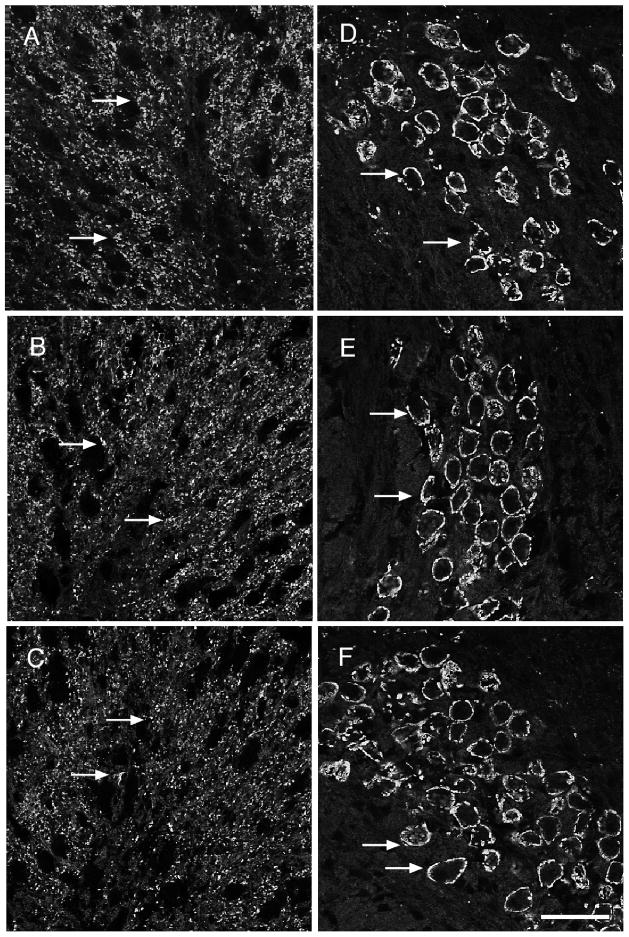
Figure 3.
Pb exposure does not affect VGLUT1 expression levels within either LSO or MNTB. A–C) Immunofluorescent staining for VGLUT1 in the LSO in response to no (A), very low (B), and low (C) Pb treatment shows no change in immunoreactivity with Pb exposure (arrows). D–F) VGLUT1 immunostaining in the MNTB in response to no (D), very low (E), and low (F) Pb exposure also demonstrates no change in expression levels (arrows). (n=5 per group). Arrows point to immunostaining. Bar = 50 μm for panels A–F.
Figure 5.
Quantification of immunostaining confirms that Pb exposure does not affect VGLUT1 (A and B), VGAT (C), or VACHT (D) expression levels in the SOC. Graphs illustrate mean ± the standard error of the mean (SEM). (n=5 per group). One-way Anova with Tukey’s all pairs comparison.
Figure 4.
Pb treatment does not affect VAChT or VGAT expression levels within the SOC. AC) VACHT immunostaining in the LSO does not change in response to no (A), very low (B), and low (C) Pb exposure (arrows). D–F) Similarly, VGAT immunostaining in the MNTB does not differ among the no (D), very Low (E), and low (F) Pb groups (arrows). (n=5 per group). Arrows point to immunostaining. Bar = 50 μm for panels A–F.
It should be noted that we found differential expression of VGAT and VAChT within central auditory nuclei. VGAT expression is found in the MNTB and but is not detectable in LSO. VAChT expression is found in the LSO and this expression pattern agrees with previous studies of VAChT distribution (Yao and Godfrey, 1998).
In summary, Pb exposure decreases the expression of VMAT 2 within LSO, but VGLUT 1, VGAT, and VAChT expression levels remain unaffected by Pb. Therefore, it appears that monoaminergic neurotransmitter systems within brainstem auditory nuclei are particularly vulnerable to low levels of Pb exposure during development.
5-HT and DβH immunostaining in central auditory nuclei decreases with Pb
The monamines dopamine, serotonin and norepeniphrine are transported into synaptic vesicles by VMAT 2 (Gopalakrishnan et al., 2007). In order to determine whether Pb differentially decreases expression of particular monoamines, tissue sections were immunolabeled with antibodies to tyrosine hydroxylase (TH, a marker for dopamine), dopamine beta-hydroxylase (DβH, a marker for norepinephrine) and serotonin (5-HT). Tyrosine hydroxylase (TH) converts tyrosine to L-Dopa, a precursor for dopamine, and is also the rate-limiting step in dopamine synthesis (Kaushik et al., 2007; Pan et al., 2006). TH immunostaining is commonly used as a marker for dopamine. Dopamine beta hydroxylase converts dopamine to norepinephrine in synaptic vesicles and is routinely used as a marker for norepinephrine expression within the brain. It has also been shown to label varicosities within auditory pathways (Behrens et al., 2002).
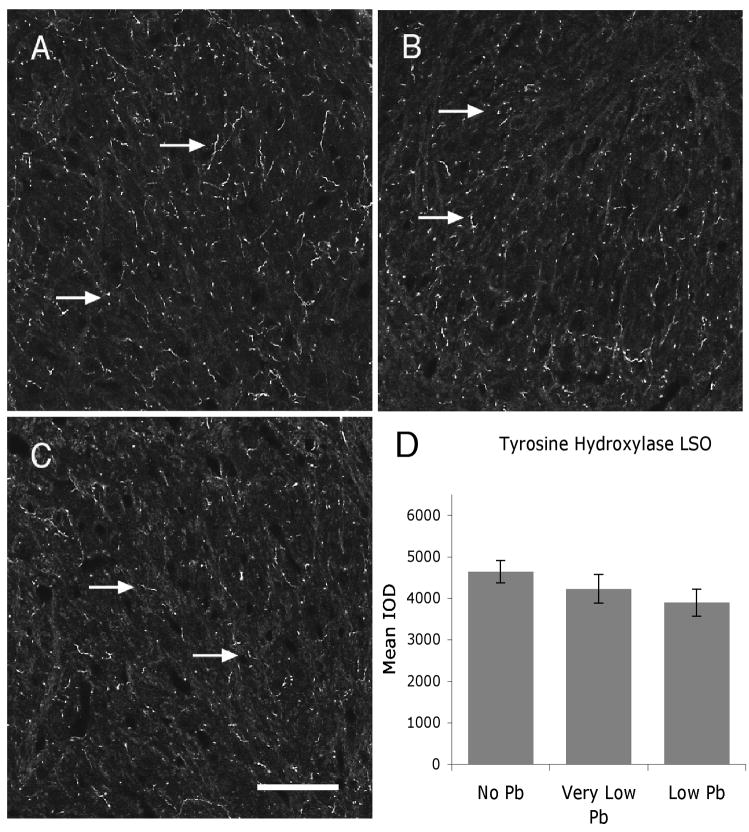
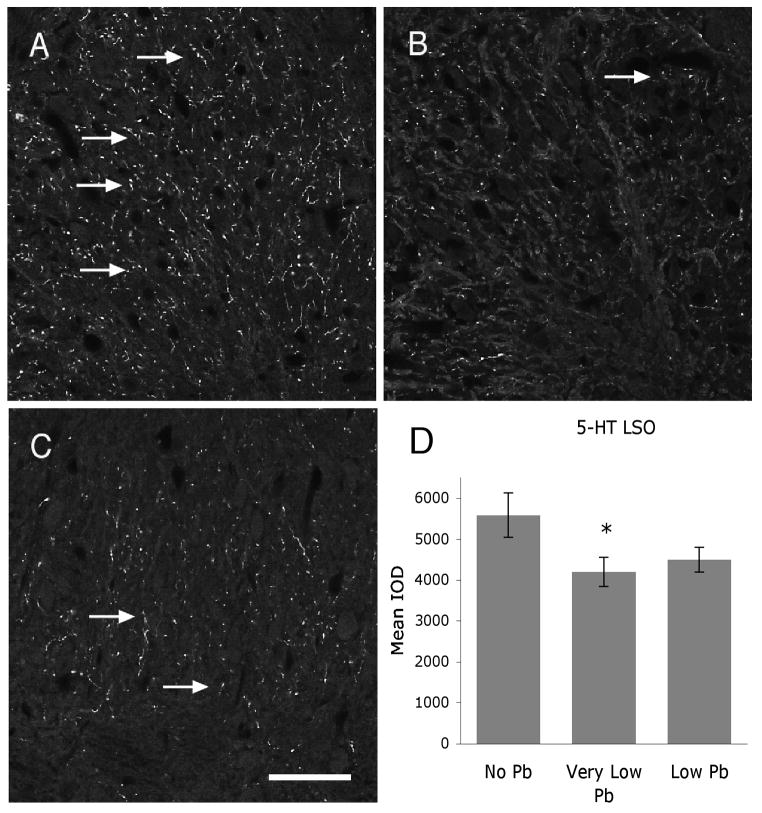
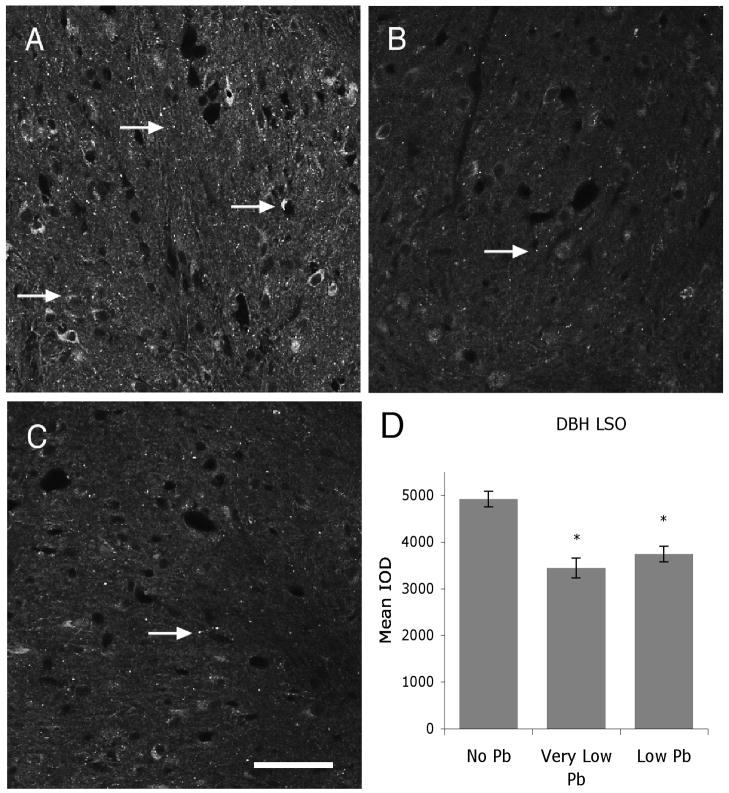
Figure 6 illustrates TH immunostaining in the LSO for all Pb treatment groups. Pb does not cause a decrease in TH immunolabel at either the very low or low dose of Pb (Figure 6), suggesting that dopamine expression does not change with Pb exposure. In contrast, immunoreactivity for both and serotonin and DβH is significantly decreased with Pb (Figures 7 and 8). Serotonin expression is decreased approximately 29% compared to controls in the very low Pb group, while DβH expression is decreased approximately 30% in both the very low and the low Pb groups (Figures 7 and 8).
Figure 6.
TH expression levels within the LSO are not altered with Pb treatment. A–C) Immunofluorescent staining for TH in the LSO in response to no (A), very low (B), and low (C) Pb treatment shows no change in immunoreactivity (arrows). Quantification of TH immunostaining in the LSO confirms that Pb has no affect on TH immunostaining (D). Arrows point to immunostaining. Graphs illustrate mean ± the standard error of the mean (SEM). (n=5 per group). One-way Anova with Tukey’s all pairs comparison. Bar = 50 μm for panels A–C.
Figure 7.
Pb treatment decreases 5-HT expression within the LSO. A–C) Immunoreactivity for 5-HT is decreased with both very low (B) and low (C) Pb compared to no Pb controls (A) (arrows). Quantification of 5-HT immunoreactivity in the LSO confirms that this decrease is statistically significant (D). Arrows point to immunostaining. The graphs illustrate mean ± the standard error of the mean (SEM). (n=5 per group) *P < 0.05, One-way Anova with Tukey’s all pairs comparison. Bar = 50 μm for panels A–C.
Figure 8.
Pb decreases DβH expression within the LSO. A–C) Pb decreases immunoreactivity for DβH in very low (B), and low (C) Pb treatment groups compared to no Pb controls (A) (Arrows). D) Quantification of staining for DβH in the LSO cofirms that this decrease is statistically significant. Arrows point to immunostaining. The graphs illustrate mean ± the standard error of the mean (SEM). (n=5 per group) *P < 0.05, One-way Anova with Tukey’s all pairs comparison. Bar = 50 μm for panels A–C.
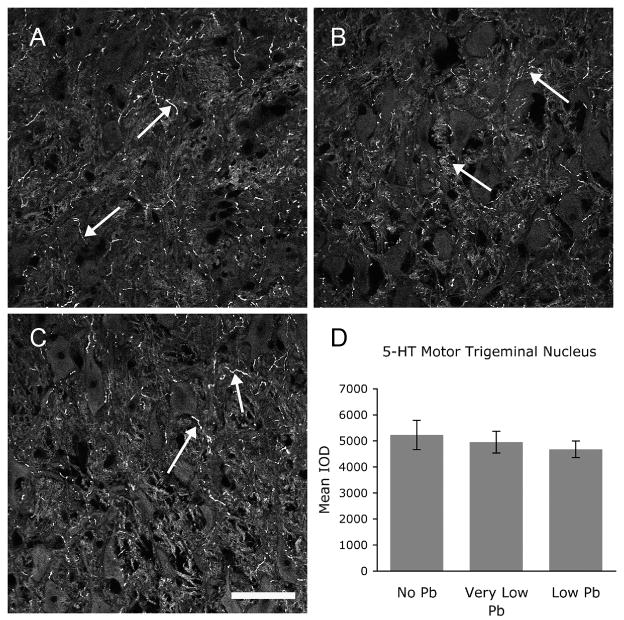
This effect of Pb appears to target central auditory nuclei within the brainstem. We also examined 5-HT immunostaining in neurons of the motor trigeminal nucleus. The motor trigeminal nucleus is a non-auditory brainstem nucleus consisting almost entirely of motor neurons located within the VBS region, near the LSO and MNTB. Motor trigeminal neurons do not show a decrease in 5-HT expression (Figure 9), demonstrating that Pb-induced decreases in 5-HT immunolabeling appears to be specific for the central auditory nuclei in the VBS region.
Figure 9.
5-HT expression levels within the motor trigeminal nucleus are not altered with Pb treatment. A–C) Immunofluorescent staining for 5-HT in the motor trigeminal nucleus in response to no (A), very low (B), and low (C) Pb treatment shows no change in immunoreactivity (arrows). Quantification of 5-HT immunostaining confirms that Pb has no affect on 5-HT immunostaining in the motor trigeminal nucleus (D). Arrows point to immunostaining. Graphs illustrate mean ± the standard error of the mean (SEM). (n=5 per group). One-way Anova with Tukey’s all pairs comparison. Bar = 50 μm for panels A–C.
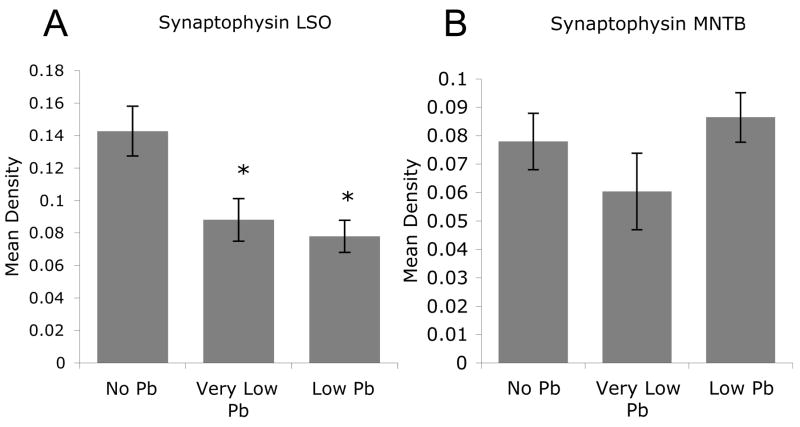
The Pb-induced decrease in VMAT 2, 5-HT, and DBH immunostaining is correlated with decreased immunoreactivity for synaptophysin
In order to determine whether the decreased expression of VMAT 2, serotonin, and DβH correlates with a loss of synapses in LSO, brainstem sections were immunolabeled with antibodies to the synaptic vesicle protein, synaptophysin. Pb exposure results in a significant decrease in synaptophysin labeling within LSO but not MNTB (Figures 10 and 11). This is particularly significant because in the mouse, LSO (but not MNTB) receives a serotonergic input (Thompson and Hurley, 2004). The fact that synaptophysin labeling decreases in LSO but not in MNTB suggests the Pb-induced loss of 5-HT and DβH immunoreactivity within LSO is correlated with a loss of synapses.
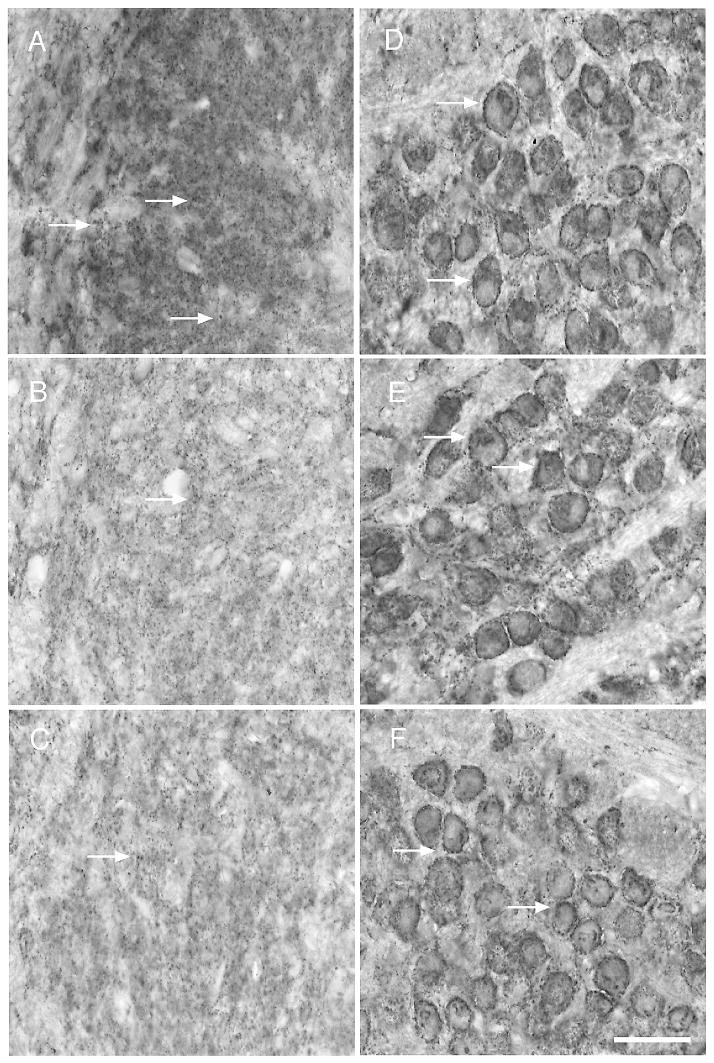
Figure 10.
Pb treatment decreases synaptophysin immunoreactivity within the LSO but has no effect on synaptophysin staining in the MNTB. A–C) Very low (B) and low (C) Pb results in decreased immunoreactivity for synaptophysin in the LSO compared to no Pb controls (A) (arrows). In contrast, MNTB synaptophysin immunostaining remains unchanged from controls (D) with very low (E) and low (F) Pb treatment. Arrows point to immunostaining. Bar = 50 μm for panels A–F.
Figure 11.
Pb treatment results in a significant decrease in synaptophysin immunoreactivity in the LSO but not the MNTB. A) Quantification of synaptophysin immunostaining in the LSO shows a significant decrease with Pb treatment. B) In contrast, quantification of synaptophysin expression in the MNTB reveals no significant decrease with Pb treatment. Graphs illustrate mean ± the standard error of the mean (SEM). (n=4 per group) *P < 0.05 One-way Anova with Tukey’s all pairs comparison.
Pb does not appear to cause the death of dorsal raphe neurons
The majority of serotonergic neurons within the brain are found in the dorsal and median raphe nuclei. Serotonergic neurons originating in the dorsal raphe innervate many areas of the brain including the auditory brainstem and inferior colliculus (Klepper and Herbert, 1991). In order to determine whether the Pb-induced loss of 5-HT, DβH, and synaptophysin is the result of loss of dorsal raphe neurons, the number of neurons was counted in a representative area within the raphe nucleus as described in the methods. Pb exposure produces no significant change in neuron number within the sample area in the dorsal raphe. The mean number of neurons counted in control dorsal raphe is 28 ± 1.8 SEM, and this number does not change significantly with either very low Pb (31 ± 2.0 SEM) or low Pb (24 ± 1.9 EM) when analyzed using a Wilcoxin-Mann-Whitney rank sum test for comparison. Thus, the Pb-induced decrease in 5-HT, DβH and synaptophysin staining does not appear to be due to the loss of dorsal raphe neurons.
Discussion
The current study demonstrates that developmental exposure to very low levels of Pb results in decreased immunoreactivity for VMAT 2, serotonin, DβH, and synaptophysin within the LSO. This effect appears to be specific for monoaminergic systems because no changes in protein expression level were observed for VGLUT1, VAChT, and VGAT within either LSO, MNTB, or the entire brainstem. In addition, immunostaining for TH did not change with Pb exposure, suggesting that dopamine levels are not altered by Pb. The loss of synpatophysin staining within LSO, but not MNTB, suggests that the decreased expression of VMAT 2, 5-HT, and DβH may be correlated with the loss of monoaminergic synapses. Studies are currently underway in our laboratory to determine whether Pb alters the formation of monoaminergic synapses and/or interferes with the vesicular loading of monoamines.
The role of the serotonin in LSO
The lateral superior olive forms part of the ascending auditory pathway to the midbrain and is thought to process interaural intensity cues for sound localization (Thompson, 2006). LSO neurons project to the inferior colliculus but a subset of neurons located within the LSO project predominantly ipsilaterally and form synapses onto inner hair cells. The functional role of this efferent system remains unknown. It has been hypothesized to modify or control binaural interactions, reduce the masking effects of background noise, protect the cochlea from noise-induced trauma, and alter the response of the cochlea to sound with changes in attention (Darrow et al., 2007; Woods and Azeredo, 1999). In the adult mouse, both ascending and descending projections that originate in the LSO receive input from the 5-HT system but LSO ascending neurons use glutamate and glycine as neurotransmitters and are not considered serotonergic (Thompson, 2006).
In addition, many 5-HT immunoreactive fibers are found in the inferior colliculus, a target for LSO neurons. Serotonin has been shown to shift first-spike latencies, neuronal spike count, temporal precision, and the interspike interval of neurons in the inferior colliculus (Hurley and Pollak, 2005a). Serotonin is therefore considered to refine the representation of acoustic stimuli within the IC. For example, in free-tailed bats, calls become more unambiguous and specific in the presence of serotonin (Hurley and Pollak, 1999) and the acoustic startle response has been shown to increase with 5-HT depletion (Woods and Azeredo, 1999).
Most of the serotonergic fibers found in the IC originate in the dorsal and median raphe nuclei (Klepper and Herbert, 1991) and, indeed, the majority of serotonergic neurons within the brain are located in the dorsal and median raphe nuclei. While serotonergic neurons innervate many areas of the brain including the auditory brainstem (Klepper and Herbert, 1991), the origin of the 5-HT fibers in murine LSO has not been fully defined.
We did not find a loss of dorsal raphe neurons following Pb exposure, so the decreased expression of 5-HT in LSO is not due to the death of dorsal raphe neurons. In addition, 5-HT expression did not decrease in the motor trigeminal nucleus following Pb exposure. We chose to analyze the motor trigeminal nucleus because it is a motor nucleus (as compared with the LSO which is sensory) and it is located in the same region of the brainstem as the LSO. The finding that Pb decreases 5-HT expression in the LSO but not the motor trigeminal nucleus suggests that central auditory nuclei such as the LSO and IC may represent a specific target for Pb. Pb has been shown to accumulate homogenously throughout the brain, and it is thought that the selective vulnerability of various brain regions to Pb is due to biochemical and molecular targets that are uniquely associated or enriched in particular brain regions (Widzowski and Cory-Slechta, 1994).
Noradrenergic Neurons and the auditory system
In addition to decreasing 5-HT expression, Pb also resulted in decreased expression of DβH. Both noradrenalin (NA) and 5-HT have been shown to modify auditory neural activity. NA applied directly to the cochlea results in increased absolute and masked auditory thresholds, indicating that NA is able to alter the ability of the auditory system to detect a signal in a noise background (Pickles, 1976). Noradrenergic nerve endings have been found within all nuclei of the SOC and in the rat, these terminals have been shown to originate in the locus coeruleus (Mulders and Robertson, 2001). These NA terminals may influence the function of the lateral olivocochlear neurons, located in the LSO, that project to the inner hair cells within the cochlea. While the function of these cells is still unknown, it is intriguing to hypothesize that the NA input to LSO may play a role in detecting a signal in a noise background, as well as to modulate attention during auditory processing. Application of NA to auditory neurons has been shown suppress spontaneous firing rate to a greater extent than the stimulus evoked discharge in cortex, suggesting that NA is able to increase the “signal to noise ratio” (reviewed in Berridge and Waterhouse, 2003). The NE system is thought to enhance cognitive function under “noisy” conditions and the presentation of extraneous auditory stimuli impairs sustained attention in rats with forebrain NE depletion, even though these same rats perform normally under non-distracting conditions (Berridge and Waterhouse, 2003).
Our finding that Pb exposure reduces DβH immunostaining within LSO is intriguing and further studies are needed to determine whether this effect is restricted to auditory nuclei or whether DβH immunostaining is decreased in other target regions of the locus coeruleus. Dysregulation of the locus coeruleous-NE system is associated with cognitive disorders such as attention deficit/hyperactivity disorder (ADHD) (Berridge and Waterhouse, 2003). Because low level Pb exposure has also been shown to be a risk factor for ADHD (Brockel and Cory-Slechta, 1998), it may be that the effect of Pb on the NE system is one potential mechanism by which Pb exposure could lead to ADHD.
Pb exposure and the Monoaminergic System
Our finding that Pb exposure alters the monoaminergic system is consistent with previous work on the effects of Pb on the CNS. Numerous studies have shown decreased function of monoamine oxidase (MAO) following exposure to lead as well as reduced NE and 5-HT levels in the brain (Devi et al., 2005; Jaya Prasanthi et al., 2005). However, the mechanism by which Pb reduces NA and 5-HT remains unknown. The current study demonstrates that Pb exposure reduces the expression of VMAT 2, the transporter that loads monamines into vesicles. The decreased expression of VMAT 2 might result in a short-term increase in levels of 5-HT and NE within the cytosol of synaptic endings, exposing them to increased degradation, and a long-term reduction in expression levels. We also found that Pb exposure results in decreased expression of the synaptic vesicle protein, synaptophysin, within LSO but not MNTB. This suggests that Pb is targeting the monoaminergic system because there is synaptic loss within LSO, but not MNTB, a nucleus that does not receive a large monaminergic input.
Our preliminary studies indicate that in the Pb-exposed LSO, the remaining 5-HT is not localized to synaptic vesicles, lending support to the hypothesis that Pb interferes with the transport of 5-HT into synaptic vesicles. Alternatively, we cannot rule out the possibility that Pb exposure might delay the development of monoaminergic synapses in LSO and the decreased expression of VMAT 2, 5-HT, and DβH could be due to delayed development of these synapses. Studies are currently underway to examine this possibility.
The role of serotonin (5-HT) and development
Serotonin has been shown to play an important role in CNS development, and highly topographically oganized sensory pathways such as the auditory, visual, and somatosensory systems appear to be specific targets of serotonin (Gaspar et al., 2003). Many of these sensory areas receive a transient but robust serotonergic innervation although the source of these serotonergic fibers are not raphe neurons. Instead, projection neurons that are largely glutaminergic take up serotonin transiently (Gaspar et al., 2003). This occurs in the barrel field region of somatosensory cortex in the postnatal rat (Lebrand et al., 1996), the retina (Upton et al., 1999), and several central auditory nuclei including the dorsal cochlear nucleus (DCN), the LSO, and the IC (Thompson, 2006; 2008). DCN, LSO and IC neurons transiently express serotonin at birth through P9. It is hypothesized that this transient accumulation of 5-HT may be necessary for the proper formation of the fibrodendritic laminae in the inferior colliculus (Thompson, 2008).
Significantly, developmental exposure to Pb alters the formation of cortical barrel fields (Wilson et al., 2000) and disrupts retinal development (Fox et al., 2008). The current study demonstrates that developmental Pb exposure results in decreased expression of VMAT2 and 5-HT within LSO. Taken together, our studies along with those of others, suggests that low level developmental Pb exposure could alter the ability of non-serotonergic neurons to take up serotonin transiently, thus altering the proper formation of sensory regions within the brain. We are currently characterizing the fibrodendritic laminae in the IC to determine whether developmental Pb exposure does indeed alter the organization of the IC.
In summary, the present study demonstrates that low level Pb exposure during development results in decreased immunoreactivity for VMAT 2, 5-HT, DβH, and synapotophysin within the murine LSO. Other neurotransmitter systems do not appear to be affected by Pb treatment as VGLUT1, VGAT, and VAChT immunoreactivity remain unchanged following developmental Pb exposure. In addition, the central auditory system appears to be a target for Pb as 5-HT expression was not significantly decreased in the motor trigeminal nucleus. The decrease in synaptophysin immunoreactivity within LSO but not MNTB suggests that there is a loss of monoaminergic synapses in this nucleus. However, further studies are needed to define the mechanism by which Pb affects the monoaminergic system within central auditory nuclei, and to determine whether low level Pb exposure modulates the patterns of connectivity that rely on serotonin within the central auditory system.
Acknowledgments
We thank Diane Brooks for her expert technical assistance with immunocytochemistry and neuronal cell counts.
Grant information: Supported by NIH NCRR P20 RR17670, NIH P20 RR015583 (DIL)
Literature Cited
- Alvarado JC, Fuentes-Santamaria V, Henkel CK, Brunso-Bechtold JK. Alterations in calretinin immunostaining in the ferret superior olivary complex after cochlear ablation. J Comp Neurol. 2004;470(1):63–79. doi: 10.1002/cne.11038. [DOI] [PubMed] [Google Scholar]
- Baer K, Burli T, Huh KH, Wiesner A, Erb-Vogtli S, Gockeritz-Dujmovic D, Moransard M, Nishimune A, Rees MI, Henley JM, Fritschy JM, Fuhrer C. PICK1 interacts with alpha7 neuronal nicotinic acetylcholine receptors and controls their clustering. Mol Cell Neurosci. 2007;35(2):339–355. doi: 10.1016/j.mcn.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens EG, Schofield BR, Thompson AM. Aminergic projections to cochlear nucleus via descending auditory pathways. Brain Res. 2002;955(1–2):34–44. doi: 10.1016/s0006-8993(02)03351-6. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Billups B. Colocalization of vesicular glutamate transporters in the rat superior olivary complex. Neurosci Lett. 2005;382(1–2):66–70. doi: 10.1016/j.neulet.2005.02.071. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114(12):1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockel BJ, Cory-Slechta DA. Lead, attention, and impulsive behavior: changes in a fixed-ratio waiting-for-reward paradigm. Pharmacol Biochem Behav. 1998;60(2):545–552. doi: 10.1016/s0091-3057(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Caminos E, Garcia-Pino E, Martinez-Galan JR, Juiz JM. The potassium channel KCNQ5/Kv7.5 is localized in synaptic endings of auditory brainstem nuclei of the rat. J Comp Neurol. 2007;505(4):363–378. doi: 10.1002/cne.21497. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003a;348(16):1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield RL, Kreher DA, Cornwell C, Henderson CR., Jr Low-level lead exposure, executive functioning, and learning in early childhood. Child Neuropsychol. 2003b;9(1):35–53. doi: 10.1076/chin.9.1.35.14496. [DOI] [PubMed] [Google Scholar]
- Chen A, Cai B, Dietrich KN, Radcliffe J, Rogan WJ. Lead exposure, IQ, and behavior in urban 5- to 7-year-olds: does lead affect behavior only by lowering IQ? Pediatrics. 2007;119(3):e650–658. doi: 10.1542/peds.2006-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol. 2004;26(3):359–371. doi: 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J Neurophysiol. 2007;97(2):1775–1785. doi: 10.1152/jn.00955.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi CB, Reddy GH, Prasanthi RP, Chetty CS, Reddy GR. Developmental lead exposure alters mitochondrial monoamine oxidase and synaptosomal catecholamine levels in rat brain. Int J Dev Neurosci. 2005;23(4):375–381. doi: 10.1016/j.ijdevneu.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull. 2001;55(5):569–578. doi: 10.1016/s0361-9230(01)00530-5. [DOI] [PubMed] [Google Scholar]
- Finkelstein Y, Markowitz ME, Rosen JF. Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Brain Res Rev. 1998;27(2):168–176. doi: 10.1016/s0165-0173(98)00011-3. [DOI] [PubMed] [Google Scholar]
- Fox DA, Kala SV, Hamilton WR, Johnson JE, O’Callaghan JP. Low-level human equivalent gestational lead exposure produces supernormal scotopic electroretinograms, increased retinal neurogenesis, and decreased retinal dopamine utilization in rats. Environ Health Perspect. 2008;116(5):618–625. doi: 10.1289/ehp.11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to 2 microg/dL. Neurotoxicology. 2006;27(5):693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer DE, Freedberg KA, Bauchner H. Management of childhood lead poisoning: clinical impact and cost-effectiveness. Med Decis Making. 1995;15(1):13–24. doi: 10.1177/0272989X9501500104. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan A, Sievert M, Ruoho AE. Identification of the substrate binding region of vesicular monoamine transporter-2 (VMAT-2) using iodoaminoflisopolol as a novel photoprobe. Mol Pharmacol. 2007;72(6):1567–1575. doi: 10.1124/mol.107.034439. [DOI] [PubMed] [Google Scholar]
- Gray L. Early lead exposure affects auditory temporal processing in chicks. Journal of Environmental Medician. 1999;1:87–93. [Google Scholar]
- Hall IC, Hurley LM. The serotonin releaser fenfluramine alters the auditory responses of inferior colliculus neurons. Hear Res. 2007;228(1–2):82–94. doi: 10.1016/j.heares.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig W, Stieler J, Boerema AS, Wolf J, Schmidt U, Weissfuss J, Bullmann T, Strijkstra AM, Arendt T. Hibernation model of tau phosphorylation in hamsters: selective vulnerability of cholinergic basal forebrain neurons - implications for Alzheimer’s disease. Eur J Neurosci. 2007;25(1):69–80. doi: 10.1111/j.1460-9568.2006.05250.x. [DOI] [PubMed] [Google Scholar]
- Hurley LM. Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain Res. 2007;1181:21–29. doi: 10.1016/j.brainres.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. J Neurosci. 1999;19(18):8071–8082. doi: 10.1523/JNEUROSCI.19-18-08071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin modulates responses to species-specific vocalizations in the inferior colliculus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005a;191(6):535–546. doi: 10.1007/s00359-005-0623-y. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin shifts first-spike latencies of inferior colliculus neurons. J Neurosci. 2005b;25(34):7876–7886. doi: 10.1523/JNEUROSCI.1178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM, Pollak GD. Serotonin in the inferior colliculus. Hear Res. 2002;168(1–2):1–11. doi: 10.1016/s0378-5955(02)00365-9. [DOI] [PubMed] [Google Scholar]
- Jaya Prasanthi RP, Hariprasad Reddy G, Bhuvaneswari Devi C, Rajarami Reddy G. Zinc and calcium reduce lead induced perturbations in the aminergic system of developing brain. Biometals. 2005;18(6):615–626. doi: 10.1007/s10534-005-2993-6. [DOI] [PubMed] [Google Scholar]
- Jones LG, Prins J, Park S, Walton JP, Leubke AE, Lurie DI. Lead exposure during development results in increased neurofilament phosphorylation, neuritic beading, and temporal processing deficits within the murine auditory brainstem. Journal of Comparative Neurology. 2008;506(6):1003–1017. doi: 10.1002/cne.21563. [DOI] [PubMed] [Google Scholar]
- Kamel NM, Ramadan AM, Kamel MI, Mostafa YA, Abo el-Naga RM, Ali AM. Impact of lead exposure on health status and scholastic achievement of school pupils in Alexandria. J Egypt Public Health Assoc. 2003;78(1–2):1–28. [PubMed] [Google Scholar]
- Kaushik P, Gorin F, Vali S. Dynamics of tyrosine hydroxylase mediated regulation of dopamine synthesis. J Comput Neurosci. 2007;22(2):147–160. doi: 10.1007/s10827-006-0004-8. [DOI] [PubMed] [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res. 1991;557(1–2):190–201. doi: 10.1016/0006-8993(91)90134-h. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep. 2000;115(6):521–529. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17(5):823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Brooks DM, Gray LC. The effect of lead on the avian auditory brainstem. Neurotoxicology. 2006;27(1):108–117. doi: 10.1016/j.neuro.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Durham D. Neuronal death, not axonal degeneration, results in significant gliosis within the cochlear nucleus of adult chickens. Hear Res. 2000;149(1–2):178–188. doi: 10.1016/s0378-5955(00)00181-7. [DOI] [PubMed] [Google Scholar]
- Maison SF, Adams JC, Liberman MC. Olivocochlear innervation in the mouse: immunocytochemical maps, crossed versus uncrossed contributions, and transmitter colocalization. J Comp Neurol. 2003;455(3):406–416. doi: 10.1002/cne.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino DM, Albalak R, Grosse S, Repace J. Second-hand smoke exposure and blood lead levels in U.S. children. Epidemiology. 2003;14(6):719–727. doi: 10.1097/01.EDE.0000081998.02432.53. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Homa DM, Matte T, Hernandez-Avila M. Active and passive smoking and blood lead levels in U.S. adults: data from the Third National Health and Nutrition Examination Survey. Nicotine Tob Res. 2005;7(4):557–564. doi: 10.1080/14622200500185264. [DOI] [PubMed] [Google Scholar]
- Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: immunocytochemical study of dopamine-beta-hydroxylase. J Comp Neurol. 1998;400(2):207–228. [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Origin of the noradrenergic innervation of the superior olivary complex in the rat. J Chem Neuroanat. 2001;21(4):313–322. doi: 10.1016/s0891-0618(01)00118-1. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Catecholaminergic innervation of guinea pig superior olivary complex. J Chem Neuroanat. 2005;30(4):230–242. doi: 10.1016/j.jchemneu.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Nosjean A, Hamon M, Darmon M. 5-HT2A receptors are expressed by catecholaminergic neurons in the rat nucleus tractus solitarii. Neuroreport. 2002;13(17):2365–2369. doi: 10.1097/00001756-200212030-00039. [DOI] [PubMed] [Google Scholar]
- Pan Y, Berman Y, Haberny S, Meller E, Carr KD. Synthesis, protein levels, activity, and phosphorylation state of tyrosine hydroxylase in mesoaccumbens and nigrostriatal dopamine pathways of chronically food-restricted rats. Brain Res. 2006;1122(1):135–142. doi: 10.1016/j.brainres.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO. The noradrenaline-containing innervation of the cochlear nucleus and the detection of signals in noise. Brain Res. 1976;105(3):591–596. doi: 10.1016/0006-8993(76)90610-7. [DOI] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101(38):13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn MD, Abelsohn A, Campbell M, Weir E. Identifying and managing adverse environmental health effects: 3. Lead exposure. Cmaj. 2002;166(10):1287–1292. [PMC free article] [PubMed] [Google Scholar]
- Sato K, Shiraishi S, Nakagawa H, Kuriyama H, Altschuler RA. Diversity and plasticity in amino acid receptor subunits in the rat auditory brain stem. Hear Res. 2000;147(1–2):137–144. doi: 10.1016/s0378-5955(00)00127-1. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000a;407(6801):189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Takamori S, Riedel D, Jahn R. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. J Neurosci. 2000b;20(13):4904–4911. doi: 10.1523/JNEUROSCI.20-13-04904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AM. “Non-serotonergic” lateral superior olivary neurons of the neonatal mouse contain serotonin. Brain Res. 2006;1122(1):122–125. doi: 10.1016/j.brainres.2006.08.126. [DOI] [PubMed] [Google Scholar]
- Thompson AM. Serotonin immunoreactivity in auditory brainstem neurons of the postnatal monoamine oxidase-A knockout mouse. Brain Res. 2008;1228:58–67. doi: 10.1016/j.brainres.2008.06.091. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Hurley LM. Dense serotonergic innervation of principal nuclei of the superior olivary complex in mouse. Neurosci Lett. 2004;356(3):179–182. doi: 10.1016/j.neulet.2003.11.052. [DOI] [PubMed] [Google Scholar]
- Tong S, von Schirnding YE, Prapamontol T. Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ. 2000;78(9):1068–1077. [PMC free article] [PubMed] [Google Scholar]
- Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev. 2005;49(3):529–554. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Trussell LO. Modulation of transmitter release at giant synapses of the auditory system. Curr Opin Neurobiol. 2002;12(4):400–404. doi: 10.1016/s0959-4388(02)00335-5. [DOI] [PubMed] [Google Scholar]
- Upton AL, Salichon N, Lebrand C, Ravary A, Blakely R, Seif I, Gaspar P. Excess of serotonin (5-HT) alters the segregation of ispilateral and contralateral retinal projections in monoamine oxidase A knock-out mice: possible role of 5-HT uptake in retinal ganglion cells during development. J Neurosci. 1999;19(16):7007–7024. doi: 10.1523/JNEUROSCI.19-16-07007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Woltjer RL, Cimino PJ, Pan C, Montine KS, Zhang J, Montine TJ. Proteomic analysis of neurofibrillary tangles in Alzheimer disease identifies GAPDH as a detergent-insoluble paired helical filament tau binding protein. Faseb J. 2005;19(7):869–871. doi: 10.1096/fj.04-3210fje. [DOI] [PubMed] [Google Scholar]
- Weihe E, Tao-Cheng JH, Schafer MK, Erickson JD, Eiden LE. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93(8):3547–3552. doi: 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widzowski DV, Cory-Slechta DA. Homogeneity of regional brain lead concentrations. Neurotoxicology. 1994;15(2):295–307. [PubMed] [Google Scholar]
- Wilson MA, Johnston MV, Goldstein GW, Blue ME. Neonatal lead exposure impairs development of rodent barrel field cortex. Proc Natl Acad Sci U S A. 2000;97(10):5540–5545. doi: 10.1073/pnas.97.10.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishcamper CA, Coffin JD, Lurie DI. Lack of the protein tyrosine phosphatase SHP-1 results in decreased numbers of glia within the motheaten (me/me) mouse brain. J Comp Neurol. 2001;441(2):118–133. [PubMed] [Google Scholar]
- Woods CI, Azeredo WJ. Noradrenergic and serotonergic projections to the superior olive: potential for modulation of olivocochlear neurons. Brain Res. 1999;836(1–2):9–18. doi: 10.1016/s0006-8993(99)01541-3. [DOI] [PubMed] [Google Scholar]
- Yao J, Hersh LB. The vesicular monoamine transporter 2 contains trafficking signals in both its N-glycosylation and C-terminal domains. J Neurochem. 2007;100(5):1387–1396. doi: 10.1111/j.1471-4159.2006.04326.x. [DOI] [PubMed] [Google Scholar]
- Yao W, Godfrey DA. Immunohistochemical evaluation of cholinergic neurons in the rat superior olivary complex. Microsc Res Tech. 1998;41(3):270–283. doi: 10.1002/(SICI)1097-0029(19980501)41:3<270::AID-JEMT10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Zhou M, Hank Simms H, Wang P. Increased gut-derived norepinephrine release in sepsis: up-regulation of intestinal tyrosine hydroxylase. Biochim Biophys Acta. 2004;1689(3):212–218. doi: 10.1016/j.bbadis.2004.03.008. [DOI] [PubMed] [Google Scholar]