Abstract
Meso- and submesoscales (fronts, eddies, filaments) in surface ocean flow have a crucial influence on marine ecosystems. Their dynamics partly control the foraging behavior and the displacement of marine top predators (tuna, birds, turtles, and cetaceans). In this work we focus on the role of submesoscale structures in the Mozambique Channel in the distribution of a marine predator, the Great Frigatebird. Using a newly developed dynamic concept, the finite-size Lyapunov exponent (FSLE), we identified Lagrangian coherent structures (LCSs) present in the surface flow in the channel over a 2-month observation period (August and September 2003). By comparing seabird satellite positions with LCS locations, we demonstrate that frigatebirds track precisely these structures in the Mozambique Channel, providing the first evidence that a top predator is able to track these FSLE ridges to locate food patches. After comparing bird positions during long and short trips and different parts of these trips, we propose several hypotheses to understand how frigatebirds can follow these LCSs. The birds might use visual and/or olfactory cues and/or atmospheric current changes over the structures to move along these biologic corridors. The birds being often associated with tuna schools around foraging areas, a thorough comprehension of their foraging behavior and movement during the breeding season is crucial not only to seabird ecology but also to an appropriate ecosystemic approach to fisheries in the channel.
Keywords: frigatebird, finite-size Lyapunov exponent, Mozambique Channel, submesoscale
In the oligotrophic open ocean mesoscale and submesoscale oceanic turbulence, which spans spatiotemporal scales from one to hundreds of kilometers and from hours to weeks, strongly modulates the structure, biomass, and rates of marine pelagic ecosystems. Eddies can stimulate the primary productivity (1, 2), affect plankton community composition (3–5), or play a significant role in exchange processes in the transitional area between the coast and offshore by transporting organic matter and marine organisms from the coast to the open ocean and vice versa (6). In view of the strong influence of eddies on physical and biogeochemical properties, it is not surprising that higher-level predators concentrate around them, where prey can be found. In fact, all investigations on the relationship between eddies and top-predator communities, using satellite imagery observations, have shown strong ties between them (7, 8). Upper predators particularly used the boundary between 2 eddies (9–12). The key point is that interactions between eddies generate strong dynamic interfaces (13) and make them a complex and energetic physical environment. In these interfaces the energy of the physical system is available to biologic processes, increasing the trophic energy of the biologic system (8). Eddies and associated structures therefore have a crucial ecologic significance, especially in tropical and subtropical regions, characterized by low mixing during winter, inferring weak supply of nutrients to the photic zone (11).
Most previous work dealing with the influence of eddies on top-predator distribution show the necessity of concentrating on submesoscale (<10 km) to fully appreciate the role of eddy–eddy interfaces on biologic production (11). Many different studies confirm that submesoscale tracer patches and filaments are strongly related to interactions between mesoscale surface eddies (1, 14). Despite this, studies on top predators using remote sensing have only used sea surface height as an indicator of eddy activity, which does not resolve submesoscale structures such as filaments, where production should be concentrated. In addition, a fundamental question remains: how do top predators find these zones of higher productivity? This is particularly difficult to understand for central-place foragers, such as seabirds, that breed on land but have to make continuous return trips between feeding zones and the colony where they care for their chick or egg. The additional difficulty in the case of eddies is that the location of production zones moves continuously.
In the West Indian Ocean, the Mozambique Channel (MC) can be considered a natural laboratory to study interactions between biologic and physical processes at mesoscale in oligotrophic areas (subtropical region) because of the transient activity of eddies. Indeed, mesoscale dynamics of the MC have been well described by previous works using remote sensing data, modeling, and in situ observations (15–17). Mesoscale activity is dominant in 2 areas, the central part of the MC and south of Madagascar (17, 18). Weimerskirch et al. (10) have shown the main role of mesoscale eddies on the foraging strategy of Great Frigatebirds. These birds fly hundreds or thousands of kilometers from the colony in a few days and spend their entire foraging trips in flight, being unable to sit on the water or enter the water column. Bird pathways are preferentially associated with eddies in the MC during their long trips and especially with the edge of eddies, avoiding their core (10). However, it is not clear where exactly they forage in the eddy system and whether and how they locate the zones of high production. The aim of the present study is to describe the fine-scale activity occurring at the edge of eddies and other submesoscale structures and to quantify the role of these on a top predator's foraging movements. We also try to understand how and why these predators might locate these structures.
For the physical environment, we have used horizontal velocity fields computed from satellite altimetry products (19). We have applied to them a recently developed Lagrangian technique, the finite-size Lyapunov exponent (FSLE), which allows computing from marine surface velocity field data, mixing activity and coherent structures that control transport at specified scales (20). FSLEs measure how fast fluid particles separate to a specified distance. Lagrangian coherent structures (LCSs) (e.g., transport barriers, filamental structures, or vortex boundaries) are identified as ridges (locations containing the maximum values) of Lyapunov exponent fields (21–24). Dispersion rates of tracer particles can be calculated by integrating trajectories toward the future (forward) or toward the past (backward), giving rise to 2 different quantifiers, FSLEf and FSLEb, respectively, containing complementary information (see Materials and Methods). Ridges of FSLEb attract neighboring trajectories, whereas FSLEf repel them. This is why we call them attracting and repelling LCSs, respectively. Sometimes, especially for plotting, it is convenient to write FSLEb and FSLEf as having negative and positive values, respectively, and expressions such as |FSLE| refer simultaneously to both types of exponents. For the marine top predators, we have used Argos positions of Great Frigatebirds from the colony on Europa Island in the MC during August and September 2003. Additional details are given in Materials and Methods.
In this study we test whether seabird positions during their foraging trips are related to dynamic structures. This is performed in different contexts: during short and long trips, day and night, and during the outward part of their foraging trips and the return back to the colony. We finally discuss which foraging strategy these top predators might use to locate prey patches.
Results
Seabird Locations During Trips and FSLE Fields.
We compare here the locations of the LCSs identified as ridges in FSLE maps and measured bird positions during August and September 2003. We will see that the latter are not random but correlated with the former.
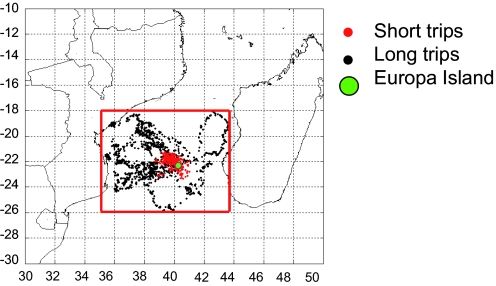
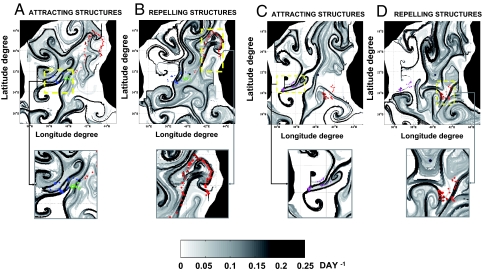
First, Fig. 1 shows Argos positions of Great Frigatebirds during long trips (black points) and short trips (red points) between August 18 and September 30, 2003. Locations of seabirds during long trips superimposed on FSLEs fields (September 24 to October 6, 2003) are shown in Fig. 2. During the week of September 24, bird 11377 (green circles) is located on high FSLEb values (the attracting LCSs), as is bird 16255 (blue circles). The positions of bird 8023 (red circles) seem to be linked to fluid repelling structures (the ridges of FSLEf) instead. For bird 8023, at the beginning of travel, the trajectory is rectilinear in the northeast direction and then follows the repelling mushroom-like structures. Foraging patches (triangles), where birds reduce flying speed, seem to exhibit the same distribution as the birds' moving positions. During the week of October 6 the movements of bird 8023 are mostly on repelling structures (Fig. 2D), as during the week of September 24, and perhaps also on some attracting structures. The important point is that any of both types of LCSs is more visited than locations outside. The positions of bird 19827 (magenta circles) are well superimposed on fluid attracting structures (ridges of FSLEb) but not on repelling ones. These 2 examples of the overlay of seabird movement and foraging positions on FSLE fields during long trips show that the locations of birds tend to overlay on LCSs, either on attracting (Fig. 2 A and C) or repelling ones (Fig. 2 B and D).
Fig. 1.
Argos locations of Great Frigatebirds during long trips (black points) and short trips (red points) in the MC, between August 18 and September 30, 2003. The green point denotes Europa Island.
Fig. 2.
Overlays of seabird positions on FSLE maps. (A and C) Backward integration in time for FLSE computation (d−1). (B and D) Forward integration in time (d−1). A and B, week of September, 24, 2003. C and D, week of October, 6, 2003. Circles represent seabird trajectories and triangles foraging patches. Each color represents the tag of a different bird (red, tag 8023; blue, tag 16255; green, tag 11377; magenta, tag 19827).
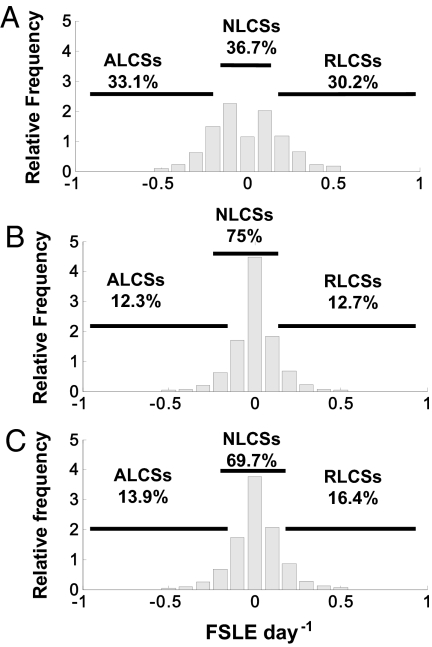
To put the above observations in quantitative form, we specified a threshold defining a significant presence of LCSs: |FSLE| > 0.1 d−1. It corresponds to mixing times smaller than 1 month. This value is chosen because it is a typical value for Lyapunov exponents in different areas of the globe (14, 20) and because regions where the Lyapunov exponents are larger already have the shape of 1-dimensional lines (see Fig. 2). The distributions of FSLEs in the whole MC and the central part and in areas crossed by seabirds were tested for conformity to the normal distribution using the Kolmogorov-Smirnov (KS) sample test, and they all are clearly non-normal. Histograms of relative frequency of FSLE in the whole MC, the central part, and in areas visited by seabirds are shown in Fig. 3. In the whole MC and the central part, Lagrangian structures detected by |FSLE| > 0.1 day−1 represent a minority of locations, occupying ≤30% of the total area. However, in areas crossed by frigatebirds >60% of the birds are on LCSs. Five KS 2-sample (KS-2) tests comparing the distributions of FSLEs in the whole MC and in the central part with the distribution of FSLEs on areas visited by seabirds during long and short trips were performed. The tests confirmed that distributions of FSLEs in areas crossed by seabirds are highly different from those found over the whole area and the central part (P < 0.0001 for both long and short trips). Distribution patterns provide clear evidence that Great Frigatebirds are not randomly distributed throughout the FSLE range (both backward and forward) and that seabirds move over specific areas rich in LCSs, despite the area occupied by LCSs being small. Nearly two-thirds of the birds' positions are on LCSs, even though only ≤30% of the whole area or the central part (Fig. 3) contain high |FSLE| and are then occupied by LCSs. These numbers were further checked by χ2 analyses using the 1-tailed G-test for goodness of fit (log-likelihood ratio), which clearly show that there are significant differences between positions of birds on LCSs and on other structures (Table 1) (G-test, P < 0.001); this confirms again that seabird positions are located more on LCSs (|FSLE| > 0.1 day −1) than outside during long and short trips, despite the small area occupied by LCSs (Fig. 3). An additional test checking the relationship between bird positions at a given week t and the LCSs computed for that week and for the following weeks, t + 1, t + 2, … t + 9, is described in the supporting information (SI) Methods. The association of bird tracks and LCSs, measured by the significance of a G-test, is highest for the LCSs of week t and decreases with the time lag to the other weeks (pt + 1 = 0.81 > pt + 3 = 0.19 > pt + 5 = 0.12) (Table S1).
Fig. 3.
Histograms of relative frequency of FSLEs with percentage of attracting (ALCSs) and repelling LCSs (RLCSs). Positive values refer to FSLEf and negative to FSLEb. (A) Areas crossed by seabirds (long and short trips); (B) in the whole MC; and (C) in the central part (16°–24°S/30°–45°E).
Table 1.
Absolute frequency of seabird positions on LCSs and on no Lagrangian structures for long and short trips per week and result of the G-test for goodness of fit
| Week | All trips |
Long trips |
Short trips |
|||
|---|---|---|---|---|---|---|
| LCSs: |FSLE| > 0.1 day−1 | |FSLE| < 0.1 day−1 | LCSs: |FSLE| > 0.1 day−1 | |FSLE| < 0.1 day−1 | LCSs: |FSLE| > 0.1 day−1 | |FSLE| < 0.1 day−1 | |
| 1 | 38 | 9 | 19 | 7 | 19 | 2 |
| 2 | 78 | 40 | 55 | 12 | 23 | 28 |
| 4 | 208 | 85 | 147 | 54 | 61 | 31 |
| 5 | 167 | 109 | 137 | 84 | 30 | 25 |
| 6 | 120 | 77 | 89 | 51 | 31 | 26 |
| 7 | 79 | 55 | 72 | 32 | 7 | 23 |
| 8 | 53 | 34 | 53 | 34 | — | — |
| 9 | 61 | 59 | 61 | 59 | — | — |
| 10 | 55 | 31 | 45 | 24 | 10 | 7 |
| 14 | 35 | 12 | 35 | 12 | — | — |
| 15 | 10 | 5 | 10 | 5 | — | — |
| % | 63.7 | 36.3 | 65.9 | 34.1 | 56.0 | 44.0 |
| G-test (log-likelihood ratio) | ||||||
| n | 1420 | 1097 | 323 | |||
| k | 11 | 11 | 7 | |||
| df | 10 | 10 | 6 | |||
| G | 28.119 | 30.613 | 32.057 | |||
| P | 0.00173 | 0.001 | 0.000 | |||
One-tailed tests. Null hypothesis Ho: Seabird positions share equally LCSs (|FSLE| > 0.1 day−1 and on no LCSs. α = 5%.
FSLE Distributions over Different Types of Flights.
We performed several statistical tests to determine whether there are statistically significant differences among travel/foraging locations, outgoing/return trips, and day/night flights.
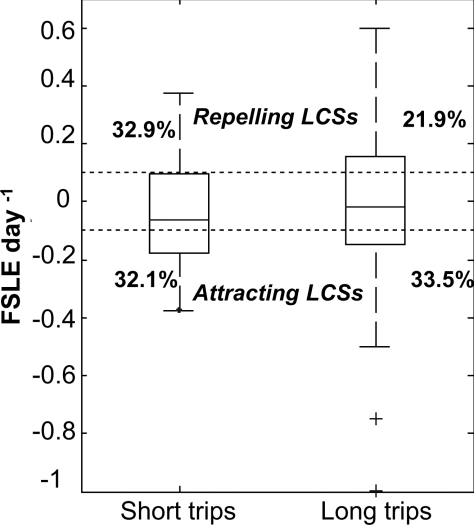
Boxplots of FSLEs on seabird positions during long and short trips are presented in Fig. 4. The range of variation of FSLE is clearly more dispersed during long trips than short trips, and the median between both kinds of trips is similar. Furthermore, distributions are clearly different between long and short trips, as confirmed by a KS-2 test (P ≪ 0.001). Indeed, 65.9% of seabird positions during long trips and 56% during short trips are on LCSs (Table 1). During long trips, Great Frigatebirds forage during a longer time and so cover a larger range of variation of FSLE values than during short trips. One-tailed G-test for goodness of fit confirms that there is a difference between the number of seabird locations on FSLE ridges and outside the ridges (Table 1) (G = 30.613, P = 0.001, df = 10 for long trips; and G = 32.057, P ≪ 0.001, df = 6 for short trips).
Fig. 4.
Box plots of the distribution of FSLEs during short and long trips. The upper and lower ends of the center box indicate the 75th and 25th percentiles of the data; the center of the box indicates the median. Suspected outliers appear in a box plot as individual points (+) outside the box. Dotted lines represent the threshold for detection of LCSs.
KS-2 tests show that the distribution of the birds between attracting and repelling LCSs displays no statistically significant difference during long trips (P > 0.05) but differs during short trips (P < 0.01). During short trips birds follow the attracting LCSs more than the repelling ones. The analyses clearly demonstrate that seabirds follow the FSLE ridges during their foraging trips, but more during long trips than during short trips. This result emphasizes the probable difference in Great Frigatebird behavior during long vs. short trips.
Boxplots of FSLE show that patterns of distribution of FSLE are not very different between flying and foraging positions (Fig. S1). Distributions of FSLEs are statistically similar for foraging and crossed areas (KS-2 test, P = 0.29 for long trips and P = 0.51 for short trips) but differ from FSLE distribution in the whole area (KS test, P < 0.0001). During long trips 69.6% of seabird positions during flying and 62% during foraging are on LCSs (Fig. S1); these figures are 61.8% and 66.7%, respectively, during short trips. During flying and foraging seabirds split almost equally between repelling and attracting structures (G-test, P > 0.05) (Table S2). All of this indicates that seabirds seem to prefer being on ridges of FSLE both for travel and foraging.
We have also investigated differences in seabird distributions in relation to FSLEs between the outward and return parts of the trip (Fig. S2 A and C). KS-2 test shows that there is no significant difference of seabird distribution during long trips (KS-2, P > 0.01) and during short trips (P > 0.05), between the outward and return parts of the trip. For all types of trips (short and long) there is no significant difference of seabird positions, either on repelling or attracting flow structures, during the outward and return parts of the trip (G-test, P > 0.05) (Table S3).
Great Frigatebirds feed mainly during daytime (10). We therefore examined whether we could identify differences between day- and nighttime distribution of seabirds. Boxplots of seabird distribution on FSLE between day and night show that patterns of distribution of FSLEs are similar during day and night during short (Fig. S2B) and long trips (Fig. S2D). The range of variation of FSLE during long trips is, however, more dispersed at night than during short trips. KS-2 test shows that there is no significant difference between FSLE distributions visited by birds during day and night (P > 0.05 during long or short trips). The probability for the frigatebirds to fly over attracting or repelling structures during day and night is statistically similar (G-test, P > 0.05) for long trips but may be different for short trips (G-test, P = 0.025) (Table S3). During daytime short trips, seabirds may follow the attracting structures more than the repelling ones.
Discussion
Because eddies affect all stages of the marine ecosystem, they are determinant for the triad “enhancement–concentration–retention” identified by Bakun (8, 25). From upwelling-driven processes at the center of cyclonic eddies (1, 2), or from other processes at the boundaries between eddies (13), local enrichment and new production have been observed. The cyclic circulation in vortices also produces retention of larvae and other planktonic organisms in their core, whereas concentration occurs in the convergence zones located at the boundary between them, which are detected by FSLEs.
Transport barriers and filament generation by interaction between eddies induce horizontal and vertical biogeochemical and biologic enhancement (13). FSLEs seem very well suited to detect such transport barriers, vortex boundaries, and filaments at meso- and submesoscale (20, 26) and to study the link with the ecologic behavior of marine top predators. However, a word of caution is required about the spatial resolution we used. Indeed, the FSLEs are computed from satellite altimetry products (19), with a spatial resolution of 1/4° interpolated here onto a 1/40° grid. This interpolation might induce some bias in the data. However, FSLEs, because of the averaging effect produced by computing them by integrating over trajectories that extend in time and space, are rather robust against noise and uncertainties in velocity data (26, 27) (see also SI Methods). The velocity field used here has been validated, and the correlation with velocities from Lagrangian drifting buoy data in the MC was satisfactory (SI Methods and Fig. S3). Furthermore, Argos positioning of birds is not of equivalent quality. Some positions have a margin of error of a few hundred meters, whereas others have an error margin of more than 1 km. Definite improvements would be to reduce interpolation by using an original higher-resolution velocity field and to obtain more precise bird locations.
In the central part of the MC, it is known that the boundary of eddies is very energetic and allows the aggregation of top-predator foraging, especially Great Frigatebirds (10), which preferentially stay in this part of the channel. To date it was believed that Great Frigatebirds used edges of eddies mainly for food because these areas are rich in forage species and associated top predators [especially tuna and dolphins (28)]. Superimposing Great Frigatebird positions on FSLE fields shows that their spatial distribution is linked to eddies and more generally to the different types of LCSs, and not only for foraging but also for traveling. Observations are in agreement with the histograms and KS tests, which demonstrate that seabirds are not randomly distributed in relation to attracting and repelling LCSs.
However, analysis of location of seabirds during long and short trips shows that the percentage of positions on LCSs is different between both kinds of trips (Table 1). During long trips, birds seem to take full measure of the LCSs, whereas on short trips they do not take full advantage of them. This difference between long and short trips is probably due to the behavior of seabirds. During short trips birds have to bring food frequently to their chick, so they feed in areas where prey are easily accessible, close to Europa Island. They used preferentially attracting structures during daytime, probably because these structures are conducive to the aggregation of prey. During long trips birds avoid areas near Europa Island, probably because the foraging yield is less rich than that of more distant waters and/or because of strong interspecific competition near the island (10). However, birds preferentially follow the LCSs in both cases.
In addition, seabirds follow LCSs not only for their foraging but also for their traveling movements. The distributions of FSLEs during the outward and inbound journeys to the colony indicate that they exhibit the same flying behavior before and after their foraging activity. Furthermore, the fact that the distribution of visited FSLEs is identical during day and night indicate that they are able to use these LCSs to move during periods of darkness. Frigatebirds move continuously during day and night at an average altitude of 200 m and never completely stop moving when they forage, but they come to the sea surface to eat only during daytime (10). If they used these structures only for food availability, then the distribution of FSLEs for areas crossed by birds should be different between day and night. This is not the case. This means that frigatebirds do not go to FSLEs ridges only to forage but that they follow them most of the time as cues to eventually find prey patches there.
It is relatively easy to understand why the attracting LCSs could be places for prey accumulation, given that horizontal flow will make passively advected organisms close to these lines approach them. More puzzling is the role of the repelling LCSs, which are also preferred locations for the frigatebirds. First we should mention that at the vortex edges, lines of the attracting and repelling types are very close and nearly tangent. Thus, it may be the case that birds' positions located at repelling lines are simultaneously also located on attracting ones; in SI Methods we explain that a position is said to be on an LCS if it is closer to it than 0.025°. Thus, if the attracting and repelling LCSs are close enough, the same bird position may be attributed to both structures. We have determined that, among the 30.2% of bird positions that were found on repelling coherent structures, 53.7% of these were in fact visiting both structures, and thus the interpretation is that they are associated to vortex edges (or to other structures in which both types of lines are tangent). For the remaining fraction that does not seem to be associated to these edges, we believe that the 3-dimensional dynamics of the flow close to these structures gives the clue for their association to bird positions. Note that FSLE values have been calculated on the basis of the 2-dimensional surface flow, and the FSLE methodology identifies these regions as places of filament and submesoscale structure formation by horizontal advection. But there is growing evidence (29, 30) of strong links between submesoscale structures from different origins and vertical motions. Thus, in an indirect manner, the calculated LCSs may be indicating the places in the ocean where vertical upwelling and/or downwelling of nutrients and organisms could occur. This is obviously important for the birds and may explain why they prefer to fly and to forage on top of them. The role of these LCSs in biologic activity is rather complex and may vary depending on the area and scale of study. For instance, Rossi et al. (31) found an inverse relationship between mixing activity (high FSLEs) and phytoplankton stocks in very productive areas, such as coastal eastern boundary upwelling.
The above arguments linking LCSs and vertical motion can be more easily justified for the attracting LCS case, because the vorticity involved in the interaction between vertical and horizontal motion will also tend to be aligned with these structures (30). But we note that in flows consisting of slowly moving eddies, we are close to the so-called integrable situation in which a large proportion of tangencies between attracting and repelling structures is expected (as indeed observed). As a consequence, it may happen that a bird starts a trip by following an attracting LCS, loses its surface signal, and finds itself on top of a repelling one simply by continuing its previous path in a more-or-less straight way. We stress, however, that all explanations we give to the observed relationship between LCSs and bird paths contain a number of hypotheses that need additional research.
One may ask how frigatebirds “follow” the LCSs during day and night. Several hypotheses can be put forward.
First, because frigatebirds use atmospheric currents, especially to gain altitude by soaring and then glide over long distances (32), we can suppose that the coupling between the ocean and the atmosphere at meso- and submesoscale generates atmospheric currents followed by seabirds. Indeed some investigators (33–36) emphasize the role of local air–sea feedbacks arising from ocean mesoscale features. For example, Chelton et al. (36) showed that an ocean–atmosphere coupling is observed in the California Current System during summer. They conclude that sea surface temperature fronts generated by mesoscale activity (eddies and upwelling) have a clear influence on the perturbation of summertime wind stress curl and divergence. In the MC, mesoscale eddies and their interaction would force the atmosphere and generate air currents favorable to Great Frigatebirds, which might take advantage of the wind to spend the least possible energy in flight.
Second, we cannot exclude that birds may follow visual or, more likely, olfactory cues. Foraging behavior of seabirds is complex and results from a number of behavioral parameters, such as sight, smell (37, 38), memory effect (39), and environmental parameters [chlorophyll concentration (10) or wind speed and direction]. Nevitt et al. (40) suggest that seabirds use olfaction to track high concentrations of odor compounds, such as dimethyl sulfide (DMS), and sight when they locate prey patches. The use of models of odor transport suggests that olfaction plays a role in foraging behavior (40). Structures detected using FSLEs are dynamic and, as mentioned above, may induce vertical mixing favorable to phytoplankton enhancement (41, 42) and their patchy distribution. The grazing of phytoplankton by zooplankton induces the production of DMS (43), which is very attractive for different species of seabirds (44). Even if there is no study on the role of olfaction in Great Frigatebird foraging behavior, we can hypothesize that they use olfaction to detect DMS and productive areas and find food patches. The interaction between the ocean and the atmosphere at submesoscale and wind may allow the dispersion of the DMS or other odors and favor their detection by seabirds that follow LCSs until they see a patch of prey. These LCSs could be viewed as moving habitat facilitating movement of seabirds. Indeed, frigatebirds might use these odorous corridors to move between food patches with efficacy.
Whatever the cue used by frigatebirds to locate and follow these LCSs, our results provide the first evidence that a top predator tracks these FSLE ridges to locate food patches. It allows us to better understand how top predators search prey and why they are able to concentrate precisely at LCSs. Because these structures are mobile, a simple memory is not sufficient for a central-place forager to return to a productive prey area. Predators could thus take a general bearing where eddies are likely to be found (e.g., to the northwest in the MC for a colony located in the central MC) and then move until they cross an FSLE ridge, which they will follow until they encounter a prey patch. Because they are unable to sit on the water, frigates are often in association with subsurface top predators to forage. We can suppose that if frigatebirds track LCSs to locate prey, it is possible that they are associated with tuna schools around foraging areas (10). Thus, understanding the rationale behind their localization is crucial not only in seabird ecology but also in the detection of the presence of tuna schools. This kind of multidisciplinary approach opens up interesting prospects in the management of ecosystems and fisheries and can be useful in the ecosystemic approach to fisheries, especially to better characterize temporary tuna habitats in the MC. Future work is to identify the responsible mechanism by which an aerial predator may spot and follow LCSs.
Materials and Methods
In this part we provide a brief overview of the methodology; further details for each section are given in SI Methods.
Great Frigatebirds.
Europa (22.3° S, 40.3° E) is 1 of the 2 colonies (with Aldabra) of Great Frigatebirds in the West Indian Ocean. The island is located in the central part of the MC. Great Frigatebirds have the ability to undertake long-range movements out of the breeding season (10), but they behave as central-place foragers when breeding. Their diet is composed essentially of flying fish and Ommastrephid squids (10), but Great Frigatebirds are also kleptoparasites, meaning they can steal prey from others. One of their particularities is that they cannot wet their feathers or dive into the water to feed. They forage mainly through association with tuna and dolphin schools, which bring prey to the surface.
To track movements of frigatebirds, 8 birds were tracked with satellite transmitters and altimeters between August 18 and September 30, 2003, resulting in 1864 Argos positions. The mean time between each position is 0.07 days, with a minimum of 0.001 days and a maximum of 1.1 days. All seabird positions from a given week were collocated on the time and space grid on which the FSLEs were calculated (with 0.025° resolution).
LCSs by FSLEs: FSLE Method.
Oceanic variability in surface velocities is not probably sensed directly by Great Frigatebirds but rather indirectly via transported substances. This calls for a Lagrangian perspective on the problem. Thus, we quantify horizontal transport processes and LCSs by the Lagrangian technique of FSLE (45), which is specially suited to study the stretching and contraction properties of transport in geophysical data (20). Because of their Lagrangian character, FSLEs describe submesoscale details that cannot be detected by other means, like the inspection of the sea level anomaly maps of the marine surface.
The calculation of the FSLE goes through computing the time, τ, at which 2 tracer particles initially separated at a distance δ0, reach a final separation distance δf, following their trajectories in the marine surface velocity field. At position x and time t the FSLE is given by:
We follow the trajectories for 200 days, so that if τ is larger than this, we define λ = 0. It is clear that the FSLEs depend critically on the choice of 2 length scales: the initial separation, δ0, and the final one, δf. δ0 has to be close to the intergrid spacing among the points x on which the FSLEs will be computed (20). In our case we calculate FSLE on all of the points of a latitude–longitude grid with a spacing of δ0 = 1/40° = 0.025°. On the other hand, because we are interested in mesoscale structures, δf is chosen as δf = 1° (i.e., separation of approximately 110 km). In this respect, the FSLE represents the inverse time scale for mixing up fluid parcels between the grid and the characteristic scales of the MC eddies. Maps of FSLE are calculated weekly. An alternative to FSLE is the finite-time Lyapunov exponents (22, 46). At the scales and parameters we are working no significant differences are expected for the locations of LCS by any of the 2 methods.
The time integration of the particle trajectories can be performed in 2 ways: forward and backward in time. For the backward computation, maximum values of FSLE organize in lines that are good approximations of the so-called unstable manifolds of hyperbolic points, which for our purposes are lines toward which neighboring fluid trajectories, while escaping from hyperbolic points, approach at long times (20, 23, 24). In consequence they are called attracting LCSs. FSLEs computed integrating trajectories toward the future (i.e., forward) take large values on lines (stable manifolds) from which neighboring trajectories appear to be repelled (repelling LCSs). These lines of maximum separation or convergence rates, or “ridges,” delineate fluid domains with quite distinct origin and characteristics. Such lines strongly modulate the fluid motion when reaching maximum values, and they act as transport barriers for particle trajectories, thus constituting a powerful tool for predicting fronts generated by, for example, passive advection, eddy boundaries, and material filaments. Other studies (20, 26, 27, 31, 42) have demonstrated the adequacy of the FSLE to characterize horizontal mixing and transport structures in the marine surface, as well as its usefulness when correlating with tracer fields like temperature or chlorophyll.
Supplementary Material
Acknowledgments.
We thank the 2 anonymous reviewers for their helpful comments on the manuscript. A Ph.D. fellowship for E.T.K was provided by the Institut de Recherche pour le Développement and the University Pierre and Marie Curie. Ph.D. financial support for V.R was provided by the Direction Générale de l'Armement. The Laboratoire d'Etudes en Géophysique et Océanographie Spatiale contribution is supported by Centre National d'Etudes Spatiales funding. The Instituto de Física Interdisciplinar y Sistemas Complejos contribution is supported by Ministerio de Ciencia e Innovación and le Fonds Européen de Développement Régional through project FISICOS (FIS2007–60327), and by Consejo Superior de Investigaciones Cientificas through the Intramural Frontier Projects OCEANTECH. H.W.'s contribution was supported by the REMIGE project funded by Agence Nationale de la Recherche (ANR 2005 Biodiv-011).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811034106/DCSupplemental.
References
- 1.McGillicuddy J, et al. Influence of mesoscale eddies on new production in the Sargasso Sea. Nature. 1998;394:263–266. [Google Scholar]
- 2.Oschlies A, Garcon V. Eddy-induced enhancement of primary production in a model of the North Atlantic Ocean. Nature. 1998;394:266–268. [Google Scholar]
- 3.Owen RW. Fronts and eddies in the sea: Mechanisms, interactions and biological Effects. In: Owen RW, editor. Fronts and Eddies in the Sea. London: Academic; 1981. [Google Scholar]
- 4.Kang J, Kim W, Chang K, Noh J. Distribution of plankton related to the mesoscale physical structure within the surface mixed layer in the southwestern East Sea. Korea J Plankton Res. 2004;26:1515–1528. [Google Scholar]
- 5.Mackas D, Tsurumi M, Galbraith M, Yelland D. Zooplankton distribution and dynamics in a North Pacific Eddy of coastal origin: II. Mechanisms of eddy colonization by and retention of offshore species. Deep-Sea Res II. 2005;52:1011–1035. [Google Scholar]
- 6.Moore T, Matear R, Marra J, Clementson L. Phytoplankton variability off the Western Australian Coast: Mesoscale eddies and their role in cross-shelf exchange. Deep-Sea Res II. 2007;54:943–960. [Google Scholar]
- 7.Polovinia J, Howell E, Kobayashi D, Seki M. The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Prog Oceanogr. 2001;49:469–483. [Google Scholar]
- 8.Bakun A. Fronts and eddies as key structures in the habitat of marine fish larvae: Opportunity, adaptive response and competitive advantage. Scientia Marina. 2006;70:105–122. [Google Scholar]
- 9.Nel D, et al. Exploitation of mesoscale oceanographic features by grey-headed albatross Thalassarche chrysostoma in the southern Indian Ocean. Mar Ecol Prog Ser. 2001;217:15–26. [Google Scholar]
- 10.Weimerskirch H, Le Corre M, Jaquemet S, Potier M, Marsac F. Foraging strategy of a top predator in tropical waters: Great Frigatebirds in the Mozambique Channel. Mar Ecol Prog Ser. 2004;275:297–308. [Google Scholar]
- 11.Hyrenbach K, Veit R, Weimerskirch H, Hunt G., Jr Seabird associations with mesoscale eddies: The subtropical Indian Ocean. Mar Ecol Prog Ser. 2006;324:271–279. [Google Scholar]
- 12.Domokos R, Seki MP, Polovinia JJ, Hawn DR. Oceanographic investigation of the American Samoa albacore (Thunnus alalunga) habitat and longline fishing grounds. Fish Oceanogr. 2007;16:555–572. [Google Scholar]
- 13.Lima I, Olson D, Doney S. Biological response to frontal dynamics and mesoscale variability in oligotrophic environments: Biological production and community structure. J Geophys Res. 2002;107:3111. [Google Scholar]
- 14.Abraham ER, Bowen MM. Chaotic stirring by a mesoscale surface ocean flow. Chaos. 2002;12:373–381. doi: 10.1063/1.1481615. [DOI] [PubMed] [Google Scholar]
- 15.De Ruijter WPM, Ridderinkhof H, Lutjeharms R, Schouten M, Veth C. Observations of the flow in the Mozambique Channel. Geophys Res Lett. 2002;29:1502. [Google Scholar]
- 16.Ridderinkhof H, de Ruijter WPM. Moored current observations in the Mozambique Channel. Deep-Sea Res II. 2003;5:1933–1955. [Google Scholar]
- 17.Schouten MW, de Ruijter WPM, van Leeuwen PJ, Ridderinkhof H. Eddies and variability in the Mozambique Channel. Deep-Sea Res II. 2003;50:1987–2003. [Google Scholar]
- 18.Tew Kai E, Marsac F. Patterns of variability of sea surface chlorophyll in the Mozambique Channel: A quantitative approach. J Mar Syst. 2009;77:77–88. [Google Scholar]
- 19.Sudre J, Morrow R. Global surface currents: A high resolution product for investigating ocean dynamics. Ocean Dyn. 2008;58:101–118. [Google Scholar]
- 20.d'Ovidio F, Fernandez V, Hernández-García E, López C. Mixing structures in the Mediterranean Sea from Finite-Size Lyapunov Exponents. Geophys Res Lett. 2004;31:L17203. [Google Scholar]
- 21.Haller G, Yuan G. Lagrangian coherent structures and mixing in two-dimensional turbulence. Physica D. 2000;147:352–370. [Google Scholar]
- 22.Haller G. Lagrangian structures and the rate of strain in a partition of two-dimensional turbulence. Phys Fluids. 2001;13:3365–3385. [Google Scholar]
- 23.Joseph B, Legras B. Relation between kinematic boundaries, stirring and barriers for the Antarctic polar vortex. J Atmosph Sci. 2002;59:1198–1212. [Google Scholar]
- 24.Koh T-Y, Legras B. Hyperbolic lines and the stratospheric polar vortex. Chaos. 2002;12:382–394. doi: 10.1063/1.1480442. [DOI] [PubMed] [Google Scholar]
- 25.Bakun A. Patterns in the Ocean: Oceanic Processes and Marine Population Dynamics. San Diego, CA: University of California Sea Grant; 1996. in cooperation with Centro de Investigaciones Biologicas de Noroeste, La Paz, Baja California Sur, Mexico. [Google Scholar]
- 26.D'Ovidio F, Isern-Fontanet J, López C, Hernández-García E, García-Ladona E. Comparison between Eulerian diagnostics and Finite-Size Lyapunov Exponents computed from altimetry in the Algerian basin. Deep-Sea Res I. 2009;56:15–31. [Google Scholar]
- 27.Haza A, Poje AC, Özgökmen TM, Martin P. Relative dispersion from a high-resolution coastal model of the Adriatic Sea. Ocean Modelling. 2008;22:48–65. [Google Scholar]
- 28.Jaquemet S, Le Corre M, Weimerskirch H. Seabird community structure in a coastal tropical environment: Importance of natural factors and fish aggregating devices (FADs) Mar Ecol Prog Ser. 2004;268:281–292. [Google Scholar]
- 29.Mahadevan A, Tandon A. An analysis of mechanisms for submesoscale vertical motion at ocean fronts. Ocean Modelling. 2006;14:241–256. [Google Scholar]
- 30.Klein P, Lapeyre G. The oceanic vertical pump induced by mesoscale eddies. Ann Rev Mar Sci. 2009;1:351–375. doi: 10.1146/annurev.marine.010908.163704. [DOI] [PubMed] [Google Scholar]
- 31.Rossi V, Lopez C, Sudre J, Hernandez-Garcia E, Garçon V. Comparative study of mixing and biological activity of the Benguela and Canary upwelling systems. Geophys Res Lett. 2008;35:L11602. [Google Scholar]
- 32.Weimerskirch H, Chastel O, Barbraud C, Tostain O. Frigatebirds ride high on thermals. Nature. 2003;421:333–334. doi: 10.1038/421333a. [DOI] [PubMed] [Google Scholar]
- 33.Xie S. Satellite observations of cool ocean–atmosphere interaction. Bull Am Meteor Soc. 2004;85:195–209. [Google Scholar]
- 34.Chelton D, Schlax MG, Freilich MH, Milliff RF. Satellite measurements reveal persistent small-scale features in ocean winds. Science. 2004;303:978–983. doi: 10.1126/science.1091901. [DOI] [PubMed] [Google Scholar]
- 35.Seo H, Miller A, Roads J. The Scripps Coupled Ocean–Atmosphere Regional (SCOAR) model, with applications in the eastern Pacific sector. J Clim. 2007;27:381–401. [Google Scholar]
- 36.Chelton D, Schlax MG, Samelson RM. Summertime coupling between sea surface temperature and wind stress in the California Current System. J Phys Oceanogr. 2007;37:495–517. [Google Scholar]
- 37.Nevitt GA. Olfactory foraging by Antarctic procellariiform seabirds: Life at high Reynolds numbers. Biol Bull. 2000;198:245–253. doi: 10.2307/1542527. [DOI] [PubMed] [Google Scholar]
- 38.Nevitt GA, Bonadonna F. Seeing the world through the nose of a bird: New developments in the sensory ecology of procellariiform seabirds. Mar Ecol Prog Ser. 2005;287:292–295. [Google Scholar]
- 39.Davoren GK, Montevecchi WA, Anderson JT. Distributional patterns of a marine bird and its prey: Habitat selection based on prey and conspecific behaviour. Mar Ecol Prog Ser. 2003;256:229–242. [Google Scholar]
- 40.Nevitt GA, Losekoot M, Weimerskirch H. Evidence for olfactory search by wandering albatross, Diomedea exulans. Proc Natl Acad Sci USA. 2008;105:4576–4581. doi: 10.1073/pnas.0709047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin A. Phytoplankton patchiness: The role of lateral stirring and mixing. Prog Oceanogr. 2003;57:125–174. [Google Scholar]
- 42.Lehahn Y, d'Ovidio F, Lévy M, Heyfetz E. Stirring of the northeast Atlantic spring bloom: A Lagrangian analysis based on multisatellite data. J Geophys Res. 2007;112:C08005. [Google Scholar]
- 43.Dacey JWH, Wakeham SG. Oceanic dimethylsulfide: Production during zooplankton grazing on phytoplankton. Science. 1986;233:1314–1316. doi: 10.1126/science.233.4770.1314. [DOI] [PubMed] [Google Scholar]
- 44.Nevitt GA, Veit RR, Kareiva P. Dimethyl sulphide as a foraging cue for Antarctic procellariiform seabirds. Nature. 1995;376:680–682. [Google Scholar]
- 45.Aurell E, Boffetta G, Crisanti A, Paladin G, Vulpiani A. Predictability in the large: An extension of the concept of Lyapunov exponent. J Phys A. 1997;30:1–26. [Google Scholar]
- 46.Beron-Vera FJ, Olascoaga MJ, Goni GJ. Oceanic mesoscale eddies as revealed by Lagrangian coherent structures. Geophys Res Lett. 2008;35:L12603. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.