Abstract
The differentiation of naïve CD4 T cells into specific effector subsets is controlled in large part by the milieu of cytokines present during their initial encounter with antigen. Cytokines that drive differentiation of the newly described Th17 lineage have been characterized in vitro, but the cytokines that prime commitment to this lineage in response to infection in vivo are less clear. Listeria monocytogenes (Lm) induces a strong Th1 response in wildtype mice. By contrast we demonstrate that in the absence of IL-12p40 (or IFN-γ) and type I IFN receptor signaling, the Th1 antigen-specific CD4 T cell response is virtually abolished and replaced by a relatively low magnitude Th17-dominated response. This Th17 response was dependent on Tgf-β and IL-6. Despite this change in CD4 T cell response, neither the kinetics of the CD4 and CD8 T cell responses, the quality of the CD8 T cell response, nor the ability of CD8 T cells to mediate protection were affected. Thus generation of protective CD8 T cell immunity was resilient to perturbations that replace a strong Th1-dominated to a reduced magnitude Th17-dominated antigen-specific CD4 T cell response. publisher disclaimer
Keywords: T cell, bacterial infection, cell differentiation, cytokines
INTRODUCTION
Naïve CD4 T cells have the ability to differentiate into distinct effector lineages each characterized by the production of a unique profile of effector cytokines. Until recently, these CD4 T cell effector lineages have included Th1 cells that produce IFN-γ important for immunity to intracellular pathogens, and Th2 cells that produce IL-4, IL-5 and IL-13 important for immunity to parasitic infections. The newly elucidated Th17 lineage produces IL-17A/F, IL-21, IL-22, and GMCSF (1, 2) and fulfills a niche that provides immunity against extracellular bacterial or fungal pathogens, like Citrobacter, Klebsiella, or Candida (3-6). However, uncontrolled activation of antigen-specific Th17 cells has been implicated in the pathogenesis of autoimmune diseases, including multiple sclerosis and rheumatoid arthritis in humans and EAE and adjuvant arthritis in mice (1, 7). Determining the biological parameters that govern the differentiation program of CD4 T cells into each specific effector lineage is critical for the design of vaccines and other immune-modulatory strategies to ensure that only the desired lineage of effector CD4 T cell is generated, while simultaneously avoiding potentially detrimental immune responses.
At least three inter-related signals are required for the activation and differentiation of naïve CD4 into specific effector cell lineages. The first (TCR binding to a specific peptide-MHC complex) and second (physical interaction between co-stimulatory receptors on the T cell and antigen presenting cell) signals lead to T cell activation; whereas, the third signal (the presence or absence of specific inflammatory cytokines) plays a more critical role in the differentiation of activated T cells into specific effector cell lineages (8). Moreover, the effector cytokines produced by each specific lineage of effector CD4 T cells further reinforces the differentiation of other naïve T cells into that lineage, while simultaneously inhibiting differentiation into other cell lineages. For example, IFN-γ produced by Th1 cells promotes T cell differentiation to Th1 lineage by inducing IL-12 receptor-β2 and T-bet expression and inhibits Th2 differentiation by repressing GATA-3 and IL-4 (9). Conversely, IL-4 produced by Th2 cells induces GATA-3 expression, thereby inducing IL-4 production by other T cells and inhibiting IL-12 receptor expression (10). Characterized primarily in vitro with T cells activated in a non-antigen specific manner, Th17 differentiation requires the presence of Tgf-β and IL-6, and the absence of IFN-γ (3, 11, 12). However, the general applicability of these results to the actual signals that are produced and are important for differentiation of antigen-specific Th17 CD4 T cells during infection in vivo is unclear.
Experimental Listeria monocytogenes (Lm) infection is a well-characterized model by which to examine priming of antigen-specific T cells in vivo (13). Following infection with either wildtype (WT) or live attenuated Lm strains that retain the ability to gain access to the cell cytoplasm, a protective T cell response is readily detected and characterized by the expansion of antigen-specific, IFN-γ-producing Th1 CD4 and CD8 effector T cells. For Lm infection, antigen-specific CD8 T cells confer the majority of the protective effects, whereas CD4 T cells have an important role in the generation of long-lived memory CD8 T cells (14-16). Using this infection model, we have recently demonstrated that priming antigen-specific CD4 T cells for IFN-γ production requires either IL-12 or type I IFNs, while priming antigen-specific CD8 T cells requires neither IL-12 nor type I IFNs (17). Furthermore, for CD4 T cells activated in the absence of IL-12 and type I IFNs, the lack of IFN-γ production is not associated with a reciprocal production of Th2 cytokines such as IL-4 or IL-13 (17). Accordingly, in the present study, we examined the possibility that Lm infection in the absence of both IL-12P40 and IFN-IR signaling could prime a Th17-dominated response. After comparing the relative expression of IFN-γ and IL-17 by antigen-specific CD4 T cells in wildtype, IL-12p40 deficient, IFN-IR-deficient, and mice deficient in both IL-12p40 and IFN-IR, our studies indicate that the presence of either IL-12p40 or IFN-I is required for Th1 differentiation of naïve CD4 T cells. In the absence of both IL-12 and IFN-IR signaling, the normally robust antigen-specific Th1 CD4 T cell response is replaced by a Th17-dominated response that is of significantly lower magnitude. Using this model for priming of antigen-specific Th17 cells, we further characterized the specific cytokine milieu required for in vivo Th17 CD4 T cell differentiation, the dynamics of antigen-specific Th17 T cell expansion and contraction after infection, and the impact a drastically skewed CD4 Th response plays on CD8 T cell immunity.
MATERIALS AND METHODS
Mice
IL-12p40-deficient (P40-/-) mice obtained from The Jackson Laboratory had been backcrossed 11 times to B6 before use. Type I IFN receptor-deficient (IFN-IR-/-) mice backcrossed to B6 mice for 12 generations were obtained from Dr. Kaja Murali-Krishna (University of Washington). Mice deficient in both IL-12p40 and IFN-IR (P40-/- IFN-IR-/- mice) were generated by intercrossing P40-/- and IFN-IR-/- mice (17). Mice were housed in a specific pathogen free facility at the University of Washington. All experiments were performed under IACUC approved protocols.
Listeria monocytogenes
The recombinant Lm strain, Lm-OVA, and Lm-OVA ΔactA derived from this strain through targeted deletion in the actA gene have been described (17, 18). For infections, Lm were grown to early log phase (OD600 0.1) in brain heart infusion media (Becton Dickinson Company) at 37°C, washed, and diluted with saline to 200 μl final volume and injected intravenously into mice.
Reagents, in vitro cultures, and cell staining
For in vivo depletion, 1.0 mg of purified rat anti-mouse IFN-γ (XMG1.2), anti-mouse IL-6 receptor (15A7), anti-mouse Tgf-β (1D11.16.8), or 0.5 mg of purified rat anti-mouse CD4 (GK1.5), anti-mouse CD8 (2.43), or the corresponding rat IgG isotype control antibodies were injected intraperitoneally one day prior to Lm infection. For in vitro culture, splenocytes were plated into 96-well round bottom plates (5 × 106 cells/ml), and stimulated with the indicated peptides (10-6 M) for 5 hours (intracellular cytokine staining) or 72 hours (culture supernatants) as described (17). For intracellular cytokine staining, Brefeldin-A (BD GolgiPlug reagent) was added to cell cultures prior to peptide stimulation. For some experiments, the CD8 T cell response to OVA257-264 was examined with H-2Kb dimerX loaded with OVA257-264 peptide according to the manufacturer's instructions (BD Bioscience). The concentration of IFN-γ and IL-17 in splenocyte culture supernatants was quantified by ELISA using reagents from R&D Systems.
Statistics
The differences in percentages and numbers of cytokine producing cells, cytokine concentrations in culture supernatants, and geometric mean numbers of recoverable CFUs between groups of mice were evaluated by using the Student's t test with P < 0.05 taken as statistically significant (Graph Pad, Prism software).
RESULTS
Lm primes a low magnitude Th17-dominated response in the absence of both IL-12 and IFN-IR
After primary Lm infection, IL-12p40 and type-I IFN signaling each play non-essential roles in priming naive CD4 T cells for IFN-γ production. However, combined defects in both IL-12p40 and type-I IFN signaling completely abrogate IFN-γ production and Th1 differentiation by antigen-specific CD4 T cells (17). For the optimal study of the adaptive T cell response using mice with targeted defects in immune response pathways known to play either protective or detrimental roles in innate susceptibility to WT Lm infection, we used infection with attenuated Lm strains with targeted deficiency in actA to prime adaptive T cell immunity and normalize antigen load after infection. This defect eliminates the actin-recruitment protein in Lm thereby preventing the bacterium from establishing a productive infection by preventing spread from an infected cell into neighboring cells. We previously demonstrated that infection of control mice or mice with targeted defects in IL-12, IFN-IR, or both IL-12 and IFN-IR with the same inoculum of Lm ΔactA results in comparable numbers of recoverable bacteria (representative of antigen load) in the first 24 hours after infection with similar kinetics of bacterial clearance within the first week after infection, whereas this is not the case with WT Lm (17, 19-23). Accordingly, Lm ΔactA was used to further investigate the individual and combined roles of IL-12P40 and IFN-IR in T cell differentiation after in vivo infection.
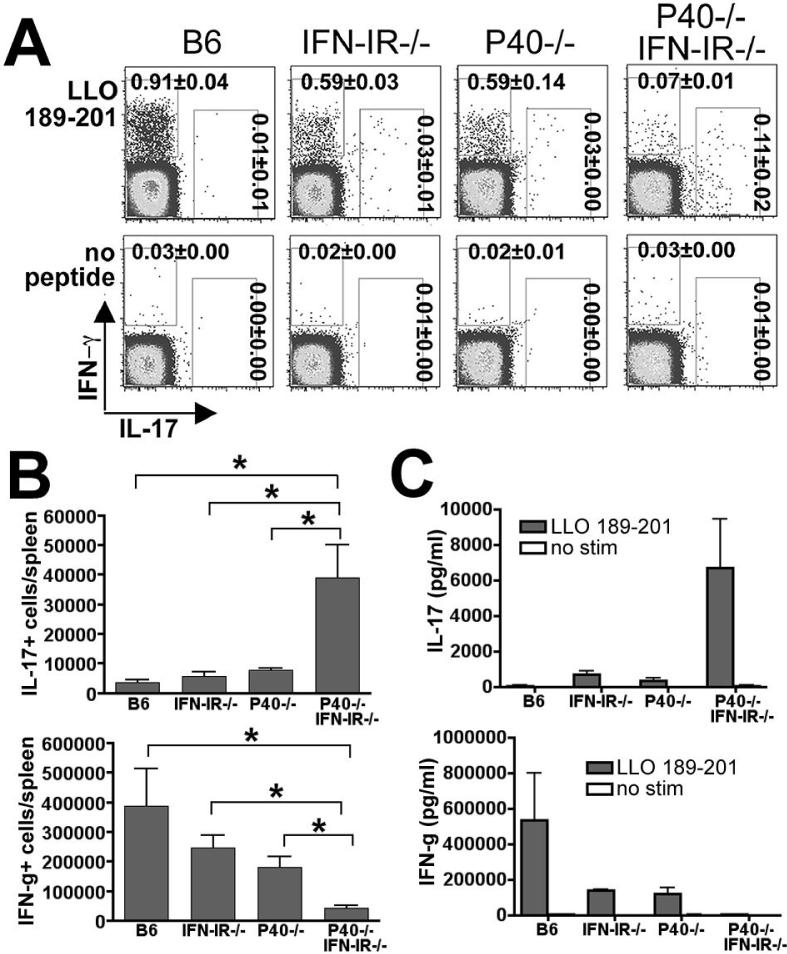
Splenocytes from B6, IFN-IR-/-, P40-/-, and P40-/-IFN-IR-/- mice were harvested and stimulated with the Lm-specific MHC class II-restricted peptide, LLO189-201, and the antigen-specific response was quantified beginning 8 days after infection (17). At this time point after infection with Lm ΔactA, no recoverable Lm CFUs could be detected in the organs of any mice. While only background levels of IL-17 producing cells were detected in B6, P40-/-, and IFN-IR-/- mice, Lm-specific IL-17-producing CD4 T cells were readily detectable in P40-/-IFN-I R-/- mice (Fig. 1A, B). Consistent with our previous studies, there was a drastic reduction in numbers of IFN-γ-producing CD4 T cells in P40-/-IFN-IR-/- mice compared with B6, P40-/-, or IFN-IR-/- mice (17). Despite the relatively low frequency and numbers of IL-17 producing CD4 cells observed in response to LLO189-201 peptide stimulation in splenocytes from P40-/-IFN-I R-/- mice compared with the frequency of IFN-γ producing cells from control mice, there was a highly significant increased frequency of IL-17 producing CD4 T cells in P40-/-IFN-IR-/- compared with B6 (10-fold increase, P < 0.001), P40-/- or IFN-IR-/- mice (4-fold increases, P < 0.005). The concentrations of IL-17 and IFN-γ in splenocyte culture supernatants after in vitro peptide stimulation corroborated this apparent shift from a Th1-dominated CD4 T cell response in B6 mice to a Th17-dominated CD4 T cell response in P40-/- IFN-IR-/- mice (Fig. 1C). Taken together, these data indicate that in the absence of both IL-12P40 and IFN-IR, the normally robust Th1 antigen-specific CD4 T cell response is virtually abolished and replaced by a Th17-dominated response of relatively low magnitude.
Figure 1.
Th17 response in the absence of both IL-12 and IFN-IR. A. FACS plot indicating IL-17 and IFN-γ production in a CD4+ cell gate among splenocytes from mice day 8 after infection with 106 Lm-OVA ΔactA and in vitro stimulation with the Lm-specific MHC class II peptide LLO189-201 or no peptide control. B. Total numbers of IL-17- or IFN-γ-producing, LLO189-201-specific CD4 T cells per mouse spleen as quantified by intracellular cytokine staining. C. Concentration of IL-17 and IFN-γ in splenocyte culture supernatants 72 hours after stimulation with the LLO189-201 peptide or no peptide. These data represent 6-10 mice for each experimental group combined from three independent experiments. Bar, standard error. * P < 0.05.
IFN-γ and IFN-I inhibit Th17 differentiation in response to infection in vivo
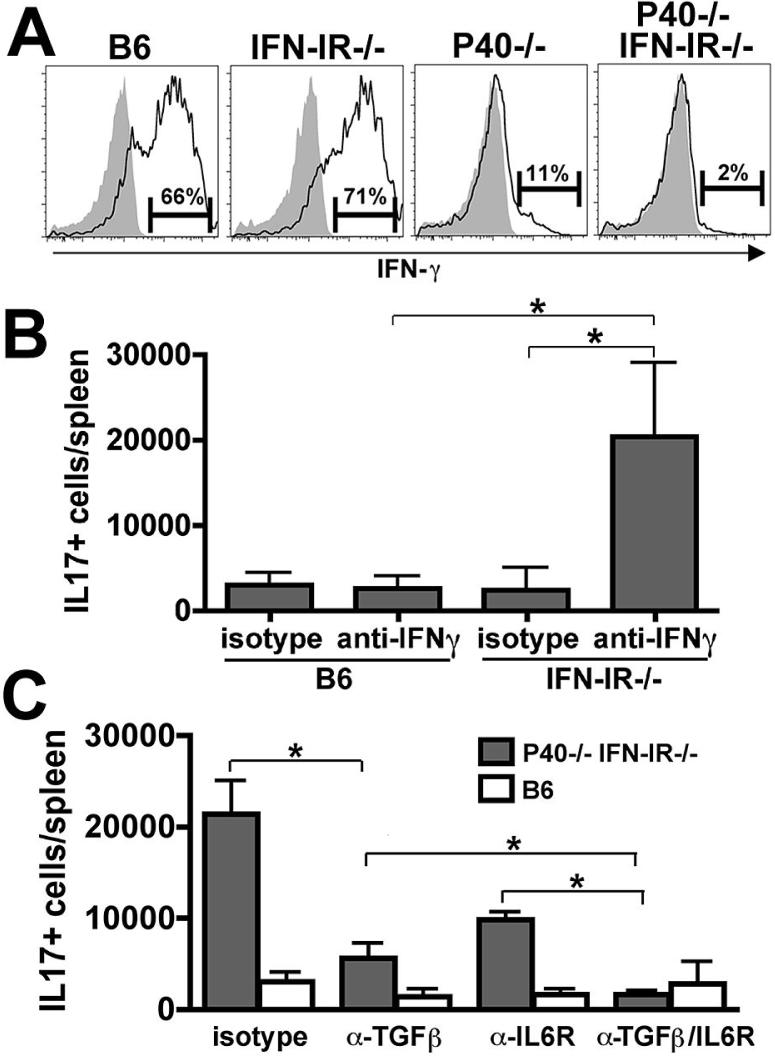
IFN-γ strongly inhibits Th17 differentiation in vitro (24, 25). Since infection with either Lm or Lm ΔactA triggers the production of IFN-γ by NK1.1+ cells in the first 24 hours after infection, the IFN-γ these cells produce might be a key factor that inhibits CD4 Th17 differentiation while facilitating Th1 differentiation in wildtype mice (26, 27). Moreover, since both IL-12 and IFN-I can induce IFN-γ production by NK cells (28, 29), we reasoned that a loss of early IFN-γ production might contribute to the observed deviation from a Th1 to a Th17 response in mice with defects in these cytokine pathways. To address this question, we first assessed production of IFN-γ by NK1.1+ cells 24 hours after infection with Lm. Similar to control B6 mice, NK1.1+ cells were the predominant cell type that produced IFN-γ after Lm infection in P40-/- or IFN-IR-/- mice (data not shown). Compared to B6 controls, P40-/- mice had a drastic reduction in the percentage of NK1.1+ cells that produced IFN-γ (Fig. 2A). Whereas no defect was apparent in IFN-IR-/- mice, IFN-γ-producing NK1.1+ cells were essentially absent in P40-/- IFN-IR-/- mice. These findings demonstrate a previously uncharacterized synergistic role for IFN-I and IL-12P40 in priming NK1.1+ cells for IFN-γ production, which in turn suppresses the development of IL-17 producing cells and enhances the generation of IFN-γ producing Th1 cells.
Figure 2.
IFN-γ or IFN-I inhibits Th17 differentiation. A. Histogram plot indicating the percentage of NK1.1+ cells that produce IFN-γ 24 hours after infection with 106 Lm-OVA ΔactA (open histograms) or in the absence of infection (shaded histograms) for the indicated groups of mice. B. Total number of IL-17-producing CD4 T cells after stimulation with LLO189-201 peptide on day 8 after infection with 106 Lm-OVA ΔactA in B6 or IFN-IR-/- mice pretreated with anti-IFN-γ or isotype control antibody. C. Total numbers of IL-17-producing CD4 T cells after stimulation with LLO189-201 peptide on day 8 after infection with 106 Lm-OVA ΔactA as quantified by intracellular cytokine staining in P40-/-IFN-IR-/- (shaded bars) or B6 (open bars) mice pretreated with either rat IgG control (isotype), anti-Tgf-β, anti-IL-6 receptor, or both anti-Tgf-β and anti-IL-6 receptor antibodies. These data represent 6-8 mice for each experimental group combined from two independent experiments. Bar, standard error. * P < 0.05
Since CD4 T cells from IFN-γ receptor-deficient mice have no obvious defects in antigen-specific IFN-γ-production and Th1-differentiation (30), the absence of IFN-γ alone is not likely to account for the shift from a Th1 to a Th17 response in P40-/-IFN-IR-/- mice. To test the possibility that a combined deficiency in both IFN-γ and IFN-IR accounts for this shift, we examined CD4 T cell differentiation in IFN-IR-/- and control B6 mice treated with anti-IFN-γ or isotype control antibody one day prior to infection with Lm-OVA ΔactA. Treatment of IFN-IR-/- mice but not B6 mice with anti-IFN-γ led to the generation of IL-17-producing cells, whereas few to no such cells were detected in IFN-IR-/- treated with the isotype control antibody (Fig. 2B). Taken together, these results demonstrate that Th17 CD4 T cell differentiation during Lm infection requires the abrogation of both IFN-γ and IFN-IR.
Tgf-β and IL-6 are required for Th17 differentiation in P40-/-IFN-IR-/- mice
Whereas IFN-γ inhibits Th17 differentiation, both Tgf-β and IL-6 are required for Th17 differentiation in vitro (3, 31). To determine if these two cytokines are also required for Th17 differentiation in response to infection in vivo, we treated P40-/-IFN-IR-/- and control B6 mice with antibodies to Tgf-β, the IL-6 receptor, or both or with isotype control antibodies prior to infection with Lm-OVA ΔactA. In P40-/-IFN-IR-/- mice, IL-6 receptor blocking antibody alone diminished the IL-17 response by ~50% (P < 0.05 compared with isotype control), and depletion of Tgf-β led to an even more dramatic reduction (~70%, P < 0.05 compared with isotype control) while depletion of both abolished this response (Fig. 2C). These results indicate that during Lm infection Tgf-β and IL-6 synergize for optimal Th17 CD4 T cell differentiation when inhibitory signals from IL-12P40 and IFN-IR are eliminated.
Dynamics of antigen-specific Th17 compared with Th1 CD4 T cells
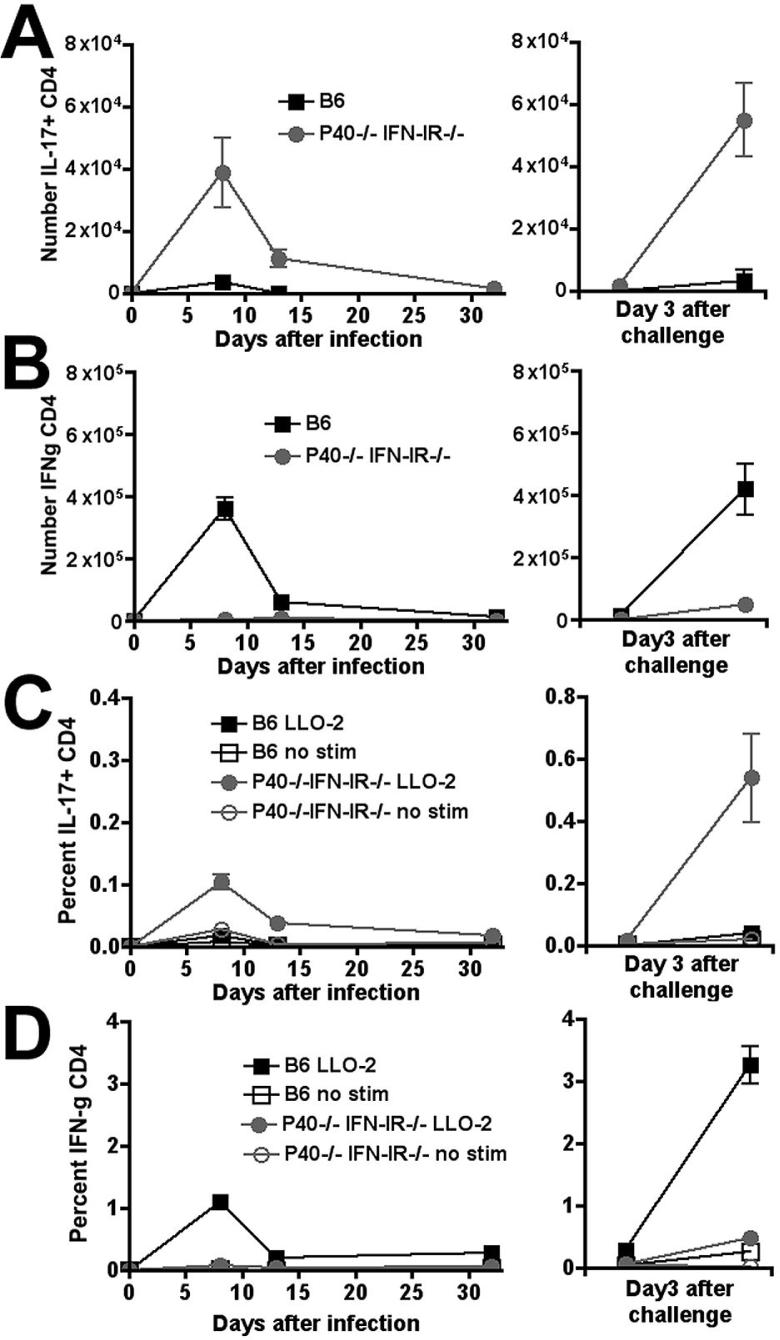
To determine how the shift from a relatively high magnitude Th1 response to a relatively low magnitude Th17 response alters the dynamics of antigen-specific CD4 T cells, we compared the percentage and numbers of CD4 T cells that produce either IL-17 or IFN-λ in response to in vitro peptide restimulation 8, 14, and 32 days after primary infection with Lm-OVA ΔactA and 3 days after secondary infection with Lm-OVA (Fig. 3). Remarkably, despite an apparent ~ 90% reduction in the overall magnitude of the total CD4 T cell response, the kinetics of expansion, contraction and re-expansion after secondary infection by IL-17-producing CD4 T cells in P40-/-IFN-IR-/- mice was virtually identical to that observed for IFN-λ-producing CD4 T cells in B6 mice. Moreover, the shift from a Th1-dominated response in B6 mice to a Th17-dominated response in P40-/-IFN-IR-/- mice observed at day 8 was maintained at all time points examined after primary infection and after secondary antigen challenge.
Figure 3.
Total number (A, B) and percentage (C, D) of IFN-γ- (B, D) producing CD4 T cells per spleen after stimulation with the MHC class II peptide LLO189-201 on days 8, 14, or 32 after primary infection with 106 Lm-OVA ΔactA (left sided panels), or on day 3 after challenge with 105 Lm-OVA in mice inoculated with Lm-OVA ΔactA 32 days prior to challenge (right sided panels). These represent 8-12 mice per time point combined from three independent experiments. Bar, standard error.
Antigen-specific CD8 T cells primed in the absence of IL-12p40 and IFN-IR
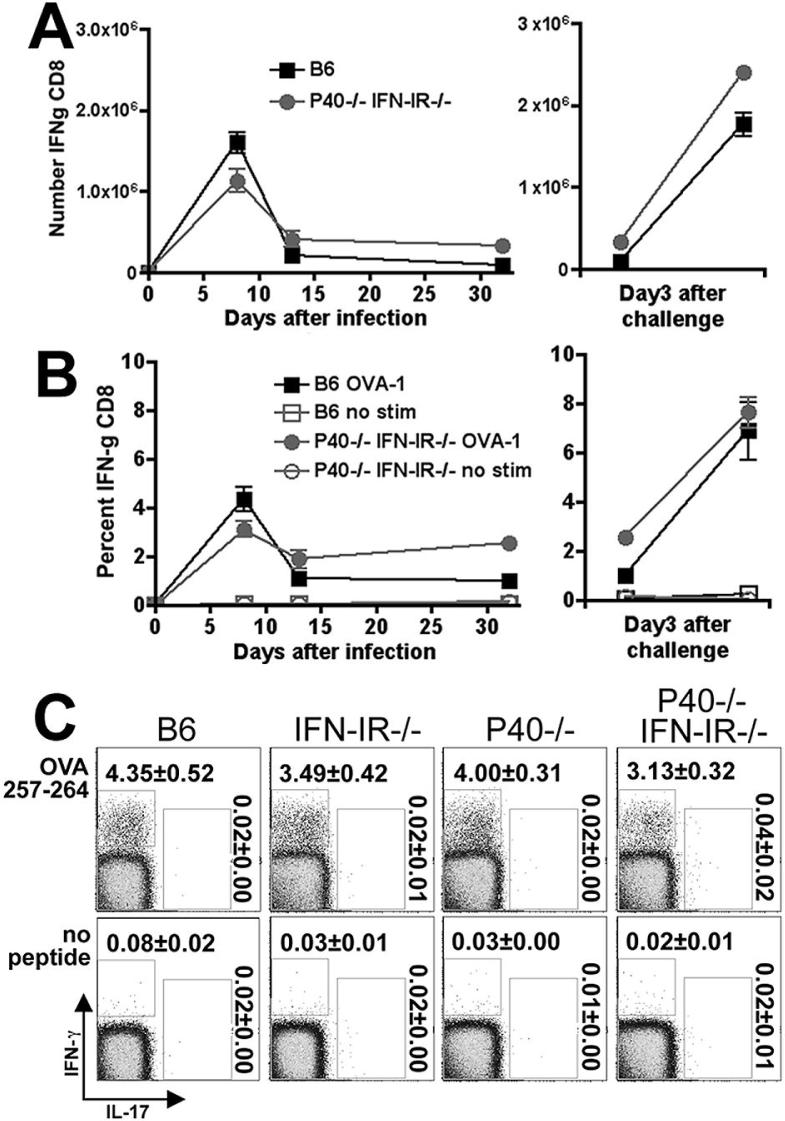
Our previous studies indicate that IL-12P40 and IFN-IR together are not required for priming antigen-specific CD8 T cells since mice with combined defects in both IL-12P40 and IFN-IR compared with control mice have similar numbers of antigen-specific CD8 T cells at the peak of the immune response after primary Lm infection (17). However since CD4 T cells are required to sustain CD8 T cells into the memory phenotype, we sought to examine how replacing a normally robust antigen-specific Th1 CD4 response with a relatively low magnitude Th17 response in P40-/-IFN-IR-/- mice would influence the dynamics of antigen-specific CD8 T cells. Measuring IFN-λ production after in vitro restimulation with the MHC class I OVA257-264 peptide by CD8 T cells from both P40-/-IFN-IR-/- and B6 control mice revealed that both the total number and percentage of antigen-specific CD8 T cells decreased from peak levels by day 14, and were maintained to day 32 after primary infection with Lm-OVA ΔactA (Fig. 4A,B). In response to rechallenge with Lm-OVA day 32 after primary infection, the numbers and percentage of IFN-g producing CD8 T cells readily re-expanded to the same degree in both P40-/-IFN-IR-/- and B6 mice (Fig. 4A,B). Importantly although IFN-g was readily produced by CD8 T cells from P40-/-IFN-IR-/- and control mice, these cells in response to antigen restimulation did not produce levels of IL-17 above background during any of these time points (Fig. 4C and data not shown). Therefore, the number and percentage of antigen-specific CD8 T cells in P40-/-IFN-IR-/- and B6 mice was similar, but in contrast to the CD4 T cells, CD8 T cells from P40-/-IFN-IR-/- mice produced IFN-λ but not IL-17.
Figure 4.
Total number (A) and percentage (B) of IFN-γ-producing CD8 T cells per spleen after stimulation with the MHC class I peptide OVA257-264 on days 8, 14, or 32 after primary infection with 106 Lm-OVA ΔactA (left sided panels), or on day 3 after challenge with 105 Lm-OVA in mice inoculated with Lm-OVA ΔactA 32 days prior to challenge (right sided panels). C. FACS plot indicating IL-17 and IFN-γ production in a CD8+ cell gate among splenocytes from mice day 8 after infection with 106 Lm-OVA ΔactA after in vitro stimulation with the MHC class I peptide OVA257-264 or no peptide control. These data represent 9-12 mice per time point combined from three independent experiments. Bar, standard error.
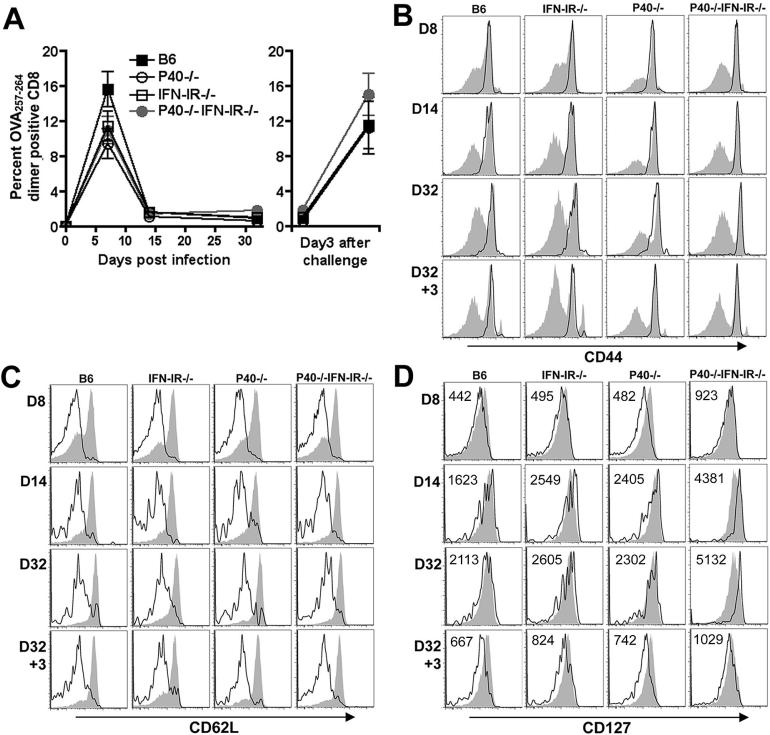
Of note, the rate of contraction after day 8 by IFN-g producing CD8 T cells was dampened in P40-/-IFN-IR-/- compared with B6 mice resulting in a modest (~50%) increase in both the numbers and percentage of antigen-specific CD8 T cells on days 14 and 32 despite an ~25% reduction in numbers and percentage on day 8. Accordingly, in additional experiments, we sought to further characterize the phenotype and activation status of the antigen-specific CD8 T cells in P40-/-IFN-IR-/- compared with control mice. For these experiments, antigen-specific CD8 T cells were identified among total splenocytes by staining with H-2Kb dimer loaded with OVA257-264 peptide. Consistent with results obtained by quantifying the CD8 T cell response after in vitro peptide restimulation, the antigen-specific CD8 T cell response quantified by OVA257-264 dimer staining was similar in P40-/-IFN-IR-/- mice compared with P40-/-, IFN-IR-/-, or B6 control mice; and was found to peak on day 8, contract by day 14 after primary infection, and readily re-expand following antigen re-challenge (Fig. 5A). Phenotypic characterization using the surface markers CD44 and L-selectin (CD62L) revealed no significant difference among antigen-specific CD8 T cells primed in P40-/-IFN-IR-/- mice compared with control mice (Fig. 5B, C). By day 8 and extending through day 32 after primary infection, and day 3 after rechallenge, the majority of antigen-specific CD8 T cells in B6, IFN-IR-/-, P40-/-, or P40-/-IFNIR-/- mice are effectors (CD44high CD62Llow) (32). Interestingly, beginning day 14 and through day 32 after primary infection, antigen-specific T cells in all groups of mice upregulated CD127 (IL-7 receptor α-chain) expression (Fig. 5D). However at each time point, the level of CD127 expression was modestly, but consistently higher for antigen-specific T cells in P40-/-IFN-IR-/- mice compared with each control group further indicating differentiation into functional long-lived memory cells (33).
Figure 5.
A. Percentage of antigen-specific CD8 T cells among splenocytes after primary infection with 106 Lm-OVA ΔactA (left sided panel), or on day 3 after challenge with 105 Lm-OVA in mice inoculated with Lm-OVA ΔactA 32 days prior to challenge (right sided panel) as determined by staining with OVA257-264 dimer. Bar, standard error. These data are from 6 mice per time point per group from two independent experiments. Expression of CD44 (B), CD62L (C), and CD127 (D) among OVA257-264 dimer positive antigen-specific (open histograms) compared with non-antigen specific (filled histograms) CD8 T cells at the indicated time points after primary infection with 106 Lm-OVA ΔactA (day 8, 14, and 32), and secondary challenge with 105 Lm-OVA (day 3). The mean fluorescent intensity for CD127 expression among dimer positive antigen-specific T cells is indicated.
Protective immunity to Lm challenge during a Th17-dominated CD4 T cell response
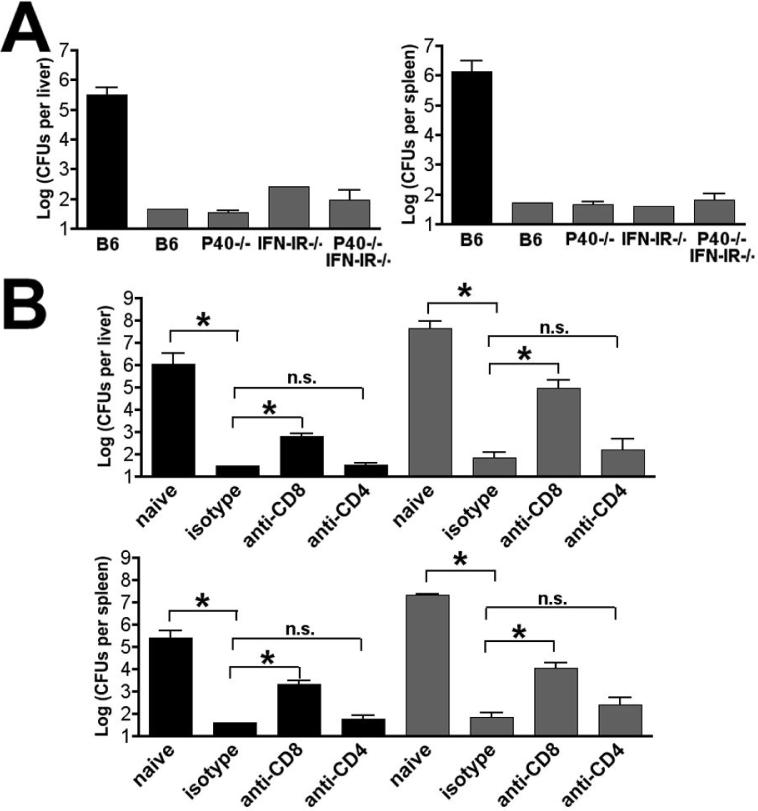
To determine if replacement of a robust Th1-dominated to a relatively low magnitude Th17-dominated CD4 T cell response compromised protective immunity to Lm infection, B6, P40-/-, IFN-IR-/-, and P40-/-IFN-IR-/- mice were primed with Lm-OVA ΔactA, challenged on day 32 with 105 CFUs (one LD50) of Lm-OVA, and CFU were enumerated 3 days later (Fig. 6A). Compared with naïve B6 mice, all groups of mice that had been primed with Lm-OVA ΔactA were highly protected and for the majority of mice in each group, CFUs were below the limits of detection in both the liver and spleen. Because CD8 T cells provide the predominant source of protective adaptive immunity to Lm infection in wildtype mice, we hypothesized that the relatively normal CD8 T cell response present in P40-/-IFN-IR-/- mice may also mediate protection. Alternatively, the CD8 T cells generated in such an environment might be less “fit” and perhaps under these circumstances Th17 CD4 T cells could play a larger role in protective immunity. To examine these possibilities, P40-/-IFN-IR-/- and B6 mice that had been previously primed with Lm-OVA ΔActA were depleted of CD4 or CD8 T cells or treated with an isotype control antibody prior to challenge with Lm-OVA. In both P40-/-IFN-IR-/- and B6 mice, depletion of CD8 T cells significantly reduced protective immunity, whereas depletion of CD4 T cells did not (Fig. 6B). These results indicate that CD8 T cells play an important role in protective adaptive immunity in P40-/-IFN-IR-/- mice, and that replacement of a Th1-dominated response present in normal mice to Th17-dominated response of relatively low magnitude does not impair the ability of CD8 T cells to confer protection to Lm infection.
Figure 6.
Protective immunity in the absence of both IL-12 and IFN-I. A. Numbers of recoverable Lm CFUs in the liver and spleen day 3 after challenge with 105 Lm-OVA in either naïve B6 mice (black bar) or the indicated mice that had been primed with 106 Lm-OVA ΔactA 32 days prior to challenge. B. Numbers of recoverable Lm CFUs in the liver and spleen day 3 after challenge with 105 Lm-OVA in either B6 (black bar) or P40-/-IFN-IR-/- (shaded bar) mice not previously infected with Lm (naïve) or infected with 106 Lm-OVA ΔactA 32 days prior to challenge and treated with anti-CD8, anti-CD4, or isotype control antibody one day prior to challenge. These data represent 8-10 mice for each experimental group combined from two independent experiments. Bar, standard error. * P < 0.05, n.s., not significant.
DISCUSSION
Since the conditions that promote Th1 differentiation of CD4 T cells (IL-12 and type I IFNs) also prime CD8 T cells to differentiate into antigen-specific effectors, immunity to many intracellular pathogens that prime a Th1-dominated CD4 T cell response can be mediated by the concerted actions of these two effector cell types or be dominated by one or the other (17, 34, 35). In Lm infection, effector CD8 T cells play the dominant role in protection (13), and here we used Lm infection to examine the requirement for a Th1-dominated CD4 T cell response to generate protective antigen-specific CD8 T cells. Our results demonstrate although the antigen-specific Th1 response in P40-/-IFN-IR-/- mice following Lm infection is virtually abolished, the protective capacity of antigen-specific CD8 T cell response remains intact. Thus, activation of CD8 T cells capable of conferring protective immunity to an intracellular pathogen requires neither IL-12, type I IFN signaling, nor help from a Th1-dominated CD4 T cell response.
The newly described Th17 lineage of CD4 T cells is believed to fill a void in the functional repertoire of adaptive T cells by providing immunity to extracellular bacterial and fungal pathogens not covered by traditional Th1 or Th2 CD4 T cells. Although some important parameters that drive Th17 differentiation of CD4 T cells after activation have been established in vitro, little is known regarding the development of these cells in response to infection in vivo. Our results demonstrate that in the absence of both IL-12p40 and IFN-IR signaling, the Th1-dominated response normally primed by Lm infection is replaced with a Th17-dominated response of lower magnitude. The generation and expansion of IL-17-producing, antigen-specific CD4 T cells required both the absence of IFN-γ (achieved either using neutralizing antibodies to IFN-γ or by the absence of IL-12p40) and IFN-IR signaling and the presence of IL-6 and Tgf-β. Thus the generation of antigen-specific Th17 cells in response to infection in vivo required the same cytokine milieu as others have described after T cell activation in vitro (3, 12, 24, 25). Our findings are also consistent with those demonstrating an in vivo role for Tgf-β in the generation of Th17 cells in the gut and in experimental autoimmune encephalomyelitis (36). We extend those findings by showing that both Tgf-β and IL-6 are required to generate antigen-specific Th17 cells after in vivo Lm infection, and demonstrate that the kinetics with which antigen-specific Th17 and Th1 CD4 T cells expand and contract after primary infection and re-expand after secondary infection are similar. Lastly, our findings that antigen-specific CD8 T cells are primed and expand after primary Lm infection, re-expand and confer protective immunity to Lm rechallenge in the absence of both IL-12p40 and IFN-IR indicate that other “signal 3” molecules for CD8 T cell are triggered during Lm infection. Of interest in this regard is IL-21. A recent study has demonstrated that IL-21 provided with microsphere beads coated with MHC + peptide (signal 1) and B7-1 for co-stimulation (signal 2) can along with IL-12 and type I-IFN provide the needed third for activation for activation of naive CD8 T cells (37). Moreover for naive CD4 T cells, IL-21 can initiate alternative IL-6 and IL-23-independent, ROR-gt-dependent pathways to induce Th17 CD4 T cell differentiation (31, 38, 39). Determining whether IL-21 or an as yet undescribed cytokine(s) can support the differentiation and maintenance of protective CD8 T cells in response to infection in vivo is an important area for future investigation.
Acknowledgments
The authors gratefully acknowledge funding support from the following sources: NICHD/NIHK08HD51584, Infectious Disease Society of America Young investigator award, Puget Sound Partners for Global Health, and March of Dimes Basil O'Conner research award.
Footnotes
Publisher's Disclaimer: Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org
REFERENCES
- 1.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 2.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 3.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 4.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 7.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 10.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 12.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 14.Harty JT, Bevan MJ. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 16.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J Immunol. 2007;178:4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 19.Tripp CS, Gately MK, Hakimi J, Ling P, Unanue ER. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- 20.Brombacher F, Dorfmuller A, Magram J, Dai WJ, Kohler G, Wunderlin A, Palmer-Lehmann K, Gately MK, Alber G. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int Immunol. 1999;11:325–332. doi: 10.1093/intimm/11.3.325. [DOI] [PubMed] [Google Scholar]
- 21.Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auerbuch V, Brockstedt DG, Meyer-Morse N, O'Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, Perry AK, Nguyen BO, Lane TF, Taniguchi T, Miller JF, Cheng G. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;200:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 26.Chang SR, Wang KJ, Lu YF, Yang LJ, Chen WJ, Lin YH, Chang HH, Wang SL. Characterization of early gamma interferon (IFN-gamma) expression during murine listeriosis: identification of NK1.1+ CD11c+ cells as the primary IFN-gamma-expressing cells. Infect Immun. 2007;75:1167–1176. doi: 10.1128/IAI.01026-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Way SS, Wilson CB. Cutting edge: immunity and IFN-gamma production during Listeria monocytogenes infection in the absence of T-bet. J Immunol. 2004;173:5918–5922. doi: 10.4049/jimmunol.173.10.5918. [DOI] [PubMed] [Google Scholar]
- 28.Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7:279–291. doi: 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman LA, Hunter CA. Regulatory pathways involved in the infection-induced production of IFN-gamma by NK cells. Microbes Infect. 2002;4:1531–1538. doi: 10.1016/s1286-4579(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 30.Haring JS, Harty JT. Aberrant contraction of antigen-specific CD4 T cells after infection in the absence of gamma interferon or its receptor. Infect Immun. 2006;74:6252–6263. doi: 10.1128/IAI.00847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou, Ivanov L, II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 32.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 33.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 34.Shen H, Slifka MK, Matloubian M, Jensen ER, Ahmed R, Miller JF. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci U S A. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 36.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Casey KA, Mescher MF. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J Immunol. 2007;178:7640–7648. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- 38.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 39.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]