Abstract
Antibodies to phosphatidylcholine (PtC), a common constituent of mammalian and bacterial cell membranes, represent a large proportion of the natural antibody repertoire in mice. Previous studies of several mouse strains (e.g., C57BL/6) have shown that anti-PtC antibodies are mainly encoded by the VH11 and VH12 immunoglobulin heavy chain variable region gene families. We show here, however, that VH11 and VH12 encode only a small proportion of the anti-PtC antibodies in BALB/c mice. Instead, VHQ52-encoded antibodies predominate in this strain. In addition, two-thirds of the cells expressing VHQ52 family genes use a single gene (which, interestingly, has been previously shown to predominate in the anti-oxazolone response). We also show here that in anti-PtC antibodies from all strains, the distinctive antigen-binding sites associated with VHQ52 differ substantially from those associated with VH11 and VH12. That is, VHQ52-containing transcripts preferentially use the joining region JH4 rather than JH1 and exhibit more diverse complementarity-determining region 3 (CDR3) junctions with more N-region nucleotide additions at the gene segment junctions. Thus, the VH gene family that predominates in the anti-PtC repertoire differs among mouse strains, whereas the distinctive VHDJH rearrangements (CDR3, JH) associated with each VH gene family are similar in all strains. We discuss these findings in the context of a recent hypothesis suggesting that CDR3 structure, independent of VH framework, is sufficient to define the specificity of an antibody.
Consistent with the major role natural antibodies play in the innate defense against invading pathogens, antibodies to phosphatidylcholine (PtC, a ubiquitous membrane phospholipid found in both bacterial and mammalian membranes) have recently been shown to protect against acute peritonitis in a murine cecal ligation and puncture model (1). B-1 cells, which produce many natural antibodies, are the sole producers of natural anti-PtC in the mouse (2, 3). Cells producing these antibodies comprise 5–15% of B-1 cells in spleen and peritoneal cavity and bind to PtC on fluorochrome-containing liposomes (3, 4). Thus, they are readily detectable by flow cytometry with a fluorescence-activated cell sorter (FACS).
Previous studies have shown that anti-PtC antibodies are largely encoded by VH11 and VH12 in combination with the Vκ9 and Vκ4 light chains, respectively (5–11). Other VH families, such as VHQ52, VHJ558, and VH7183, have been detected at very low frequencies (10, 12–14). We have revisited this issue by sorting individual PtC-binding cells from several mouse strains and by using a single-cell reverse transcriptase (RT)-PCR method (with a promiscuous primer that amplifies VH genes from all VH families) to more precisely define the Ig heavy chain variable region sequences these cells express. Our findings agree with the predominance of VH11 (5–11, 15) in C57BL/6 mice and with the predominance of VH12 in other mouse strains (we demonstrate it here in C.B-17). However, we surprisingly find that another gene family, VHQ52, predominates among anti-PtC in BALB/c mice.
We consider the mechanisms underlying these strain differences elsewhere (J.A.W., K.J.S., and L.A.H., unpublished work) and focus here, instead, on comparison of the full rearrangement sequences [joining region (JH) and diversity region (D) usage, N-region addition, etc.] of the large number of anti-PtC antibodies isolated in the course of these genetic studies.
Examination of these anti-PtC sequences demonstrates that each of the three variable region gene families has distinctive antigen-binding site characteristics. We show that anti-PtC antibodies encoded by the VHQ52 gene family are more diverse with respect to N-region addition and other complementarity-determining region 3 (CDR3) features than those encoded by VH11 and VH12 gene families. Furthermore, we show that VHQ52 is mainly associated with JH4, whereas VH11 and VH12 are mainly associated with JH1. This close association between VH gene family and CDR3 structure challenges the recently proposed idea that CDR3 structure per se is sufficient to define antibody specificity (16). Thus, we discuss our findings in the context of this model as it applies to anti-PtC and other natural antibodies.
MATERIALS AND METHODS
Mice.
C57BL/6J (Ighb), BALB/cnHz (Igha), and C.B-17/Hz (BALB/c.Ighb) females, 5–6 months of age, were bred and housed at Stanford or were a kind gift from R. Riblet (Torrey Pines Institute for Molecular Studies, San Diego, CA).
FACS Reagents and Staining.
Reagents, cell preparation, staining, and FACS sorting methods were described previously (17). For single-cell sorting, cells were stained with fluorescein-encapsulated liposomes (PtC-liposomes) (3, 4, 15), phycoerythrin-conjugated anti-IgM (331.12), and allophycocyanin-conjugated anti-CD5 (Ly-1, 53–7). Dead cells were excluded by propidium iodide.
For FACS measurement of VH-family frequencies, cells were stained with PtC-liposomes, anti-VH11 (VH11Id.6e9) (18) (kind gift from G. Haughton, Univ. of North Carolina), anti-VH12 (CH27Id.5c5) (also from G. Haughton), anti-IgD (11-26), propidium iodide, anti-IgMa (DS-1), anti-IgMb (AF6-78), and antibodies specific for T cells, macrophages, and granulocytes conjugated to the same fluorochrome (Dump) (PharMingen). Samples from the latter studies were analyzed on a flow cytometer modified at Stanford to simultaneously detect eight fluorochromes (19).
Single-Cell Sorting and Sequence Analyses.
PtC-binding cells were first bulk-sorted either from individual C57BL/6 and C.B-17 mice or from a pool of either 5 or 9 BALB/c mice and then resorted to deposit individual cells into tubes for single-cell sequence analysis, and FACS phenotype information was collected for individually sorted cells. These methods, and methods used for RT-PCR amplification and sequencing of VH, and the sequence analysis software tools, have all been described (15, 20, 21). Sequences were aligned to a germ-line database that we have assembled (A.B.K., J.D.M., K.J.S., and L.A.H., unpublished work).
Detection of Antibody Production by PtC-Binding Cells.
Peritoneal cells pooled from 13 three-month-old BALB/c female mice were stained with PtC-liposomes, anti-CD5, and anti-IgM (see above) and sorted on the basis of level of staining with PtC-liposomes. PtC-bright, -intermediate and -negative sorted populations and mock-sorted total peritoneal cells were put into culture in RPMI medium 1640 supplemented with 10% fetal calf serum, l-glutamine (290 μg/ml), penicillin (100 units/ml), and streptomycin (70 μg/ml)) for 48 hr at a density of 106 cells per ml in the presence or absence of Salmonella typhosa lipopolysaccharide (LPS) (50 μg/ml). Sort purity was 98% for PtC-bright, 69% for PtC-intermediate, and 68% for PtC-negative cells. Cells were then resuspended at a concentration of 5 × 106 cells per ml and tested for the ability to lyse bromelain-treated mouse erythrocytes (BrMRBC) in a complement-dependent plaquing assay (PFC) as previously described (22).
RESULTS
Anti-PtC Antibodies in BALB/c, C57BL/6, and C.B-17 Use the Same VH Families, Although at Markedly Different Frequencies.
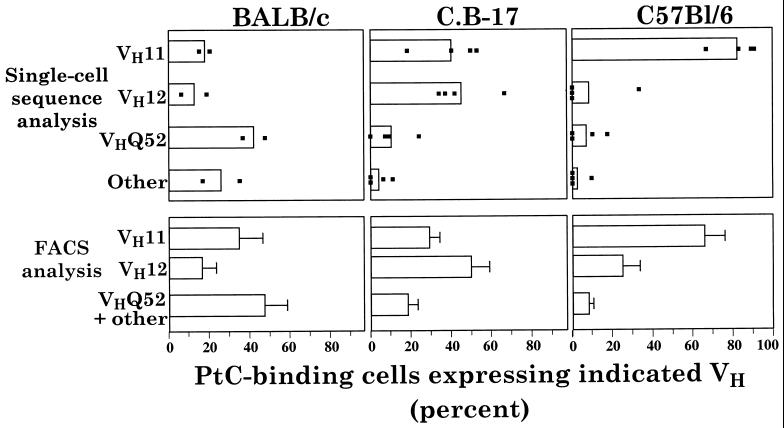
Consistent with previous findings, our single-cell RT-PCR and sequencing studies show that anti-PtC antibodies in C57BL/6 mice are predominantly VH11-encoded (15). Surprisingly, however, anti-PtCs from BALB/c mice are predominantly encoded by the VHQ52 family (Fig. 1 Upper; Table 1). C.B-17 mice, which are congenic to BALB/c but carry an IgHb chromosome region similar to C57BL/6, show a third anti-PtC pattern: VH12 predominates; VH11 is common; and VHQ52 is rare. VHJ558 family genes are used equally by all strains, at frequencies below the other three gene families. Despite these differences, representatives of each VH family are found among the anti-PtC antibodies in all strains (Fig. 1, Table 1).
Figure 1.
VH gene family expression among PtC-binding B cells. (Upper) Bars represent the average VH family representation based on single-cell data from individually sorted PtC-binding cells. VH genes other than VH11, VH12, and VHQ52 are designated “Other.” Number of FACS-sorted individual (single) cells analyzed by RT-PCR and sequencing for each strain: 43 and 58, from two BALB/c pools (5 and 9 animals); 19, 17, 33 and 36, from four C.B-17 mice; and 15, 12, 10, and 10 from 4 C57BL/6 mice. Each point in the figure represents the VH family frequency found for a single mouse or pool of mice (BALB/c). (Lower) Bars with standard deviations represent the VH family representation among all PtC-binding cells determined by FACS for individual mice and averaged for the figure. Number of mice analyzed: 12 BALB/c, 8 C.B-17, and 6 C57BL/6.
Table 1.
VH family representation in PtC-binding cells
| Strain* | VH rearrangements
|
||
|---|---|---|---|
| Family | Total no. | Unique no. | |
| BALB/c | VH1 (J558) | 11 | |
| (n = 101) | VH10 (DNA4) | 2 | |
| VH8 (3609) | 1 | ||
| VH8 (3609N) | 1 | ||
| VH12 | 14 | 12 | |
| VH3 (3660) | 3 | ||
| VH3 (3660b) | 2 | ||
| VH7 (S107) | 1 | ||
| VH11 | 18 | 5 | |
| VH2 (Q52) | 44 | 43 | |
| VH5 (7183) | 4 | ||
| C.B-17 | VH1 (J558) | 1 | |
| (n = 36) | VH6 (J606) | 1 | |
| VH12 | 13 | ||
| VH11 | 17 | 15 | |
| VH2 (Q52) | 4 | 3 | |
The VH gene frequencies in anti-PtC in the three strains are mirrored by data from multiparameter FACS studies in cells that were stained with fluorochrome-coupled monoclonal antibodies to VH11 and VH12 (Fig. 1). In initial studies confirming the specificity of these antibodies, cells costained with PtC-liposomes and each of the antibodies coupled to a different fluorochrome were FACS-sorted and analyzed by RT-PCR and sequencing. Results showed that all cells stained with anti-VH11 expressed VH11 sequences (19/19); all cells stained with anti-VH12 expressed VH12 sequences (18/18); and all PtC-binding cells that failed to bind either anti-VH11 or anti-VH12 expressed VH genes from other families. Furthermore, roughly two-thirds of these latter cells, which were sorted from BALB/c mice, expressed VHQ52. FACS staining and analysis of peritoneal cells from individual BALB/c, C.B-17, and C57BL/6 mice reveal a VH gene representation pattern among PtC-binding cells that is largely similar to the pattern obtained by single-cell RT-PCR (Fig. 1 Lower). The FACS data most likely constitute the more accurate view because more cells per animal and more animals per strain were examined.
VHQ52 PtC-Binders Secrete Anti-PtC Antibodies.
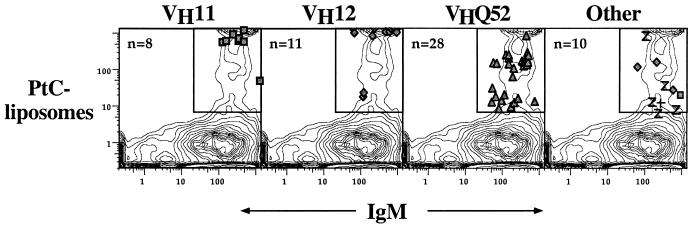
VHQ52 PtC-binding cells tend to bind fewer PtC-liposomes than cells expressing VH11 anti-PtC (ref. 15 and Fig. 2). To determine whether VHQ52 PtC-binding cells secrete anti-PtC antibodies, we FACS-sorted three fractions of PtC-binding cells (negative, intermediate, and bright PtC-binding cells) from BALB/c, stimulated the sorted cells in vitro with lipopolysaccharide, and tested for anti-PtC secretion in a standard plaquing assay (complement-dependent lysis of bromelain-treated mouse erythrocytes). Results showed that the intermediate and bright anti-PtC populations were equivalent with respect to plaque-forming cell (PFC) activity (3 PFC/100 PtC-binding cells cultured in both cases; equivalent to the overall PtC-binding population).
Figure 2.
VHQ52 expression is enriched among intermediate PtC-binders. The FACS phenotype of individual BALB/c PtC-binding cells is overlaid on the FACS plot for the B cell population from which the cells were sorted. The PtC-binding cells were sorted from a pool of 9 BALB/c mice. The three most frequently expressed VH families (Q52, VH11, and VH12), are displayed separately from other VH families (VHJ558, VH10, VH3660, VH7183). Specialized FACS hardware and software developed at Stanford were used to determine the precise phenotype of cells that were sorted and subsequently analyzed by single-cell RT-PCR and sequencing.
In addition, as expected, PFC activity in these populations was enriched at least 7-fold in comparison with the activity in the negative PtC-binding population (0.4 PFC/100 cells cultured), which may have included a few very dull PtC-binding cells. Thus, like VH11 and VH12 anti-PtCs, anti-PtCs encoded by VHQ52 are functional antibody-producing cells whose activity is readily detectable in the standard anti-PtC PFC assay.
PtC-Binding Cells Mainly Express a Single Germ-Line Gene from Each of the Predominant VH Families.
Although the VHQ52 family is the third-largest VH family (reviewed in ref. 23), a single VHQ52 germ-line gene, MMU53526 also known as VHOX-1, predominates among VHQ52 PtC-binding cells. This gene, which represents 68% of VHQ52 anti-PtC in BALB/c, has previously been shown to encode the dominant idiotype produced by BALB/c (Igha) in response to phenyloxazolone (30) and phthalate (24). Consistent with evidence that VHOx-1 does not predominate in this response in similarly immunized Ighb mice, a recent study failed to detect the germ-line gene encoding the VHOX-1 sequence in C57BL/6 mice (24). However, this finding represents either a technical failure or a genetic difference among Ighb mice, since VHOX-1 is present in VHQ52 anti-PtC antibodies from C.B-17 and C57BL/6, which both carry IgHb chromosomes. In contrast to the tendency to use a single germ-line gene in each of the predominant VH gene families in anti-PtC, seven different VHJ558 genes are used in anti-PtC antibodies.
Within the VH11 family, which is estimated to have only 1 or 2 germ-line genes, PtC-binding cells from C57BL/6 and BALB/c mice express the same VH11 germ-line gene (MMIGVHAB) (15). VH11 PtC-binding cells from C.B-17 mice also use only a single VH11 germ-line gene; however, it differs slightly in sequence from that used in C57BL/6 and BALB/c mice—i.e., by conserved nucleotide changes in codons 29 [framework region 1 (FR1)] and 84 (FR3). Thus, at the amino acid level, C.B-17, BALB/c, and C57BL/6 mice all express the same VH11 product.
A single germ-line gene (MUSIGHAAM) is also expressed in VH12 anti-PtC and, like VH11, the germ-line gene expressed in C57BL/6 and BALB/c mice differs slightly from that expressed in C.B-17. However, while the nucleotide changes in codons 20 (FR1), 62 (CDR2), and 73, 74, 79, and 80 (FR3) of this VH12 germ-line gene result in conserved amino acids, a change in codon 21 (FR1) results in a change from alanine (nonpolar hydrophobic) to threonine (polar but uncharged). Therefore, C.B-17 mice express a VH12 anti-PtC antibody highly homologous to but nevertheless distinct from that expressed by BALB/c and C57BL/6.
JH Segment Usage and CDR3 Characteristics Differ According to the Associated VH.
Although there are strain-specific differences in the VH expression frequencies among PtC-binding cells, characteristic JH and CDR3 regions are associated with each of the predominant VH gene families. The characteristics of these rearrangements are documented in the sections that follow, in which we analyze sequences obtained for each VH gene family independent of strain of origin. Repeated rearrangements occur frequently among PtC-binding cells (15); however, these are counted only once in the following analyses, which are based on data from 48 VHQ52, 27 VH11, 28 VH12, and 12 VHJ558 anti-PtC sequences.
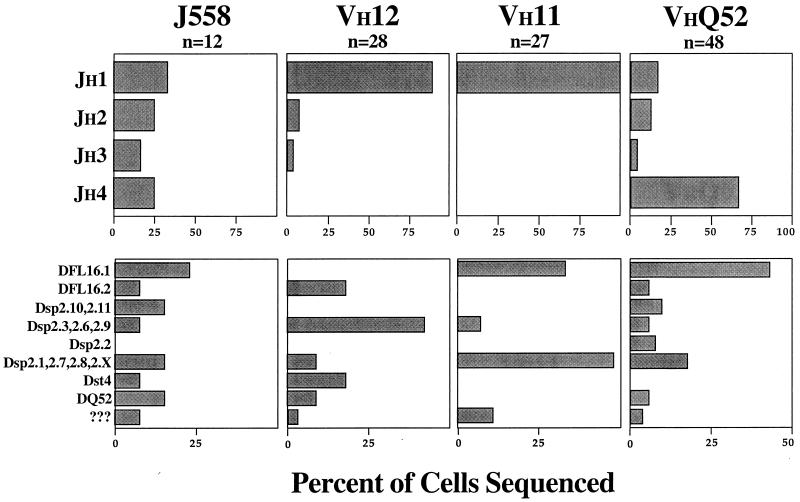
JH Usage.
VH11 and VH12 preferentially use JH1 (89% and 100%, respectively), whereas VHQ52 preferentially uses JH4 (68%). In contrast, VHJ558 uses all four JH segments in roughly equivalent amounts (17–33%) (Fig. 3). D segment usage also differs among the families, with VH11 and VHQ52 being more similar than VH12 (Fig. 3).
Figure 3.
JH and D gene usage differ for the four most frequently expressed VH families among anti-PtC antibodies. Analyses shown are based on unique sequences combined from all three strains of mice (BALB/c, C57BL/6, C.B-17). Data are expressed as percentage of cells sequenced within each VH family. Some D segments are at times indistinguishable from each other and are therefore grouped; several could not be identified and are grouped as “???”. Reading frame usage is not significantly different among the VH families (not shown). There is no significant difference between VH12 and VH11 for JH usage (Kruskal–Wallis, P = 0.09). JH usage of VH11 and VH12 significantly differs from VHJ558 and VHQ52 (Kruskal–Wallis, P < 0.0002 for all combinations). The usage of VHQ52 and VHJ558 significantly differ from one another (P = 0.02).
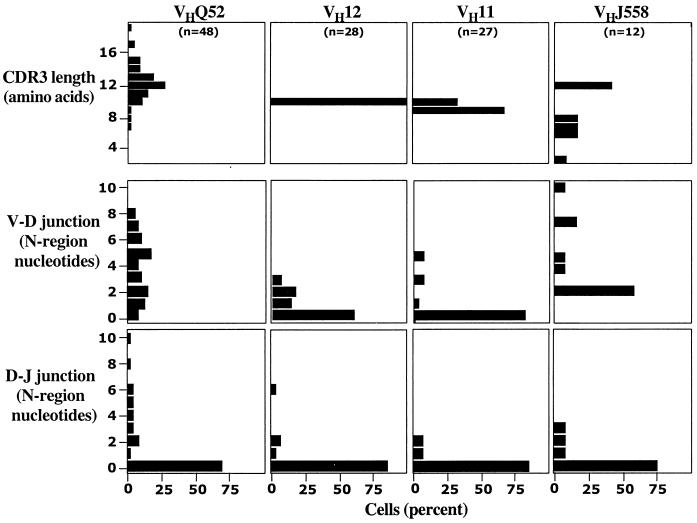
CDR3 Diversity.
Most of the anti-PtC antibodies that have N-region additions at one or both junctions are encoded by VHQ52 genes, whereas VH11 and VH12 often have no insertions at all (Table 2; Fig. 4). Consistent with this observation, VHQ52 CDR3 length is extremely heterogeneous, whereas VH11 and VH12 have homogeneous CDR3 lengths (Fig. 4).
Table 2.
N-region addition in PtC-binding cells
| VH in BALB/c, C.B-17, and C57BL/6 PtC-binding cells
|
N-region addition at the V–D/D–J junction, % of sequences
|
||||
|---|---|---|---|---|---|
| Family | no. | 0/0 | 0/>1 | >1/0 | >1/>1 |
| VH11 | 27 | 67 | 15 | 19 | 0 |
| VH12 | 28 | 54 | 7 | 32 | 7 |
| VHJ558 | 12 | 50 | 8 | 25 | 17 |
| VHQ52 | 48 | 8 | 0 | 60 | 31 |
| Total PtC-binders | 115 | 37 | 6 | 40 | 17 |
Figure 4.
CDR3 length and N-region addition differ among the four predominant VH families expressed in the anti-PtC repertoire. Frequency distributions for N-region nucleotides at the V–D and D–J junctions, and CDR3 length (number of amino acids) are shown. Differences in CDR3 length among the VH families are all significant (Kruskal–Wallis, P < 0.001). The only significant differences (P < 0.01) in number of N-region insertions were between VHQ52 and both VH11 and VH12 at the V–D junction (P < 0.0001).
CDR3 Homology.
The conserved CDR3 amino acid sequence (codons 95–102) in VH11 and VH12 anti-PtC differ as previously reported (18, 25). VHQ52 sequences differ from those in either VH11 or VH12 and are conserved only from positions 100H to 102 [Y100H Y100I (A/Y)100J M100K D101 (Y/V)102], which are derived from the JH4 gene segment. VHJ558 sequences differ from those in VHQ52 but are also conserved from positions 100H to 102 [Y100H W100I Y100J F100K D101 (V/Y)102]. The positions 100H to 102 are conserved among the VHJ558, VH11, and VH12 families because of their predominant use of JH1 (Fig. 3) and may represent a PtC-binding motif. However, the amino acids conserved at these positions are not the same as those conserved among the VHQ52 (JH4) sequences.
Comparison of derived amino acid sequences of the entire length of the VHJ558, VH11, VH12 and VHQ52 germ-line genes (including the CDR1, CDR2 and FW regions) did not reveal any unexpected amino acid motifs shared among all PtC-binding antibodies.
Junctional Sequence Overlap.
Sequences lacking N-region nucleotides often exhibit sequence overlap (homology) such that either coding end participating in a joint could have contributed the observed nucleotides at the joint. Consistent with this, VH11 and VH12 commonly lack N-region insertions (Table 2) and tend to have more joints with sequence homology—e.g., of the VH11 N-less sequences, two or more nucleotides of sequence overlap occurs at 68% (15/22) of the V–D junction, and 70% (16/23) of the D–J junctions.
DISCUSSION
Recent studies have shown directly that the innate production of antibodies to PtC plays a central role in protection against acute peritonitis in a murine cecal ligation and puncture model (1). These antibodies, which also react with PtC revealed on mouse erythrocyte membranes by protease treatment, are produced by B-1 cells that constitute a major fraction (5–15%) of the B-1 population in the peritoneal cavity and the spleen (3). Two VH gene families, VH11 and VH12, have been shown to participate or predominate among anti-PtC antibody produced in the mouse strains studied thus far (C57BL/6-related mice, SM/J, NZB, BALB/c) (5, 7–12, 26). These genes are expressed in anti-PtC hybridomas and B cell tumors originating in these animals. The IgH they encode have largely been characterized from these sources.
Here, we use FACS-sorting and single-cell RT-PCR and sequencing methods to characterize the IgH expressed by B-1 cells that bind PtC. We confirm the VH11 predominance in C57BL/6 and demonstrate that VH12 predominates among anti-PtC in C.B-17 (BALB/c.Ighb). Further, we introduce a third gene family to the anti-PtC repertoire, VHQ52, and show that it predominates among anti-PtCs in BALB/c mice. However, despite this dramatic differential predominance, we find all three VH gene families represented among anti-PtCs in each strain. In addition, we find that each VH family is associated with a characteristic JH, DH, and CDR3 region regardless of whether it is the major or minor component of the anti-PtC repertoire in a particular strain.
An earlier study identified VHQ52 as well as the other VH genes as components of the anti-PtC repertoire in BALB/c mice (5) but examined the relative frequencies of these genes indirectly, by hybridoma analysis. This previous study failed to recognize the predominance of VHQ52 described here, either for methodological reasons or perhaps because the substrains of BALB/c or the maintenance conditions of the mice differ from our own. The genetic, immune, and developmental mechanisms underlying the differential VH predominance that we observe will be considered elsewhere, in the context of evidence that bears directly on these issues. We report these finding here mainly to show that, in all strains, a given VH is typically associated with a characteristic CDR3 region.
Specifically, although VH representation in anti-PtC differs among mouse strains, in all strains we find that VH11 and VH12 anti-PtCs are mainly associated with JH1 and with CDR3 regions that are homogeneous with respect to length. In contrast, VHQ52 in anti-PtC is mainly associated with JH4 and with heterogeneous CDR3s that vary substantially in length. This finding suggests that, to be successful as PtC-binding antibodies, anti-PtC encoded by VH11 and VH12 must associate with substantially different JH and CDR3 structures than anti-PtC encoded by VHQ52.
Taken at face value, these findings conflict with a recent proposal by Davis et al. (16) that VH genes contribute very little to antibody specificity. These authors point out that the antigen-binding capabilities of the diverse CDR3 structures generated in association with a single VH should be sufficient, in combination with somatic mutation and antigen selection, to generate the entire range of antibody specificities in the murine repertoire. We do not disagree in principle; however, the selective association of VH and CDR3 structures in anti-PtC that we have demonstrated suggests more complexity than this model allows.
This contradiction is readily resolved by recognizing that innate antibodies to antigens such as PtC must exist prior to antigen encounter and hence cannot necessarily rely on somatic mutation and selection as a means of defining specificity (for an antigen yet to be encountered). Thus, because B-1 cells are the primary producers of innate antibodies present in circulation (29), we suggest that the model of Davis et al. is suitable for B-2 cells, whereas a model that allows germ-line VH genes to influence antibody specificity is more appropriate for B-1 cells. Evidence that VH usage (20, 27) and repertoire-shaping B cell development mechanisms (28, 29) differ between B-1 and B-2 cells is consistent with this dual model, as is the idea that many VH genes expressed in B-1 cells have evolved to provide innate protection against invading pathogens (29).
Acknowledgments
We thank Ometa Herman for expert technical assistance, Garry Nolan for critical reading of the manuscript, Nicole Baumgarth for helpful discussions, the Stanford Protein and Nucleic Acid Facility for sequencing help, Cindy Merrill and Jan Hilson for their contributions to the early phase of this single-cell project, and David Parks and the Stanford FACS facility for help with specialized FACS instrumentation. This work was supported in part by Grants CA 42509 and AI 34762 from the National Institutes of Health. K.J.S. was supported by National Institutes of Health Grant EY 07106.
ABBREVIATIONS
- PtC
phosphatidylcholine
- FACS
fluorescence-activated cell sorter
- PFC
plaque-forming cells
- RT-PCR
reverse transcriptase–PCR
- VH
immunoglobulin heavy chain variable region
- D
diversity region
- JH
joining region
- CDR3
VH complementarity-determining region 3
Footnotes
References
- 1.Boes M, Prodeus A P, Schmidt T, Carroll M C, Chen J. J Exp Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayakawa K, Hardy R R, Honda M, Herzenberg L A, Steinberg A D, Herzenberg L A. Proc Natl Acad Sci USA. 1984;81:2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercolino T J, Arnold L W, Hawkins L A, Haughton G. J Exp Med. 1988;168:687–698. doi: 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercolino T J, Arnold L W, Haughton G. J Exp Med. 1986;163:155–165. doi: 10.1084/jem.163.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poncet P, Huetz F, Marcos M A, Andrade L. Eur J Immunol. 1990;20:1583–1589. doi: 10.1002/eji.1830200726. [DOI] [PubMed] [Google Scholar]
- 6.Carmack C E, Shinton S A, Hayakawa K, Hardy R R. J Exp Med. 1990;172:371–374. doi: 10.1084/jem.172.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reininger L, Ollier P, Poncet P, Kaushik A, Jaton J C. J Immunol. 1987;138:316–323. [PubMed] [Google Scholar]
- 8.Reininger L, Kaushik A, Izui S, Jaton J C. Eur J Immunol. 1988;18:1521–1526. doi: 10.1002/eji.1830181008. [DOI] [PubMed] [Google Scholar]
- 9.Hardy R R, Carmack C E, Shinton S A, Riblet R J, Hayakawa K. J Immunol. 1989;142:3643–3651. [PubMed] [Google Scholar]
- 10.Pennell C A, Arnold L W, Haughton G, Clarke S H. J Immunol. 1988;141:2788–2796. [PubMed] [Google Scholar]
- 11.Pennell C A, Mercolino T J, Grdina T A, Arnold L W, Haughton G, Clarke S H. Eur J Immunol. 1989;19:1289–1295. doi: 10.1002/eji.1830190721. [DOI] [PubMed] [Google Scholar]
- 12.Poncet P, Reininger L, Freitas A, Holmberg D, Dighiero G, Coutinho A. Res Immunol. 1989;140:255–264. doi: 10.1016/0923-2494(89)90058-8. [DOI] [PubMed] [Google Scholar]
- 13.Kaushik A, Mayer R, Fidanza V, Zaghouani H, Lim A, Bona C, Dighiero G. J Autoimmun. 1990;3:687–700. doi: 10.1016/s0896-8411(05)80036-8. [DOI] [PubMed] [Google Scholar]
- 14.Kaushik A, Schulze D H, Bonilla F A, Bona C, Kelsoe G. Proc Natl Acad Sci USA. 1990;87:4932–4936. doi: 10.1073/pnas.87.13.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidl K J, MacKenzie J D, Wang D, Kantor A B, Kabat E A, Herzenberg L A, Herzenberg L A. Int Immunol. 1997;9:689–702. doi: 10.1093/intimm/9.5.689. [DOI] [PubMed] [Google Scholar]
- 16.Davis M M, Lyons D S, Altman J D, McHeyzer-Williams M, Hampl J, Boniface J J, Chien Y. Ciba Found Symp. 1997;204:94–100. doi: 10.1002/9780470515280.ch7. [DOI] [PubMed] [Google Scholar]
- 17.Kantor A B, Stall A M, Adams S, Herzenberg L A, Herzenberg L A. Proc Natl Acad Sci USA. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold L W, Spencer D H, Clarke S H, Haughton G. Int Immunol. 1993;5:1365–1373. doi: 10.1093/intimm/5.11.1365. [DOI] [PubMed] [Google Scholar]
- 19.Roederer M, De Rosa S, Gerstein R, Anderson M, Bigos M, Stovel R, Nozaki T, Parks D, Herzenberg L, Herzenberg L. Cytometry. 1997;29:328–339. doi: 10.1002/(sici)1097-0320(19971201)29:4<328::aid-cyto10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 20.Kantor A B, Merrill C E, Herzenberg L A, Hillson J L. J Immunol. 1997;158:1175–1186. [PubMed] [Google Scholar]
- 21.Kantor A B, Merrill C E, Hillson J L. In: Immunochemistry and Molecular Immunology, Handbook of Experimental Immunology. Herzenberg L A, Blakwell, Herzenberg L A, Weir D M, editors. Vol. 1. Boston: Blackwell; 1998. pp. 13.1–13.6. [Google Scholar]
- 22.Cunningham A J. Nature (London) 1974;252:749–751. doi: 10.1038/252749a0. [DOI] [PubMed] [Google Scholar]
- 23.Kofler R, Geley S, Kofler H, Helmberg A. Immunol Rev. 1992;128:5–21. doi: 10.1111/j.1600-065x.1992.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 24.Winter D, Diamond M, Abu-hadid M, Falkenberg S, Bankert R. J Immunol. 1995;155:2445–2452. [PubMed] [Google Scholar]
- 25.Arnold L W, Haughton G. Ann N Y Acad Sci. 1992;651:354–359. doi: 10.1111/j.1749-6632.1992.tb24635.x. [DOI] [PubMed] [Google Scholar]
- 26.Mercolino T J, Locke A L, Afshari A, Sasser D, Travis W W, Arnold L W, Haughton G. J Exp Med. 1989;169:1869–1877. doi: 10.1084/jem.169.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tornberg U C, Holmberg D. EMBO J. 1995;14:1680–1689. doi: 10.1002/j.1460-2075.1995.tb07157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasserman R, Li Y S, Shinton S A, Carmack C E, Manser T, Wiest D L, Hayakawa K, Hardy R R. J Exp Med. 1998;187:259–264. doi: 10.1084/jem.187.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantor A B, Herzenberg L A. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 30.Kaartinen M, Solin M L, Makela O. EMBO J. 1989;8:1743–1748. doi: 10.1002/j.1460-2075.1989.tb03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]