Abstract
Animal populations act as reservoirs for emerging diseases. In order for transmission to be self-sustaining, a pathogen must have a basic reproduction number > 1. Following a founding transmission event from an animal reservoir to humans, a pathogen has not yet adapted to its new environment and is likely to have an < 1. However, subsequent evolution may rescue the pathogen from extinction in its new host. Recent applications of branching process theory investigate how the emergence of a novel pathogen is influenced by the number and rates of intermediate evolutionary steps. In addition, repeated contacts between human and reservoir populations may promote pathogen emergence. This article extends a stepping-stone model of pathogen evolution to include reservoir interactions. We demonstrate that the probability of a founding event culminating in an emerged pathogen can be significantly influenced by ongoing reservoir interactions. While infrequent reservoir interactions do not change the probability of disease emergence, moderately frequent interactions can promote emergence by facilitating adaptation to humans. Frequent reservoir interactions promote emergence even with minimal adaptation to humans. Thus, these results warn against perpetuated interaction between humans and animal reservoirs, as occurs when there are ecological or environmental changes that bring humans into more frequent contact with animal reservoirs.
Keywords: reservoirs, infectious diseases, branching processes, zoonosis, emergence
1 Introduction
Animal reservoirs play a significant role in emergence and transmission of human infectious diseases (Wolfe et al., 2007). A range of both domesticated and wild animal species act as reservoirs from which zoonotic diseases arise (Weiss, 2001). Recent zoonotic diseases include both vector borne diseases, such as West Nile fever (Estrada-Franco et al., 2003) and directly transmitted diseases such as H5N1 influenza (Horimoto and Kawaoka, 2001) and SARS (Martina et al., 2003).
The first event in the process of disease emergence is a founding zoonotic transmission event from an animal reservoir to a human host. Founding events are likely to occur when ecological and environmental changes bring us into contact with reservoirs from which we have previously been isolated. The chance that a founding zoonotic transmission event will lead to an epidemic depends on the epidemiology of the pathogen, the evolutionary process, and the ecological relationships between humans and the reservoir species. For modeling purposes, we assume that founding events coincide with significant mutations in the pathogen that allow it to survive within a human host, and that these mutations distinguish the founding strain from wildtype strains of pathogen. However, even when founding events occur, they may not cause a sustained epidemic. The introduced pathogen strain is usually poorly adapted for transmission and is unlikely to cause health problems beyond the index case. However, mutation may rescue a poorly adapted pathogen by transforming it into a new strain with elevated transmission among humans (Gomulkiewicz and Holt, 1995; Antia et al., 2003).
The basic reproduction number of a pathogen is the expected number of new cases caused by the average infection in a susceptible population (Anderson and May, 1991). When < 1, a transmission chain is not self-sustaining and will die out. If the pathogen evolves into a strain with a basic reproduction number > 1, there is a positive probability, called the probability of emergence, that the transmission chain will lead to a self-sustaining epidemic.
Branching process theory has recently been used to investigate how the emergence of a pathogen is influenced by the number and rates of the mutational events required to reach > 1 (Antia et al., 2003; Iwasa et al., 2004). Both theory and empirical observation have shown that partially-adapted strains often become extinct before they mutate to fully-adapted strains with self-sustaining transmission. However, if humans also transmit partially-adapted strains to animal species, these animal species may act as temporary refuges that facilitate evolution. We investigate whether these interactions are sufficient to influence the emergence probability.
In this paper, we analyze a reducible multitype branching process model of transmission among human and reservoir populations. Using perturbation theory, elasticity analysis, and numerical root-finding methods, we demonstrate that the probability of a founding event culminating in a newly emergent disease can be significantly influenced by ongoing reservoir interactions. Infrequent reservoir interactions may contribute negligibly to emergence. If reservoir interactions are frequent, a pathogen will behave like a vector-borne disease and may cause significant public health problems even without pathogen adaptation. When transmission from human to reservoir is frequent but transmission from reservoir to human is infrequent, reservoir interactions increase the emergence probability several fold without facilitating the persistence of partially-adapted strains. This is the situation of greatest public-health risk because there will be few herald-cases as the pathogen adapts to humans. While we focus on animal reservoir to human transmission, our results are equally applicable to emergence from one animal species to another, for example H5N1 from wildfowl to domestic poultry. Such disease emergence in new animal species may then increase the likelihood of emergence to humans.
The next section describes a reducible branching-process model of pathogen evolution in the presence of interactions with an animal reservoir. The third section undertakes a mathematical analysis of said model. The final section describes the significance and potential applications of our results.
2 Model Description
We focus on the transmission chains following a founding zoonotic transmission event, when prevalence is low and before acquired immunity becomes significant. In the initial stage of an outbreak, transmission events are approximately independent. Thus, the stochastic process of pathogen evolution and transmission can be modeled using a multitype Galton–Watson branching process (Athreya and Ney, 1972; Mode, 1971).
We distinguish among different types of transmission. The first transmission event from an animal reservoir to a human population is a “founding zoonosis”. We assume that the “founding zoonosis” coincides with mutations that allow the pathogen to infect a human host successfully. Following a founding event, the pathogen can be transmitted from one human to another directly (direct transmission). There may also be transmission from infected humans to animals in the reservoir population (homonotic transmission) and subsequent transmission from the reservoir back into the human population (zoonotic transmission). We refer to the transmission path from humans to the reservoir and back to humans as “indirect transmission”. Transmission from humans to the reservoir and from the reservoir to humans constitute “reservoir interactions”.
We model the number of direct transmission events in successive generations using a Poisson distribution with mean Ri per case for each type i (Feller, 1968). Indirect transmission is incorporated by including homonotic transmission of human-adapted strains from humans to the reservoir (ρ), direct transmission within the reservoir (κ), and zoonotic transmission from the reservoir to humans (σ) (Fig. 1). We model the number of homonotic transmission events per human case as a Poisson-distributed random variable with intensity ρ, independent of pathogen strain. We model the number of secondary infections in the reservoir per reservoir case using a Poisson distribution with intensit κ. Conditional on the mutation rate, all mutations are probably equally likely in human and reservoir infections, but human-adaptive mutations arising in the reservoir should be significantly less likely to survive the transmission bottle neck in reservoir animals than in humans. Thus, we do not include mutation in the reservoir. Finally, we model the number of zoonotic transmission events per reservoir case as a Poisson-distributed random variable with intensity σ, independent of pathogen strain. In general, transmission intensities are the product of the contact rate and the probability of transmission per contact, integrated over the duration of infection within the individual. Thus, while we treat them as independent parameters, they are not independent biologically. For instance, perturbations that shorten the duration of infection of a reservoir case will decrease both σ and κ.
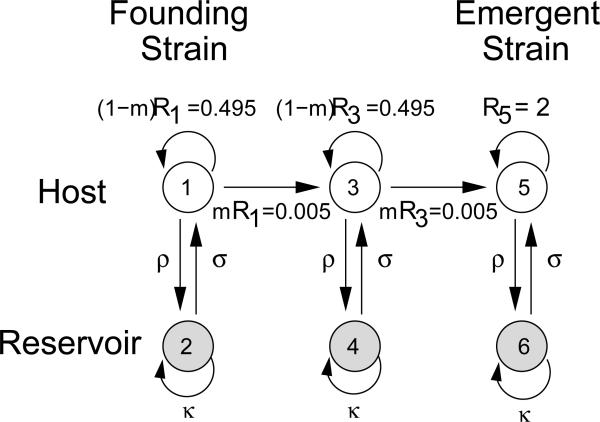
Figure 1.
Digraph of a stepping-stone model of pathogen evolution with a reservoir. Types 1, 3, and 5 represent pathogen strains in humans while types 2, 4, and 6 represent strains in an animal reservoir. Types 1 and 2 are the same pathogen strain, types 3 and 4 are the same strain, and types 5 and 6 are the same strain. Type 1 is the strain of the founding event that has evolved from the wildtype pathogen. An epidemic emerges if an infinite number of cases occur. Frequent reservoir interactions can facilitate the process by slowing extinction at each evolutionary step. Pre-emergent strains each have a transmission intensity of 0.5 in humans, while the emergent strain has a transmission intensity of 2. The process is reducible because there is no path from type 5 to type 1 (see Section 3). The model parameters are the pre-emergence direct transmission R1 = R3, the emergent direct transmission R5, the mutation probability m, homonotic transmission ρ, reservoir transmission κ, and zoonotic transmission σ.
An emerging disease that has long persisted in a reservoir will be near dynamic and evolutionary equilibrium in the reservoir. At equilibrium, the effective reproductive number of reservoir-endemic strains must be 1, and mutant strains diverging from the evolutionary equilibrium will likely have effective reproductive numbers less than 1. Thus, within the reservoir population, human-adapted strains are likely to have either neutral (κ = 1) or reduced (κ < 1) transmissibility compared with reservoir-endemic strains. In influenza, for instance, transmissibility is constrained by the structure of receptor-binding sites, and adaptation to humans is likely to decrease transmissibility in avian reservoirs. However, in pathogens with complex life histories, adaptation to humans may less significantly alter reservoir transmissibility. Both cases will be considered.
Underlying human-reservoir interactions is a landscape that defines possible mutation pathways and the transmissibility of each mutant within the functional constraints of the pathogen. For simplicity, we employ a stepping-stone landscape in which two mutation events occur in sequential order following the founding event (Antia et al., 2003). The probability that a mutation occurs during direct transmission that increases transmissibility is m. After two mutations, the transmission intensity is sufficient to allow emergence. To see how large an effect reservoirs can have on emergence, we assume that the intermediate strain has the same direct transmission intensity as the founding strain (R3 = R1). In cases where R1 < R3 < R5, adaptation will be more likely, and the need for reservoir facilitation will be reduced. The assumption that R1 = R3 indicates that the first mutation event subsequent to founding is neutral, so we might expect it to occur with equal likelihood in the reservoir. However, we expect that although overall mutation rates are comparable in humans as in reservoirs, as a human-adaptive step, conditioned on the mutation occurring, a mutation will be more likely to result in a transmission in humans than in the reservoir. Consequently, we neglect mutation within the reservoirs. We note that adding such mutation would further increase the potential for emergence.
3 Mathematical Analysis
The number of secondary infections per transmission generation is described by the probability generating function (PGF) of a Poisson process
| (1) |
where M is the matrix of one step transmission intensities, is a vector with elements between zero and one, is the vector with all elements equal to one, and exponentiation operates component-wise. For the stepping-stone model depicted in Fig. 1,
| (2) |
A property of Poisson processes is that the matrix M is also the expectation matrix of the process, i.e. , Mij is the expected number of new infections of type i produced by an infection of type j. Iteration of the PGF in (1) describes the evolution of the multi-type branching process over successive generations of transmission. Transmission generations do not generally correspond to time, because many generations of infection may overlap in time when the duration of infection is long. Since we are concerned with the total probabilities of extinction and emergence, considering generations of transmission is sufficient. However, branching processes with an explicit time representation would be needed to investigate the time to extinction or emergence.
Another important property of our stepping-stone model is that evolutionary mutations are assumed to be irreversible, making the model reducible, i.e. at least one state does not communicate to all other states. For i to communicate to j, there must be a path in the process digraph (Fig. 1) from i to j. Algebraically, type i is said to communicate with type j if there is an integer power of the expectation matrix with a positive ji entry, i.e. there exists an integer t > 0 such that (Mt)ji > 0. A process where all states communicate with each other is called irreducible. An irreducible version of our stepping-stone model can be constructed by allowing mutations in the opposite directions. However, our reducible model has the particular advantage of permitting us to study certain conditional emergence probabilities. Some of the theorems often applied to irreducible processes are not applicable to reducible processes. In particular, the basic reproduction number and emergence probabilities behave differently between reducible and irreducible processes. Although theoretical models have made extensive use of irreducible branching processes, this is not the case for reducible processes (although see Pötscher (1985) and Harris (1963)). The following two subsections discuss the calculation of the basic reproductive number and emergence probabilities for the reducible process under consideration.
3.1 Asymptotic Growth Rate and
The asymptotic growth rate λ per iteration of an irreducible discrete-time multitype branching process is the unique positive eigenvalue of the expectation matrix M (Athreya and Ney, 1972). Irreducible branching processes with a finite number of types are classified subcritical if λ < 1, critical if λ = 1, and supercritical if λ > 1. If a process is subcritical, it will go extinct. If a process is supercritical, there is a positive probability that it will emerge. Since our branching process model measures the number of new transmissions per generation, λ also represents the asymptotic expected number of new transmission events per generation. Thus, . Please note that the basic reproductive number of the process , , is different from the direct transmission rates, Ri, defined in Section 2.
Unlike that of an irreducible branching process, the expectation matrix of a reducible branching process may have more than one positive eigenvalue. The of a reducible process must be defined as the largest positive eigenvalue of the expectation matrix, but the same classification rules as for irreducible processes can be applied. In the case of Eq. (2), there are three fundamental subprocesses that would be irreducible in the absence of mutation (m = 0). The three subprocesses correspond to each particular strain and are represented by the 2 × 2 blocks along the diagonal of Eq. (2). Since m is small, the positive eigenvalue for each subprocess is a small perturbation of
| (3) |
Given that the final strain does not mutate and we assume R1 = R3 << R5, the largest eigenvalue of matrix M is equal to
| (4) |
With some algebraic manipulation, we can show that our stepping-stone model is supercritical provided that
| (5) |
a condition that is universally satisfied whenever R5 > 1 (Figure 1).
3.2 Emergence Probabilities
Let εij represent the event that a transmission chain started by a single infected individual of type i produces an infinite number of infections of type j, and let ϕij = P[εij] be the probability of that event, which we call the emergence probability of type j starting from type i. Let represent the vector of probabilities that a transmission chain started by a single infected individual of each type produces an infinite number of individuals of type j. If type i communicates to type j, ϕij ≥ 0, but if i does not communicate to j, ϕij = 0. Using standard methods (Feller, 1968), we can show that for each type j, is a solution of the system of equations
| (6) |
For irreducible processes, the probability of producing an infinite population of any type is independent of the final type. If one population type were to become infinite, all other communicating populations would also become infinite. In a reducible process, however, it is possible for some populations to be infinite while others remain finite.
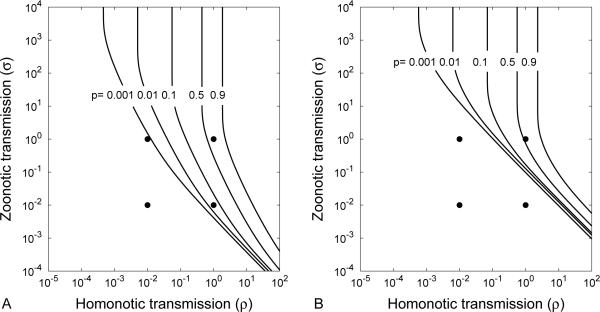
Eq. (6), together with specific knowledge of cases where ϕij = 0, is sufficient to determine all of the emergence probabilities. We are specifically interested in p = ϕ15, the probability that a founding event of type 1 leads to an infinite number of infections of type 5 (Fig. 1). Eq. (6) can be solved using fixed-point iteration methods, but for our model, convergence can require thousands of evaluations. By alternating Newton's method steps that accelerate convergence with fixed-point steps that stabilize convergence, we can efficiently solve for p, and investigate how the reservoir interaction parameters ρ and σ contribute to emergence (Fig. 2).
Figure 2.
Contour plots of the emergence probability, p = ϕ15 as a function of the homonotic transmission intensity ρ and the zoonotic transmission intensity σ, for (A) κ = 0.99, and (B) κ = 0.79. Circles mark the locations of the elasticities shown in Table 1 (See Section 3.3). In the absence of any reservoir interactions, the pathogen has a low probability of emergence (p = ϕ15 = 7.75 × 10−5). The probability for emergence increases significantly if the intensity of zoonotic transmission (σ) is sufficient for the human-adapted strain to become established. Reduced reservoir transmission intensity (ρ) significantly diminishes emergence when zoonotic transmission is slow. The parameter values are m = 0.01, R1 = R3 = 0.5, and R5 = 2.
We can use to determine conditional emergence probabilities. In particular, using Bayes’ theorem, we can show that the probability of an infinite number of cases of type 3 given that there are an infinite number of cases of type 5 is
| (7) |
The probability that the number of cases of type 5 becomes infinite while the number of cases of type 3 stays finite is
| (8) |
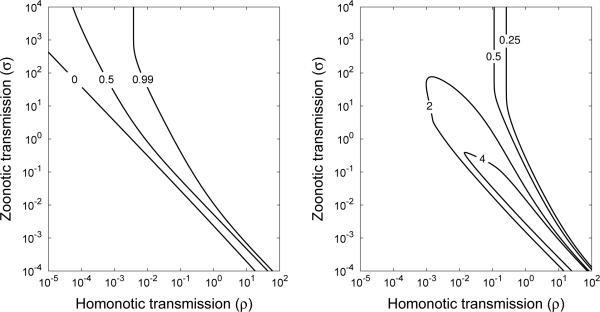
These surfaces are shown in Figure 3a and 3b, respectively.
Figure 3.
A. The emergence probability of type 3 given that type 5 emerges (Eq. (7)). B. How many times more likely type 5 is to emerge while type 3 becomes extinct, compared to the emergence probability of type 5 in the absence of reservoir effects (calculated from Eq. (9)). For large zoonotic transmission and homonotic transmission intensities, type 5 is unlikely to emerge without type 3 also emerging. For small homonotic transmission and zoonotic transmission intensities, the probability of emergence is about the same as it would be in the absence of a reservoir. Reservoir effects are most important when homonotic transmission is common but zoonotic transmission is rare. This is the parameter region of highest public health risk, since there will have been little opportunity for development of control measures based on partially-adapted strains prior to emergence. For example, if ρ = 1 and σ = 0.01, the probability of emergence of type 5 without the emergence of type 3 is more than 4 times as great as it would have been without reservoir interactions. Parameter values were κ = 0.99, and m = 0.01.
Comparing Figure 3 and Figure 2a, we discover that there are two competing routes to emergence: an evolution-only route and a reservoir-interaction route. For small homonotic transmission or zoonotic transmission intensities in Figure 3a, all partially-adapted strains become extinct. Any pathogen emergence seen in this region of Figure 2a must result from adaptation to humans. However, for the relatively large homonotic transmission and zoonotic transmission intensities where we are most likely to see an infinite number of cases of the emergent strain (Figure 2a), we are also likely to see an infinite number of cases of partially-adapted strains (Figure 3a). The region of large contour values in Figure 3b, calculated as
| (9) |
highlights the scenarios where reservoir effects increase the emergence probability of the emergent strain. Here, is the probability of emergence for the model without reservoir effects (ρ = σ = 0).
When reservoir interactions are frequent, all pathogen strains may persist indefinitely. Even the partially-adapted strains are likely to emerge. Thus, the completion of all evolutionary steps is critical to the emergence of a pathogen only in the absence of frequent reservoir interactions. However, if homonotic transmission intensity is large and inversely proportional to zoonotic transmission intensity, reservoir interactions can increase the emergence probability several fold without sustaining partially adapted strains (Figure 3b). Thus, the combination of evolution and reservoir interactions is crucial for only a narrow band of interaction frequencies.
3.3 Elasticity Analysis
Elasticity analysis was employed to determine how local changes in parameter values affect emergence probabilities. For example, the elasticity
| (10) |
measures the proportional change of the emergence probability p with respect to proportional changes in the mutation probability m. Elasticities can be calculated by numerical derivative-approximations or by the analytical methods described in Appendix A. Elasticities for all parameters are shown in Table 1 for certain parameter values.
Table 1.
Elasticities for the emergence probability p at the points shown in Fig. 2 and with respect to parameters m, ρ, σ, κ, R1, R3, and R5. The elasticity is the local percentage change in emergence probability per percent change in the parameter value. The exceptionally large elasticities to the reservoir transmission intensity in the cases where κ = .99, ρ = 0.01, σ = 1, and κ = 0.99, ρ = 1, σ = 0.01, correspond to the steep transitions in the emergence probability. Parameter values m = 0.01, R1 = R3 = 0.5, R5 = 2.
| Parameter values | p = ϕ15 | m | ρ | σ | κ | R1 | R3 | R5 |
|---|---|---|---|---|---|---|---|---|
| ρ = σ = 1, κ = 0.99 | 6.6 × 10−1 | 0.0 | 0.5 | 0.1 | 0.2 | 0.2 | 0.0 | 0.0 |
| ρ = 1, σ = 0.01, κ = 0.99 | 3.6 × 10−2 | 0.0 | 2.7 | 1.8 | 92.1 | 2.6 | 0.1 | 0.0 |
| ρ = 0.01, σ = 1, κ = 0.99 | 6.3 × 10−4 | 0.6 | 1.9 | 1.2 | 47.4 | 2.4 | 0.7 | 0.2 |
| ρ = σ = 0.01, κ = 0.99 | 8.0 × 10−5 | 2.0 | 0.0 | 0.0 | 2.9 | 2.0 | 2.0 | 0.7 |
| ρ = σ = 1, κ = 0.79 | 6.4 × 10−1 | 0.0 | 0.6 | 0.2 | 0.2 | 0.3 | 0.0 | 0.0 |
| ρ = 1, σ = 0.01, κ = 0.79 | 9.6 × 10−5 | 2.0 | 0.2 | 0.2 | 0.8 | 2.1 | 2.1 | 0.6 |
| ρ = 0.01, σ = 1, κ = 0.79 | 9.4 × 10−5 | 2.0 | 0.2 | 0.2 | 0.7 | 2.1 | 2.0 | 0.7 |
| ρ = σ = 0.01, κ = 0.79 | 7.8 × 10−5 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 0.7 |
We find that elasticities with respect to parameters m, ρ, σ, κ, R1, R3, and R5 are all positive, showing that increases in any of these parameters increases the emergence probability, as should be expected. The table indicates that the emergence probability increases with the mutation probability and transmissibility when homonotic transmission and zoonotic transmission are rare or reservoir transmission intensity is reduced. In the cases where κ = .99, ρ = 0.01, σ = 1, and κ = 0.99, ρ = 1, σ = 0.01, elasticities are exceptionally large with respect to κ ( and , respectively) because of steep transitions in the emergence probability (Fig. 2a). This indicates that the rapid rise in emergence probabilities depends critically on the persistence of the adapted strain in the reservoir populations. More moderate elasticities occur with regard to R1 provided that reservoir interactions are not extremely frequent (ρ = σ = 1). This further highlights the role that persistence of a strain plays in the ultimate emergence of a fully-adapted strain.
Some of the elasticities are correlated. The R1-elasticity is uniformly greater than the R3-elasticity and the R3-elasticity is greater than the R5-elasticity, indicating that changes in transmission at early stages are more important than at later stages. When the elasticity with respect to κ is large, the elasticities with respect to the zoonotic and homonotic transmission intensities σ and ρ also increase. This is reasonable since persistence within the reservoir only enhances the emergence probability through reservoir interactions. Also, when the R1-elasticity dominates the κ-elasticity, the m-elasticity is elevated. This relationship arises because higher direct transmission rates and increased mutation rates both increase transitions across evolutionary steps. Finally we notice that the mutational elasticity is about 2 under weak indirect transmission because of the two steps in our stepping-stone model. Generally, the mutational elasticity of emergence should be approximately equal to the number of steps in the model.
3.4 The Founding Subprocess
There are three distinct contour behaviors in Fig. 2: contours with a slope of about −1, contours with a slope of about −2, and vertical contours. This pattern can be deciphered by reconsidering the subprocesses corresponding to each strain in the absence of mutation (m = 0), and in particular that of the founding strain (Types 1 and 2 in Figure 1). The growth of the subprocess is governed by Eq. (3), so that the reservoir interactions make the subprocess critical when ρσ = (1 − R1)(1 − κ) and supercritical when
| (11) |
The linear features appearing in the contours of Figure 2 can be understood by considering the PGF of this irreducible subprocess, given by
| (12) |
where 0 < R1 < 1, 0 ≤ κ ≤ 1, ρ > 0, and σ > 0. The founding subprocess is irreducible so the emergence probabilities are dependent only on the initial state. From Eq. (6), the probability of emergence ϕ11 of a chain from a founding zoonotic transmission event of type 1 without evolution satisfies
| (13) |
With sufficiently large σ, the first term in Eq. (13) will be close to zero when treated as a function of ϕ11 and the extinction probability solves
| (14) |
independent of σ or κ. For small ρ, ϕ11 is also small and can be approximated by
| (15) |
The vertical portion of the contours in Figure 2 are explained by Eq. (15).
When the zoonotic transmission intensity σ is small, we can perform a perturbation analysis by expanding the left side of Eq. (13) near ϕ11 ≈ 0. This gives
| (16) |
Solving for the emergence probability under the condition that ρ α 1/σ, we find that to highest order,
| (17) |
Eq. (17) explains the linear portion of the contours with −1 slope in Figure 2. However, Eq. (17) is singular in the case of a neutral reservoir (κ = 1). Taking κ = 1 in Eq. (13) and using a multidimensional Taylor expansion in ρ and σ, we reveal a third limit,
| (18) |
Eq. (18) explains the intermediate region of contour linearity seen in Fig. 2. Since lowering the reservoir transmission intensity will increase the extinction probability, Eq. (18) provides an upper bound on the emergence probability for all κ < 1.
Emergence of the founding strain implies the eventual emergence of descendent strains, ϕ11 ≤ ϕ15. Thus, Eqs. (15), (17), and (18) together provide approximations for a lower bound on the emergence probability. As pointed out above, the relevance of the three approximations can be seen in the linear portions of the contours in Figure 2. If we solve for σ in Equations (17) and (18), we get equations that are functions of ρ to the −1 and −2 powers, respectively. So the portions with −1 slope in Figure 2's log-log plots are the influence of Eq. (17), and the portions with −2 slope are the influence of Eq. (18). Moreover, since Eq. (15) is independent of σ, the vertical portions of the contours are the influence of this expression. These results are summarized in Table 2. If the emergence probability in the absence of reservoir effects significantly exceeds one of these approximations, then the emergence probability is already substantial and the inclusion of reservoir effects will not dramatically increase said probability.
Table 2.
Asymptotic approximations of the emergence probability in the simplified model. The log-log contour slope is the slope of contour lines when the equation is plotted in terms of ρ vs. σ with logarithmic axes.
| ϕ11 | σ | ρ | κ | log-log contour slope |
|---|---|---|---|---|
| large | small | independent | ∞ | |
| small | ∝ 1/σ | < 1 | −1 | |
| small | small | 1 | −2 |
4 Results and Discussion
We have employed generating function methods to calculate the probability that a founding zoonotic transmission will become an epidemic disease. Increases in the contact frequency between humans and the reservoir are likely to increase both the homonotic transmission and the zoonotic transmission processes simultaneously, but the roles of the homonotic transmission intensity ρ and the zoonotic transmission intensity σ are asymmetric.
If there is insufficient homonotic transmission, indirect transmission through the reservoir will not contribute to emergence because partially-adapted strains become extinct in the human population before they can leak into the reservoir. Given sufficient homonotic transmission, we found a transition in the emergence probability as a function of the product of the homonotic transmission and zoonotic transmission intensities (Eq. (17)). If zoonotic transmission is rare or the reservoir transmissibility of human-adapted strains is low, the reservoir will contribute few secondary infections, and the emergence probability will be the same as that of a model without a reservoir. If indirect transmission is sufficiently common, interactions with the reservoir may significantly increase the emergence probability (Figure 3). Indirect transmissions can be particularly important in situations with near-neutral reservoirs that allow human-adapted strains to persist. Similarly, Kepler and Perelson (1998) found that the presence of a within-host anatomical sanctuary provides more opportunities for drug-resistance to accumulate during an HIV infection.
Emergence can occur through two routes: a predominantly evolutionary route and a predominantly reservoir-interaction route. Within our model, emergence occurs through evolution when the final strain emerges, but emergence occurs through reservoir-interactions when partially-adapted strains also emerge. When reservoir interactions are infrequent (where Eq.(11) is false), emergence can only occur through evolution (Fig. 3a). Frequent reservoir interactions promote emergence of partially-adapted strains. Only for the thin band within the 2-contour of Fig. 3b will reservoir interactions significantly increase the chance of emerging along the evolutionary route. However most of this band lies above the 0.5-contour of Fig. 3a, indicating that even within the band, a given emergence event is more likely to have followed the reservoir-interaction route than the evolution route. Allowing intermediate strains to have improved transmission (R1 < R3 < R5) will primarily contribute to emergence through evolution, as would inclusion of mutation within the reservoir for the parameter values we have chosen (results not shown). In our model, emergence through reservoir interactions always implies the eventual evolution to sustainable direct transmission. However, there are many examples of vector-borne diseases that have coexisted with man for thousands of years but can not be transmitted directly among humans. More research is needed to understand this evolutionary bifurcation, but our work suggests that the model proposed by Wolfe et al. (2007) may be incomplete.
One possible criticism of our model is that the reservoir-route to emergence requires the generation of large numbers of infections within the reservoir. If wild-type strains are endemic in the reservoir, it may seem that there isn't room for a large number of infections generated by interactions with humans. Indeed, the reservoir-route to emergence may be restricted in reservoir populations where most individuals are infected or resistant. However, the level of endemicity is usually positively correlated to the overall transmission rate. The creation of a new transmission route (indirect transmission through humans) may significantly increase the equilibrium level of endemicity. In addition, the new transmission route may provide a selective advantage to founding strains within the reservoir despite a reduced direct transmission intensity in the reservoir. Thus, density-dependent effects within the reservoir do not necessarily impose large constraints on the reservoir-route to emergence. The role of reservoir density-dependence will vary depending on the specific biology.
While we have presented our results for a stepping-stone landscape, we have obtained similar results for multi-loci landscapes with and without reversible mutation. In a multi-loci landscape, several loci can evolve independently, each contributing to the pathogen's transmissibility. One drawback of multi-loci landscapes is that the number of evolutionary states grows exponentially with the number of loci, but for small numbers of loci they provide a mechanistically motivated landscape for pathogen evolution. Pathogen evolution depends greatly on the shape of the governing landscape, but our results suggest that the effects of reservoir interactions are similar for different landscapes.
Our model can be extended in various ways. First, transmission events are generally not Poisson-distributed. Recent work has noted that tail shape can have an important influence on emergence risk (Lloyd-Smith et al., 2005). The importance of contact distribution has yet to be explored in the context of reservoir interactions. Second, when population-mixing is weak or a large proportion of the population has been previously exposed, transmission events are not independent. If transmission events are not independent, PGF methods can only provide an approximation. Related assumptions of spatially-explicit population structure and finite-population sizes also break the independence assumption. The theory of contact processes provides significant insight into emergence in spatially structured populations (Liggett, 1999). Simulation methods can often estimate emergence probabilities when transmission events are not independent.
This research has focused on the probability of emergence per founding event. Naturally, when attempting to determine the risk that disease-emergence poses to a community, we must also account for the frequency of founding events which will be positively correlated to the frequency of reservoir interactions. Over an infinite time horizon, recurrent founding events ensure emergence, and it is not immediately clear how to evaluate future risk in this context. Still, our analysis highlights the potential importance of the ecological interactions among species to the evolution of emerging diseases. Frequent interactions between human and reservoir populations can dramatically increase the chance of emergence. Although our results are not quantitatively precise predictions of disease emergence, they provide qualitative insights into the importance of frequently contacted reservoir populations, whether domestic or wild, to disease emergence. In some circumstances, the risks posed by pathogen evolution may be reduced by identifying pathogen reservoirs and making appropriate policy decisions to limit human–reservoir interactions. Surveillance of human populations in frequent contact with reservoir populations is paramount. While this analysis is applied to emerging diseases in humans, it is also applicable to problems in ecology and conservation such as the potential transmission of anthrax from ruminants to chimpanzees (Leendertz et al., 2004).
Models that incorporate ideas from both evolutionary ecology and epidemiology generate predictions that could not be made by either discipline alone (Galvani, 2003). Melding epidemiology with evolutionary ecology has widespread potential to assess both the immediate epidemiological impact and the longer-term evolutionary repercussions of control strategies for emerging diseases. Future work may include a variety of evolutionary parameter relationships with strain-dependence, periodic and stochastic environments, spatially explicit population structure, and extensions to describe vector-born pathogens.
5 Acknowledgments
Discussions with Hong Qian contributed greatly to the initiation of this work. The authors thank J. Medlock, A. Lloyd, and the participants of the 2006 PIMS “Bridging the scales of disease dynamics” workshop for constructive discussion, and two anonymous referees for helpful comments. Portions of this work were performed under the auspices of the U.S. Department of Energy under contract DE-AC52-06NA25396. TCR was supported in part by NIH grants AI28433 and RR06555 (ASP) and the Human Frontiers Science Program grant RPG0010/2004. APG was supported by a fellowship from the Institute for Advanced Studies in Berlin and the Notsew Orm Sands foundation.
A Analytic Sensitivity Calculation
Elasticities of the emergence probabilities can be calculated from analytic formulas for the sensitivities
| (19) |
Sensitivity dϕ/dm can be derived by differentiating
| (20) |
and solving the linear system
| (21) |
evaluated at
| (22) |
(Dorman et al., 2004). Here, we are employing the Magnus–Neudecker notation for multivariable calculus, so ∂s/∂s is equivalent to the identity matrix I. Eq. (21) arises as the O(Δm) expansion of Eq. (20) with m = m0 + Δm. For irreducible critical processes, is a double root of Eq. (6), making Eq. (21) singular. So Eq. (21) must be supplemented with the additional condition
| (23) |
evaluated at
| (24) |
This corresponds to the O(Δm2) term of the expansion of Eq. (20). The Fredholm alternative applied to Eq. (23) supplies the additional constraints on ds/dm to uniquely identify the sensitivities and elasticities for many critical processes. An alternative interpretation of this method is as the multivariable equivalent of L'Hôpital's rule.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford University Press; New York, NY: 1991. [Google Scholar]
- Antia R, Regoes RR, Koella JC, Bergstrom CT. The role of evolution in the emergence of infectious diseases. Nature. 2003 December;426:658–661. doi: 10.1038/nature02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athreya KB, Ney PE. Branching Processes. Springer-Verlag; New York, NY: 1972. [Google Scholar]
- Dorman KS, Sinsheimer JS, Lange K. In the garden of branching processes. SIAM Review. 2004;46(2):202–229. [Google Scholar]
- Estrada-Franco JG, Navarro-Lopez R, Beasley DW, Coffey L, Carrara A-S, da Rosa AT, Clements T, Wang E, Ludwig GV, Cortes AC, Ramirez PP, Tesh RB, Barrett AD, Weaver SC. West Nile virus in Mexico: Evidence of widespread circulation since July 2002. Emerging Infectious Diseases. 2003 December;9(12):1604–1607. doi: 10.3201/eid0912.030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller W. An introduction to probability theory and its applications. 3rd Edition Wiley; New York, NY: 1968. [Google Scholar]
- Galvani A. Epidemiology meets evolutionary ecology. Trends in Ecology and Evolution. 2003;18:132–139. [Google Scholar]
- Gomulkiewicz R, Holt RD. When does evolution by natural selection prevent extinction? Evolution. 1995;49:201–207. doi: 10.1111/j.1558-5646.1995.tb05971.x. [DOI] [PubMed] [Google Scholar]
- Harris TE. The Theory of Branching Processes. Springer–Verlag; 1963. [Google Scholar]
- Horimoto T, Kawaoka Y. Pandemic threat posed by avian influenza A viruses. Clinical Microbiology Reviews. 2001 January;14(1):129–+. doi: 10.1128/CMR.14.1.129-149.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa Y, Michor F, Nowak MA. Evolutionary dynamics of invasion and escape. Journal of Theoretical Biology. 2004;226:205–214. doi: 10.1016/j.jtbi.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Kepler TB, Perelson AS. Drug concentration facilitates the evolution of drug resistance. Proceedings of the National Academy of Sciences. 1998;95:11514–11519. doi: 10.1073/pnas.95.20.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leendertz FH, Ellerbrok H, Boesch C, Couacy-Hymann E, Mtz-Rensing K, Hakenbeck R, Bergmann C, Abaza P, Junglen S, Moebius Y, Vigilant L, Formenty P, Pauli G. Anthrax kills wild chimpanzees in a tropical rainforest. Nature. 2004 July 22;430:451–452. doi: 10.1038/nature02722. [DOI] [PubMed] [Google Scholar]
- Liggett TM. Stochastic interacting systems: contact, voter, and exclusion processes. Springer; New York: 1999. [Google Scholar]
- Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina BEE, Haagmans BL, Kuiken T, Fouchier RAM, Rimmelzwaan GF, van Amerongen G, Peiris JSM, Lim W, Osterhaus ADME. Virology: SARS virus infection of cats and ferrets. Nature. 2003 October;425:915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mode CJ. Multitype Branching Processes: Theory and Applications. American Elsevier; New York, NY: 1971. [Google Scholar]
- Pötscher BM. Moments and order statistics of extinction times in multitype branching processes and their relation to random selection models. Bulletin of Mathematical Biology. 1985;47(2):263–272. [Google Scholar]
- Weiss RA. Animal origins of human infectious disease. Philophical Transactions of the Royal Society of London. 2001;356:957–977. doi: 10.1098/rstb.2001.0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]