Abstract
Background and Aims
We determined the effects of acute and chronic calorie restriction with either a low-fat, high-carbohydrate diet or a low-carbohydrate diet on hepatic and skeletal muscle insulin sensitivity.
Methods
Twenty-two obese subjects (body-mass index, 36.5±0.8kg/m2) were randomized to a high-carbohydrate (>180g/d) or low-carbohydrate (<60g/d) energy-deficit diet. A euglycemic–hyperinsulinemic clamp, muscle biopsies, and magnetic resonance spectroscopy were used to determine insulin action, cellular insulin signaling and intrahepatic triglyceride content before, after 48 h, and after ~11 wks (7% weight loss) of diet therapy.
Results
At 48 h, intrahepatic triglyceride content decreased more in the low-carbohydrate than the high-carbohydrate diet group (29.6±4.8% vs. 8.9±1.4%; P<0.05), but was similar in both groups after 7% weight loss (low-carbohydrate diet, 38.0±4.5% vs. high-carbohydrate diet, 44.5±13.5%). Basal glucose production rate decreased more in the low-carbohydrate than the high-carbohydrate diet group at 48 h (23.4±2.2% vs. 7.2±1.4%, P<0.05) and after 7% weight loss (20.0±2.4% vs. 7.9±1.2%, P<0.05). Insulin-mediated glucose uptake did not change at 48 h, but increased similarly in both groups after 7% weight loss (48.4±14.3%, P<0.05). In both groups, insulin-stimulated phosphorylation of Jun N-terminal kinase decreased by 29±13% and phosphorylation of Akt and insulin receptor substrate -1 increased by 35±9% and 36±9%, respectively, after 7% weight loss (all p<0.05).
Conclusion
Moderate calorie restriction causes temporal changes in liver and skeletal muscle metabolism; 48 h of calorie restriction affects the liver (intrahepatic triglyceride content, hepatic insulin sensitivity, and glucose production), whereas moderate weight loss affects muscle (insulin-mediated glucose uptake and insulin signaling).
Insulin resistance is the most common metabolic complication associated with obesity, and is associated with an increased risk of developing nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes1, 2. A reduced calorie diet is a primary therapy for insulin-resistant obese persons, because even moderate diet-induced weight loss (5%–10% of body weight) decreases intrahepatic triglyceride content (IHTG) and improves hepatic and skeletal muscle insulin sensitivity3–9. However, the effect of brief calorie restriction (CR) (≤3d) is confusing because short-term therapy with a very-low calorie diet (≤800 kcal/d) improves insulin action10, 11, whereas short-term fasting induces insulin resistance12, 13.
The mechanism responsible for the apparent discrepancy between severe and complete CR on insulin action is not clear, but it is possible that differences in total carbohydrate intake could be responsible. Data from studies that used the hyperinsulinemic-euglycemic clamp technique to assess insulin action found that short-term CR with low carbohydrate intake (0–50 g/d) is associated with a decline in hepatic and skeletal muscle insulin sensitivity14, 15, whereas short-term CR with adequate carbohydrate intake (100 g/d) is associated with an increase in both hepatic and skeletal muscle insulin sensitivity4. We previously found that carbohydrate restriction, not total energy restriction, is responsible for initiating the lipolytic response to fasting; providing daily energy requirements by infusing a lipid emulsion (carbohydrate restriction) resulted in the same increase in lipolytic rate that occurred after complete fasting16. The summation of these data suggest that short-term CR with a low-carbohydrate (LC) diet could have adverse effects on insulin sensitivity because of increased FFA release into the circulation, which can cause both hepatic17, 18 and skeletal muscle19 insulin resistance.
The current recommended dietary guidelines for treating obesity is to reduce daily energy intake by 500–1000 kcal20. Although, both low-carbohydrate (LC) and high-carbohydrate (HC), low-fat diets are frequently used to lose weight, it is not known whether the acute and chronic effects of CR on IHTG content and insulin action in liver and muscle differs between diets. Therefore, the purpose of the present study was to evaluate the acute and chronic metabolic effects of a 1000 kcal/d deficit HC (≥180g/d) or LC (≤50g/d) diet in obese insulin-resistant subjects. A euglycemic-hyperinsulinemic clamp procedure, in conjunction with stable isotope tracer infusion, was performed to assess hepatic and muscle insulin sensitivity, vastas lateralis muscle samples were obtained to determine the concentration of key factors that regulate skeletal muscle insulin sensitivity, and magnetic resonance spectroscopy was used to determine IHTG content after short-term CR (48 h) and moderate (7%) weight loss. We hypothesized that, compared with an energy-deficit HC diet, consuming an energy-deficit LC diet has adverse effects on insulin action.
METHODS
Subjects
Twenty-two obese subjects (4 men and 18 women; 43.6±2.5 years old, BMI=36.5±0.8 kg/m2) participated in this study. All subjects completed a medical evaluation, which included a history and physical examination, standard blood and urine tests, an electrocardiogram, and a 2-h oral glucose-tolerance test (OGTT). All subjects were considered insulin-resistant, defined as homeostasis model assessment of insulin resistance (HOMA-IR) value >3.021. In addition, 63% of subjects had impaired glucose tolerance based on a plasma glucose concentration between 140 and 199 mg/dL at 2 h after a 75 g oral glucose load22. Subjects who had diabetes, a history of excessive alcohol consumption, liver disease, or evidence of other serious illnesses or organ dysfunction, and subjects who smoked tobacco products or took medications that are known to alter glucose metabolism were excluded from the study. All subjects were weight stable (≤2% change in body weight) and had been sedentary (<1 h of exercise per week) for at least 3 months before being enrolled in the study.
The study was approved by the Human Studies Committee of Washington University School of Medicine in St. Louis, MO. Written informed consent was obtained from each subject before their participation in this study.
Experimental Design
Body Composition Assessments
Total body fat mass (FM) and fat-free mass (FFM) were determined by using dual-energy x-ray absorptiometry (DXA, Hologic QDR 4500, Waltham, MA) 23. Total abdominal, subcutaneous abdominal, and intra-abdominal fat volumes were quantified by using magnetic resonance imaging (MRI, Siemens Vision 1.5 Tesla imager). Intrahepatic triglyceride (IHTG) content was determined by using proton magnetic resonance spectroscopy (MRS) with a 1.5T scanner (Magneton Vision Scanner; Siemens, Erlanger, Germany)24; three 2 × 2 × 2 voxels were analyzed for each subject and the values were averaged for data analyses. These body composition assessments were made at baseline (before diet intervention), after 48 h of CR with either a HC or LC diet, and after subjects lost 7% of their initial body weight and were weight stable for 4 weeks.
Euglycemic-hyperinsulinemic clamp procedure
Subjects were admitted to the inpatient unit of the General Clinical Research Center (GCRC) on two separate occasions. A euglycemic-hyperinsulinemic clamp procedure, in conjunction with stable isotopically labeled tracer infusion, was performed at baseline (before diet intervention), after 48 h of CR with either a HC or LC diet, and after subjects lost 7% of their initial body weight and were weight stable for 4 weeks. Subjects were instructed to abstain from exercise and to maintain their regular diet for at least 3 days and to abstain from caffeine and alcohol for at least 24 h before each admission. Female subjects were studied during the follicular phase of their menstrual cycle.
During the first GCRC admission subjects were admitted for 4 days. In the evening on the day of admission, subjects consumed a standard meal, containing 15 kcal/kg FFM and 55% of total energy as carbohydrates, 30% as fat, and 15% as protein at ~1800 h and then fasted (except for water) and rested in bed until completion of the clamp procedure the next day. The following morning, at 0600 h, a catheter was inserted into an antecubital vein of one arm to infuse stable isotopically labeled glucose, insulin and dextrose; another catheter was inserted in a contralateral hand vein, which was placed in a thermostatically controlled (65°C) box to obtain arterialized blood25. At 0630 h, resting energy expenditure was determined by using a metabolic measuring cart (Delta Trac; SensorMedics, Yorba Linda, CA). At ~0700 h, after a blood sample was obtained to determine the background glucose enrichment, a primed, continuous infusion of [6,6-2H2]glucose was started and maintained for 7 h. At 210 min after starting the tracer infusion, insulin was infused at a rate of 40 mU·m2 body surface area (BSA)−1·min−1 for 210 min (initiated with a two-step priming dose of 160 mU·m2 BSA−1·min−1 for 5 min followed by 80 mU·m2 BSA−1·min−1 for 5 min). Dextrose (20%), enriched with [6,6-2H2]glucose to ~2.5% to minimize changes in plasma glucose enrichment26, was infused at a variable rate to maintain euglycemia (plasma glucose concentration of 5.6 mM). The infusion rate of [6,6-2H2]glucose was decreased by 75% during the clamp procedure to account for the expected decline in hepatic glucose production. Blood samples were taken every 10 min during the last 30 min of the basal period and the clamp procedure to determine plasma glucose TTR and concentration and plasma insulin concentration during basal conditions and insulin infusion. A muscle biopsy from the vastus lateralis was taken at 240 min (i.e., 30 min after starting the insulin infusion) to assess specific cellular factors involved in insulin sensitivity. The tissue was immediately frozen in liquid nitrogen and then stored at −80°C until final analyses.
Diet intervention
After completing the first insulin clamp procedure, subjects were randomized to treatment with either a low-calorie HC diet or an LC diet. The energy content of the HC and LC diets were designed to provide a 1000 kcal daily energy deficit, based on an estimated daily energy requirement (calculated as 1.3 times measured resting energy expenditure); the average total daily energy intake was ~1100 kcal. The HC diet provided ≥180 g carbohydrates (CHO) per day and ~65% of total daily energy intake as CHO, 20% as fat, and 15% as protein; the LC diet provided ≤60 g CHO per day and ~10% of daily energy intake as CHO, 75% as fat, and 15% as protein.
Subjects remained in the GCRC until the second insulin clamp procedure and body composition assessment were completed. All food was provided by the GCRC metabolic kitchen and subjects’ food intake was monitored. On the first day of the diet intervention (i.e., the day of the first clamp procedure), the calorie and CHO contents of the diet were adjusted to account for the glucose calories infused during the clamp procedure. On the third morning in the GCRC, the insulin clamp procedure was repeated after 48 h of consuming either a low-calorie HC or low-calorie LC diet. After completing the second insulin clamp procedure, the calorie and CHO contents of the diet were again adjusted to account for the glucose calories infused during the clamp procedure. The following morning (day 4 in the GCRC), IHTG content and body composition were evaluated and subjects were then discharged from the GCRC.
All subjects received detailed dietary instructions by a registered dietician and were instructed to follow the HC and LC diet until they lost 7% of their total body weight. Subjects received weekly individual or group behavior therapy and diet education with a registered dietician and experienced behavior counselor to enhance dietary compliance. Once subjects achieved a 7% body weight loss (on average after 6±1 wks), total calorie intake was adjusted to maintain a constant body weight and prevent further weight loss. After being weight stable at their new body weight for at least 4 weeks, subjects were readmitted to the GCRC and the insulin clamp procedure and body composition analyses were repeated.
Sample Analyses
Plasma substrate and hormone concentrations
Plasma glucose concentration was determined by using an automated glucose analyzer (YSI 2300 STAT Plus, Yellow Spring Instrument Co., Yellow Springs, OH). Plasma insulin and leptin concentrations were measured by using radioimmunoassay and enzyme-linked immunosorbent assay kits were used to measure plasma adiponectin concentrations (Linco Research, St Louis, MO). The relative changes in plasma 3-hydroxybutyrate concentrations at 48 h and ~11 wks of CR compared with baseline values were determined by using a gas chromatography-mass spectrometry platform, as described previously27
Plasma glucose isotopic enrichment
Plasma glucose tracer to tracee ratio (TTR) was determined by using gas chromatography-mass spectrometry (Agilent Technologies/HP 6890 Series GC System–5973 Mass Selective Detector, Hewlett-Packard, Palo Alto, CA), after preparing the heptafluorobutyryl derivative of glucose and selectively monitoring ions at m/z 519 and 52128.
Muscle Akt/PKB, IRS-1, and JNK 1 phosphorylation were determined by using Western blotting analyses (Muscle Akt/PKB, and JNK 1 phosphorylation) and immunoprecipitation (IRS-1 phosphorylation). Muscle samples were homogenized in lysis buffer (50 mM Tris, 150 mM NaCl, and 1% NP40), containing a cocktail of protease and phosphatase (NaF and NaVO4) inhibitors29. Protein content was quantified and then 60 μg protein was electrophoresed by SDS-PAGE and transferred to nitrocellulose membranes. Blots were probed with polyclonal antibodies directed against total Akt/protein kinase B (PKB) (Amersham Biosciences, Pittsburgh, PA), Akt/PKB phosphorylated at serine 473 (Amersham Biosciences, Pittsburgh, PA), total c-Jun N-terminal kinase (JNK; EMD Biosciences, San Diego, CA), and JNK phosphorylated at threonine 183 (EMD Biosciences, San Diego, CA). To evaluate IRS-1 tyrosine phosphorylation, IRS-1 was immunoprecipitated from 500 μg of protein using a polyclonal antibody against IRS-1 (gift of Mike Mueckler) prior to SDS-PAGE and immunoblotting with an antibody directed against phosphotyrosine (Cell Signaling, Danvers, MA) or IRS-1 (gift of Mike Mueckler). The intensity of bands obtained by Western blotting analyses was quantified by digitizing the autoradiographic images and using Image Processing and Analysis in Java Program (ImageJ, National Institutes of Health, Version 1.36b). The intensity of the phosphorylated forms of the proteins were corrected for total content of that protein and normalized to the baseline value (i.e., before intervention); therefore, values are expressed as percentage change from baseline.
Calculations
Total (endogenous and exogenous) glucose rate of appearance (Ra) in plasma during basal conditions and the clamp procedure were calculated by dividing the glucose tracer infusion rate by the average plasma glucose TTR between 180 and 210 minutes (basal) and 390 and 420 min (clamp)30. Basal, endogenous glucose Ra was calculated by subtracting the glucose tracer infusion rate from total glucose Ra. It was assumed that glucose Rd was equal to total glucose Ra.
The homeostasis model assessment of insulin resistance (HOMA-IR) was determined by dividing the product of plasma glucose concentration (in mM) and plasma insulin concentration (in mU/L) by 22.521. Hepatic insulin sensitivity index was assessed as the reciprocal of the Hepatic Insulin Resistance Index, which is calculated as the product of the basal hepatic glucose production rate (in μmol·kg FFM−1·min−1) and fasting plasma insulin concentration (in mU/L) 31, 32. Skeletal muscle insulin sensitivity was determined by evaluating the ability of insulin to stimulate skeletal muscle glucose uptake, assessed as the relative increase in whole-body glucose Rd during insulin infusion compared with baseline values.
Statistical Analysis
A two-way analysis of variance with repeated measures was used to compare between and within group differences in the changes in outcome measures from baseline to 48 hours and from baseline to 7% weight loss. Tukey’s post-hoc procedure was used to locate differences, if a significant main effect was found. The relationship between the percent change in intra-abdominal fat volume and the percent change in IHTG content and HISI were assessed by using linear regression analysis. A P-value of ≤0.05 was considered statistically significant. Data are expressed as means ±SEM. All data were analyzed using SAS (8.2, Cary, NC).
RESULTS
Study subject characteristics
Baseline metabolic variables and body composition measurements were not different between subjects randomized to the HC and LC diet groups (Table 1). Fifty percent of subjects in the HC diet group and 58% of subjects in the LC diet group had nonalcoholic fatty liver disease, defined as IHTG content >5.6% 33.
Table 1.
Baseline body composition and metabolic characteristics of the study subjects
| High-carbohydrate diet group (n=11) | Low-carbohydrate diet group (n=11) | All subjects (n=22) | |
|---|---|---|---|
| Age (yrs) | 45.4 ±4.0 | 41.8 ± 3.1 | 43.6 ± 2.5 |
| Body weight (kg) | 101.0 ± 4.1 | 101.9 ± 4.0 | 101.5 ± 2.8 |
| BMI (kg/m2) | 36.9 ± 1.2 | 36.1 ± 1.0 | 36.5 ± 0.8 |
| Fat-free mass (kg) | 57.2 ± 3.1 | 57.9 ± 3.2 | 57.6 ± 2.2 |
| Fat mass (kg) | 41.7 ± 2.4 | 42.1 ± 1.7 | 41.9 ± 1.4 |
| Fat mass (% body weight) | 42.3 ± 1.9 | 42.3 ± 1.4 | 42.3 ± 1.1 |
| Total abdominal fat volume (cm3) | 5625 ± 233 | 5753 ± 321 | 5686 ± 191 |
| Subcutaneous abdominal fat volume cm3) | 4010 ± 243 | 4208 ± 385 | 4105 ± 219 |
| Intra-abdominal fat volume (cm3) | 1556 ± 234 | 1544 ± 221 | 1550 ± 158 |
| Intrahepatic triglyceride content (%) | 11.2 ± 2.9 | 12.4 ± 2.9 | 11.8 ± 2.0 |
| Plasma glucose (mg/dL) | 96.8 ± 2.7 | 101.5 ± 4.5 | 99.1 ± 2.6 |
| Plasma insulin (μU/mL) | 15.5 ± 2.8 | 18.7 ± 2.4 | 17.1 ± 1.8 |
| Plasma triglyceride (mg/dL) | 138.9 ± 17 | 147.7 ± 21.0 | 143.5 ± 13 |
| HDL-cholesterol (mg/dL) | 45.2 ± 2.7 | 44.1 ± 3.7 | 44.6 ± 2.2 |
| LDL-cholesterol (mg/dL) | 93.3 ± 5.5 | 96.7 ± 7.3 | 95.0 ± 4.5 |
Values are means ± SEM
Dietary compliance
Changes in plasma 3-hydroxybutyrate concentrations during CR suggest that study subjects in both the HC and LC diet groups were compliant with their dietary assignment. In subjects randomized to CR with an HC diet, plasma β-hydroxybutyrate increased ~2-fold at 48 h of CR (P=0.02) and returned to baseline values at 11 wks of CR. In subjects randomized to CR with a LC diet, plasma 3-hydroxybutyrate increased ~10-fold at 48 h of CR (P<0.0001) and remained 10-fold greater than baseline at 11 wks of CR (P=0.002).
Body weight and body composition
Short-term CR caused a similar decrease in body weight at 48 h with either diet (Table 2) (mean weight loss for both groups combined=2.0±0.2%, P<0.0001). Long-term weight loss after completing the diet intervention was also similar in both groups (Table 2) (mean weight loss for both groups combined at ~11 wks of dieting=7.5% ± 0.4% P<0.0001). The time to achieve 7% weight loss was not different between the AC diet group (6.2±1.0 wks) and the LC diet group (5.9±1.0 weeks).
Table 2.
Percent change from baseline in body weight and metabolic variables after 48 h and 11 wks (7% weight loss) of calorie restriction (CR) in subjects consuming a high carbohydrate (HC) or low-carbohydrate (LC) diet.
| Percent change after 48 h CR | Percent change after ~11 weeks CR | |||
|---|---|---|---|---|
| HC | LC | HC | LC | |
| Body weight | −1.6± 0.2** | −2.2 ± −0.2** | −7.3 ± 0.6** | −7.6 ± 0.5** |
| Plasma glucose | −2.6 ± 2.3 | −9.8 ± 2.4*, # | −6.2 ± 1.6* | −8.9 ± 3.0* |
| Plasma insulin | −22.0±5.1* | −33.9 ± 6.4** | −22.0 ± 5.7* | −38.4 ± 5.2**,# |
| C-Peptide | −14.4 ± 3.5* | −26.3 ± 4.5** | −12.0 ± 3.1* | −25.3 ± 3.6** |
| Free fatty acids | 13.9 ± 6.2* | 32.1 ± 8.0* | −1.5 ± 9.9 | −1.5 ± 7.5 |
| HOMA-IR | −23.8 ± 5.9* | −40.3 ± 6.1**,# | −27.1 ± 5.1** | −44.0 ± 4.7**,# |
Values are means ± SEM.
Value significantly different from baseline value:
p<0.05,
P<0.001.
Value significantly different from value in HC group,
P<0.05.
HOMA-IR: Homeostasis model assessment of insulin resistance
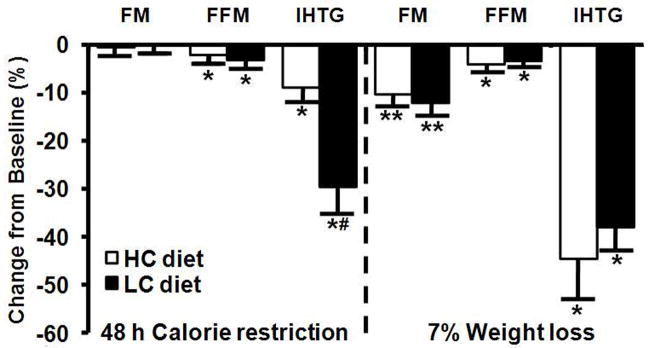
Changes in body FM and FFM at ~11 wks of dieting and 7% weight loss were not different between the HC and LC groups (the average decreases in FM, FFM, and intra-abdominal fat volume in all subjects were 11.3 ± 0.9%, 3.8 ± 0.6%, and 12.0 ± 2.8%, respectively; all P<0.001) (Figure 1). Calorie restriction with either the HC or LC diet caused a progressive decrease in IHTG content. The relative decrease in IHTG was ~3 times greater in the LC group than in the HC group at 48 h of CR, but was not different between groups after ~11 wks of CR (~7% weight loss) (Figure 1). There was not a significant relationship between percent change in intra-abdominal fat volume and the percent change in IHTG (R2=0.001, P>0.05).
Figure 1.
Changes in body composition and intrahepatic triglyceride (IHTG) content after 48 h (2% weight loss) and ~11 weeks (7% weight loss) of calorie restriction in obese subjects consuming either a high-carbohydrate or low-carbohydrate 1000 kcal/d deficit diet. Values are means ± SEM. Value significantly different from baseline value; *P<0.05, **P<0.001; #Value significantly different from corresponding high-carbohydrate diet group, P<0.05. FM=Fat Mass, FFM= Fat-free Mass
Plasma adipokine and hepatic enzymes concentrations
Plasma leptin concentration decreased similarly in both groups after 48 h (10.8±3.6%) decrease from baseline in combined groups, P<0.01) and ~11 wks (19.4±6.8%) decrease from baseline in combined groups, P<0.01) of CR. Plasma adiponectin concentrations decreased in both groups after 48 h (8.8±3.5% decrease from baseline in combined groups, P<0.05) and tended to increase after ~11 wks (12.1±7.2% increase from baseline in combined groups, P>0.05) of CR.
Plasma ALT and AST concentrations did not change after 48 h and ~11 wks of CR in either the HC or LC diet groups. In the combined groups, plasma ALT concentrations were 29.2 ± 2.4, 31.1 ± 3.4 and 33.4 ± 5.2 IU/L and plasma AST concentrations were 25.5 ± 2.0, 28.0 ± 2.9, and 26.2 ± 2.8 IU/L at baseline, 48 h and 11 wks of CR, respectively.
In vivo measures of insulin sensitivity and glucose homeostasis
Plasma glucose, c-peptide, and insulin concentrations
Calorie restriction caused a decline in plasma glucose, c-peptide and insulin concentrations both after 48 h and ~11 wks (~7% weight loss) of dieting in the HC and LC groups (Table 2). There was a trend toward a greater decrease in both plasma glucose, c-peptide and insulin concentrations in the LC group than the HC group after both short-term and long-term dieting. However, only the decrease in plasma glucose concentration after 48 h of CR and the decrease in plasma insulin concentration after 7% weight loss were significantly different between groups.
Homeostasis model assessment of insulin resistance
HOMA-IR improved in both groups after 48 h of CR and did not change further after ~11 wks of dieting (~7% weight loss) (Table 2). However, the decrease in HOMA-IR was greater in the LC than the AC diet group both after 48 h of CR and 7% weight loss (Table 2).
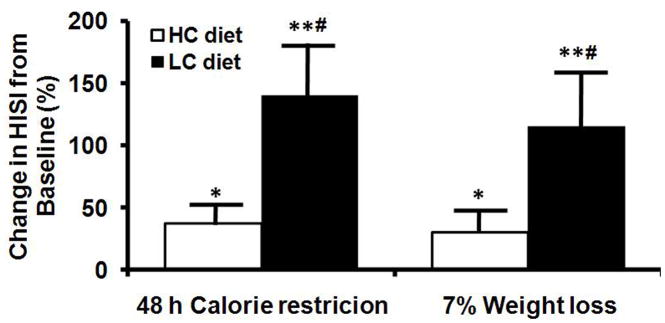
Hepatic Insulin Sensitivity Index
Hepatic insulin sensitivity increased after 48 h of CR in both the AC and LC groups, but did not improve further after 11 wks of CR (7% weight loss) (Figure 3, top panel). However, the improvement in hepatic insulin sensitivity was greater in the LC than the AC group, after both 48 h CR and 7% weight loss (Figure 3A). There was not a significant correlation between percent changes in IHTG content and HISI value (R2=0.083, P>0.05).
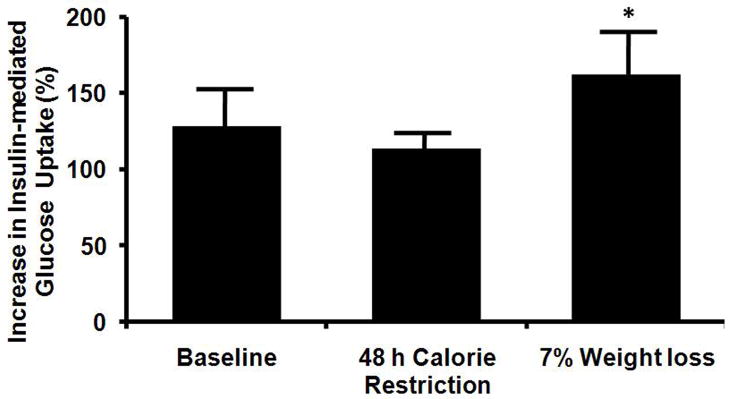
Figure 3.
Hepatic insulin sensitivity index (HISI) (top panel) in subjects consuming ether a high-carbohydrate or low-carbohydrate diet and changes in insulin mediated glucose uptake, an index of skeletal muscle insulin sensitivity, in both groups combined (bottom panel) after 48 h and ~11 wks (7% weight loss) of calorie restriction. Value significantly different from baseline value: * P<0.05, ** P<0.001. Value significantly different from value in HC group, # P<0.05.
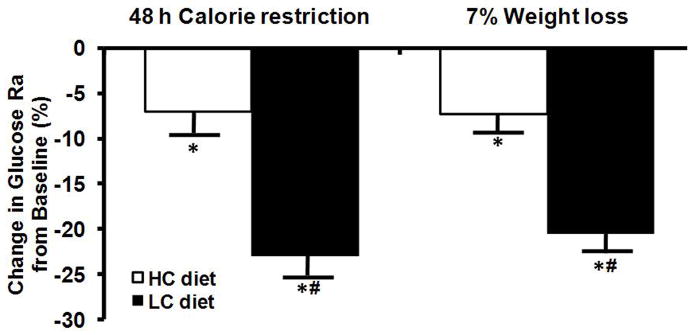
Basal glucose kinetics
Basal glucose Ra decreased after 48 h of CR in both the AC and LC groups, but was not different between groups and did not change further with more prolonged CR and 7% weight loss. Glucose Ra in the combined groups were 13.8±0.4, 12.0±0.4, and 12.2±0.3 μmol/kg FFM/min at baseline, and at 48 h and 11 wks of CR, respectively (p<0.001 for each CR value compared with baseline value). The decline in basal glucose Ra was greater in the LC than the AC group after both short-term (48 h) and long-term (~11 wks, 7% weight loss) CR (Figure 2).
Figure 2.
Relative changes in basal glucose Rate of appearance (Ra) in plasma after 48 h of calorie restriction and 7% weight loss. Values are means ± SEM. *Value significantly different from baseline value; P<0.001. # Value significantly different from value in AC group; P<0.001.
Insulin-mediated glucose uptake
Plasma insulin concentrations during the clamp procedure were not different between the HC and LC groups at any time point during the study. However, plasma insulin concentrations after 48 h (84.3± 3.4 μU/mL) and 11 wks (84.5±.2μU/mL) of CR were ~10% lower than values at baseline (95.4± 3.3μU/mL; p<0.0001). Glucose Rd values during insulin infusion was similar in both groups: 30.0 ± 2.6, 25.0 ± 1.4, and 31.1 ± 2.5 μmol/kg FFM/min at baseline, and at 48 h and 11 wks of CR for the combined groups, respectively. The relative increase in glucose Rd during insulin infusion was not greater at 48 h of CR than at baseline before CR in either diet group. However, the relative increase in glucose Rd during insulin infusion was greater after 7% weight loss than at baseline in both diet groups. Both short-term (48 h) and long-term (11 wks, 7% weight loss) CR caused similar changes in insulin-mediated increases in glucose Rd in the AC and LC groups, so the data from both groups are combined (Figure 3, bottom panel).
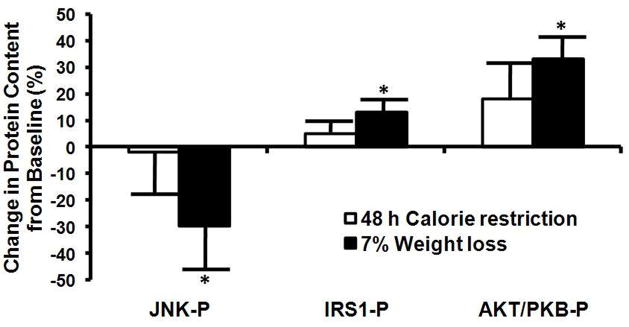
Cellular insulin signaling in skeletal muscle
At 48 h of CR, skeletal muscle phosphorylation of Tyr183 JNK, Tyr IRS1, and Ser473 Akt/PKB content assessed after insulin stimulation (30 min of insulin infusion) was not significantly different than baseline (before CR) in either diet group (Figure 4). However, at 11 weeks of CR (7% weight loss), insulin-stimulated skeletal muscle phosphoTyr IRS1, and phosphoSer473 Akt/PKB content increased, whereas phosphoTyr183 JNK content decreased, compared with baseline in both diet groups. Changes in phosphorylation status of Tyr183 JNK, Tyr IRS1, and Ser473 Akt/PKB were similar in the HC and LC groups, so the data from both groups are combined in Figure 4.
Figure 4.
Changes in phosphoTyr183 JNK, phosphoTyr IRS, and phosphoSer473 Akt/PKB protein levels in vastus lateralis muscle biopsies obtained after 30 min of insulin infusion during a euglycemic-hyperinsulinenmic clamp procedure after 48 h and ~11 wks (7% weight loss) of calorie restriction. Values are corrected for total JNK, IRS1, and Akt/PKB protein content and normalized (=0) to values from baseline samples (day 0). Values are means± SEM. Value significantly different from corresponding baseline value, *p<0.05.
DISCUSSION
An energy-deficit diet is the cornerstone of therapy for obesity. However, the most appropriate macronutrient composition of diet therapy needed to improve metabolic health remains controversial. In the present study, we carefully evaluated the longitudinal metabolic effects of short-term (48 h; 2% weight loss) and longer-term (11 wks; 7% weight loss) calorie restriction (1000 kcal/d energy deficit) with either a high- or low- carbohydrate diet in obese, insulin-resistant but non-diabetic adults. Our data demonstrate that short-term CR caused a rapid decrease in IHTG content, increase in hepatic insulin sensitivity, and decrease in endogenous glucose production rate, whereas longer-term CR and moderate 7% weight loss improved skeletal muscle insulin sensitivity, in conjunction with an increase in cellular insulin signaling. In addition, short-term CR with a low-carbohydrate diet caused a greater change in liver fat content and metabolic function than short-term CR with a high-carbohydrate diet. These data underscore the complexity of the metabolic effects of CR with diets that differ in macronutrient composition, and demonstrate temporal differences among organ systems in the adaptive response to CR itself and subsequent weight loss.
Our results refute our original hypothesis that a LC diet will cause insulin resistance because of increased adipose tissue lipolytic rates and excessive FFA release into the bloodstream. In fact, we found that LC intake rapidly caused a greater reduction in IHTG content, improvement in hepatic insulin sensitivity, and decrease in endogenous glucose production rate than consumption of an isocaloric low-fat diet. The mechanism responsible for the early beneficial effects on liver metabolism is not known, but is probably related to the greater decrease in plasma insulin concentrations in subjects consuming the low-carbohydrate diet. The decline in circulating insulin likely decreased IHTG because of enhanced lipolysis of IHTG and hepatic fatty acid oxidation14, and decreased hepatic glucose production because of hepatic glycogen depletion17 and decreased glycogenolysis4, 34. These metabolic alterations are similar to the physiologic adaptations that occur during the early response to starvation, which are also triggered by a reduction in carbohydrate intake16. However, in contrast with data obtained from studies evaluating the metabolic effects of brief fasting12–14, we did not detect a significant decline in skeletal muscle insulin sensitivity after 48 h of CR with a low-carbohydrate diet.
Weight loss, but not short-term CR, was necessary to increase skeletal muscle insulin-mediated glucose disposal. The improvement in muscle insulin sensitivity we observed in vivo is explained by enhanced cellular insulin signaling (increased insulin stimulated IRS-1 tyrosine and Akt/PKB serine phosphorylation) detected after 7% weight loss but not after 48 h of CR. These results are consistent with data from a study conducted in subjects with type 2 diabetes that found insulin-stimulated Akt/PKB did not change after 2 days of CR35. In addition, our data suggest that the mechanism responsible for the increase in insulin signaling involves down-regulation of JNK, which inhibits IRS-1 serine phosphorylation and the proximal component of the insulin signaling cascade36. Therefore, these findings demonstrate that the increase in JNK associated with obesity and type 2 diabetes is responsive to nutritional manipulation and can be normalized by weight loss.
Nonalcoholic fatty liver disease is associated with insulin resistance37, 38 and is an important risk factor for diabetes39. We previously found a linear inverse correlation between IHTG content and insulin sensitivity in both liver and skeletal muscle38. In the present study, dietary manipulation of IHTG content allowed us to dissociate the interrelationships among IHTG and insulin sensitivity in liver and skeletal muscle. After 48 h of CR, IHTG content decreased by ~20%, which was associated with a decrease in basal glucose production rate and an increase in hepatic insulin sensitivity, whereas skeletal muscle insulin sensitivity did not change. Continued CR until subjects lost 7% of initial body weight caused a further decrease in IHTG content, without a further decrease in basal glucose production or improvement in hepatic insulin sensitivity. However, 7% weight loss up-regulated skeletal muscle insulin signaling and increased muscle insulin sensitivity. These data support the notion of a causal link between steatosis and hepatic insulin resistance. The mechanism responsible for the link between IHTG content and hepatic insulin sensitivity is unknown, but could be related to an accumulation of intracellular fatty acid metabolites, which can antagonize the effects of insulin signaling on endogenous glucose production40
Our data provide new insights into the potential mechanism responsible for the marked improvement in glycemic control observed within days after Roux-en-y gastric bypass (RYGP) surgery in obese patients with type 2 diabetes41. For example, in one study, 90% of patients were able to discontinue all diabetes medications and maintain normal glycemia at discharge from the hospital 6 days after RYGP surgery, before much weight loss occurred42. These observations have led to the hypothesis that diversion of ingested nutrients from the upper gastrointestinal tract has beneficial effects on glucose homeostasis, possibly because of an altered incretin response to meals43. However, our results suggest that the rapid decrease in liver fat and improvement in hepatic insulin sensitivity that occur after brief CR can completely explain the early improvement in glucose homeostasis observed after bariatric surgery. Food intake is limited after RYGP surgery, and patients usually consume less than 250 kcal/d for several days after the operation41. Therefore, the marked postoperative reduction in calorie intake, itself, likely has profound effects on hepatic fat content and metabolism40. Moreover, the decrease in calorie intake makes it is unlikely that diversion of ingested nutrients from the upper gastrointestinal tract has an important effect on glucose metabolism.
In summary, the data from this study demonstrate that the effect of moderate calorie restriction in obese subjects with either a low-fat or low-carbohydrate diet on metabolic function is a continuum, with differential effects on specific organ systems. Brief (48 h) CR and minimal weight loss (~2% of initial body weight) primarily affects the liver, manifested by a decrease in IHTG content, an increase in hepatic insulin sensitivity, and a decrease in endogenous glucose production, whereas longer (~11 wks) CR and moderate weight loss (~7% of initial body weight) primarily affects skeletal muscle, manifested by an increase in muscle insulin-mediated glucose uptake and enhanced cellular insulin signaling. These findings help explain the rapid improvement in glucose homeostasis observed after low-calorie diet therapy and bariatric surgery.
Acknowledgments
The authors thank Joan Heins for providing dietary and behavioral therapy, Adewole Okunade and Freida Custodio for their technical assistance, the staff of our General Clinical Research Center and Intensive Research Unit for their help in performing the studies, and the study subjects for their participation.
Grant Support: This publication was made possible by Grant Number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and National Institutes of Health grants DK 37948, DK 56341 (Clinical Nutrition Research Unit), RR-00036 (General Clinical Research Center), and RR-00954 (Biomedical Mass Spectrometry Resource). No conflicts of interest exist.
Nonstandard abbreviations used
- FFA
free fatty acid
- FM
fat mass
- FFM
fat-free mass
- HISI
hepatic insulin sensitivity index
- IHTG
intrahepatic triglyceride
- Ra
rate of appearance
- Rd
rate of disappearance
- SAAT
subcutaneous abdominal adipose tissue
- TTR
tracer-to-tracee ratio
- VAT
visceral adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–17. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415. [PubMed] [Google Scholar]
- 4.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77:1287–93. doi: 10.1210/jcem.77.5.8077323. [DOI] [PubMed] [Google Scholar]
- 5.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–8. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiikkainen M, Bergholm R, Vehkavaara S, Rissanen A, Hakkinen AM, Tamminen M, Teramo K, Yki-Jarvinen H. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 2003;52:701–7. doi: 10.2337/diabetes.52.3.701. [DOI] [PubMed] [Google Scholar]
- 7.Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care. 1994;17:30–6. doi: 10.2337/diacare.17.1.30. [DOI] [PubMed] [Google Scholar]
- 8.Markovic TP, Jenkins AB, Campbell LV, Furler SM, Kraegen EW, Chisholm DJ. The determinants of glycemic responses to diet restriction and weight loss in obesity and NIDDM. Diabetes Care. 1998;21:687–94. doi: 10.2337/diacare.21.5.687. [DOI] [PubMed] [Google Scholar]
- 9.Lara-Castro C, Newcomer BR, Rowell J, Wallace P, Shaughnessy SM, Munoz AJ, Shiflett AM, Rigsby DY, Lawrence JC, Bohning DE, Buchthal S, Garvey WT. Effects of short-term very low-calorie diet on intramyocellular lipid and insulin sensitivity in nondiabetic and type 2 diabetic subjects. Metabolism. 2008;57:1–8. doi: 10.1016/j.metabol.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care. 1991;14:802–23. doi: 10.2337/diacare.14.9.802. [DOI] [PubMed] [Google Scholar]
- 11.Jazet IM, Pijl H, Frolich M, Romijn JA, Meinders AE. Two days of a very low calorie diet reduces endogenous glucose production in obese type 2 diabetic patients despite the withdrawal of blood glucose-lowering therapies including insulin. Metabolism. 2005;54:705–12. doi: 10.1016/j.metabol.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Bergman BC, Cornier MA, Horton TJ, Bessesen DH. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. Am J Physiol Endocrinol Metab. 2007;293:E1103–11. doi: 10.1152/ajpendo.00613.2006. [DOI] [PubMed] [Google Scholar]
- 13.Duska F, Andel M, Kubena A, Macdonald IA. Effects of acute starvation on insulin resistance in obese patients with and without type 2 diabetes mellitus. Clin Nutr. 2005;24:1056–64. doi: 10.1016/j.clnu.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest. 1987;79:207–13. doi: 10.1172/JCI112785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svanfeldt M, Thorell A, Brismar K, Nygren J, Ljungqvist O. Effects of 3 days of "postoperative" low caloric feeding with or without bed rest on insulin sensitivity in healthy subjects. Clin Nutr. 2003;22:31–8. doi: 10.1054/clnu.2002.0589. [DOI] [PubMed] [Google Scholar]
- 16.Klein S, Wolfe RR. Carbohydrate restriction regulates the adaptive response to fasting. Am J Physiol. 1992;262:E631–6. doi: 10.1152/ajpendo.1992.262.5.E631. [DOI] [PubMed] [Google Scholar]
- 17.Boden G, Cheung P, Stein TP, Kresge K, Mozzoli M. FFA cause hepatic insulin resistance by inhibiting insulin suppression of glycogenolysis. Am J Physiol Endocrinol Metab. 2002;283:E12–9. doi: 10.1152/ajpendo.00429.2001. [DOI] [PubMed] [Google Scholar]
- 18.Mittelman SD, Bergman RN. Inhibition of lipolysis causes suppression of endogenous glucose production independent of changes in insulin. Am J Physiol Endocrinol Metab. 2000;279:E630–7. doi: 10.1152/ajpendo.2000.279.3.E630. [DOI] [PubMed] [Google Scholar]
- 19.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–65. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institutes of Health, National Heart, Lung, and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults-The Evidence Report. Obesity Research. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 21.Mathews DR, Hosker JP, Redenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and Beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2005;28:S37–42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 23.Genton L, Hans D, Kyle UG, Pichard C. Dual-energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18:66–70. doi: 10.1016/s0899-9007(01)00700-6. [DOI] [PubMed] [Google Scholar]
- 24.Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. American Journal of Psychiatry. 1971;127:89–94. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 25.Jensen MD, Heiling VJ. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism. 1991;40:406–9. doi: 10.1016/0026-0495(91)90152-m. [DOI] [PubMed] [Google Scholar]
- 26.Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987;36:914–24. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- 27.Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L, Hanson RW, Kalhan SC, Ryals JA, Milburn MV. Analysis of the adult human plasma metabolome. Pharmacogenomics. 2008;9:383–97. doi: 10.2217/14622416.9.4.383. [DOI] [PubMed] [Google Scholar]
- 28.Patterson BW. Use of stable isotopically labeled tracers for studies of metabolic kinetics: an overview. Metabolism. 1997;46:322–9. doi: 10.1016/s0026-0495(97)90260-2. [DOI] [PubMed] [Google Scholar]
- 29.Cresci S, Wright LD, Spratt JA, Briggs FN, Kelly DP. Activation of a novel metabolic gene regulatory pathway by chronic stimulation of skeletal muscle. Am J Physiol. 1996;270:C1413–20. doi: 10.1152/ajpcell.1996.270.5.C1413. [DOI] [PubMed] [Google Scholar]
- 30.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–30. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 31.Gastaldelli A, Miyazaki Y, Pettiti M, Buzzigoli E, Mahankali S, Ferrannini E, DeFronzo RA. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. J Clin Endocrinol Metab. 2004;89:3914–21. doi: 10.1210/jc.2003-031941. [DOI] [PubMed] [Google Scholar]
- 32.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–13. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–8. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 34.Christiansen MP, Linfoot PA, Neese RA, Hellerstein MK. Effect of dietary energy restriction on glucose production and substrate utilization in type 2 diabetes. Diabetes. 2000;49:1691–9. doi: 10.2337/diabetes.49.10.1691. [DOI] [PubMed] [Google Scholar]
- 35.Jazet IM, Ouwens DM, Schaart G, Pijl H, Keizer H, Maassen JA, Meinders AE. Effect of a 2-day very low-energy diet on skeletal muscle insulin sensitivity in obese type 2 diabetic patients on insulin therapy. Metabolism. 2005;54:1669–78. doi: 10.1016/j.metabol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 37.Deivanayagam S, Mohammed BS, Vitola BE, Naguib GH, Keshen TH, Kirk EP, Klein S. Nonalcoholic fatty liver disease is associated with hepatic and skeletal muscle insulin resistance in overweight adolescents. Am J Clin Nutr. 2008;88:257–262. doi: 10.1093/ajcn/88.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, Muscle, and Adipose Tissue Insulin Action Is Directly Related to Intrahepatic Triglyceride Content in Obese Subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anna Ludovica Fracanzani LV, Bugianesi Elisabetta, Andreoletti Marco, Colli Agostino, Vanni Ester, Bertelli Cristina, Fatta Erika, Bignamini Daniela, Marchesini Giulio, Fargion Silvia. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 40.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 41.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–50. doi: 10.1097/00000658-199509000-00011. discussion 350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–81. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 43.Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]