Abstract
Chronic ethanol feeding decreases expression of adiponectin by adipocytes and circulating adiponectin. Adiponectin treatment during chronic ethanol feeding prevents liver injury in mice. Chronic ethanol feeding also increases oxidative and endoplasmic reticulum (ER) stress in adipose tissue. Here we tested the hypothesis that supplemental taurine, an amino acid that functions as a chemical chaperone/osmolyte and enhances cellular anti-oxidant activity, would prevent ethanol-induced decreases in adiponectin expression and attenuate liver injury. Serum adiponectin concentrations decreased as early as 4–7 days after feeding rats a 36% ethanol diet. This rapid decrease was associated with increased oxidative, but not ER, stress in subcutaneous adipose tissue. Taurine prevented ethanol-induced oxidative stress and increased inflammatory cytokine expression in adipose tissue. Ethanol feeding also rapidly decreased expression of transcription factors regulating adiponectin expression (C/EBPα, PPARγ and PPARα) in subcutaneous adipose tissue. Taurine prevented the ethanol-induced decrease in C/EBPα and PPARα normalizing adiponectin mRNA and serum adiponectin concentrations. In the liver, taurine prevented ethanol-induced oxidative stress and attenuated TNF-α expression and steatosis, at least in part, by increasing expression of genes involved in fatty acid oxidation.
In conclusion
In subcutaneous adipose tissue, taurine decreased ethanol-induced oxidative stress and cytokine expression, as well as normalized expression of adiponectin mRNA. Taurine prevented ethanol-induced decreases in serum adiponectin; normalized adiponectin was associated with a reduction in hepatic oxidative stress, TNF-α expression and steatosis. Taken together, these data demonstrate that taurine has important protective effects against ethanol-induced tissue injury in both adipose and liver.
Keywords: Adiponectin, Ethanol feeding, Taurine
Adiponectin, an adipokine primarily secreted by adipose tissue, comprises about 0.01% of total serum protein (1). It circulates in three oligomeric forms: high molecular weight (HMW), middle molecular weight (MMW) and low molecular weight (LMW). Adiponectin interacts with two receptors, AdipoR1 and AdipoR2, which are expressed at high levels in skeletal muscle and liver, respectively (1). Adiponectin has important metabolic functions regulating both glucose and lipid homeostasis. Adiponectin stimulates fatty acid oxidation and decreases triglyceride accumulation in both liver and skeletal muscle (1). Serum adiponectin concentrations are decreased in both human and murine models of obesity, insulin resistance and type 2 diabetes (2;3). Adiponectin also has potent anti-inflammatory functions, acting to decrease the production of inflammatory cytokines, such as TNF-α and IL-6, by macrophages and adipocytes (1;4).
Chronic alcohol consumption results in the development of alcoholic liver disease, characterized by the development of hepatic steatosis and an increase in the expression of a number of inflammatory mediators, including cytokines, reactive oxygen species and nitrogen species (5). Recent studies have implicated ethanol-induced changes in the expression of adipokines in mediating the pathophysiological effects of ethanol in the liver. Chronic ethanol exposure decreases serum adiponectin concentration in mice (6–8) and rats (9;10). Ethanol-induced decreases in circulating adiponectin are associated with lower adiponectin mRNA in subcutaneous adipose tissue, as well as a reduced rates of adiponectin secretion by subcutaneous adipocytes (10). Importantly, treatment of mice with adiponectin during chronic ethanol exposure prevents the development of ethanol-induced liver injury (7), in part by increasing fatty acid oxidation in the liver, thus preventing ethanol-induced steatosis, as well as decreasing TNF-α expression (7).
While the mechanisms by which ethanol feeding decreases adiponectin expression and secretion are not well understood, a growing body of evidence indicates that adipose tissue is an important target for ethanol action. For example, chronic ethanol impairs insulin-stimulated glucose uptake (11) and disrupts the hormonal regulation of lipolysis (12). Chronic ethanol increases macrophage infiltration and inflammatory cytokine expression (13) and causes endoplasmic reticulum (ER) stress in adipose tissue (8). These changes in adipose tissue in response to chronic ethanol are similar to the increased numbers of infiltrating macrophages, increased proinflammatory cytokine expression (14;15), as well as ER stress (16), observed in adipose tissue in models of obesity and insulin resistance.
“Chemical chaperones” is a term given to a group of low molecular weight osmolytes which stabilize protein conformation, thus improving the folding of proteins within the ER (17). Treatment of ob/ob mice with chemical chaperones, such as taurodeoxycholate, reduced ER stress in the liver and restored normal glucose homeostasis (18). Given the parallels between the impact of obesity-induced insulin resistance and chronic ethanol exposure on adipose tissue, both resulting in decreased adiponectin expression, as well as the development of ER stress and inflammation, here we have tested the ability of taurine, an endogenous sulfur containing amino acid that functions both as a chemical chaperone/osmolyte and enhances cellular anti-oxidant activity (19;20), to prevent the effects of ethanol on adipose tissue in rats. Here we report that chronic ethanol feeding for 1 week depleted taurine from subcutaneous adipose tissue, increased inflammatory cytokine expression and decreased adiponectin expression. Supplementation with taurine restored the concentration of taurine in adipose tissue and normalized cytokine and adiponectin expression. Importantly, taurine supplementation to rats also attenuated ethanol-induced steatosis, oxidative stress and TNF-α expression in the liver. These data indicate that the development of therapeutic interventions targeting the effects of ethanol on adipose tissue may be efficacious in the prevention of ethanol-induced liver injury.
Materials and Methods
Animals
The ethanol feeding model used in this study has been previously described (10). See Supplemental Information for further details on the ethanol feeding protocol.
Plasma and tissue metabolite measurements
Adiponectin ELISA was performed as previous described (10). Plasma ethanol was assayed using enzymatic assay kits (Diagnostic Chemicals, Oxford, CT). Plasma ALT, hepatic Oil Red O staining and total triglycerides were performed as previously described (21). The concentration of hepatic malondialdehyde (MDA) was measured by ELISA using a kit from Cayman Chemical Company (Ann Arbor, MI).. Plasma and tissue taurine concentrations, as well as tissue glutathione concentrations, were measured using a modified version of the HPLC method of Cross, et al. (22) (See Supplemental Information for further details).
Quantitative Real-time PCR
Real-time PCR was carried out as previously described (10). Primers are shown in Supplemental Table 1.
Reverse-transcription PCR for XBP-1 mRNA splicing
1μl of cDNA prepared from adipose tissue was amplified using Platinum Taq DNA polymerase (Invitrogen, Carlsbad CA) and primers shown in Table 1. The conditions for amplication of rat/mouse XBP-1 were as follows: 93°C for 5 minutes, then 40 cycles of amplification (95°C for 30 seconds, 51°C for 30 seconds and 72°C for 45 seconds) with the final extension at 72°C for 10 minutes. PCR products were separated by 2% agarose gel and visualized under UV light by ethidium bromide staining.
Adipose tissue homogenates and Western blotting
The procedure for preparation of tissue homogenates and Western blotting were as previously described (21), except that 0.2 g of subcutaneous adipose tissue was homogenized in 5 ml/g tissue lysis buffer using an electric tissue grinder.
Immunohistochemistry
Immunohistochemical analysis of 4-HNE adducts, TNF-α and ED2 were carried out in formalin-fixed, paraffin-embedded sections of adipose and liver using standard techniques (see Supplemental Information for more details).
Gel filtration and quantification of adiponectin oligomers
Adiponectin oligomers in 50 μl serum were separated by Fast Protein Liquid Chromatography (FPLC) (23) and quantified by ELISA.
Data analysis
In all the figures, data were analyzed by general linear models followed by least square means analysis of differences between groups (SAS, Carey, IN). Adjustments for multiple group comparisons were made using the Tukey-Kramer test (SAS, Carey, IN). Analysis was performed on data collected over multiple feeding trials.
Results
Adiponectin circulates in three oligomeric complexes; HMW adiponectin is thought to mediate the insulin-sensitizing functions of adiponectin (1). Chronic ethanol feeding to rats for 4 weeks decreases serum adiponectin (9;10). FPLC separation revealed both HMW and MMW oligomers in rat serum (Figure 1A); both HMW and MMW oligomers were decreased after chronic ethanol feeding for 4 weeks (Figure 1A). Interestingly, this ethanol-mediated decrease in adiponectin was an early response to ethanol exposure, with serum adiponectin concentrations reduced as early as 4 days after ethanol feeding (Figure 1B).
Figure 1. Effects of ethanol feeding on serum adiponectin concentration and adiponectin oligomer distribution.
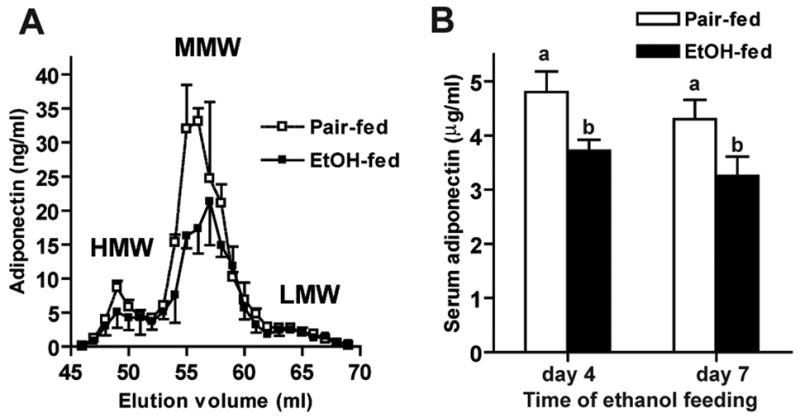
(A) Rats were allowed free access to an ethanol-containing diet or pair-fed a control diet for 4 weeks. Adiponectin oligomers in serum from pair-fed and ethanol-fed rats were separated by FPLC and quantified by adiponectin ELISA. Values represent means ± SEM, n=3. (B) Rats were allowed free access to an ethanol-containing diet or pair-fed control diet for 4–7 days. Serum adiponectin concentrations were measured by ELISA. Values represent means ± SEM, n=9–14. Values with different superscripts are different from each other, p<0.05..
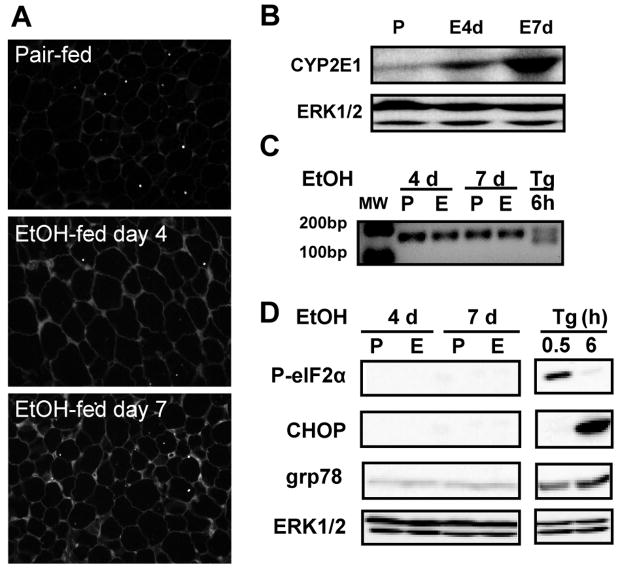
In order to understand the mechanisms for ethanol-induced decreases in serum adiponectin, we focused on the early impact of ethanol on adipose tissue. Ethanol feeding rapidly increased oxidative stress in adipose tissue, as evidenced by the accumulation of immunoreactive 4-HNE adducts (Figure 2A). Oxidative stress was associated with an increase in the expression of CYP2E1 protein in adipose tissue after 7 days of ethanol exposure (Figure 2B).
Figure 2. Early effects of ethanol feeding include oxidative stress, but not ER stress, in subcutaneous adipose tissue.
Rats were allowed free access to an ethanol-containing diet or pair-fed a control diet for 4 and 7 days. (A) Formalin-fixed subcutaneous adipose sections were stained with an antibody against 4-HNE protein adducts. (B) Immunoreactive CYP2E1 was measured in subcutaneous adipose tissue homogenates by Western blotting. ERK1/2 was used as loading control. The values (means ± SEM, arbitrary units of density for CYP2E1/ERK1/2, n=4) were 0.16 ± 0.04 for pair-fed, 0.40 ± 0.08 at day 4 and 0.57 ± 0.14 at day 7. CYP2E1 expression was increased at day 7 compared to pair-fed, p<0.05. (C) XBP-1 splicing was detected by RT-PCR. (D) Immunoreactive phospho-eIF2α, CHOP and grp78 in subcutaneous adipose tissue homogenates were measured by Western blotting. ERK1/2 was used as loading control. Images are representative of 3 (A) and 4–6 (B, C and D) rats in each experimental group. P: pair-fed, E: ethanol-fed, Tg: thapsigargin.
Chronic ethanol feeding for 4–6 weeks induces ER stress in the liver (24) and adipose tissue (8). Ethanol-induced ER stress is associated with decreased adiponectin expression in adipose tissue (8). In contrast to the impact of 4-week ethanol feeding on ER stress in adipose tissue (8), chronic ethanol feeding for 4–7 days did not activate typical ER stress markers in subcutaneous adipose tissue, including the splicing of XBP-1 mRNA (Figure 2C), phosphorylation of eIF2α, increased expression of CHOP and grp78 (Figure 2D). RAW 264.7 macrophages treated with 2 μM thapsigargin, a drug which induces ER stress, were used as positive controls (Figure 2C and D). These data suggested that oxidative stress, but not ER stress, was associated with decreased serum adiponectin at the early stage of ethanol feeding.
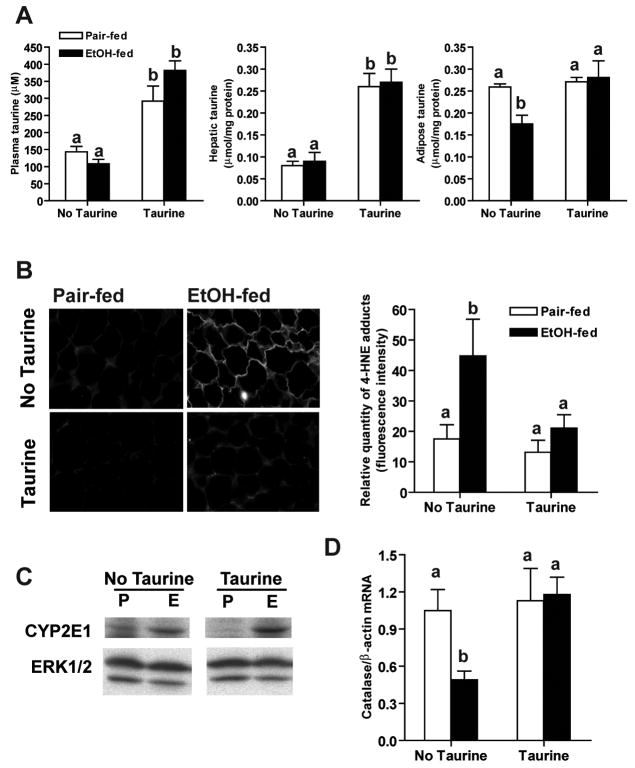
If ethanol-induced oxidative stress in adipose tissue contributes to decreased adiponectin expression, then treatment to prevent oxidative stress would be expected to ameliorate the effects of ethanol on adiponectin expression. In order to test this hypothesis, rats were allowed free access to ethanol-containing diets, or pair-fed control diets, supplemented or not with 30 g/L taurine for 1 week. Body weight and food intake did not differ among groups (Supplemental Table 2). Ethanol feeding increased plasma ethanol concentrations in rats supplemented or not with taurine (Supplemental Table 2). Ethanol feeding did not affect taurine concentrations in plasma or liver; supplementation with taurine increased taurine content in both plasma and liver (Figure 3A). In contrast, ethanol feeding depleted taurine in subcutaneous adipose tissue; taurine supplementation restored normal taurine content in adipose tissue (Figure 3A). In subcutaneous adipose tissue, taurine prevented the ethanol-induced increase in 4-HNE protein adducts (Figure 3B). In contrast, taurine had no effect on ethanol-induced increases in CYP2E1 (Figure 3C). In other models, taurine prevents/attenuates oxidative stress by increasing cellular anti-oxidant activities, such as catalase and SOD2 (19;20). Here ethanol feeding decreased catalase mRNA in subcutaneous adipose tissue, suggesting that ethanol impaired cellular anti-oxidant functions. Treatment with taurine prevented this decrease in catalase mRNA (Figure 3D). Neither ethanol nor taurine changed the SOD2 mRNA in subcutaneous adipose tissue (data not shown).
Figure 3. Taurine prevented ethanol-induced oxidative stress in subcutaneous adipose.
Rats were allowed free access to an ethanol-containing diet or pair-fed a control diet supplemented or not with 30g/L taurine. (A) Taurine concentration in plasma, liver and subcutaneous adipose tissue was measured by HPLC. Values represent means ± SEM, n=4–7. Values with different superscripts are different from each other, p<0.05. (B) Formalin-fixed subcutaneous adipose sections were stained with an antibody against 4-HNE protein adducts. The relative quantity of 4-HNE adducts was measured using the Image J program. Value represent means ± SEM, n=8–10. Values with different superscripts are different from each other, p<0.05. (C) Immunoreactive of CYP2E1 in subcutaneous adipose tissue homogenates was measured by Western blotting. ERK1/2 was used as loading control. Images are representative of 4 rats in each experimental group. The values (means ± SEM, arbitrary units of density for CYP2E1/ERK1/2, n=5–6) were 0.13 ± 0.02 for pair-fed, 0.08 ± 0.02 for pair-fed/taurine, 0.47 ± 0.09 for ethanol-fed and 0.39 ± 0.0.12 for ethanol-fed/taurine. CYP2E1 expression was increased by ethanol feeding with or without taurine supplementation compared to pair-fed, p<0.05. (D) Catalase mRNA level in subcutaneous adipose tissue was measured by real-time PCR and then normalized to β-actin mRNA level. Values represent means ± SEM, n=6. Values with different letters are significantly different from each other, p<0.05.
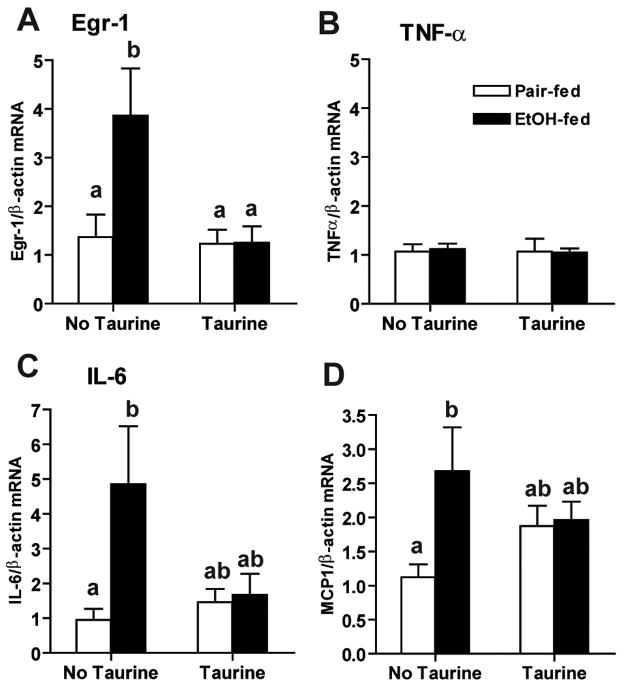
Expression of inflammatory cytokines and chemokines is regulated by different transcription factors that are sensitive to the redox state of the cell, including NF B, Egr-1 and AP-1 (25). Since chronic ethanol feeding increases the expression of inflammatory cytokines in adipose tissue (13), we next investigated the impact of taurine supplementation on ethanol-induced expression of cytokines and chemokines, as well as the redox-sensitive transcription factor, Egr-1, in subcutaneous adipose tissue. Ethanol feeding for 1 week increased mRNA for Egr-1, as well as IL-6 and MCP-1, in subcutaneous adipose tissue (Figure 4). Taurine supplementation prevented these ethanol-induced increases in Egr-1, IL-6 and MCP-1 expression. In contrast, there was no increase in TNF-α mRNA at 1 week of ethanol feeding (Figure 4B).
Figure 4. Taurine prevented ethanol-induced increases in Egr-1 and IL-6 mRNA in subcutaneous adipose.
Rats were allowed free access to an ethanol-containing diet or pair-fed a control diet supplemented or not with 30g/L taurine. Egr-1 (A), TNF-α (B), IL-6 (C) and MCP1 (D) mRNA levels in subcutaneous adipose tissue were measured by real-time PCR and then normalized to β-actin mRNA level. Values represent means ± SEM, n=6–8. Values with different letters are significantly different from each other, p<0.05.
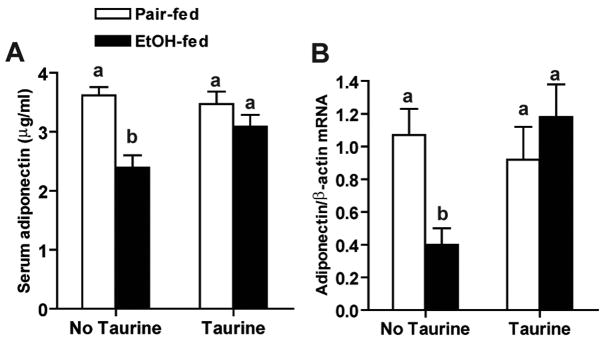
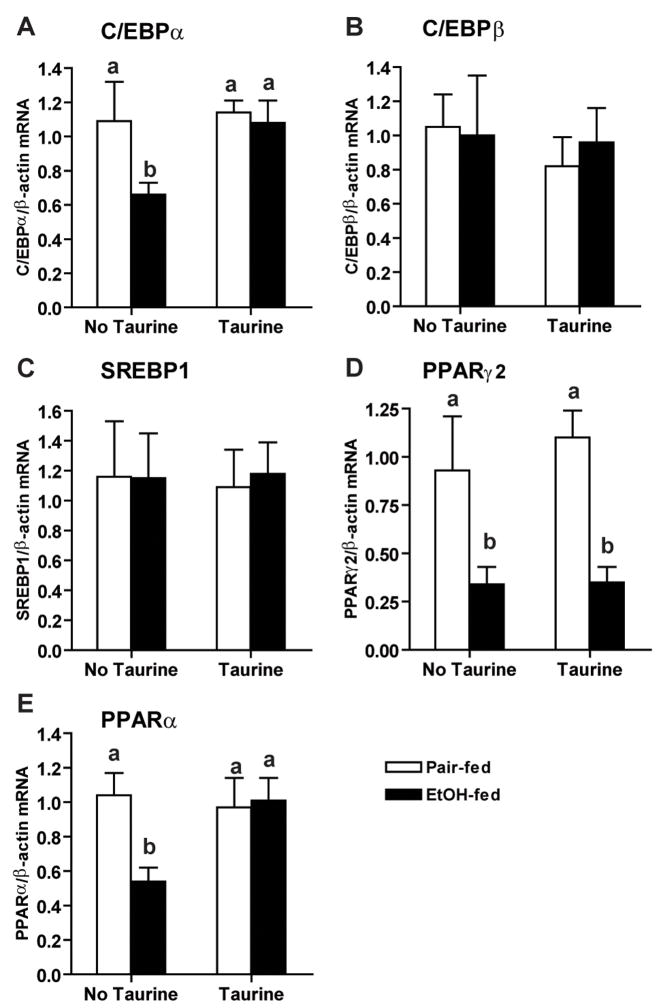
One week of ethanol feeding decreased both serum adiponectin and adiponectin mRNA in subcutaneous adipose tissue (Figure 5). Importantly, taurine supplementation restored adiponectin mRNA in adipose, as well as serum adiponectin concentration (Figure 5). Multiple transcription factors, including C/EBPα, C/EBPβ, PPARγ and SREBP1, have been identified as regulators of adiponectin transcription (26–28). Since several of these transcription factors are also sensitive to regulation by the redox state of the cell (25), we next investigated the impact of taurine supplementation on regulators of adiponectin transcription in adipose tissue. Ethanol feeding differentially regulated the expression of transcription factors controlling adiponectin expression. C/EBPα mRNA (Figure 6A), as well as PPARγ2 mRNA (Figure 6D), were decreased after 1 week of ethanol feeding in subcutaneous adipose tissue. Taurine prevented the ethanol-induced decrease in C/EBPα, but not PPARγ2, mRNA. In contrast, ethanol feeding had no effect on expression of C/EBPβ or SREBP1 mRNA in subcutaneous adipose tissue (Figure 6B, 6C).
Figure 5. Taurine prevented ethanol-induced decreases in adiponectin expression.
Rats were allowed free access to an ethanol-containing diet or pair-fed a control diet supplemented or not with 30g/L taurine. (A) Serum adiponectin concentrations were measured by ELISA. Values represent means ± SEM, n=11–12. Values with different letters are significantly different from each other, p<0.05. (B) Adiponectin mRNA levels in subcutaneous adipose tissues were measure by real-time PCR and then normalized to β-actin mRNA level. Values represent means ± SEM, n=6–7. Values with different letters are significantly different from each other, p<0.05.
Figure 6. Taurine prevented the decrease of C/EBPα and PPARα mRNA in subcutaneous adipose tissue after ethanol feeding.
Rats were allowed free access to an ethanol-containing diet or pair-fed a control diet supplemented or not with 30g/L taurine. C/EBPα (A), C/EBPβ (B), SREBP1c (C), PPARγ2 (D) and PPARα (E) mRNA levels in subcutaneous adipose tissue were measured by real-time PCR and then normalized to β-actin mRNA level. Values represent means ± SEM, n=5–8. Values with different letters are significantly different from each other, p<0.05.
PPARα is also implicated as a regulator of adiponectin expression. Treatment with fenofibrate, a PPARα agonist, increases adiponectin gene expression in adipose and its circulating level in both human and rodents (29;30). Ethanol feeding decreased PPARα mRNA in subcutaneous adipose tissue and taurine supplementation prevented this reduction (Figure 6E). Taken together, these data suggested that taurine prevented the ethanol-induced decrease in adiponectin mRNA, at least in part, by regulating C/EBPα and PPARα.
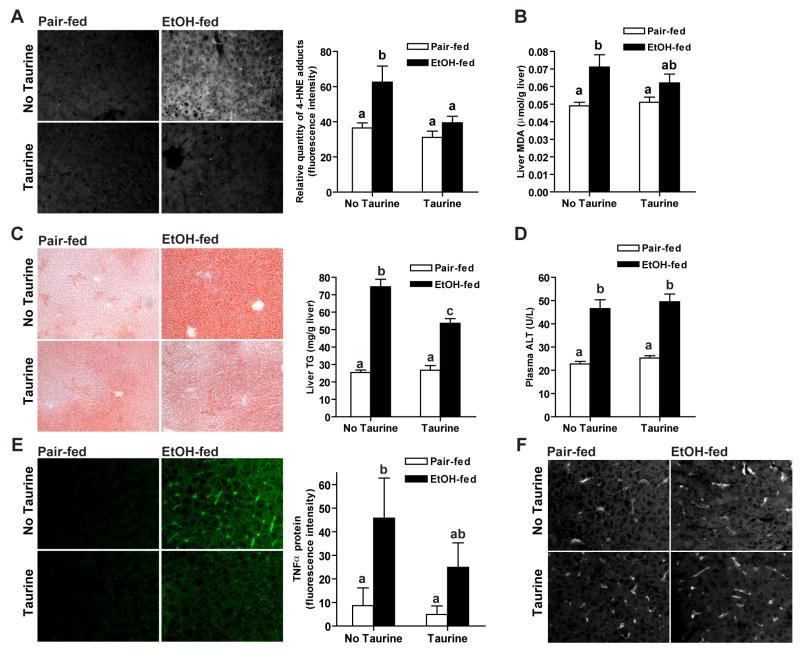
Since taurine ameliorated the impact of ethanol on adipose tissue, we next investigated the impact of taurine on ethanol-induced liver injury. Ethanol feeding increased 4-HNE adduct formation (Figure 7A), as well as the concentration of malondialdehyde (Figure 7B). Taurine supplementation prevented the impact of ethanol feeding on the accumulation of 4-HNE adducts and attenuated the increase in malondialdehyde. Ethanol feeding also increased hepatic triglyceride content (Figure 7C) and plasma ALT activity (Figure 7D) by 1 week. Taurine supplementation attenuated hepatic triglyceride accumulation (Figure 7C), but did not prevent ethanol-induced increases in plasma ALT (Figure 7D). Hepatic TNF-α expression was also increased after ethanol feeding (Figure 7E), in a pattern characteristic of predominant expression by resident macrophages (ED2 positive Kupffer cells) in the liver (Figure7F). Taurine supplementation partially reduced ethanol-induced TNF-α expression in liver (Figure 7E). In addition, neither ethanol feeding nor taurine changed the mRNA levels of catalase, SOD2, AdipoR1 and AdipoR2 in the liver (data not shown).
Figure 7. Taurine prevented/attenuated ethanol-induced oxidative stress and hepatic steatosis.
Rats were allowed free access to an ethanol-containing diet or pair-fed a control diet supplemented or not with 30g/L taurine. (A) Formalin-fixed liver sections were stained with an antibody against 4-HNE protein adducts. The relative quantity of 4-HNE adducts was measured using the Image J program. Values represent means ± SEM, n=4. (B) Concentration of hepatic malondialdehyde (MDA) was measured by ELISA. Values represent means ± SEM, n=7–8. (C) Frozen liver OCT sections were prepared and stained in Oil Red O. Images were taken at 100x magnification. Total triglyceride content in the liver was quantified using the Trinder reagent. Values represent means ± SEM, n=11. (D) Plasma ALT activity was measured by enzymatic assay. Values represent means ± SEM, n=7–8. (E/F) Formalin-fixed liver sections were stained with antibody against TNF-α (E) or ED2 (F), a marker for macrophages. The relative quantity of TNF-α was measured using the Image J program. Values represent means ± SEM, n=4. For all panels, values with different letters are significantly different from each other, p<0.05.
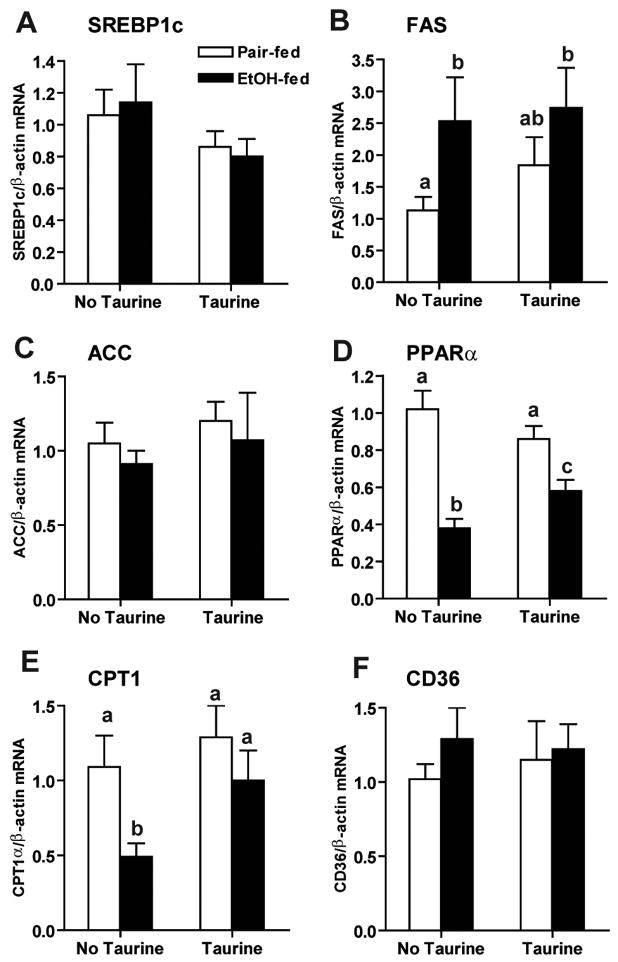
In order to investigate the mechanism by which taurine attenuated ethanol-induced hepatic steatosis, we measured the expression of genes involved in hepatic fatty acid metabolism. Chronic ethanol feeding for 4–6 weeks increases hepatic fatty acid synthesis via activation of SREBP1 and induction of FAS (31). While ethanol feeding for 1 week did not increase SREBP1 mRNA in the liver (Figure 8A), FAS mRNA was increased. Taurine supplementation attenuated this increase (Figure 8B). Neither ethanol feeding nor taurine affected hepatic ACC mRNA level (Figure 8C). Chronic ethanol feeding has also been shown to decrease fatty acid oxidation by down-regulating the expression of PPARα and the PPARα-regulated gene, including CPT1α, the rate limiting enzyme in fatty acid oxidation (32). Similar to these long-term effects of ethanol, here, we found that ethanol feeding for 1 week decreased both PPARα and CPT1α mRNA in the liver (Figure 8D/E); taurine supplementation attenuated these changes. Neither ethanol feeding nor taurine changed CD36 mRNA in the liver (Figure 8F).
Figure 8. Taurine partially reversed ethanol-induced decrease in fatty acid oxidation.
SREBP1 (A), FAS (B), ACC (C), PPARα (D), CPT1α (E) and CD36 (F) mRNA levels in liver were measured by real-time PCR and then normalized to β-actin mRNA level. Values represent means ± SEM, n=5–6. Values with different letters are significantly different from each other, p<0.05.
Discussion
Here we show that ethanol feeding depletes taurine from adipose tissue of rats and that supplementation with taurine prevented multiple effects of ethanol on adipose tissue, acting to decrease ethanol-induced oxidative stress and inflammatory cytokine expression, as well as normalize expression of adiponectin mRNA in subcutaneous adipose tissue. The restoration of adiponectin mRNA expression in adipose was due, at least in part, due to the ability of taurine to prevent ethanol-induced decreases in the expression of C/EBPα and PPARα, two important transcription factors regulating adiponectin expression. Importantly, taurine supplementation prevented ethanol-induced decrease in serum adiponectin. Normalization of adiponectin in response to taurine supplementation was associated with an attenuation of ethanol-induced hepatic oxidative stress, steatosis and TNF-α expression. Taken together, these data demonstrate that taurine has important protective effects against ethanol-induced tissue injury in both liver and adipose tissue.
Chronic ethanol feeding for 4–6 weeks decreases serum adiponectin concentration (6;7;9;10). Here we report that decreased serum adiponectin is a rapid response to ethanol feeding, with reduced adiponectin observed as early as 4 days after the start of ethanol feeding. This rapid decrease in adiponectin was associated with an early development of oxidative stress, but not ER stress, in subcutaneous adipose tissue during ethanol feeding.
Adiponectin expression is sensitive to oxidative stress in other pathophysiological conditions, such as obesity and metabolic syndrome (1). Adipose tissue expresses CYP2E1 (33), an ethanol-metabolizing enzyme that contributes to oxidative stress, particularly during ethanol metabolism. We report that ethanol-induced oxidative stress in adipose tissue was associated with increased expression of CYP2E1 in adipose tissue (Figure 2). While CYP2E1 has long been considered a major contributor to ethanol-induced oxidative stress in liver (5), the present data are consistent with the hypothesis that CYP2E1 expression also contributes to ethanol-induced toxicity in adipose tissue. Ethanol feeding decreased taurine content in adipose tissue; this decrease was ameliorated by taurine supplementation. Importantly, taurine supplementation protected adipose tissue from ethanol-induced oxidative stress. In particular, taurine prevented ethanol-induced decreases in catalase expression in adipose, consistent with previous reports that taurine enhances cellular anti-oxidant activity (19;20). In contrast, taurine prevented ethanol-induced oxidative stress in the liver without affecting hepatic expression of catalase or SOD2. Moreover, neither ethanol feeding nor taurine changed the mRNA levels of AdipoR1 and AdipoR2 in the liver, suggesting the ability of the liver to respond to adiponectin was not affected by either 1 wk of ethanol feeding or taurine supplementation. Taken together, these data suggest that taurine prevented ethanol-induced oxidative stress in the liver, at least in part, by normalizing serum adiponectin concentrations.
Increased expression of inflammatory cytokines in adipose tissue is a hallmark of the metabolic syndrome (34). Ethanol feeding also rapidly increases the expression of pro-inflammatory cytokines and chemokines in adipose tissue (Figure 4). Longer periods of ethanol exposure also leads to the recruitment of macrophages to the adipose tissue, further contributing to local inflammation in adipose tissue (13). Taurine supplementation prevented the early increase in IL-6 expression in response to ethanol feeding. This protective effect was associated with a normalization in the expression of Egr-1, a redox sensitive transcription factor regulating the expression of a number of stress-responsive genes (35). Interestingly, egr-1 −/− mice are protected from ethanol-induced liver injury (36). The current studies suggest that ethanol-induced expression of Egr-1 in adipose tissue may be important to the long-term pathophysiological effects of ethanol.
Taurine supplementation also normalized the expression of transcription factors regulating adiponectin expression. While ethanol feeding decreased expression of C/EBPα PPARα and PPARγ2 (Figure 6), taurine only prevented the decrease in C/EBPα and PPARα mRNA. Importantly, these taurine-dependent effects were sufficient to restore adiponectin mRNA and plasma adiponectin in ethanol-fed rats. Oxidative stress decreases C/EBPα DNA binding activity in adipocytes (37), while TNF-α decreases both C/EBPα and PPARγ2 activity (34). Taken together, these results suggest that it is likely that the ability of taurine to protect adipose tissue from ethanol-induced oxidative stress contributes both to decreased inflammation in adipose tissue, as well as sustained expression of adiponectin mRNA during ethanol feeding.
Chronic ethanol feeding increases hepatic fatty acid synthesis and decreases fatty acid oxidation (38), as well as increases in the expression of inflammatory cytokines (25). Adiponectin is an important regulator of hepatic triglyceride metabolism, acting to increase fatty acid oxidation, and also has potent anti-inflammatory effects (1). In mice, supplementation of exogenous adiponectin during ethanol exposure increased activity of CPT1 and decreased hepatic steatosis and TNF-α expression (7). Here we find a similar response to taurine supplementation during ethanol feeding to rats. Taurine prevented the ethanol-induced decrease in serum adiponectin, decreased hepatic TNF-α expression, and attenuated ethanol-induced decreases in expression of genes involved in the regulation of fatty acid oxidation in liver, including PPARα and CPT1α (Figure 8). These changes in hepatic gene expression were associated with reduced ethanol-induced steatosis in taurine-supplemented rats. Interestingly, taurine had no effect on plasma ALT activity, an indicator of hepatocyte injury, suggesting that taurine protects from only some of the toxic effects of ethanol in the liver. Our experimental design cannot distinguish direct effects of taurine on hepatic function from indirect effects of taurine on the liver via changes in adiponectin expression in adipose tissue. However, the protective effect of taurine supplementation on ethanol-induced steatosis and the lack of effect on plasma ALT are consistent with reports that adiponectin is more important in the regulation of hepatic triglyceride content, rather than plasma ALT, in models of diet-induced obesity (1).
The current study demonstrated a preventative role of taurine in ethanol-induced changes in adipose tissue and an attenuation in the development of ethanol-induced hepatic steatosis. Taurine has also been shown to have therapeutic effects in ethanol-induced liver injury in rats: taurine supplementation accelerates the reduction in hepatic steatosis observed upon ethanol withdrawal after 4 weeks of ethanol exposure (39). Taurine is considered a non-toxic amino acid and is easily supplemented into daily diets. Indeed, taurine is widely used as a nutritional supplement in infant formulas and energy drinks(40;41). Taken together, these data suggest that taurine may be a useful nutritional supplement to attenuate ethanol-induced steatosis. Further, these data suggest that adipose tissue can be a potentially important target for therapeutic interventions to prevent ethanol-induced fatty liver.
Acknowledgments
We thank Brian T. Pratt and Emmanuelle Ogier for their technical support.
Grants
This work was supported in part by NIH grant AA011876 to LEN and HL52234 and HL71907 to DWJ.
Abbreviation
- 4HNE
4-hydroxynonenal
- C/EBPα/β
CCAAT/enhancer binding protein α/β
- PPARα/γ
peroxisome proliferator-activated receptor α/γ
- CPT1α
carnitine palmitoyltransferase 1a
- AdipoR1
adiponectin receptor 1
- AdipoR2
adiponectin R2
- TNF-α
tumor necrosis factor α
- IL-6
interleukin-6
- ER
endoplasmic reticulum
- ALT
alanine aminotransferase
- CYP2E1
cytochrome P450 2E1
- XBP-1
X-box binding protein 1
- eIF2α
alpha subunit of translational initiation factor 2 in eukaryotes
- CHOP
C/EBP homologous protein
- grp78
glucose regulated protein 78KD
- SOD2
superoxide dismutase 2
- Egr-1
Early growth response factor 1
- MCP-1
macrophage chemoattractant protein 1
- SREBP1
sterol regulatory element binding protein-1
- FFA
fatty acid synthase
- ACC
acetyl-coA carboxylas
- CD36
fatty acid translocase
Reference List
- 1.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 2.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20(6):1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 3.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271(18):10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 4.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol. 2005;288(5):R1220–5. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 5.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43(2 Suppl 1):S63–74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 6.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112(1):91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42(3):568–77. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Z, Zhou Z, Deaciuc I, Chen T, McClain CJ. Inhibition of adiponectin production by homocysteine: a potential mechanism for alcoholic liver disease. Hepatology. 2008;47(3):867–79. doi: 10.1002/hep.22074. [DOI] [PubMed] [Google Scholar]
- 9.Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF{alpha} production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol. 2006 doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Sebastian BM, Nagy LE. Chronic ethanol feeding to rats decreases adiponectin secretion by subcutaneous adipocytes. Am J Physiol Endocrinol Metab. 2007;292(2):E621–8. doi: 10.1152/ajpendo.00387.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poirier LA, Rachdaoui N, Nagy LE. GLUT4 vesicle trafficking in rat adipocytes after ethanol feeding: regulation by heterotrimeric G-proteins. Biochem J. 2001;354(Pt 2):323–30. doi: 10.1042/0264-6021:3540323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, et al. Chronic Ethanol and Triglyceride Turnover in White Adipose Tissue in Rats: Inhibition of the anti-lipolytic action of insulin after chornic ethanol contributes to increased triglyceride degradation. J Biol Chem. 2007;282(39):28465–73. doi: 10.1074/jbc.M705503200. [DOI] [PubMed] [Google Scholar]
- 13.Kang L, Sebastian BM, Pritchard MT, Pratt BT, Previs SF, Nagy LE. Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines. Alcohol Clin Exp Res. 2007;31(9):1581–8. doi: 10.1111/j.1530-0277.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- 14.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 16.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 17.Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1(2):109–15. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev. 2001;17(5):330–46. doi: 10.1002/dmrr.229. [DOI] [PubMed] [Google Scholar]
- 20.Bouckenooghe T, Remacle C, Reusens B. Is taurine a functional nutrient? Curr Opin Clin Nutr Metab Care. 2006;9(6):728–33. doi: 10.1097/01.mco.0000247469.26414.55. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Medof ME, et al. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology. 2007;132(3):1117–26. doi: 10.1053/j.gastro.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cross JM, Sandoval FJ, Roje S. An HPLC-based fluorometric assay for cobalamin-independent methionine synthase. Anal Biochem. 2007;360(1):157–9. doi: 10.1016/j.ab.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Xu A, Chan KW, Hoo RL, Wang Y, Tan KC, Zhang J, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280(18):18073–80. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 24.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124(5):1488–99. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 25.Nagy LE. Molecular aspects of alcohol metabolism: transcription factors involved in early ethanol-induced liver injury. Annu Rev Nutr. 2004;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]
- 26.Seo JB, Moon HM, Noh MJ, Lee YS, Jeong HW, Yoo EJ, et al. Adipocyte determination- and differentiation-dependent factor 1/sterol regulatory element-binding protein 1c regulates mouse adiponectin expression. J Biol Chem. 2004;279(21):22108–17. doi: 10.1074/jbc.M400238200. [DOI] [PubMed] [Google Scholar]
- 27.Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52(7):1655–63. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 28.Park SK, Oh SY, Lee MY, Yoon S, Kim KS, Kim JW. CCAAT/enhancer binding protein and nuclear factor-Y regulate adiponectin gene expression in adipose tissue. Diabetes. 2004;53(11):2757–66. doi: 10.2337/diabetes.53.11.2757. [DOI] [PubMed] [Google Scholar]
- 29.Choi KC, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, et al. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem Biophys Res Commun. 2005;336(3):747–53. doi: 10.1016/j.bbrc.2005.08.203. [DOI] [PubMed] [Google Scholar]
- 30.Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Kim JA, et al. Additive beneficial effects of fenofibrate combined with candesartan in the treatment of hypertriglyceridemic hypertensive patients. Diabetes Care. 2006;29(2):195–201. doi: 10.2337/diacare.29.02.06.dc05-1418. [DOI] [PubMed] [Google Scholar]
- 31.You M, Crabb DW. Molecular mechanisms of alcoholic fatty liver: role of sterol regulatory element-binding proteins. Alcohol. 2004;34(1):39–43. doi: 10.1016/j.alcohol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol. 2004;34(1):35–8. doi: 10.1016/j.alcohol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Yoshinari K, Sato T, Okino N, Sugatani J, Miwa M. Expression and induction of cytochromes p450 in rat white adipose tissue. J Pharmacol Exp Ther. 2004;311(1):147–54. doi: 10.1124/jpet.104.067066. [DOI] [PubMed] [Google Scholar]
- 34.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–77. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pritchard MT, Nagy LE. Ethanol-induced liver injury: potential roles for egr-1. Alcohol Clin Exp Res. 2005;29(11 Suppl):146S–150S. doi: 10.1097/01.alc.0000189286.81943.51. [DOI] [PubMed] [Google Scholar]
- 36.McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced Fatty liver injury in mice. Gastroenterology. 2005;128(7):2066–76. doi: 10.1053/j.gastro.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pessler-Cohen D, Pekala PH, Kovsan J, Bloch-Damti A, Rudich A, Bashan N. GLUT4 repression in response to oxidative stress is associated with reciprocal alterations in C/EBP alpha and delta isoforms in 3T3-L1 adipocytes. Arch Physiol Biochem. 2006;112(1):3–12. doi: 10.1080/13813450500500399. [DOI] [PubMed] [Google Scholar]
- 38.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G1–6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 39.Kerai MD, Waterfield CJ, Kenyon SH, Asker DS, Timbrell JA. Reversal of ethanol-induced hepatic steatosis and lipid peroxidation by taurine: a study in rats. Alcohol Alcohol. 1999;34(4):529–41. doi: 10.1093/alcalc/34.4.529. [DOI] [PubMed] [Google Scholar]
- 40.Militante JD, Lombardini JB. Treatment of hypertension with oral taurine: experimental and clinical studies. Amino Acids. 2002;23(4):381–93. doi: 10.1007/s00726-002-0212-0. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton EJ, Berg HM, Easton CJ, Bakker AJ. The effect of taurine depletion on the contractile properties and fatigue in fast-twitch skeletal muscle of the mouse. Amino Acids. 2006;31(3):273–8. doi: 10.1007/s00726-006-0291-4. [DOI] [PubMed] [Google Scholar]