Abstract
Oxidants are well-recognized for their capacity to reduce the phosphorylation of the mammalian target of rapamycin (mTOR) substrates, eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) and p70 S6 kinase 1 (S6K1), thereby hindering mRNA translation at the level of initiation. mTOR functions to regulate mRNA translation by forming the signaling complex mTORC1 (mTOR, raptor, GβL). Insulin signaling to mTORC1 is dependent upon phosphorylation of Akt/PKB and the inhibition of the tuberous sclerosis complex (TSC1/2), thereby enhancing the phosphorylation of 4E-BP1 and S6K1. In this study we report the effect of H2O2 on insulin-stimulated mTORC1 activity and assembly using A549 and bovine aortic smooth muscle cells. We show that insulin stimulated the phosphorylation of TSC2 leading to a reduction in raptor-mTOR binding and in the quantity of proline-rich Akt substrate 40 (PRAS40) precipitating with mTOR. Insulin also increased 4E-BP1 co-precipitating with mTOR and the phosphorylation of the mTORC1 substrates, 4E-BP1 and S6K1. H2O2, on the other hand, opposed the effects of insulin by increasing raptor-mTOR binding and the ratio of PRAS40/raptor derived from the mTOR immunoprecipitates in both cell types. These effects occurred in conjunction with a reduction in 4E-BP1 phosphorylation and the 4E-BP1/raptor ratio. siRNA-mediated knockdown of PRAS40 in A549 cells partially reversed the effect of H2O2 on 4E-BP1 phosphorylation but not on S6K1. These findings are consistent with PRAS40 functioning as a negative regulator of insulin-stimulated mTORC1 activity during oxidant stress.
Introduction
High concentrations of oxygen-derived free radicals generated in the cellular microenvironment during inflammatory processes have the capacity to alter vital metabolic processes including protein synthesis. Reports from our laboratory and others indicate that reactive oxygen species (ROS) modulate mRNA translation at the level of initiation by influencing the formation of the eukaryotic initiation factor complex 4F (eIF4F), a heterotrimeric complex of the cap-binding protein eIF4E, the large scaffolding protein, eIF4G, and the RNA helicase eIF4A [1–4]. This complex facilitates the binding of the 40S ribosomal subunit to the 7-methyl-GTP cap at the 5′ terminus of the mRNA [5]. Under nutrient poor conditions, eIF4E is sequestered by eIF4E-binding proteins (4E-BPs), which compete with eIF4G for a common eIF4E binding site[6]. Upon nutrient and growth factor stimulation, 4E-BP1 is phosphorylated in a canonical fashion by the serine/threonine kinase mammalian target of rapamycin (mTOR), thereby releasing eIF4E to foster cap-dependent initiation [6].
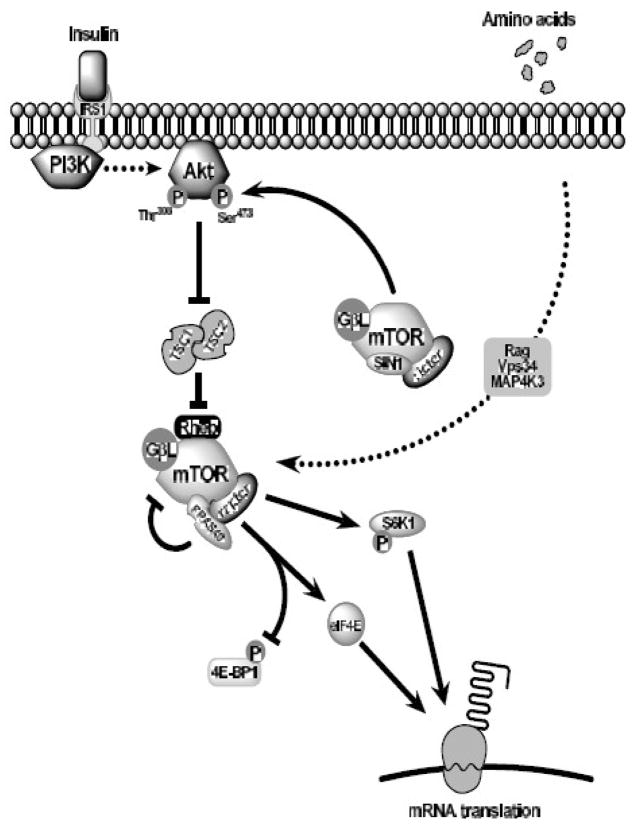
Functional activation of mTOR is dependent upon its ability to form the multi-protein complexes mTORC1 and mTORC2. The composition of mTORC1 includes raptor and GβL, while mTORC2 replaces raptor with rictor and adds SIN1 in addition to GβL [7]. Within mTORC1, raptor serves as a scaffolding protein, allowing the binding of substrates and facilitating their phosphorylation [8]. Notable mTORC1 substrates include the eIF4E-binding proteins (4E-BP1-3) and S6 kinase 1 (S6K1) which bind raptor through conserved TOR signaling (TOS) motifs [7]. Insulin signaling to mTORC1 involves cell surface insulin receptors and the subsequent activation of the phosphatidylinositol-3′ kinase-Akt (also known as protein kinase B, PKB) pathway. Stimulation of Akt/PKB, in turn, phosphorylates and inactivates the tuberous sclerosis complex (TSC1–TSC2), reducing GTPase activity toward Rheb (Ras homologue enhanced in brain) [9]. GTP-bound Rheb, in turn, activates mTORC1 through a yet to be defined mechanism [10]. This complex interplay of signaling events is represented diagrammatically in Figure 1.
Fig. 1.
mTOR signaling cascade. Insulin combines with the insulin receptor (IRS1) to recruit and activate phosphatidylinositol-3′kinase (PI3K). PI3K phosphorylates Akt at Thr308, which when fully activated, relieves the tonic repression of the TSC1/2 complex, reducing its GTPase activity for Rheb. GTP-loaded Rheb in turn, activates mTORC1 (mTOR, raptor, GβL) enabling the phosphorylation of 4E-BP1 and S6K1 and promoting mRNA translation. PRAS40, an insulin-sensitive component of mTORC1, may compete with 4E-BP1 and S6K1 for raptor binding, and in so doing, repress mTORC1 activity. Amino acid-mediated stimulation of mTORC1 bypasses the PI3K-Akt pathway, utilizing Rag proteins, Vps34, and MAP4K3 to stimulate mTORC1 activity distal to TSC1/2. The complex, mTORC2 (mTOR, rictor, GβL), phosphorylates Akt on Ser473, contributing to the full activation of Akt.
Unlike insulin, amino acid-mediated stimulation of mTORC1 activity is independent of Akt/PKB and TSC1/2. Although previous evidence suggested Vps34 and MAP4K3 (mitogen-activated protein kinase kinase kinase kinase 3) as actuators of amino acid effects under specific conditions, the recent report that Rag proteins are both essential and sufficient for mTORC1 activation appears to have clarified the mechanism [11–13]. These small GTPases appear to bind mTORC1 and mediate its localization. Stimulation of Rag activity brings the mTORC1 complex in proximity to membranes containing GTP-bound Rheb, thereby facilitating Rheb binding and mTORC1 activation [13].
Further complexity of mTORC1 regulation was added with the identification of the proline-rich Akt substrate of 40 kDa (PRAS40) as a component of mTORC1 and potential competitive inhibitor of substrate binding [14, 15]. Like 4E-BP1 and S6K1, PRAS40 contains a TOS motif and binds raptor [15]. Insulin stimulation in the presence of amino acids leads to Akt/PKB-dependent phosphorylation of PRAS40 at Thr246 by Akt/PKB leading to its dissociation from raptor (See Fig. 1) [15, 16]. Decreasing PRAS40 expression with shRNA increases 4E-BP1:raptor binding while PRAS40 overexpression has the opposite effect [15]. These observations have nurtured the theory that insulin-stimulated release of PRAS40 from mTORC1 “frees” TOS binding sites to allow increased 4E-BP1 and S6K1 binding [15, 17]. The recent observation that phorbol esters activate mTORC1 independent of changes in PRAS40 phosphorylation and raptor binding suggests that control of mTORC1 activity is likely to be stimulus specific [18].
Although all aerobic organisms are exposed to ROS, during pathologic conditions such as ischemia-reperfusion and stroke, atherosclerosis, diabetes mellitus, and lung injury, oxidant concentrations may exceed endogenous scavenging capacity leading to cell injury or death. With respect to H2O2,concentrations within the local cellular microenvironment have been estimated to exceed 300 μM during ischemia-reperfusion [19]. Similar levels have been found to reduce mTORC1 activity in cultured cells [3]. The initial down-regulation of mTORC1 activity may be beneficial through conservation of cellular energy necessary for the repair of oxidative damage. When sufficiently prolonged or severe, however, diminished mTORC1 activity may stimulate apoptosis [20].
Despite current evidence indicating that oxidants reduce mTORC1 activity downstream of TSC1–TSC2, the mechanism remains unclear [3]. As such, we designed the present study to delineate the impact of H2O2 on insulin- and leucine-stimulated mTORC1 activity and mTORC1/2 assembly and substrate phosphorylation. Our results reveal that H2O2 impairs insulin-stimulated mTORC1 assembly in both the human lung epithelial cell line, A549, and in normal bovine aortic smooth muscle cells. Moreover, our findings suggest that H2O2 reduces 4E-BP1 phosphorylation, in part, by fostering the competitive binding of PRAS40 with raptor.
Materials and Methods
Reagents and Supplies
Antibodies were purchased from the corresponding suppliers: 4E-BP1, non-phosphorylated Thr46 4E-BP1, S6K1, S6K1 (Thr389), mTOR, mTOR (Ser2448), S6rp, S6rp (Ser235/236), Akt, Akt (Ser473), Akt (Thr308), raptor, TSC1, and, TSC2 (Thr1462), TSC2 from Cell Signaling Technologies (Beverly, MA); S6K1, mTOR, rictor, raptor (immunoprecipitation-specific) and SIN-1 from Bethyl Laboratories (Montgomery,TX); PRAS40 and PRAS40 (Thr246) from Invitrogen/Biosource (Carlsbad, CA); TSC2 and α-tubulin from Santa Cruz Biotechnology (Santa Cruz, CA); and β-actin from Sigma Chemical (St. Louis, MO). Anti-mouse and rabbit HRP-IgG and chemiluminescence detection was obtained from GE Healthcare Bio-Science (Piscataway, NJ). Pre-cast gels were purchased from Invitrogen or Jule Biotechnologies (Milford, CT). Complete, EDTA-free protease inhibitor tablets were purchased from Roche Applied Science (Indianapolis, IN). The remaining chemicals were obtained from Fisher Scientific (Pittsburgh, PA).
Cell culture and conditions
The human lung adenocarcinoma cell line, A549, (American Type Culture Collection, Manassas, VA) were grown in Dulbecco’s Minimal Essential Medium (DMEM; Invitrogen) supplemented with 10% FBS, 50 IU/ml of penicillin, 50 μg/ml streptomycin, and 2 mM glutamine. Early passage (p3–5) bovine aortic smooth muscle cells (a gift from Dr. Kathleen Martin) were established as previously described and grown in DMEM + 10% FBS + 50 IU/ml of penicillin + 50 μg/ml streptomycin + 2 mM glutamine [21]. Both cell types were seeded into 6-well plates at a density of 20k/cm2 and allowed to attach overnight. The following day, medium was replaced with leucine- and serum-free DMEM (Millipore; Billerica, MA). Twenty four hours later, wells were treated varying concentrations of H2O2 followed by stimulation with 174 nM insulin, 0.8 mM leucine (1X for DMEM), or both for the times listed.
Immunoblotting
Cell monolayers were rinsed with PBS, trypsinized, and lysed in RIPA lysis buffer [50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1 mM Na2EDTA; 0.5% NP-40; 1 mM Na3VO4; 1 mM PMSF (final concentrations); EDTA-free protease inhibitors]. Lysates were briefly sonicated on ice, supernatants collected following centrifugation at 1000 × g, and total protein determined by the bicinchoninic acid assay. Equal amounts of protein were resolved on either bis-Tris or Tris-glycine gels. Resolved proteins were transferred to PVDF membranes and incubated overnight at 4°C with primary antibodies [all at 1:1000, except Akt (Ser308 1:500); non-phosphorylated Thr46 4E-BP1 (1:2000), S6rp (1:2000), α-tubulin (1:5000), and β-actin (1:5000)] in 5% non-fat dry milk blocking buffer. Following rinsing, membranes were incubated with the corresponding HRP-conjugated secondary antibody (1:5000) and the signal detected by chemiluminescence. Values were normalized to β-actin or α-tubulin expression.
Immunoprecipitation
Following treatment, monolayers were rinsed in cold PBS and lysed in CHAPS buffer [0.3% CHAPS; 40 mM HEPES, pH 7.5; 120 mM NaCl; 1 mM Na2EDTA; 10 mM Na4HP2O7.10H2O; 10 mM glycerophosphate; 50 mM NaF; 1.5 mM Na3VO4; 1 mM PMSF (final concentrations); EDTA-free protease inhibitors] on ice. Lysates were subsequently prepared in a manner analogous to that for immunoblotting without sonication. Goat anti-rabbit BioMag beads (300 μl - Qiagen; Valencia, CA) were mixed with 3.5 μl mTOR (Cell Signaling) or 6 μl TSC2 (Santa Cruz) antibodies or equivalent quantities of rabbit IgG for 4 hours at 4°C. Beads were rinsed with 3 volumes of elution buffer and then boiled in sample buffer to dissociate complexed proteins. Immunoprecipitates were loaded onto Tris-glycine gels and separated by electrophoresis. Control studies illustrated that substitution of mTOR or TSC2 primary antibodies with rabbit IgG eliminated precipitation of mTOR and TSC2 proteins and co-precipitating proteins.
Cell transfection
A549 cells in DMEM at 80% confluency were used for transfection. Cells were trypsinized, centrifuged, and suspended in Nucleofector T (1×106 cells/100 μl; Amaxa). For each 100 μl of cells, 1 μg of siRNA directed against PRAS40 [Sense: r(GGG CAU UAG UGA UAA UGG A)dTdT and antisense r(UCC AUU AUC ACU AAU GCC C)dAdG; Qiagen], pmax GFP (Amaxa), or scrambled siRNA (Qiagen) was added. Cells were electroporated in the Amaxa Nucleofector I device using program X-01 designed for A549 cells. Cells were then reseeded into 6-well plates and returned to the incubator. Control studies revealed >80% GFP transfection and minimal cell death using 1–3 μg siRNA.
Statistics
All studies were performed a minimum of 3 times. The effects of leucine, insulin, time, and H2O2 and potential interactions were tested with 2- or 3-way analysis of variance (ANOVA) with Fisher’s LSD post-hoc analysis to determine individual differences. Data are listed as mean ± standard error and the level of significance set at p<0.05.
Results
Leucine and insulin-stimulated mTORC1 activity is suppressed by H2O2
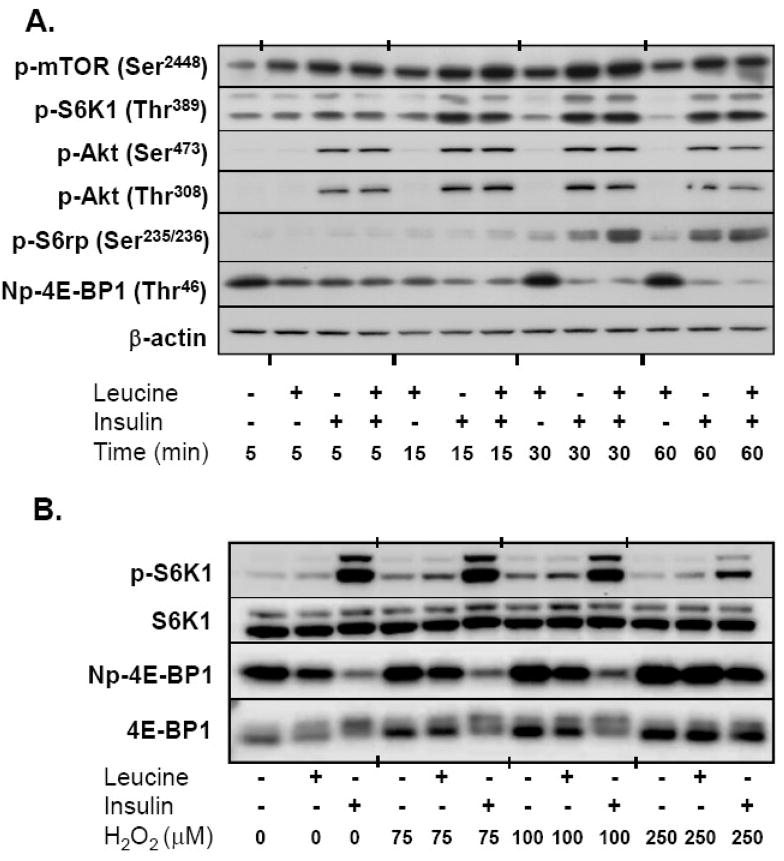
To study the effects of oxidants on mTOR activity, we first conducted experiments to determine the sensitivity of A549 cells to leucine withdrawal. Using the phosphorylation of 4E-BP1 and S6K1 as functional readouts of mTORC1 activity, we found that A549 cells were resistant to leucine-deprivation, requiring a 24-hour withdrawal period in order to observe a consistent decrease in 4E-BP1 and S6K1 phosphorylation with deprivation. Following the starvation period, incubation with leucine induced a rapid increase in the phosphorylation of mTOR at Ser2448 and the mTORC1 substrate, 4E-BP1 (decreased intensity using the non-phosphorylated Thr46 4E-BP1 antibody), without altering Akt or S6K1 phosphorylation or total kinase expression. This effect was transient, reaching a maximum between 5 and 15 minutes. Insulin stimulation was conducted using the 174 nM concentration commonly found in standard cell culture supplements. Addition of insulin, alone or in conjunction with leucine increased mTOR, 4E-BP1, S6K1, S6rp, and Akt phosphorylation within 5–15 minutes resulting in sustained activation for at least 60 minutes, again without altering the total protein expression of each kinase (See Fig 2A).
Fig. 2.
mTORC1 signaling and activity in response to leucine, insulin, and H2O2. (A) Time course of nutrient-mediated mTORC1 signaling. Serum and leucine-starved A549 cells were incubated with 0.8 mM leucine, 174 nM insulin, or both for 5–60 min. Equal amounts of whole cell lysates were separated by SDS PAGE, transferred to PVDF membranes, and immunoblotted with phosphorylation (p)-specific antibodies. Figure is illustrative of immunoblots from three independent experiments. Basal levels of phosphorylation (no insulin or leucine) were unchanged over the course of the experiment and therefore represented only at 5 min. Np-4E-BP1 = non-phosphorylated 4E-BP1 at Thr46. (B) Effect of H2O2 on the phosphorylation of downstream mTORC1 targets. Serum and leucine-starved cells were incubated with varying concentrations of H2O2 15 min prior to the addition of leucine and insulin for an additional 30 min. Whole cell lysates were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with S6K1, S6K1 Thr389 (p-S6K1), 4E-BP1, and Np-4E-BP1 antibodies. Immunoblot is representative of three independent experiments.
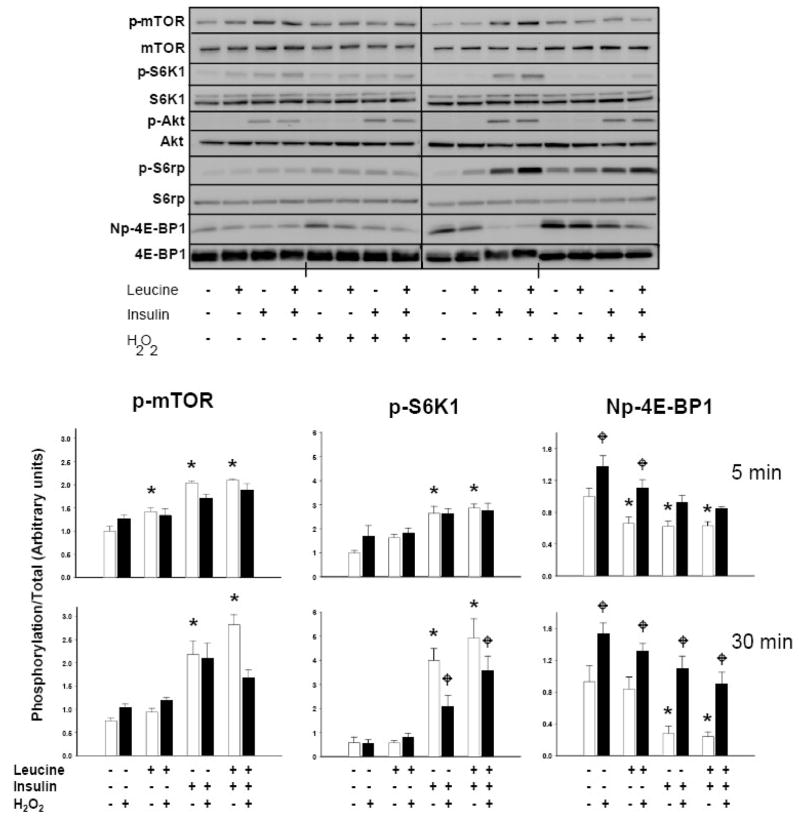
We next sought to determine the concentrations of H2O2 necessary to alter leucine and insulin-induced mTORC1 activation. Preliminary studies indicated that 75 μM was required to minimally reduce insulin-stimulated 4E-BP1 phosphorylation at 30 minutes, but that concentrations in excess of 100 μM were required to diminish the leucine+insulin-induced phosphorylation of 4E-BP1 and S6K1 (Fig 2B). Changes in phosphorylation at 5 minutes were only observed using concentrations of at least 250 μM (not shown). In order to best study the effects of oxidant stress on mTORC1 activity, all subsequent experiments were conducted with 250 μM H2O2. As anticipated, leucine, insulin, and leucine+insulin significantly enhanced the phosphorylation of mTOR, S6K1, and 4E-BP1. We also observed that H2O2 reduced the phosphorylation of 4E-BP1 and S6K1 in a time-dependent manner (p<0.05). Post-hoc analysis revealed that H2O2 reduced leucine-induced phosphorylation of 4E-BP1 at 5 and 30 minutes without altering the phosphorylation of mTOR or S6K1. In cells stimulated with insulin or leucine+insulin, H2O2 decreased the phosphorylation of 4E-BP1 and S6K1 at 30 minutes, while leucine+insulin, but not insulin alone, decreased mTOR phosphorylation. Peroxide failed to inhibit insulin-induced phosphorylation of Akt at Thr308 at either time point (Fig 3).
Fig 3.
Effect of H2O2 on leucine- and insulin-stimulated mTORC1 signaling. Serum and leucine-starved cells were treated with 250 μM H2O2 or vehicle for 15 min prior to incubation with leucine (0.8 mM), insulin (174 nM), or both for 5 and 30 min. Whole cell lysates were then separated by SDS- PAGE, transferred to PVDF membranes, and immunoblotted with mTOR, p-mTOR (Ser2448), S6K1, p-S6K1 (Thr389), Akt, p-Akt (Thr308), S6rp, p-S6rp (Ser235/236), 4E-BP1, and Np-4E-BP1 antibodies. Upper immunoblot is representative of 5 independent experiments. Histograms quantify changes in the phosphorylation of mTOR, S6K1, and 4E-BP1 as determined by densitometry (density of phosphorylation/density of total kinase, n=5). Columns represent means and bars standard error (SE). As determined by 2-way ANOVA, “*”denotes statistical significance (p<0.05) of leucine, insulin, or both vs. no leucine+insulin vehicle control at 5 or 30 min; “ ” identifies statistically significant effect of H2O2 (p<0.05) under a given nutrient condition.
” identifies statistically significant effect of H2O2 (p<0.05) under a given nutrient condition.
H2O2 fails to hinder insulin-stimulated TSC1/TSC2 binding
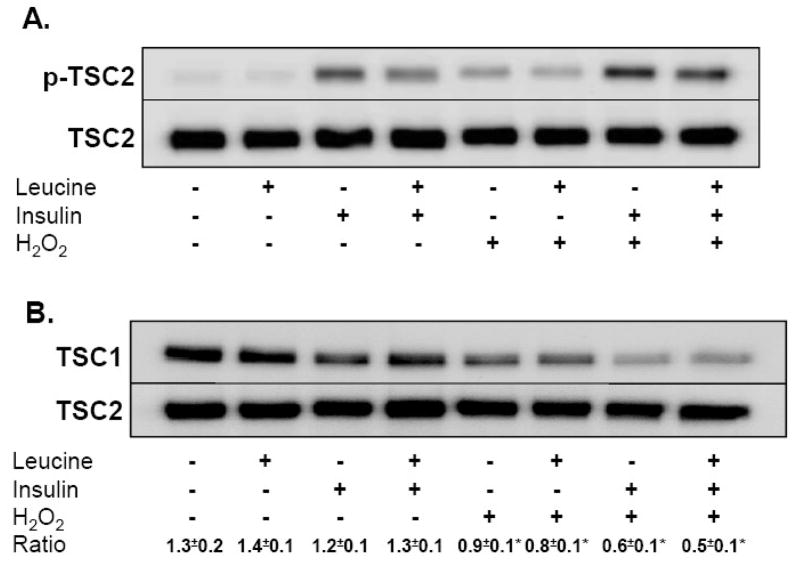
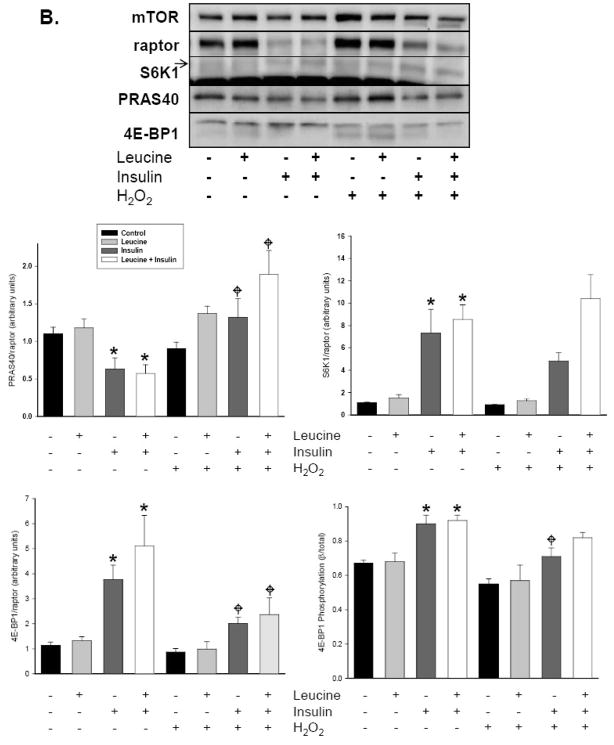
Akt phosphorylation is dependent upon phosphorylation at both Ser473 by mTORC2 and at Thr308 by 3-phosphoinositide-dependent protein kinase-1 [22]. Once activated, Akt directly phosphorylates TSC2 on Thr1462, repressing the GAP activity of TSC2 and increasing Rheb-GTP levels [23, 24]. The integrity of TSC2-TSC1 complexes also regulates Rheb-GAP activity and recent information indicates that ERK activation leads to TSC1–TSC2 dissociation and enhanced mTORC1 activity [25]. Given that our laboratory has previously shown that peroxide potently augments ERK 1/2 activity and phosphorylation in A549 cells, we were interested in the potential for TSC1/2 to modulate mTORC1 activity in response to oxidant stress [1]. Using both immunoblotting and immunoprecipitation, we observed that leucine failed to alter TSC2 phosphorylation at Thr1462 or TSC1/2 binding (Fig 4A). Insulin and insulin combined with leucine, however, dramatically increased TSC2 phosphorylation without altering TSC1/2 binding ratios. Treatment of cells with H2O2 increased TSC2 phosphorylation and reduced TSC1/2 binding under all conditions, with cells treated with leucine+insulin demonstrating greater decline in TSC1/2 binding than in control cells (Fig 4B). Although not true activity assays per se, these results indicate that H2O2 actually represses TSC1/2, a process which should augment Rheb.GTP levels and enhance mTORC1 function.
Fig 4.
Impact of H2O2 on the tuberous sclerosis complex. A549 cells starved of serum and leucine were treated with 250 μM H2O2 or vehicle for 15 min prior to incubation with leucine (0.8 mM), insulin (174 nM), or both for 30 min. (A) TSC2 phosphorylation. Whole cell lysates separated by SDS-PAGE were immunoblotted with TSC2 and p-TSC2 (Thr1462) antibodies. Figure shows a representative immunoblot taken from three independent experiments. (B) TSC1/2 complex assembly. Cells treated with H2O2 as described above were lysed in non-reducing CHAPS buffer after 30 minutes of nutrient stimulation. Intact TSC1/2 complexes were separated from whole cell lysates using a rabbit anti-TSC2 antibody and anti-rabbit magnetic beads. Complex components were then separated by SDS-PAGE, transferred to PVDF membranes, and probed with TSC1 and TSC2 antibodies. Densitometric ratios of TSC1/TSC2 were derived from 3 independent experiments and expressed as mean ±SE. “*” denotes a significant difference (p<0.05) between vehicle control and H2O2 as determined by 2-way ANOVA.
H2O2 impairs insulin-stimulated mTORC1 assembly
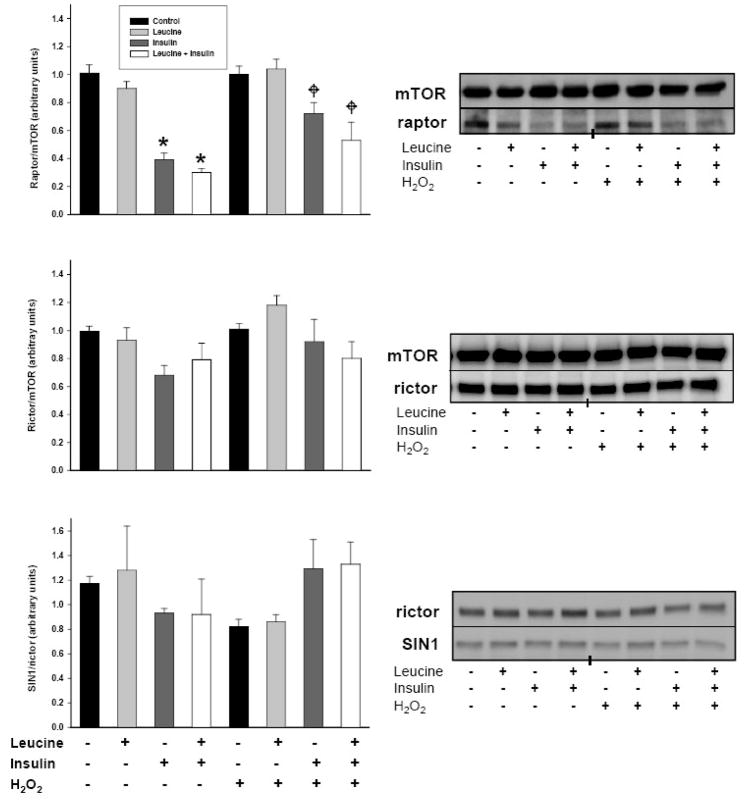
mTOR kinase activity is dependent upon the formation of two complexes, mTORC1 (mTOR, raptor, GβL) and mTORC2 (mTOR, rictor, GβL). Within mTORC1, raptor binds 4E-BP1 and S6K1 through the TOR signaling motif (TOS), an event required for the canonical phosphorylation of 4E-BP1 and the activation of S6K1 [26, 27]. In preliminary studies, we found that rapamycin reduced raptor-mTOR binding in insulin-stimulated A549 cells but had no effect of rictor-mTOR binding. To assess changes in mTORC1/2 formation attributable to peroxide, cells were stimulated with leucine, insulin, or both for 30 minutes. As illustrated in Figure 5, insulin and leucine+insulin reduced the amount of raptor co-precipitating with mTOR when compared with control and leucine stimulated cells. When cells were treated with H2O2, the effects of insulin appeared blunted, but remained less than in control or leucine treated cells. Within mTORC2, the association of rictor with mTOR was unresponsive to leucine, insulin or both or to the presence of H2O2. The rictor binding partner, stress-activated protein kinase interacting protein 1 (SIN1), has been shown to be required for mTORC2 function on Akt phosphorylation at Ser473 [28]. In the current study, the rictor-SIN1 association was somewhat variable, but statistically unchanged by leucine, insulin, or the combination or the presence of H2O2.
Fig. 5.
Modulation of mTORC1 and mTORC2 assembly by H2O2. Serum and leucine starved cells were lysed in CHAPS non-reducing buffer following treatment with 250 μM H2O2 and stimulation with leucine (0.8 mM), insulin (174 nM), or both for 30 min. Equal amounts of protein were then incubated with an anti-mTOR antibody and immune complexes separated using magnetic beads. Intact complexes were resolved via SDS-PAGE, transferred to PVDF membranes, and immunoblotted for mTOR, rictor, raptor, and SIN1. Immunoblots are representative of 6 individual experiments and histograms depict relative densities of each complex component relative to its binding partner. Data are presented as means ±SE. “*” identifies statistically significant differences between insulin or leucine+insulin and vehicle control, while “ ” signifies an effect of H2O2.
” signifies an effect of H2O2.
H2O2 alters insulin-stimulated mTOR-substrate associations
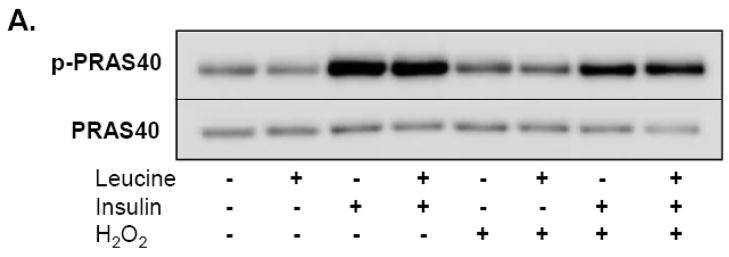
Recent work has shown that PRAS40 functions as a negative regulator of mTORC1 by binding to raptor through a TOS motif [15, 17, 29]. Evidence further suggests that PRAS40 competes for raptor binding with 4E-BP1 and S6K1 and that these events may be due in part to alterations in Akt induced phosphorylation of Thr246 in response to insulin [15, 29]. In an effort to determine if H2O2-induced reductions in insulin-stimulated 4E-BP1 and S6K1 phosphorylation could be regulated by PRAS40, we determined the impact of H2O2 on PRAS40 phosphorylation and on PRAS40, S6K1, and 4E-BP1 content in mTOR immunoprecipitates. For each mTOR immunoprecipitate, the ratio of PRAS40 and 4E-BP1 relative to mTOR-precipitated raptor was determined to assimilate the substrate content within mTORC1. As shown in Figure 6A, insulin and leucine+insulin increased PRAS40 phosphorylation at Thr246 compared to vehicle control and leucine while pre-treatment with H2O2 partially blunted this effect. Insulin and leucine+insulin also increased S6K1/raptor and 4E-BP1/raptor ratios and the relative phosphorylation of bound 4E-BP1 (β/α+β bands) within mTORC1 (Fig. 6B). Pre-treating cells with 250 μM H2O2 attenuated these effects. Peroxide also reduced the 4E-BP1/raptor quotient by nearly half in response to insulin and leucine+insulin; an effect accompanied by diminished 4E-BP1 phosphorylation. Likewise, H2O2 increased the PRAS40/raptor ratio 2–3 fold in insulin and leucine+insulin-stimulated cells. Statistical analysis further revealed a significant interaction between the stimuli (leucine, insulin, leucine+insulin) and H2O2, demonstrating that H2O2 reversed insulin-mediated decreases in PRAS40 binding (p<0.001). Little, if any, change was observed in the S6K1/raptor ratio in response to H2O2.
Fig. 6.
H2O2 alters PRAS40 phosphorylation and the quantity of PRAS40 and 4E-BP1 co-precipitating with mTOR. (A) Whole cell lysates separated by SDS-PAGE were transferred to PVDF membranes, and immunoblotted with antibodies directed against PRAS40 and phosphorylated-PRAS40 (Thr246). Image represents representative immunoblot from 4 separate experiments. (B) mTOR immunoprecipitates were separated on 6% Tri-glycine gels, transferred to PVDF, and immunoblotted with mTOR, raptor, S6K1, PRAS40, and 4E-BP1 antibodies as described in Methods. A representative immunoblot of 5 experiments is shown. Arrow indicates S6K1 immunoreactive band while the dark band in the lower half of the S6K1 immunoblot represents heavy chain IgG. Relative protein abundance was determined by densitometry and ratios of S6K1/raptor, 4E-BP1/raptor, and PRAS40/raptor are shown in corresponding histograms. For 4E-BP1 phosphorylation, the slowest migrating (β), highest phosphorylated band of 4E-BP1 was divided by total 4E-BP1 (α+β). Data are presented as means ±SE. “*” identifies statistically significant differences between insulin or leucine+insulin and vehicle control, while “ ” signifies an effect of H2O2.
” signifies an effect of H2O2.
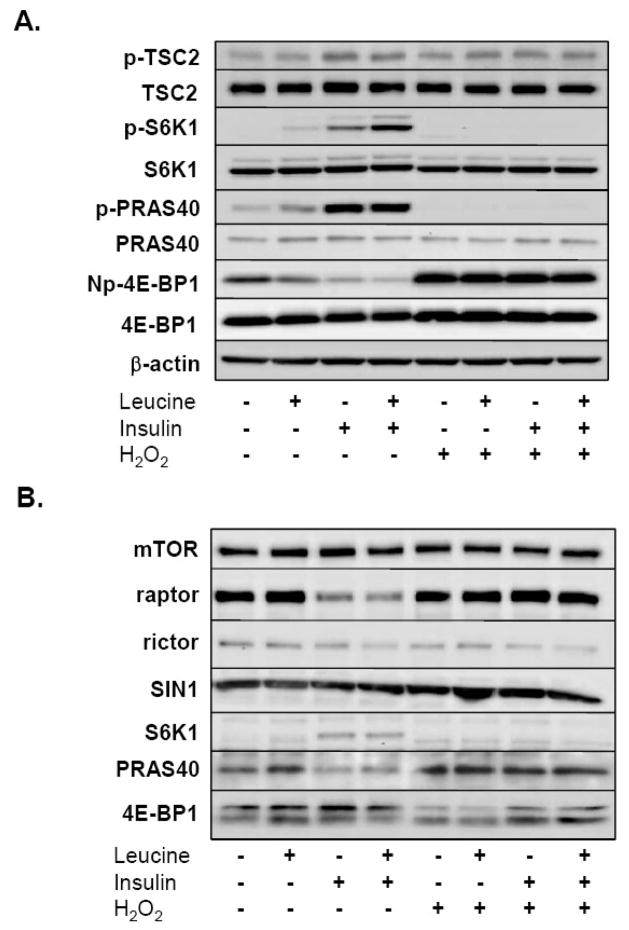
Because A549 cells are a transformed, malignant cell line, we repeated the analysis of key signaling events using early passage, primary bovine aortic smooth muscle cells (BASMC). As shown in Figure 7A, 250 μM H2O2 reduced the phosphorylation of the mTORC1 substrates, 4E-BP1 and S6K1, and the phosphorylation of PRAS40. The phosphorylation of TSC2, on the other hand, was minimally effected by H2O2, in contrast to our observation in A549 cells. Nonetheless, both insulin and leucine+insulin reduced the amount of raptor co-precipitating with mTOR while the pre-treatment with H2O2 blunted this effect (Fig. 7B). In each of the three trials performed, H2O2 also increased the quantity of PRAS40, and diminished S6K1 and 4E-BP1, co-precipitating with raptor. As observed in A549 cells, H2O2 also appeared to decrease the relative phosphorylation of 4E-BP1 within mTORC1 in BASMC. In summary, these findings illustrate that H2O2 impairs insulin-stimulated mTORC1-substrate associations in addition to mTORC1 assembly.
Fig. 7.
Effect of H2O2 on insulin-induced mTOR signaling and mTORC1 formation in bovine aortic smooth muscle cells. Serum and leucine-starved BASMC were treated with 250 μM H2O2 or vehicle for 15 min prior to incubation with leucine (0.8 mM), insulin (174 nM), or both for 30 min. (A) Whole cell lysates were then separated by SDS- PAGE, transferred to PVDF membranes, and immunoblotted with TSC2, p-TSC2 (Thr1462), S6K1, p-S6K1 (Thr389), PRAS40, phosphorylated-PRAS40 (Thr246), 4E-BP1, Np-4E-BP1, and β-actin antibodies. The figures shows representative immunoblots obtained from 3 experiments illustrating the effect of leucine, insulin, or both, with and without H2O2. (B) Equal amounts of protein were also incubated with an anti-mTOR antibody and immune complexes separated using magnetic beads. Intact complexes were resolved via SDS-PAGE, transferred to PVDF membranes, and immunoblotted for mTOR, rictor, raptor, and SIN1, PRAS40, S6K1, and 4E-BP1. The figure depicts the assembly of mTORC1 and mTORC2 in BASMC and is representative of the findings from 3 individual experiments.
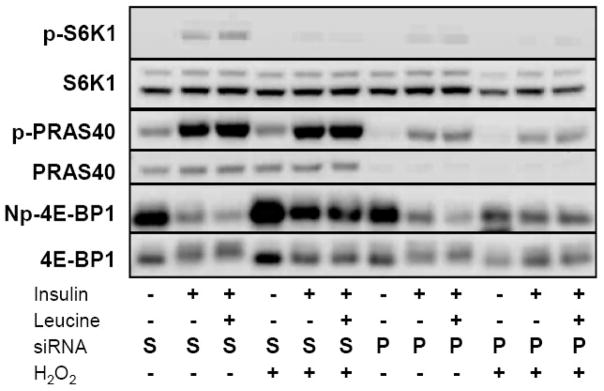
PRAS40 knockdown enhances the phosphorylation of 4E-BP1
Based upon our immunoprecipitation data, we sought to determine if reductions in PRAS40 content would restore insulin-stimulated mTORC1 activity. To this effect, we transfected cells with siRNA directed against PRAS40 using a sequence previously shown to inhibit PRAS40 expression [29]. Our preliminary studies indicated that 1 and 3 μg of siRNA effectively reduced PRAS40 expression by approximately 80% compared to non-transfected cells. By comparison, cells transfected with transfection reagent alone or siRNA without transfection reagent showed no identifiable change in PRAS40 expression. In addition, neither scrambled nor PRAS40 siRNA altered insulin-induced Akt phosphorylation (not shown). As illustrated in Figure 8, transfection with PRAS40 siRNA potently reduced PRAS40 protein and decreased the degree of insulin- and leucine+insulin-mediated PRAS40 phosphorylation at Thr246 compared to scrambled siRNA controls. Knockdown of PRAS40 partially restored H2O2-mediated reductions in 4E-BP1 phosphorylation at Thr46 as demonstrated by a decrease in density of the immunoreactive band representing non-phosphorylated (Np) 4E-BP1 (Thr46). In agreement with previous studies, PRAS40 knockdown also diminished insulin-stimulated S6K1 phosphorylation regardless of the presence of H2O2 [15, 17].
Fig. 8.
Role of PRAS40 in H2O2-mediated reduction in 4E-BP1 phosphorylation. A549 cells were transfected with siRNA directed against PRAS40 (P) or scrambled (S) siRNA using the Amaxa system. Serum and leucine starved transfected cells were then treated with 250 μM H2O2 for 15 min prior to stimulating with leucine (0.8 mM) or insulin (174 nM) for an additional 30 min. Cells were then harvested, lysates separated by SDS-PAGE, and immunoblotted with antibodies directed against S6K1, PRAS40, 4E-BP1, S6K1 (Thr389); PRAS (Thr246), and non-phosphorylated (Np) 4E-BP1 (Thr46). Immunoblot is representative of 3 independent experiments.
Discussion
Oxygen-derived free radicals are well-recognized suppressors of global protein synthesis. Several reports have documented the capacity for high concentrations of H2O2 to inhibit mRNA translation at the level of initiation, in part by reducing the phosphorylation and activity of the eIF4E repressor protein, 4E-BP1 [2, 30, 31]. mTOR, when complexed with raptor within the mTORC1 complex, is the only known kinase for 4E-BP1. mTORC1 function is sensitive to both direct stimulation by amino acids (leucine) and to receptor-mediated signaling from growth factors such as insulin. Amino acids signal to mTORC1 downstream of TSC1/2, by stimulating Rag GTPases to bind raptor and alter the intracellular localization of mTORC1 with respect to Rheb [13]. Insulin, through activation of the PI3K-Akt pathway, leads to phosphorylation and inactivation of TSC2, thereby promoting Rheb.GTP cycling to activate mTORC1 [10]. In turn, activation of mTORC1 increases the binding of 4E-BP1 and S6K1 to raptor thereby promoting phosphorylation of these substrates [32, 33]. The current study demonstrates that H2O2 impairs insulin-stimulated mTORC1 assembly and the association of mTOR with 4E-BP1.
Low to moderate concentrations of H2O2 commonly induce the phosphorylation of Akt at Ser473 [34]. In the A549 cells utilized in the present investigation, 250 μM H2O2 failed to alter insulin-induced Akt phosphorylation, despite inhibiting both 4E-BP1 and S6K1 phosphorylation. Maintenance of insulin-stimulated TSC2 phosphorylation and reduced TSC1/2 complex assembly indicates that H2O2 does not impede insulin signaling upstream mTORC1 signaling, akin to the previous report that H2O2 effects on 4E-BP1 are independent of TSC2 [35]. Aside from Akt, TSC2 activity is also regulated by adenosine monophosphate-activated kinase (AMPK), an energy sensing enzyme responsive to changes in AMP:ATP concentrations. While H2O2 was been found to promote AMPKα phosphorylation in an LKB1-dependent manner in human skin keratinocytes, A549 cells lack functional LKB1, indicating that changes in peroxide-mediate alterations in TSC1/2 activity are unlikely to involve AMPK [20, 36].
Nutrient signaling to mTORC1 is responsive to the branched chain amino acid leucine. Withdrawal of leucine from culture medium for 24 hours in the A549 cells produced only minor decreases in 4E-BP1 and mTOR phosphorylation and no discernable change in S6K1 phosphorylation. Although subsequent experiments confirmed the capacity of A549 cells to augment the phosphorylation of both mTORC1 substrates upon re-addition of all essential amino acids, the reason for the relative resistance to leucine withdrawal in these cells is unclear. Nevertheless, despite the small increase in 4E-BP1 phosphorylation in response to leucine, phosphorylation at Thr46 was still diminished in the presence of H2O2. According to previous studies, leucine stimulation would be anticipated to weaken raptor-mTOR interactions while H2O2 would do the opposite [37, 38]. In leucine-stimulated A549 cells, changes in raptor-mTOR binding were not consistently observed with or without H2O2. This suggests that H2O2 alters leucine-stimulated mTORC1 activity independent of changes in raptor binding, or alternatively, that additional mechanisms, such as phosphatase activation are involved. Peroxide-induced PKCα activation has recently been demonstrated to stimulate PP2A activity and 4E-BP1 dephosphorylation independent of Akt/PKB in intestinal epithelial cells [39]. Whether PP2A activity participates to H2O2-mediated reductions in nutrient-stimulated 4E-BP1 and S6K1 phosphorylation is currently being investigated within our laboratory.
As anticipated, insulin stimulation produced a marked increase in 4E-BP1, S6K1, and PRAS40 phosphorylation and in the association of 4E-BP1 and S6K1 with mTOR. Simultaneously, insulin lead to a reduction in the amount of PRAS40 precipitating with mTOR analogous to the effect previously reported in 3T3-L1 adipocytes [16, 32]. Insulin also increased the fraction of highly phosphorylated 4E-BP1 in the immunoprecipitates. While we did not assess the individual 4E-BP1 phosphorylation sites, others have suggested that raptor binds most tightly to poorly phosphorylated 4E-BP1 and that the sequential phosphorylation of Thr37/46 and then Thr70 is required for dissociation from raptor [8, 26]. This suggests that the increased 4E-BP1 precipitating with mTOR is likely to reflect the reduced quantity of raptor with the complex rather than the increased affinity of highly phosphorylated 4E-BP1.
Previous results indicate that H2O2 enhances raptor-mTOR interactions [37]. In the present study, our experiments produced similar findings in A549 cells in insulin-stimulated cells, but also identified specific alterations in the amount of the mTORC1 substrates co-precipitating with mTOR. In opposition to the effects of insulin, H2O2 pre-treatment increased the PRAS40/raptor ratio derived from the mTOR immunoprecipitations while diminishing the 4E-BP1/raptor ratio. The effect of H2O2 on theS6K1/raptor ratio, on the other hand, was negligible. Knockdown of PRAS40 using siRNA partially restored insulin-stimulated 4E-BP1 phosphorylation in the presence of H2O2, but reduced S6K1 phosphorylation independently from H2O2. These findings are consistent with PRAS40 serving as a direct inhibitor of 4E-BP1 binding as proposed by Wang et al [15]. Theoretically, insulin causes the release of PRAS40 from the substrate binding site on raptor and in so doing, promotes the binding of 4E-BP1 via the TOS motif [15]. Peroxide, on the other hand, reduces insulin-stimulated PRAS40 phosphorylation at Thr246. This process would be anticipated to reduce the binding of PRAS40 to 14-3-3 proteins (phosphoprotein binding proteins), allowing PRAS40 to re-associate with raptor at the expense of 4E-BP1 [17, 40]. Since PRAS40 not only binds raptor via the TOS motif, but also mTOR-raptor via the KSLP region, PRAS40 is speculated to have stronger substrate binding affinity for mTORC1 than 4E-BP1 or S6K1 [15].
Despite consistency with the published literature, the competitive inhibitor mechanism cannot fully account for the failure of H2O2 to reduce insulin-stimulated S6K1 phosphorylation. S6K1 and 4E-BP1 contain non-identical TOS motif sequences [41]. 4E-BP1 also possesses a RAIP (Arg-Ala-Ile-Pro) motif that binds raptor [41]. This region, however, is believed to play only an accessory role in mTORC1-mediated phosphorylation [26, 41]. Given that the binding affinity of S6K1 for raptor-mTOR appears weaker than for 4E-BP1, H2O2-induced modifications to RAIP motif function could account for differences in S6K1- and 4E-BP1-raptor binding [8]. Reductions in S6K1 phosphorylation, then, may be secondary to activation of PP2A, as previously discussed, or to H2O2-mediated activation of alternate mTORC1 signaling pathways such as extracellular-signal-regulated kinase (ERK) and/or p90 ribosomal S6 kinase (RSK) - kinases strongly activated by H2O2 [18].
Although the findings presented in this study indicate that H2O2 impairs insulin-stimulated mTORC1 assembly and substrate associations, some caveats to the data are noteworthy. First, insulin-mediated reductions in raptor-mTOR binding have not been reported in the literature at concentrations ranging from 0.6–100 nM [14, 15, 17]. Aside from cell-type specific differences, an alternative explanation for the findings is that high insulin concentrations activate a negative feedback loop involving IRS-1 [42]. Consequences of IRS-1 degradation may include time-dependent reductions in Akt, 4E-BP1, or S6K1 phosphorylation, events we did not observe. Nonetheless, because we did not study mTORC1 assembly prior to 30 minutes, we cannot exclude the possibility that rapid mTORC1 activation occurred in the absence of insulin-mediated reductions in raptor-mTOR binding. We were also unable to measure Rheb-mTOR and therefore cannot firmly conclude that peroxide-mediated alterations in Rheb binding or Rheb.GTP loading may impact some of our results. Likewise, it is interesting to note that the reduction in S6K1 phosphorylation in presence of H2O2 remained following PRAS40 siRNA transfection, albeit to a lesser degree. PRAS40 knockdown-associated reductions in insulin-stimulated S6K1 phosphorylation have been previously documented by two independent laboratories using different cell types [15, 17]. The finding that S6K1 phosphorylation is the not solely mediated by raptor binding in response to oxidants raises speculation that PRAS40 possesses S6K1-specific regulatory activity distinct from mTORC1, perhaps through interactions with eIF3 as proposed by Wang and colleagues [15].
Finally, although our study indicates that H2O2 alters the stoichiometry of mTORC1, it does not delineate the precise target or targets of peroxide. The demonstration of the redox-sensitive nature of the mTOR-raptor interaction suggests that oxidants could directly modify cysteine-thiols that form the basis of mTORC1 interactions [43]. In addition, peroxide may alter the phosphorylation state of mTORC1 components, such as raptor, which has recently been shown to inhibit mTORC1 function when phosphorylated on Ser722/Ser792 [44]. Future investigation must include study of these potential oxidant-induced protein modifications and their contributions to mTORC1 assembly and function.
In summary, our findings illustrate that H2O2-mediated reductions in insulin-stimulated mTORC1 activity coincide with impaired mTORC1 assembly and reciprocal changes in ratios of PRAS40/raptor and 4E-BP1/raptor derived from mTOR immunoprecipitation. We speculate the raptor ratios reflect changes in raptor substrate binding within mTORC1 consistent with a competitive inhibition mechanism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shenberger JS, Zhang L, Hughlock MK, Ueda T, Watanabe-Fukunaga R, Fukunaga R. Roles of mitogen-activated protein kinase signal-integrating kinases 1 and 2 in oxidant-mediated eIF4E phosphorylation. Int J Biochem Cell Biol. 2007;39:1828–1842. doi: 10.1016/j.biocel.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel J, McLeod LE, Vries RG, Flynn A, Wang X, Proud CG. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur J Biochem. 2002;269:3076–3085. doi: 10.1046/j.1432-1033.2002.02992.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 4.Shenton D, Smirnova JB, Selley JN, Carroll K, Hubbard SJ, Pavitt GD, Ashe MP, Grant CM. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J Biol Chem. 2006;281:29011–29021. doi: 10.1074/jbc.M601545200. [DOI] [PubMed] [Google Scholar]
- 5.Shatkin AJ. Capping of eucaryotic mRNAs. Cell. 1976;9:645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- 6.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- 8.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 9.Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 11.Gulati P, Thomas G. Nutrient sensing in the mTOR/S6K1 signalling pathway. Biochem Soc Trans. 2007;35:236–238. doi: 10.1042/BST0350236. [DOI] [PubMed] [Google Scholar]
- 12.Findlay GM, Yan L, Procter J, Mieulet V, Lamb RF. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem J. 2007;403:13–20. doi: 10.1042/BJ20061881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases Bind Raptor and Mediate Amino Acid Signaling to mTORC1. Science. 2008 doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseca BD, Smith EM, Lee VH, Mackintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007 doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Harris TE, Lawrence JC., Jr Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem. 2008;283:15619–15627. doi: 10.1074/jbc.M800723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 18.Fonseca BD, Lee VH, Proud CG. The binding of PRAS40 to 14-3-3 proteins is not required for activation of mTORC1 signalling by phorbol esters/ERK. Biochem J. 2008;411:141–149. doi: 10.1042/BJ20071001. [DOI] [PubMed] [Google Scholar]
- 19.Hyslop PA, Zhang Z, Pearson DV, Phebus LA. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res. 1995;671:181–186. doi: 10.1016/0006-8993(94)01291-o. [DOI] [PubMed] [Google Scholar]
- 20.Cao C, Lu S, Kivlin R, Wallin B, Card E, Bagdasarian A, Tamakloe T, Chu WM, Guan KL, Wan Y. AMP-activated protein kinase contributes to UV- and H2O2-induced apoptosis in human skin keratinocytes. J Biol Chem. 2008 doi: 10.1074/jbc.M804144200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, Powell RJ. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–517. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- 22.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 23.Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14(Spec No 2):R251–258. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- 24.Tee AR, Blenis J, Proud CG. Analysis of mTOR signaling by the small G-proteins, Rheb and RhebL1. FEBS Lett. 2005;579:4763–4768. doi: 10.1016/j.febslet.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Teruya-Feldstein J, Bonner P, Bernardi R, Franz DN, Witte D, Cordon- Cardo C, Pandolfi PP. Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res. 2007;67:7106–7112. doi: 10.1158/0008-5472.CAN-06-4798. [DOI] [PubMed] [Google Scholar]
- 26.Eguchi S, Tokunaga C, Hidayat S, Oshiro N, Yoshino K, Kikkawa U, Yonezawa K. Different roles for the TOS and RAIP motifs of the translational regulator protein 4E-BP1 in the association with raptor and phosphorylation by mTOR in the regulation of cell size. Genes Cells. 2006;11:757–766. doi: 10.1111/j.1365-2443.2006.00977.x. [DOI] [PubMed] [Google Scholar]
- 27.Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem. 2003;278:15461–15464. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- 28.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 29.Oshiro N, Takahashi R, Yoshino K, Tanimura K, Nakashima A, Eguchi S, Miyamoto T, Hara K, Takehana K, Avruch J, Kikkawa U, Yonezawa K. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem. 2007;282:20329–20339. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Loghlen A, Perez-Morgado MI, Salinas M, Martin ME. N-acetyl-cysteine abolishes hydrogen peroxide-induced modification of eukaryotic initiation factor 4F activity via distinct signalling pathways. Cell Signal. 2006;18:21–31. doi: 10.1016/j.cellsig.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Pham FH, Sugden PH, Clerk A. Regulation of protein kinase B and 4E-BP1 by oxidative stress in cardiac myocytes. Circ Res. 2000;86:1252–1258. doi: 10.1161/01.res.86.12.1252. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Rhodes CJ, Lawrence JC., Jr Activation of mammalian target of rapamycin (mTOR) by insulin is associated with stimulation of 4EBP1 binding to dimeric mTOR complex 1. J Biol Chem. 2006;281:24293–24303. doi: 10.1074/jbc.M603566200. [DOI] [PubMed] [Google Scholar]
- 33.Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 34.Radisavljevic ZM, Gonzalez-Flecha B. TOR kinase and Ran are downstream from PI3K/Akt in H2O2-induced mitosis. J Cell Biochem. 2004;91:1293–1300. doi: 10.1002/jcb.20037. [DOI] [PubMed] [Google Scholar]
- 35.Rolfe M, McLeod LE, Pratt PF, Proud CG. Activation of protein synthesis in cardiomyocytes by the hypertrophic agent phenylephrine requires the activation of ERK and involves phosphorylation of tuberous sclerosis complex 2 (TSC2) Biochem J. 2005;388:973–984. doi: 10.1042/BJ20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 37.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument- Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Guan L, Song K, Pysz MA, Curry KJ, Hizli AA, Danielpour D, Black AR, Black JD. PKC-mediated down-regulation of cyclin D1 involves activation of the translational repressor 4E-BP1 via a PI3K/Akt-independent, PP2A-dependent mechanism in intestinal epithelial cells. J Biol Chem. 2007 doi: 10.1074/jbc.M610513200. [DOI] [PubMed] [Google Scholar]
- 40.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Lee VH, Healy T, Fonseca BD, Hayashi A, Proud CG. Analysis of the regulatory motifs in eukaryotic initiation factor 4E-binding protein 1. Febs J. 2008;275:2185–2199. doi: 10.1111/j.1742-4658.2008.06372.x. [DOI] [PubMed] [Google Scholar]
- 42.Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006;26:63–76. doi: 10.1128/MCB.26.1.63-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarbassov DD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem. 2005;280:39505–39509. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- 44.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]