Abstract
An automated platform for development of high producing cell lines for biopharmaceutical production has been established in order to increase throughput and reduce development costs. The concept is based on the Cello robotic system (The Automation Partnership) and covers screening for colonies and expansion of static cultures. In this study, the glutamine synthetase expression system (Lonza Biologics) for production of therapeutic monoclonal antibodies in Chinese hamster ovary cells was used for evaluation of the automation approach. It is shown that the automated procedure is capable of producing cell lines of equal quality to the traditionally generated cell lines in terms of colony detection following transfection and distribution of IgG titer in the screening steps. In a generic fed-batch evaluation in stirred tank bioreactors, IgG titers of 4.7 and 5.0 g/L were obtained for best expressing cell lines. We have estimated that the number of completed cell line development projects can be increased up to three times using the automated process without increasing manual workload, compared to the manual process. Correlation between IgG titers obtained in early screens and titers achieved in fed-batch cultures in shake flasks was found to be poor. This further implies the benefits of utilizing a high throughput system capable of screening and expanding a high number of transfectants. Two concentrations, 56 and 75 μM, of selection agent, methionine sulphoximine (MSX), were applied to evaluate the impact on the number of colonies obtained post transfection. When applying selection medium containing 75 μM MSX, fewer low producing transfectants were obtained, compared to cell lines selected with 56 μM MSX, but an equal number of high producing cell lines were found. By using the higher MSX concentration, the number of cell line development projects run in parallel could be increased and thereby increasing the overall capacity of the automated platform process.
Keywords: Chinese hamster ovary cells, Automation, Cello robotic system, Cell line development, Glutamine synthetase
Introduction
A stable high expressing cell line is a prerequisite for achieving antibody yields suitable for commercial biopharmaceutical production. Chinese hamster ovary (CHO) cells are the most commonly used mammalian cells for recombinant protein production. Expression systems based on CHO cells such as the CHO dihydrofolate reductase (DHFR) system (Page and Sydenham 1991) and the CHO glutamine synthetase (GS) system (Cockett et al. 1990; Birch and Racher 2006) have now become industrial standard. The GS gene expression system (Lonza Biologics, Slough, UK) is based on CHO and NS0 cells and makes use of the metabolic pathway of glutamate and ammonium to glutamine for the selection of recombinant cells (Birch et al. 2005). Methionine sulphoximine (MSX) irreversibly binds GS and is used as a powerful selection agent when the GS gene and the gene of interest are co-expressed (Sanders and Wilson 1984; Bebbington et al. 1992; Barnes et al. 2000). Expression is enhanced by a strong viral promoter for the gene of interest (Birch et al. 2005) and does not depend on the copy number of the integrated gene (Peakman et al. 1994). Only transfectants that have stably incorporated GS in a transcriptionally active site will survive at a selection pressure of 50 μM MSX (Birch and Racher 2006). In most mammalian expression systems, including the GS system, plasmid DNA is randomly integrated into host cell chromosomes (Wurm 2004) and expression levels are thereby dependent on site of integration. A distribution curve of productivity of clones, originating from one transfection event, usually shows only a few clones that have significantly higher productivity levels. In most cases, the number of high producing clones detected depend on the number of clones screened (Carroll and Al-Rubeai 2004). The clone selection process has been referred to as the main bottleneck when generating a high producing cell line, since sub cloning and testing is usually time consuming and labour intensive. Several techniques have been developed with the intention to reduce time constraints and manpower costs during cell line development. This includes fluorescence based systems such as fluorescence-activated cell sorting, FACS (Carroll and Al-Rubeai 2004; Brezinsky et al. 2003; Sleiman et al. 2007), and more recently, the Genetix ClonePix FL system. We have chosen another approach to reduce cost and decrease labour, which is not dependent on fluorescence technology. A system capable of screening for colonies and expansion of static cultures has been established utilizing the Cello robotic system developed by The Automation Partnership, Royston, UK. The system combines optical clone screening using automated microscopy and scale up of static cultures with parallel automated cell culture and 24 h operation. The system software records all process steps and associated data in a relational database together with clone images to facilitate detailed documentation of the selection process. This is of importance from a regulatory perspective. The current work describes evaluation of the automated platform process for cell line development by comparison to the manual process. A protocol based on the GS-CHO system was applied in both processes. Different DNA constructs, encoding IgG1 human monoclonal antibodies, were used in the manual and automated processes, respectively. The relative performance of the different processes in terms of IgG titer and number of full time equivalents (FTE) was compared. The automated process was capable of generating high expressing cell lines with reduced man time demand compared to the manual process. We have also studied further possibilities to increase capacity and reduce the manual workload in cell line development. This could be achieved by reducing the number of culture plates handled for each cell line generation project in the Cello system, either by selecting fewer cell lines for scale up or by preventing growth of low producing transfectants. Firstly, the correlation between IgG titer of individual transfectants in the different evaluation steps was investigated to see if cell lines that would be high IgG producers in fed-batch culture could be predicted at an early stage in the selection process. However, the correlation was not sufficient to allow reduction of the number of transfectants selected for expansion. Secondly, the impact of selection pressure on number of colonies obtained following transfection was evaluated. By using the selection agent concentration of 75 μM MSX, the number of low producers was reduced with maintained number of high producing transfectants compared to selection with 56 μM MSX.
Materials and methods
The Cello robotic system
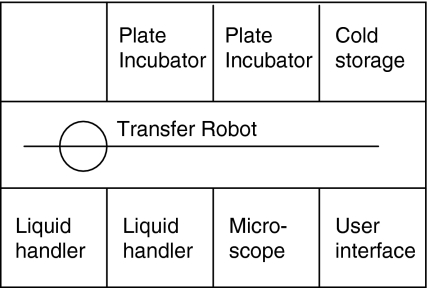
The Cello robotic system was used for automation of clone screening and handling of static cultures in the cell line development process. The system consists of a number of integrated modules positioned on either side of a central plate transfer robot. The modules include a microscope reader (MAIA Scientific, Geel, Belgium), two airflow-controlled liquid handling stations, two plate incubators and a cold storage cabinet (Fig. 1). Culture plates are prepared and placed in one of the Cello incubators. Individual wells on the culture plates are then identified by Cello software based on well number and a unique plate barcode. A generic software process, ‘life cycle’, has been established to control the Cello operations. The life cycle has been designed for our specific application and covers screening for single colonies and expansion of transfectants, handling 96-, 24-, and 6-well plates. It also includes preparation of plates for two separate at-line IgG assays that are the basis for selection of transfectants for expansion. The operator has control over the life cycle through user interface, which enables change of parameters such as pipetting volumes and starting time for a particular operation. Cello software administers processing and scheduling of the different tasks involved in the life cycle. The microscope reader takes high resolution images of transfectants growing in 96-well plates. Images are analyzed by the Cello software to determine clonality and colony size. Colonies that are identified as single colonies in a well are allowed to grow until they reach a pre-defined size and are then automatically transferred to 24-well plates. Examples of images of colonies are shown in Fig. 2. Pictures are available for manual inspection for a defined period of time and are subsequently archived to disc. Open liquid handling is performed aseptically in two laminar airflow stations. These are equipped with pipetting robots to transfer cells and medium between reservoirs and plates. Source plates are handled one at the time in the liquid-handling modules in order to minimize the risk of cross contamination. Between each transfer of cells, tips are washed with high purity water (ELIX, Millipore, Solna, Sweden) and ethanol (Solveco Steril, Rosersberg, Sweden) for decontamination. Cell culture samples in plates are prepared in Cello for at-line ELISA or HPLC IgG assay. Based on assay results, the operator decides which transfectants shall be expanded further by loading the barcode and well number of the chosen transfectants into the Cello software. Selected transfectants are cultivated for a defined period of time in 6-well plates prior to manual transfer to shake flasks. At the end of an experiment, Cello software creates a report documenting the processing history of all transfectants that have reached the 6-well plate stage, including images of the corresponding initial colonies.
Fig. 1.
Schematic drawing of the Cello robotic system showing the different modules on each side of the central plate transfer robot
Fig. 2.
Pictures of transfectants growing in 96-well plates taken with Cello microscope (MAIA Scientific). a A small single colony. b A single colony of sufficient size to be expanded. c Multiple colonies
Overview of the cell line development procedure
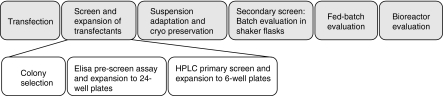
An outline of the different steps in the cell line development procedure is shown in Fig. 3. Following transfection, cell suspension was distributed over 96-well plates, which were incubated for three to four weeks, prior to colony detection. Single colonies were expanded to 24-well plates. A pre-screen ELISA assay was used to rank transfectants grown in 96-well plates with respect to IgG titer. Based on the results from this assay, cell lines were selected for expansion to 6-well plates. A primary screen of IgG titer in 24-well plates was performed on these transfectants in order to distinguish, which cell lines to expand to shake flask cultures. The selected transfectants were further evaluated in batch- and fed-batch cultures in shake flasks. The best producing cell line was further evaluated in fed-batch culture in a stirred tank bioreactor.
Fig. 3.
Schematic drawing of the cell line development process used in this study. Colony selection and expansion to 24- and 6-well format is automated in the Cello robotic system
CHO cell culture
The suspension adapted CHOK1SV host cell line (Lonza Biologics) was cultured in chemically defined, animal component free (CDACF) CD-CHO medium (Invitrogen, Carlsbad, CA, USA) supplemented with 6 mM l-glutamine (Invitrogen). Host cells were cultivated in vented cap shake flasks, sub-cultured every 2–3 days by dilution to 0.2 × 106 cells/mL and incubated at 36.5 °C, 150 rpm and 10% CO2 (Infors AG, Bottmingen, Switzerland). Cell density and viability were determined using a CEDEX cell counter (Innovatis AG, Bielefeld, Germany) using the trypan blue dye exclusion method. Transfected cells at the shake flask cultivation stage were grown in CD-CHO supplemented with 50 μM MSX (Sigma–Aldrich, St Louis, MO, USA) and cultivated following the same routine as for host cells.
Vectors, transfection and selection
CHOK1SV cells were transfected by electroporation (Gene Pulser, BioRad, München, Germany) following Lonza Biologics’ proprietary protocol. Linearized plasmid DNA encoding IgG1 heavy and light chain, with a vector backbone derived from Lonza Biologics was used for transfection. In this study, two DNA constructs were used, encoding different IgG1 human monoclonal antibodies (mAb A and mAb B). Transfected cells were diluted in CD-CHO medium and distributed over 96-well plates and incubated at 36.5 °C in a humidified incubator at 10% CO2. One day post transfection, CD-CHO medium supplemented with MSX was added to each well giving a final MSX concentration of 56 or 75 μM.
Colony detection and expansion
In the automated procedure, 96-well plates containing transfectants were loaded into the Cello system 3 weeks post transfection and repeatedly screened for cell colonies over the course of 2 weeks. Single colonies with an area exceeding 1.8 × 106 square microns were automatically selected for ELISA assay in 96-well format and further expanded to 24-well plates. The ELISA assay was applied as a pre-screen of the transfectants regarding antibody titer in the supernatant. 20–30% of the transfectants were selected for further expansion to 6-well plates. As a primary screen, IgG concentration in cell free supernatant from 14-day overgrown batch cultures in 24-well plates was analyzed, using a protein A HPLC method. 15–20% of transfectants were selected for further expansion to shake flasks. In the manual procedure, colonies were visually detected in the 96-well plates three to 5 weeks post transfection, using a Nikon TMS microscope. Supernatants from wells containing single colonies of an approximate size of 1/3 of the well were selected for ELISA assay as described above. Cell cultures were manually expanded from 96-to 24-well plates, 6-well plates and shake flasks. IgG assays and rankings were carried out as described for the automated procedure.
IgG titer evaluation of batch- and fed-batch culture in shake flasks
Cell cultures in 6-well plates were manually expanded to 30 mL cultivation volume in 125-mL shake flasks. When cell cultures reached an exponential growth pattern they were evaluated in a batch process in 50 mL cultivation volume in 250 mL shake flasks. After 14 days, IgG content was analyzed using a protein A HPLC method. The best producing cell lines were further evaluated in a 14-day fed-batch process. This represents a scale-down model of the final fed-batch bioreactor production process using CD-CHO medium and Lonza Biologics’ CDACF GS-CHO supplements and feeds (Lonza Biologics proprietary information). At harvest, cell free supernatants were analyzed for IgG concentration as above and the top cell line was chosen for further evaluation in stirred tank bioreactor.
Bioreactor cell culture
The best producing cell line from the manual procedure and the automated procedure, respectively, was evaluated in a generic fed-batch process in a stirred tank bioreactor. The cell line from the manual procedure was evaluated in 20 L cultivation volume in a stainless steel stirred tank bioreactor (Braun, Melsungen, Germany). The cell line from the automated procedure was cultured in 25 L cultivation volume in a single use bioreactor (Hyclone, Logan, UT, USA). Bioreactors were inoculated with 0.2 × 106 cells/mL and the cells were cultured for 14 days at 36.5 °C, 15% dissolved oxygen tension and pH 7.0. Agitation speed was 100 rpm in the stainless steel reactor and 125 rpm in the single use bioreactor. The cultivation processes were performed following a standard fed-batch protocol (proprietary information to Lonza Biologics). CD-CHO was used as cell culture medium and Lonza Biologics’ proprietary CDACF GS-CHO supplements and feeds were added during cultivation. Process parameters including pH, dissolved oxygen tension and temperature were controlled and monitored throughout the cultivation process. Samples were taken daily from the bioreactor and IgG titer in cell free supernatant was determined by protein A HPLC.
IgG analysis
A standard sandwich ELISA assay was used as a pre-screening step to remove non- or very low producing transfectants. Rabbit anti-human kappa light chain (Sigma–Aldrich) was used as capture antibody and polyclonal rabbit anti-human IgG horseradish peroxidase (HRP; Sigma–Aldrich) as a detection antibody. o-Phenylenediamine dihydrochloride (OPD; Sigma–Aldrich) substrate was used for oxidation of HRP and plates were analyzed at 450 nm in EL × 800 absorbance microplate reader (BioTek, Winooski, VT, USA). A protein A HPLC method was used to rank cell lines in primary and secondary screen and to determine IgG titer in fed-batch cultures in shake flasks and bioreactor evaluations. Cell free supernatant was prepared by centrifugation of cell suspensions at 2,000g for 10 min. Samples were analyzed by HPLC (Agilent, Palo Alto, CA, USA) using an Omnifit column 3 × 50 mm (Omnifit, Cambridge, UK) packed with rProtein A Sepharose™ fast flow (GE Healthcare, Uppsala, Sweden) or a MiniChrom 5 × 10 mm column (Atoll, Weingarten, Germany) packed with MabSelect SuRe Flow (GE Healthcare). Peaks were detected both at 214 and 280 nm. PBS, pH 7, was used as equilibration buffer and PBS pH 2.5 as elution buffer. Concentrations were determined by normalization against a standard curve prepared from an in-house IgG standard.
Results and discussion
Verification of automated procedure: a comparative study
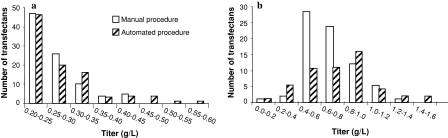
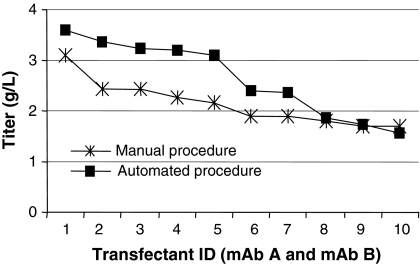
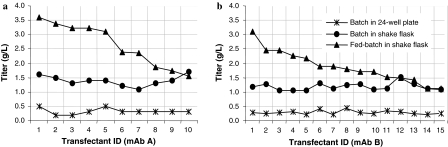
A comparative study between the automated and the manual cell line development process was performed in order to evaluate the automated procedure with respect to detection of transfectants and distribution of IgG titer at different stages during scale up. Two DNA constructs were used, encoding different IgG1 human monoclonal antibodies (mAb A and mAb B). Cell lines expressing mAb A were developed according to the proven manual process and cell lines expressing mAb B were developed following the automated process. One of the objectives for the automated procedure was to enable screening of a large number of transfectants in order to increase the probability of finding high producing cell lines (Carroll and Al-Rubeai 2004). The initial experiment was designed to screen a comparable number of transfectants in both the automated and manual procedure. In the automated procedure, 120 96-well plates were screened in the Cello robotic system and single colonies were detected by the Cello software and automatically selected for expansion. In the manual procedure, transfection was repeated and in total 160 plates were screened. Following each evaluation step, a comparable number of transfectants were selected for further expansion (Table 1). IgG titers of transfectants exceeding expression levels of 0.2 g/L in the primary screen are shown in Fig. 4a. The distribution pattern of the titers from transfectants cultured in the manual and the automated procedure are very similar with a large number of low producers and a few high producing transfectants. Figure 4b shows IgG titer distribution from the subsequent secondary screen of batch cultures in shake flasks. Most of the clones show an IgG titer of 0.4–1.2 g/L, but there are a few transfectants reaching titers exceeding 1.2 g/L. The ten best expressing cell lines according to IgG titer obtained in the secondary screen were submitted to a fed-batch evaluation in shake flasks. The cell lines generated in the automated and the manual process were comparable regarding IgG titer at harvest (Fig. 5). The IgG titer of all cell lines in this fed-batch evaluation was more than 1 g/L and the best expressing cell lines from each process showed IgG titers exceeding 3 g/L. The cell lines showing the highest IgG titers in fed-batch evaluations were evaluated in a generic fed-batch process in bioreactor, giving IgG titers of 5.0 and 4.7 g/L for cell lines from the manual and the automated process, respectively. Our results show that the automated procedure supports cell growth and survival during the cell line development process. By using this procedure, cell lines were generated giving antibody titers at the same level as cell lines generated in the manual procedure. Similar results were obtained in a second experiment (data not shown). In that experiment, transfectants expressing a human IgG4 antibody were developed according to the automated procedure and five cell lines reached antibody titers exceeding 4 g/L in generic shake flask fed-batch cultures. The effort required for clone screening and scale up in the manual and the automated process, respectively, was estimated. The time from transfection until expansion of transfectants to shake flasks is comparable in the automated and the manual procedure. However, in the automated procedure, significantly less manual labour is required. This markedly reduces the effort required for cell line development (Table 2). In our calculations, a project was defined to include transfection, screening for colonies, expansion and transfer of transfectants to shake flasks. Effort was estimated in ‘Full time equivalents’ with one FTE corresponding to 30 man hours of work per week. In a standard screen, 1.5 FTE are required for completing ten projects per year using the manual procedure. Based on the FTE requirement for our current cell line development projects, we have estimated that 1.5 FTE is needed for 20 projects per year using the automated system. Additional benefits can be achieved by enlarging colony screenings from our standard target of 400 colonies on 80 plates to 800 colonies on 160 plates. The number of completed projects could then be increased by three times using the automated compared to the manual process, without increasing the FTE number. This is possible, since the capacity of the Cello allows 160 plates to be processed per project, only requiring extra operator time for the manual at-line analysis. In comparison, if the selection process started with 160 instead of 80 plates, the manual screening and scale up procedure would require twice the man hours.
Table 1.
Number of wells or transfectants analyzed in different steps through cell line development
| Cello process | Manual process | |
|---|---|---|
| 96-well plates screened | 120 | 160 |
| Transfectants analyzed in ELISA assay | 1,083 | 792 |
| Transfectants analyzed in HPLC assay | 243 | 237 |
| Cell lines evaluated in batch culture in shake flask | 52 | 73 |
| Cell lines evaluated in fed-batch culture in shake flask | 10 | 15 |
Fig. 4.
Titer distribution of transfectants generated in manual procedure (mAb A) and automated procedure (mAb B). a IgG titer of batch cultures with IgG titer exceeding 0.2 g/L in 24-well plates. b IgG titer of batch cultures in shake flasks
Fig. 5.
IgG titer distribution from fed-batch evaluation of transfectants generated in the manual procedure (mAb A) and the automated procedure (mAb B). Transfectants are ranked and numbered according to IgG titer
Table 2.
Comparison of cell line development projects per year for manual and automated process at fixed effort
| Standard screen: target 400 clones on 80 plates | Large screen: target 800 clones on 160 plates | |||
|---|---|---|---|---|
| Manual process | Automated process | Manual process | Automated process | |
| Number of projects per year with 1.5 FTE* | 10 | 20 | 5 | 15 |
* One full time equivalent (FTE) equals 30 man hours per week
The correlation between titer in primary screen, secondary screen and fed-batch evaluation
In each screening step during cell line development, low producing transfectants are removed to decrease workload in the subsequent steps. However, still a large number of transfectants, typically 20–30%, is progressed in each step to prevent removal of potentially high expressing transfectants. In the current study, IgG titers of individual cell lines were compared for the different screening steps. Only the top ten cell lines from manual or automated screening that were assigned for evaluation in fed-batch cultures were studied. The correlation in cell line ranking was evaluated in order to investigate whether the best producers in fed-batch cultures could be predicted based on data from an earlier screen (Fig. 6). In general, titers increased when going from static cultures in the primary screen to suspension cultures in the secondary screen. This can be explained by an increased cell density in suspension culture, compared to static cultures (data not shown). Even higher IgG titers were obtained when going from batch to fed-batch culture conditions and the IgG titers were markedly more variable for different cell lines. This could be explained by a higher cell density compared to the batch evaluation but also by transfectant specific response to the feeds. Since the same variability was not observed when comparing IgG titers in the earlier screens it was not possible to predict the best producer at an earlier stage. As a result, a relatively large number of transfectants need to be expanded to fed-batch evaluation in shake flasks. Larger screens in fed-batch cultures might increase the chance of finding even better producing cell lines due to the poor predictability of titer in batch evaluations.
Fig. 6.
Comparison of IgG titer in batch culture in 24-well plates, batch culture in shake flask and fed-batch culture in shake flask. Transfectants are ranked and numbered according to IgG titer in the fed-batch evaluation. a Transfectants generated in automated procedure (mAb B). b Transfectants generated in manual procedure (mAb A)
Effect of concentration of selection agent on number of colonies and concentration of produced antibody
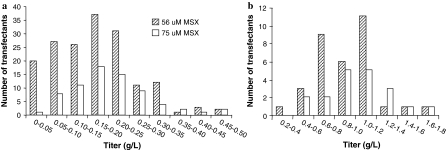
The effects of selection pressure on colony growth and number of colonies obtained during the initial selection phase in cell line development has previously been demonstrated for MSX concentrations of 25 and 50 μM (de la Cruz Edmonds et al. 2006). In our study, the effect of an even higher concentration of MSX selection agent following transfection was examined in terms of number of colonies obtained and titer distribution in primary and secondary screens. The aim was to investigate whether a higher concentration of selection agent could reduce the number of colonies obtained without affecting the probability of finding high expressing transfectants. Cells were transfected with DNA encoding mAb B. One day post transfection, selection medium was added to the cells resulting in final concentrations of 56 or 75 μM MSX, respectively. Screening in the Cello showed that 846 single colonies were obtained, for the lower concentration and 237 colonies for the higher concentration of MSX. This clearly shows that the level of MSX during the first selection phase affects the number of colonies obtained. Supernatants from single colonies were analyzed in a pre-screen ELISA assay. 170 colonies selected using the lower MSX concentration and 73 colonies selected using the higher MSX concentration, were expanded to 24-well plates. From this stage, all transfectants were cultured in medium with 75 μM MSX in order to provide equivalent selection conditions during scale up. IgG titers in the subsequent primary screen were compared for transfectants selected with the two MSX concentrations (Fig. 7a). Titers exceeding 0.3 g/L were obtained for both MSX conditions. However, the profile of the distribution curve is different. The number of low producing transfectants is markedly reduced when the higher MSX concentration is applied for selection. The transfectants that were showing the highest IgG titer in the primary screen were transferred to shake flasks. In total 33 and 19 transfectants from the lower and higher MSX concentration, respectively, were evaluated in the secondary screen in batch cultures. A comparable number of cell lines, 14 and 10 selected with low and high MSX pressure, respectively, reached titers exceeding 1.0 g/L (Fig. 7b) and the best producing cell lines from each selection condition produced IgG at similar levels (approximately 1.6 g/L). The higher MSX concentration in the initial selection phase decreased the number of colonies obtained following transfection and thereby reduced the effort required for expansion and titer evaluation in the pre-screen and primary screen. Furthermore, high producing cell lines were found to the same extent although fewer colonies were screened. By using the higher MSX concentration, more cell line projects can be run in parallel and thereby the overall capacity can be increased. Alternatively, by transfecting a larger number of cells combined with a concentration of 75 μM MSX in the initial selection still a high number of colonies could be screened. This would result in a higher number of high producing cell lines for further evaluation, which is favourable since candidate cell lines could be withdrawn at a later stage if requirements for large scale production like stability or product quality, are not fulfilled. It might be possible to further increase the MSX concentration in the initial selection phase in order to get an even more stringent selection. However, higher concentration of MSX might be toxic to the cells and lead to amplification of the GS gene, leading to poor growth and stability issues (Birch and Racher 2006).
Fig. 7.
Titer distribution of transfectants generated with two different MSX concentrations post transfection. a results of IgG titer of batch cultures in 24-well plates. b results of IgG titer of batch cultures in shake flasks
Conclusions
In this study we have demonstrated that our automated procedure for cell line development is capable of generating high producing cell lines. A comparable titer distribution was obtained for the Cello generated cell lines and for the manually generated cell lines at different stages of the cell line development process. IgG titers of 5.0 and 4.7 g/L were obtained in fed-batch evaluations in bioreactor scale of the best producing cell line from the manual and the automated process, respectively. Our automated process has a large screening capacity and three times as many cell line development projects can be run in the automated process compared to the manual process without increasing the manual workload. In addition, documentation is simplified and traceability is improved. The correlation between IgG titers obtained for best expressing cell lines in batch cultivation in 24-well plate and shake flask as well as in fed-batch culture in shake flask was unfortunately poor. This suggests that it is not possible to select top producers from data obtained early in the cell line development process. The selection pressure was shown to have impact on the number of colonies generated following transfection. When using a selection pressure of 75 μM MSX, cell lines giving high titer in batch and fed-batch culture were found to the same extent as with 56 μM MSX, although a lower number of colonies had to be screened. By using the higher MSX concentration, several cell line development projects could be run in parallel in the Cello and thereby the overall capacity of the automated process would be increased.
Acknowledgments
We would like to thank Niklas Henriksson and our colleagues in the Upstream Development Group, Bio Process R&D, AstraZeneca for their technical support. We would also like to thank Tim Ward, The Automation Partnership, for critical reading of the manuscript.
Footnotes
A. Salmén and K. Lindgren contributed equally to the work.
References
- Barnes LM, Bentley CM, Dickson AJ (2000) Advances in animal cell recombinant protein production: GS-NS0 expression system. Cytotechnology 32:109–123. doi:10.1023/A:1008170710003 [DOI] [PMC free article] [PubMed]
- Bebbington CR, Renner G, Thomson S, King D, Abrams D, Yarranton GT (1992) High-level expression of a recombinant antibody from myeloma cells using a glutamine synthetase gene as an amplifiable selectable marker. Biotechnology 10:169–175. doi:10.1038/nbt0292-169 [DOI] [PubMed]
- Birch JR, Racher AJ (2006) Antibody production. Adv Drug Deliv Rev 58:671–685. doi:10.1016/j.addr.2005.12.006 [DOI] [PubMed]
- Birch JR, Mainwaring DO, Racher AJ (2005) Use of the glutamine synthetase (GS) expression system for the rapid development of highly productive mammalian cell processes. Mod Biopharm 809:832
- Brezinsky SCG, Chiang GG, Szilvasi A, Mohan S, Shapiro RI, MacLean A, Sisk W, Thill G (2003) A simple method for enriching populations of transfected CHO cells for cells of higher specific productivity. J Immunol Methods 277:141–155. doi:10.1016/S0022-1759(03)00108-X [DOI] [PubMed]
- Carroll S, Al-Rubeai M (2004) The selection of high-producing cell lines using flow cytometry and cell sorting. Expert Opin Biol Ther 4:1821–1829. doi:10.1517/14712598.4.11.1821 [DOI] [PubMed]
- Cockett MI, Bebbington CR, Yarranton GT (1990) High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamine synthetase gene amplification. Biotechnology 8:662–667. doi:10.1038/nbt0790-662 [DOI] [PubMed]
- de la Cruz Edmonds MC, Tellers M, Chan C, Salmon P, Robinson DK, Markusen J (2006) Development of transfection and high producer screening protocols for the CHOK1SV cell system. Mol Biotechnol 34:179–190. doi:10.1385/MB:34:2:179 [DOI] [PubMed]
- Page MJ, Sydenham MA (1991) High-level expression of the humanized monoclonal antibody Campath-1H in Chinese hamster ovary cells. Biotechnology 9:64–68. doi:10.1038/nbt0191-64 [DOI] [PubMed]
- Peakman TC, Worden J, Harris RH, Cooper H, Tite J, Page SJ, Gewert DR, Bartholemew M, Crowe JS, Brett S (1994) Comparison of expression of a humanized monoclonal antibody in mouse NS0 myeloma cells and Chinese hamster ovary cells. Hum Antibodies Hybridomas 5:65–74 [PubMed]
- Sanders PG, Wilson RH (1984) Amplification and cloning of the Chinese hamster glutamine synthetase gene. EMBO J 3:65–71 [DOI] [PMC free article] [PubMed]
- Sleiman RJ, Gray PP, McCall MN, Codamo J, Sunstrom NAS (2007) Accelerated cell line development using two-colour fluorescence activated cell sorting to select highly expressing antibody-producing clones. Biotechnol Bioeng 99:578–587. doi:10.1002/bit.21612 [DOI] [PubMed]
- Wurm FM (2004) Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22:1393–1398. doi:10.1038/nbt1026 [DOI] [PubMed]