Abstract
Embryoid bodies (EBs) are primitive embryonic structures derived from differentiating embryonic stem cells (ESCs). Many techniques have been used to obtain EBs. Improving the technique of EB formation can help in achieving better results in ESCs differentiation into neurons, myocardiocytes, haemopoeitic cells, and others. We evaluated the use of Sigmacote™ as a hydrophobic substrate to improve EB formation. CCE and P19 cell lines were used to obtain EBs and retinoic acid was used to induce neural differentiation. The results revealed that Sigmacote™, as a hydrophobic substrate, can improve EB formation from ESCs. Our results demonstrate that the silicon-coating of glass petri dishes by Sigmacote™ is an easy and reproducible technique to enhance EB formation from murine ESCs and EC cells.
Keywords: Embryoid body, Hydrophobic substrate, Sigmacote, P19 cell line, CCE cell line
Introduction
Embryonic stem cells (ESCs) are pluripotent cells derived from the inner cell mass of blastocysts (Evans and Kaufman 1981; Thomson et al. 1995, 1998; Park et al. 2004). Their ability to differentiate into different types of mature cells, such as neurons, myocardiocytes, endothelial cells, and insulin secreting cells (Bain et al. 1995; Maltsev et al. 1994; Hirashima et al. 1999; Lumelsky et al. 2001) has raised the possibility that these cells can be used therapeutically to treat many diseases, such as neurodegenerative diseases, myocardial infarction, and diabetes mellitus (Bjorklund et al. 2002; Liu et al. 2000; Johkura et al. 2003; Soria et al. 2000). Many investigators have shown that different chemicals, such as retinoic acid and neurogenic medium, can induce the differentiation of ESCs into neuroepithelial cells (Gottlieb and Huettner 1999; Brustle et al. 1999; Chung et al. 2002). ESCs should first be allowed to form embryoid bodies (EBs) in culture media without inhibitory factors of differentiation, such as leukemia inhibitory factor (Yamada et al. 2002), STO cell line (Xian et al. 2003), and primary embryo fibroblast (Nishikawa et al. 1998). There are several techniques to encourage EB formation, which include the suspended drop method, the bacteriological culture petri dish method, and using semisolid substrates, such as methylcellulose. Other methods include having ESCs in direct contact with stromal cells (Keller GM 1995; Stephen et al. 2002), the use of polypropylene conical tubes (Kurosawa et al. 2003), the slow turning later vessel (STLV) method (Wang et al. 2006), and culturing ESCs on polystyrene surfaces coated with the polymer, 2-methacryloyloxyethyl phosphorylcholine (MPC) (Koike et al. 2005; Konno et al. 2005).
The drop method is the most widely used of all these techniques. However, the method is difficult to use and is not sufficiently efficient for forming EBs (Kurosawa et al. 2003; Konno et al. 2005). Furthermore, Gottlieb reported that the culturing of ESCs in a liquid suspension culture on bacteriological grade petri dishes could result in occasional toxicity or cell adhesion (Gottlieb, http://thalamus.wustl.edu/gottlieblab/gottlieb_lab_3.html).
Against this background, additional research is needed if a new, simple and efficient protocol for the formation of EBs from ESCs is to be developed. We propose a simple method for forming EBs from murine ESCs and EC cells. A hydrophobic substance known as Sigmacote was used as a substrate to form EBs. Retinoic acid was then used as an inducer of EBs to obtain neuronal cells and to confirm that these EBs are useful for further study of differentiation.
Materials and methods
P19 (EC) cell line, originally derived from a teratocarcinoma induced in C3H/HC mice (McBurney et al. 1982), is obtained from Pastur Institute, Tehran, Iran.
CCE, a mouse embryonic stem (ES) cell line derived from 129/Sv mouse strain (Robertson et al. 1986; Keller et al. 1993), is kindly provided by Dr. Ahmad-Reza Bahrami at the University of Sheffield. P19 cells were maintained in an undifferentiated state in RPMI-1640 medium (Gibco-BRL) supplemented with 10% fetal bovine serum (Gibco-BRL) in an incubator under 5% CO2. CCE cells were maintained in DMEM (Gibco-BRL) supplemented with 15% fetal bovine serum (FBS) (Gibco-BRL), 100 U/mL penicillin (Gibco-BRL), 50 μg/mL streptomycin (Gibco-BRL), 0.5 μM 2-mercaptoethanol (2ME) and 500 pM LIF (StemCell Technologies, Vancouver, BC, Canada) (Xian et al. 2003; Nishikawa et al. 1998). The cells were cultured on 60 mm petri dishes (Nunc) coated with 1% gelatin (Sigma) in phosphate buffered saline, pH 7.2–7.4. Glass petri dishes were used to evaluate the effect of Sigmacote (Sigma, SL-2) on EB formation. Siliconization was carried out in three stages. (1) Initially, the bottom of a clean dry 100 mm glass petri dish was covered with undiluted Sigmacote for 30 min at room temperature and covered to prevent the evaporation of Sigmacote. The excess Sigmacote was then poured off. (2) The dish was dried at room temperature in a sterilized hood for 30 min and washed with sterilized double distilled water to remove hydrochloric acid by product. (3) Then the dishes were dried at 100 °C in an oven for 30 min. P19 cells (1 × 105 cells/mL) were seeded onto the Sigmacote-coated dishes and maintained in the RPMI-1640 medium supplemented with 0.5 μM retinoic acid (RA) and 5% FBS (Jones-Villeneuve et al. 1982; Robertson 1987), under the same culture conditions. The cells aggregated spontaneously and the number of cell aggregates or EBs were counted 2 days later. Two days later, the medium was replaced with fresh medium containing the same concentration of RA. Then the cells were cultured for a further 2 days. The aggregated cells were plated onto tissue culture grade plastic dishes surface coated with 1% gelatin in DMEM supplemented with 10% FBS. The cultures were maintained for 12 days, during which time the medium was changed every 3 days. The cells were examined for neuronal differentiation. The CCE cell line was trypsinized and the dispersed cells (1 × 105 cells/mL) were plated on bacteriological grade petri dishes and Sigmacote-coated petri dishes. The cells were allowed to aggregate in the DMEM medium that contained 7.5% FBS without LIF and RA. The cultures were kept for 48 h, after which the number of EBs was counted. RA (0.5 μM) (Xian et al. 2003) was added for a further 2 days. The aggregated cells were transferred to plastic tissue culture grade dishes (Nunc) surface coated with 1% gelatin that contained DMEM supplemented with 10% FBS.
The differentiation of P19 and CCE cells into neuronal lineage was determined by indirect immunocytochemistry. For immunocytochemistry studies, cells were grown on glass coverslips coated with 0.1% gelatin. They were then fixed by 20-min incubation in PBS containing 4% paraformaldehyde, rinsed in PBS, and stored at 4 °C in this buffer until used. All subsequent steps of permeabilization, washing, and incubation with antibodies were performed at room temperature. Fixed cells were permeabilized for 10 min in PBS containing 0.02% triton X, blocked for 30 min in PBS goat serum (PBS containing 1% goat serum and 0.02% triton X), and incubated for 45 min with the primary antibody diluted (Nestin 1-500 and Synaptophysin 1-200, Chemicon) for 60 min with a HRP-conjugated antibody (Serotrc) diluted 1:100. PBS was used for washing between incubations. Coverslips were mounted in PBS containing 50% glycerol and were immediately examined under the microscope. Nestin and synaptophysin are special markers for neural progenitor cells and neural cells, respectively.
Statistical analysis
The data were analyzed statistically using SPSS software. The data were tested by student test.
Results
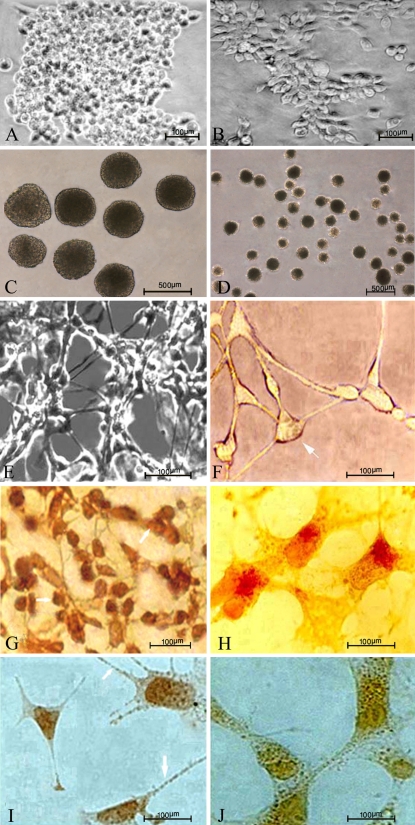
Our results demonstrated that with our new method, EBs can be prepared easily and reproducibly. Table 1 shows the number of EBs in five replicates, where the data from Sigmacote technique was compared with those of suspended drop methods for both cell lines. The number of EBs increased significantly when siliconized petri dishes were used. There was no significant difference between the two cell lines in the bacteriological grade petri dish group. Uniform EBs were formed from the CCE cell line and P19 cells after 48 hours using Sigmacote as a substrate (Fig. 1c, d). Spherical cell aggregates were called EBs on the basis of their ability to differentiate to neuroprogenitor and neural cells. Four-day-old EBs were seeded into 24-well gelatin-coated dishes and cultured for further differentiation. Twenty-four wells were seeded with EBs prepared from siliconized petri dishes. The EBs were observed under a microscope periodically, to monitor the generation of the neuroprogenitor and neural cells. On days 6–8, the percentage of the wells that generated neural progenitor and neural cells from the EBs derived from P19 cells and CCE cells that were cultured on siliconized petri dishes was 92 and 77%, respectively. The percentage of wells that generated neural progenitor and neural cells from the EBs when bacteriological-grade petri dishes were used was 90 and 74%, respectively. Figure 1 (e and f) shows neuron-like cells derived from CCE and P19 cells. These cells have extensions from the cell body and form synapse-like connections with other cells. The results of immunocytochemical evaluation indicated that differentiated cells express nestin (Fig. 1 g, h) and synaptophysin antigens (Fig. 1 i, j).
Table 1.
The number of embryoid bodies obtained following seeding of 105 cells/mL P19 and CCE cell lines on Sigmacote-coated culture dishes and bacteriological-grade petri dishes
| Experiment | ESCells (CCE) | ESCells (CCE) | ECCells (P19 Cells) | ECCells (P19 Cells) |
|---|---|---|---|---|
| Type of petri dish | Siliconized glass petri dishes | Bacteriological grade petri dishes | Siliconized glass petri dishes | Bacteriological petri dishes |
| 1 | 296 | 212 | 340 | 320 |
| 2 | 320 | 204 | 324 | 202 |
| 3 | 302 | 206 | 350 | 188 |
| 4 | 288 | 184 | 330 | 204 |
| 5 | 290 | 196 | 312 | 240 |
| Mean | 299 ± 13* | 200 ± 11 | 350 ± 15* | 230 ± 53 |
The number of EBs increased significantly when using siliconized petri dishes for both cell lines. Data are expressed as mean ± SD and obtained from five experiments for each set. (*P < 0.05 compared with the Bacteriological grade petri dishes group)
Fig. 1.
Sigmacote—coated glass petri dishes were used to form EBs from murine ES (CCE line) cells and EC (P19 line) cells. The EBs that formed differentiated into neural cells. Photomicrographs a and b show undifferentiated CCE and P19 stem cells, respectively, which were cultured as monolayer. Photomicrographs c and d show EBs derived from CCE and P19 cells, respectively, after culturing the cells for 2 days on Sigmacote™ as a substrate. EBs derived from P19 cells were cultivated from day 0 to day 4 in the presence of 0.5 μM RA. EBs derived from CCE cells were cultivated from day 0 to day 2 in the medium without RA, then on days 3 and 4 in the presence of 0.5 μM RA as the agent to induce neural differentiation. Neuron-like cells derived from CCE cells (e) and P19 cells (f) exhibited many cytoplasmic extensions from the cell body and formed synapse-like connections with the other cells. The photomicrographs g and h show DAB-immunostaining of differentiated EBs derived from CCE and P19 cells. Neural progenitor nestin positive cells derived from CCE and P19 cells, respectively. Photomicrographs i and j show synaptophysin positive neural cells differentiated from CCE and P19 cells, respectively. Magnification of d is 100×, other magnifications are 200×
Discussion
Sigmacote™ is a clear, colorless solution of a chlorinated organopolysiloxane in heptane. It reacts with surface silanol groups on glass to produce a neutral, hydrophobic, microscopically thin film. The film repels water, retards the clotting of blood or plasma, and prevents adsorption of many basic proteins. The aggregation of ESCs is very important for EB formation and for the subsequent generation of many ESC derivatives (Wang et al. 2006). Thus, we focused on establishing a new protocol for EB formation. Mouse ESCs and EC cells were cultured on siliconized surfaces using Sigmacote. Then, the morphological appearances of the formed EBs were monitored under microscope. The attachment of EBs on the siliconized surfaces was not as good as their attachment on bacteriological grade petri dishes. This is probably the reason for observed lower number of EBs in bacteriological grade petri dishe group.
The method of inducing differentiation of ESCs requires that these cells should not be allowed to attach to the surface of the culture vessel. To prevent such attachment, the suspended or hanging drop technique, in which ESCs settle to the bottom of drop, was used (Robertson 1987). In comparison with a widely used suspended drop method, our results suggest that our new technique is sufficient to promote EB formation and the differentiation of murine ESCs and EC cells into neural cells. In our study, two well-known embryonic stem cell lines were used for EB formation and differentiation. We particularly used the murine P19 embryonal carcinoma cells, which are pluripotent and more extensively characterized stem cells that can be induced to differentiate in vitro into multiple cell types, especially neural and cardiac cells (McBurney 1993; Skerjanc 1999; Van der Heyden and Defize 2003). The differentiation of P19 cells into neural cells or cardiomyocytes is initiated by allowing the cells to aggregate in suspension culture in the presence of different inducers (Skerjanc 1999).
To achieve the clinical potential of ESCs as a source of cells for cell-based technologies, methods for generating large quantities of the desired cell type must be developed (Stephen et al. 2002). We demonstrated that EBs were formed and could be differentiate into neural cells using retinoic acid after culturing murine ESCs and EC lines in siliconized glass petri dishes. This technique is suggested as an easy method for promoting EB formation and differentiation.
Contributor Information
Fardin Fathi, Phone: +98-871-6664673, FAX: +98-871-6664674, Email: farfath@yahoo.com.
Taki Altiraihi, Email: takialtr@modares.ac.ir.
Seyed Javad Mowla, Email: sjmowla@modares.ac.ir.
References
- Bain G, Kitchenes D, Yao M et al (1995) Embryonic stem cells express neuronal properties in vitro. Dev Biol 163:342–357. doi:10.1006/dbio.1995.1085 [DOI] [PubMed]
- Bjorklund LM, Sanchez-Pernaute R, Chung S et al (2002) Embryonic cells develops in to functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA 99:2344–2349. doi:10.1073/pnas.022438099 [DOI] [PMC free article] [PubMed]
- Brustle O, Jones KN, Learish RD et al (1999) Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science 285:754–756. doi:10.1126/science.285.5428.754 [DOI] [PubMed]
- Chung S, Sonntag KC, Andersson T et al (2002) Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur J Neurosci 16:1829–1838 [DOI] [PMC free article] [PubMed]
- Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotent cells from mouse embryos. Nature 292:154–156. doi:10.1038/292154a0 [DOI] [PubMed]
- Gottlieb DI, Huettner JE (1999) An in vitro pathway from embryonic stem cells to neurons and glia. Cells Tissues Organs 165:165–172. doi:10.1159/000016696 [DOI] [PubMed]
- Hirashima M, Kataoka H, Nishikawa S et al (1999) Maturation of embryonic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood 93:1253–1263 [PubMed]
- Johkura K, Cui L, Suzuki A et al (2003) Survival and function of mouse embryonic stem cell-derived cardiomyocytes in ectopic transplants. Cardiovasc Res 58:435–443. doi:10.1016/S0008-6363(02)00730-7 [DOI] [PubMed]
- Jones-Villeneuve EM, McBurney MW, Rogers KA, Kalnins VI (1982) Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol 94:253–262 [DOI] [PMC free article] [PubMed]
- Keller GM (1995) In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 7:862–869 [DOI] [PubMed]
- Keller G, Kennedy M, Papayannopoulou T et al (1993) Hematopoietic commitment during embryonic stem cells differentiation in culture. Mol Cell Biol 13:473–486 [DOI] [PMC free article] [PubMed]
- Koike M, Kurosawa H, Amano YA (2005) Round-bottom 96-well polystyrene plate coated with 2-methacryloyloxyethyl phosphorylcholine as an effective tool for embryoid body formation. Cytotechnology 47:3–10 [DOI] [PMC free article] [PubMed]
- Konno T, Akita K, Kurita K et al (2005) Formation of embryoid bodies by mouse embryonic stem cells on plastic surfaces. J Biosci Bioeng 100:88–93 [DOI] [PubMed]
- Kurosawa H, Imamura T, Koike M et al (2003) A Simple method for forming embryoid body from mouse embryonic stem cells. J Biosci Bioeng 96:409–411 [DOI] [PubMed]
- Liu S, Qu Y, Stewart TJ et al (2000) Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci USA 97:6126–6131 [DOI] [PMC free article] [PubMed]
- Lumelsky N, Blondel O, Laeng P et al (2001) Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 292:1389–1394 [DOI] [PubMed]
- Maltsev VA, Wobus AM, Rohvedel J et al (1994) Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res 75:233–244 [DOI] [PubMed]
- McBurney MW (1993) P19 embryonal carcinoma cells. Int J Dev Biol 37:135–140 [PubMed]
- McBurney MW, Jones-Villeneuve EM, Edwards MK et al (1982) Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature 299:165–167 [DOI] [PubMed]
- Nishikawa S, Nishikawa S, Hirashima M et al (1998) Progressive lineage analysis by cell sorting and culture identifies FLK1+ VEcadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125:1747–1757 [DOI] [PubMed]
- Park S, Lee YJ, Lee KS et al (2004) Establishment of human Embryonic Stem cell lines from frozen-thawed blastocysts using STO cell feeder layers. Hum Reprod 9:676–684 [DOI] [PubMed]
- Robertson EJ (1987) Teratocarcinomas and embryonic stem cells: a practical approach. Oxford, Washington DC
- Robertson E, Bradley A, Kuehn M (1986) Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature 323:445–448 [DOI] [PubMed]
- Skerjanc IS (1999) Cardiac and skeletal muscle development in P19 embryonal carcinoma cells. Trends Cardiovasc Med 9:139–143 [DOI] [PubMed]
- Soria B, Roche E, Berná G et al (2000) Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 49:157–162 [DOI] [PubMed]
- Stephen MD, Kybe M, Perlingeiro R et al (2002) Effeicency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng 78:442–453 [DOI] [PubMed]
- Thomson JA, Kalishman J, Golos TG et al (1995) Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA 92:7844–7848 [DOI] [PMC free article] [PubMed]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed]
- Van der Heyden MA, Defize LH (2003) Twenty-one years of P19 cells: what an embryonal carcinoma cell line taught us about cardiomyocyte differentiation. Cardiovasc Res 58:292–302 [DOI] [PubMed]
- Wang X, Wei G, Weiting Yu et al (2006) Scalable producing embryoid bodies by rotary cell culture system and constructing engineered cardiac tissue with es-derived cardiomyocytes in vitro. Biotechnol Prog 22:811–818 [DOI] [PubMed]
- Xian H, McNichols E, Clair AS et al (2003) A subset of ES-cell-derived neural cells marked by gene targeting. Stem Cells 21:41–49 [DOI] [PubMed]
- Yamada T, Yoshikawa M, Kanda S et al (2002) In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells 20:146–154 [DOI] [PubMed]