Abstract
Although the importance of animal cell culture for the industrial (large scale) production of pharmaceutical products is continuously increasing, the sensibility of the cells towards their cultivation environment is still a challenging issue. In comparison to microbial cultures, cell cultures which are not protected by a cell wall are much more sensitive to shear stress and foam formation. Reactor design as well as the selection of ‘robust’ cell lines is particularly important for these circumstances. Nevertheless, even ‘sensitive’ cell lines are selected for certain pharmaceutical processes due to various reasons. These sensitive cell lines have even higher requirements regarding their cultivation environment. Important characteristics for the corresponding reactor design are a high (volumetric) gas mass transfer coefficient, low volumetric power input, low shear stress, low susceptibility to bio-fouling, the ability to cultivate sticky cells and sufficient mixing properties. Membrane aeration has been a long-known possibility to meet some of these requirements, but has not often been applied in recent years. The reasons lie mainly in low gas mass transfer rates, a limited installable volume-specific membrane surface area, restrictions in scalability and problems with membrane fouling. The dynamic membrane aeration bioreactor aeration is a simple concept for bubble-free oxygen supply of such sensitive cultures. It overcomes limitations and draw-backs of previous systems. Consisting of an oscillating, centrally arranged rotor (stirrer) that is wrapped with silicone membrane tubing, it enables doubling the gas mass transfer at the same shear stress in the investigated cultivation scales of 12, 20, 100, and 200 L. Continuous cultivation at these scales allows the same product output as fed-batch cultivation does at tremendously larger reactor volumes. Apart from introducing this novel technology, the presentation comprises selected cultivation results obtained for blood coagulation factor VIII in continuous mode and a therapeutic monoclonal antibody in fed-batch mode in comparison to reference trials.
Keywords: Cell cultivation, Bubble-free bioreactor aeration, Membrane, Sticky cells, Fouling, Low shear
Introduction
Mammalian cells are being increasingly used for the production of therapeutic proteins because of their ability to properly fold and glycosylate proteins. Unlike many recombinant microbial fermentations, large-scale cell culture cultivation can be a dominant cost factor in production and therefore a field for optimization. Given the relative fragility of many cells in culture, reactor design becomes an important issue in enhancing process economics. Among the requirements of animal cells towards the cultivation environment, hydrodynamic shear stress is an important aspect to consider and to decrease as much as possible. On the other hand, sufficient mixing, e.g., by a stirrer, has to be provided to maintain homogeneous conditions inside the bioreactor and to rapidly distribute feeds such as base, medium in continuous processes or antifoam agent (Oldshue 1996; Henzler and Obernosterer 1991; Mayr 1992). Furthermore, adequate sorption of oxygen and desorption of carbon dioxide in the cultivation medium for the respiration of the cells is important given the low solubility of oxygen and the aim to decrease shear stress. Oxygenation of cells and the corresponding design of cell culture bioreactors has been a major topic of interest since the inception of cell culture technology (Aunins and Henzler 1993; Henzler and Kauling 1993; Fenge and Lüllau 2006; Aunins et al. 1986; Vorlop and Lehmann 1988; Engasser 1988). Overviews are given in Aunins and Henzler (1993), Henzler and Kauling (1993) and Fenge and Lüllau (2006).
Shear stress is not only created by agitating the medium but also during the distribution and fragmentation of gas bubbles due to the stirrer and by bubble rupture at the liquid surface (Henzler 2000). Another problem is the formation of foam, especially in serum and protein containing media, above the liquid surface. Cells floating with gas bubbles stick to the foam and are lost as productive components of the process but appear as undesired producers of by-products and cell debris when dying due to insufficient culture conditions in the foam. Therefore gas flows have to be reduced to values where a considerable cell density still can be aerated but a tolerable amount of foam is created.
Recently, bioreactor aeration using metal or ceramic microspargers has been used as a viable option for industrial-scale mammalian cell culture processes (Nehring et al. 2004; Qi et al. 2001). Microsparging which employs micron-sized bubbles for gas transfer is characterized by high interfacial area resulting in high oxygen transfer and moderate carbon dioxide removal rates. For long-term cultivation, a decrease in performance can occur due to the adsorption of substances on the sparger surface or the partial blocking of the porous surface.
Traditional obstacles to bubble aeration and microsparging including cell damage by hydrodynamic shear and excessive foaming in the bioreactor (Chisti 2000) were overcome through the addition of pluronic F-68 (Ma et al. 2004; Murhammer and Goochee 1990), a surface active agent, while a silicone-based antifoaming agent was used to control foaming in the bioreactor. However, not all cell lines can be cultivated in microsparged bioreactors, even with the addition of shear protective agents. Also, addition of surfactants can cause complications in downstream purification processes.
While bubble aeration for cell culture with standard cell lines has been intensively studied and is nowadays state-of-the-art (Chu and Robinson 2001; Farid 2006; Birch et al. 1987; Katinger et al. 1997; Sucker et al. 1994), still sensitive cell lines not tolerating bubble aeration are selected for certain pharmaceutical processes due to various reasons. There is hence a need for an effective bubble-free aeration process that introduces minimal shear in the bioreactor.
The use of tubular membranes for aeration of mammalian cell bioreactors offers a means of supplying oxygen to the cells with minimal shear introduction in the bioreactor (Côté et al. 1988, 1989; Su et al. 1992; Ahmed and Semmens 1992a, b, 1996; Catapano et al. 2004; Schneider et al. 1995; Ahmed et al. 2004; Qi et al. 2003; Lehmann et al. 1987).
A classical technical solution for a tubular membrane aeration system consists of a stator, most frequently shaped as a cylinder, which is wrapped with a certain length of a tubular, gas-permeable membrane material, and a central mechanical agitator acting within the cylindrical space defined by the stator. Such systems will be referred to as “rotor-stator-systems” or “conventional membrane aeration setup” in the following. The way by which the gas-permeable tubing is arranged on the stator and the type and dimensions of the central agitator may vary and do have a distinctive influence on the overall performance of such an aeration and mixing system. However, beyond all those technical differences, the classical rotor-stator-system is principally limited in overall mass transfer capacity. This plays an important role with increasing scale and at high cell densities of fed-batch cultures and continuous cultures where optimization is also an important issue (Kompala and Ozturk 2006). An example for a pharmaceutical product produced in continuous culture is the recombinant blood coagulation factor VIII production by Bayer Health Care. A classical rotor-stator-system is not properly scalable because during scale-up the installable tubing surface area, thus the oxygen supply capacity, only increases with the linear dimension of a reactor system squared while the fermenter volume, i.e., the capacity demand (at the same cell density), increases with the linear dimension cubed.
Tubular membrane aeration has been studied at length since its introduction into cell culture. Though appearing quite simple at first glance, tubular membrane aeration comprises a bundle of partly challenging aspects from an engineering point of view. In part, this is due to the fact that the overall mass transfer resistance is typically controlled both by liquid-side mass transfer and molecular or pore diffusion in the membrane material itself (Côté et al. 1989). In addition, profiles of total and partial pressures of the gas contained in the tubing may need attention and detailed study. Su et al. (1992) found that at fixed inlet pressure there is a critical length of the tubing beyond which the membrane surface area should be divided into separate parallel segments in order to increase the overall oxygen transfer rate.
Hollow fibers for oxygenation may be used in flow-through and dead-end mode. Two papers from Ahmed and Semmens (1992a, b) are dedicated to an experimental and theoretical analysis of the latter mode. In another paper, these authors studied the influence of the angle of inclination of a single tubular membrane towards the direction of flow (Ahmed and Semmens 1996). Transverse flow proved to be much more efficient than parallel flow. They gave correlations for the tubing Sherwood number as a function of the Reynolds number of the flow, the Schmidt number and the angle between flow and hollow fibers.
Materials suitable for tubular membrane aeration can be classified in dense film, microporous and composite materials. A good overview on materials and permeabilities was recently given by Catapano et al. (2004). While gas transport in dense film membranes is controlled by molecular diffusion and requires comparatively good gas solubility, the permeability of hydrophobic microporous membranes is based on gas diffusion through the pores. Schneider et al. (1995) compared mass transfer measured with PTFE tubing with literature data on silicone tubing and found that PTFE is better by roughly a factor of two, although that comparison was based on different reactor geometries and flow patterns. Microporous materials mostly display a better permeability compared with silicone rubber (Catapano et al. 2004). However, they suffer from the drawback that formation of bubbles at the outer surface starts at lower inside gas pressures. This aspect was recently addressed by Ahmed et al. (2004) by investigating a hollow-fiber composite tubular membrane with a 1 micron layer of pore-free polyurethane in the center of a 30 micron microporous polyethylene membrane.
Mechanical effects associated with the elastomeric nature of silicone rubber tubing may affect the overall performance of a membrane tubing aeration system. When gas pressure is imposed to an elastic tubular membrane wrapped onto a metallic cage, the flow resistance within the bends is a function of the local internal overpressure and, hence, the back pressure imposed to the system. Qi et al. (2003) studied such effects both experimentally and theoretically, comprising the influence of “loose” and “tight” wrapping of the tubing during membrane cage set-up. They measured the pressure at various points along the length of the tubing and developed a mathematical model that was able to accurately predict experimental data.
Effects of hydrodynamic stresses imposed to suspended cells have been the subject of investigations for many years (e.g., Mahnke et al. 2000). Some of theses studies are mainly concerned with the reaction of living organisms to a well-defined hydrodynamic environment.
Other authors look at this problem more from the viewpoint of local variations of hydrodynamic conditions within an agitated system. Henzler (2000) gave an excellent overview on the topic particle stress in bioreactors. He developed and applied various model particle systems (clay flocs, emulsion droplets, beads with immobilized enzymes) which react to hydrodynamic stress with changes to a measurable property (floc or droplet size, enzyme kinetics). These systems were used for intensive study of the overall particle shear created by various agitation systems and bioreactor principles, such as various impellers, bubble columns, and loop reactors.
The floc destruction kinetics method described in Henzler (2000) is the basis for assessing overall particle shear created by bubble-free cell culture aeration systems investigated in the present paper. The pivotal criterion for assessment of particle shear is the so-called equivalent floc diameter. Henzler points out that the shear load imposed to suspended cells in agitated systems may be strongly non-homogeneous and that the average volume-specific power input to an agitated system is an insufficient criterion for assessing these local effects. Typically, local power dissipation rates in close proximity to an agitator may exceed the average dissipation rate within a stirred system by a factor of 10–100. Henzler gave a correlation for this enhancement factor as a function of the agitator geometry which was derived from the overall floc destruction kinetics measured.
For membrane-aerated, mechanically agitated bioreactors (rotor-stator-systems), local shear created by the interaction of the flow with the tubular membranes needs to be considered, apart from shear caused by the agitator itself. To the best of their knowledge, the authors of the present paper are not aware of any open literature covering these aspects, although they need to be taken into account for a proper assessment of a bioreactor.
The starting point for developing the dynamic membrane aeration (DMA) system presented in this paper is the fact that in technical fermenters membrane aeration capacity provided by rotor-stator-systems may be limiting cell density and culture performance by limited oxygen supply and carbon dioxide removal at a level of overall particle (cell) stress that is acceptable from the viewpoint of cell death and by-product formation.
These limitations were foreseen by Lehmann et al. (1987) and Lehmann (1989) who developed a moving membrane aeration device that is based on a tumbling movement of the membrane cage within the bioreactor. Although this system was a breakthrough in terms of providing high oxygenation capacity under gentle agitation conditions, it did not find widespread application in the industry. This may be due, in part, to the fact that Lehmann’s solution is either based on magnetic forces to create the tumbling movement or it is dependent on a mechanical gear for generating this movement that needs to be placed within the sterile bioreactor. While the magnet solution appears only applicable for small-scale bioreactors, the internal-gear solution is probably subject to wear and might give rise to severe cleaning, sterility and maintenance issues.
Kim et al. (1996) re-addressed the moving-membrane concept by presenting a bioreactor which comprises an agitator that is wrapped with aeration tubing. Transfer and removal of gas to/from the membrane agitator is achieved via a special, custom-made mechanical seal system. The agitator rotates steadily and it remains unclear if and how baffling is achieved in this system. Without stationary elements acting as baffles, such a system should basically create a solid-body-like rotation of the liquid in the reactor with poor relative flow between the membrane tubing and the fermenter broth. The system was successfully applied for the production of tissue-type Plasminogen Activator with 1 × 107 viable cells mL−1 of a human fibroblast cell line in a 1.5 L bioreactor. No statements were made about the scalability of this system.
A drawback of conventional membrane aeration systems is their limited mass transfer capacity. This limitation, in turn, restricts the cell density and thus the space-time yield of the bioreactor. The power input cannot be increased to raise the mass transfer coefficient due to the resulting shear on the cells. In particular, cell cultivation processes with cell densities of a magnitude of 8 × 106 cells mL−1 and above are subject to mass transfer capacity limitations (depending on the scale). This applies especially to perfusion processes (with cell retention) and fed-batch processes, which achieve such cell densities.
These outline conditions give rise to the demand for an appropriate membrane aeration method capable of achieving high mass transfer coefficients with a low average power input and accordingly low shear stress. A further condition is that sufficient mixing must take place in the reactor. The mixing action must prevent sedimentation of the cells and facilitate the homogenization of any added liquids (nutrient media, buffer solution etc.) within a sufficiently short time.
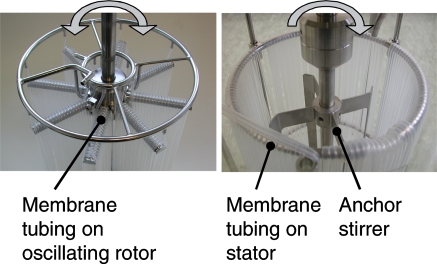
This paper presents a simple, effective, gentle and easy-to-clean DMA bioreactor system that enables an intensified bubble-free aeration of animal cell cultures. As described above, conventional rotor-stator-systems consist of a rotor (impeller) and a membrane stator. The stator can be a cylindrical body wrapped with membrane tubing, preferentially in a vertical direction. The rotor, e.g., a low-shear anchor impeller, rotates inside this membrane stator. The liquid therefore flows around the membrane tubing each time a rotor blade passes. With the new system, the functional elements of a rotor and stator are combined in a rotary oscillating membrane carrier. Both systems are illustrated in Fig. 1.
Fig. 1.
Photo of the dynamic membrane aeration (DMA) concept (left) in contrast to a conventional rotor-stator-system/membrane aeration setup (right). On the DMA, the membrane tubing is wrapped vertically around the rotor arms, which are arranged radially on the rotor shaft at the top and bottom of the bioreactor. The shaft is located in the center of the reactor and performs an oscillating rotary movement driven by an external servo motor. The flow around the membrane tubing is initiated by the relative motion between the membrane and the liquid. The two concentric rings provide membrane gas supply and removal. On the conventional membrane aeration setup, the tubing is wrapped vertically on a stator with a concentric, steadily rotating anchor stirrer
Gas is supplied to the membrane carrier from the reactor lid by way of flexible hoses. The oscillating rotary movement improves the flow around the membrane tubing and eliminates the need for rotating seals for the gas supply. The system’s hygiene and service life benefit accordingly. This functional simplification creates space for increasing the membrane area. It also facilitates scaling up. A wrapping device enables easy wrapping of each rotor arm separately from the bioreactor.
By way of example, experimental results are indicated below for a 100 and 200 L model system (non-sterilizable DMA reactor for investigation of mass transfer, power input, shear stress, and mixing times). Furthermore DMA cell cultivation results are presented in comparison to conventional membrane aeration systems at the 20 and 200 L scale and results summarized for the 12 L scale.
Materials and methods
DMA cell culture bioreactors and model system
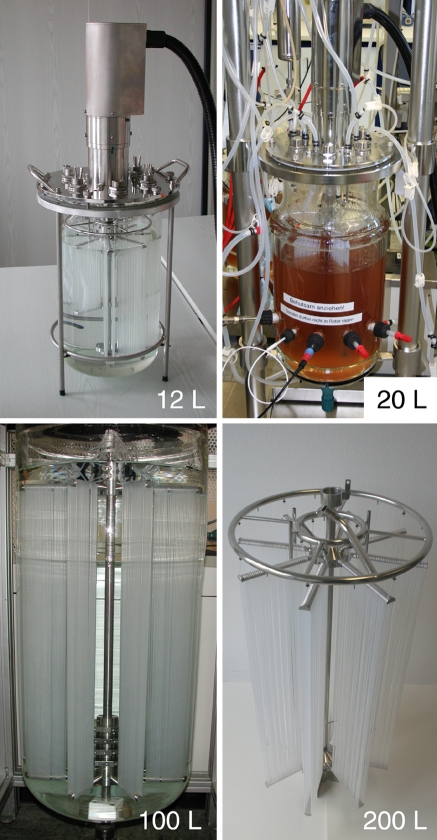
Figure 2 contains photographs of the investigated DMA cell culture bioreactors and a model system in various scales.
Fig. 2.
Photographs of the investigated DMA scales: 12, 20, 100 and 200 L (filling volume). The 12, 20 and 200 L systems are cell culture bioreactors (12 L shown without reactor peripherals, 20 L with peripherals and cultivation in progress and 200 L just DMA rotor). The 100 L system, in contrast, is a model system for the characterization concerning mass transfer, shear stress, power input and mixing number
The 12 and 20 L DMA scale are powered by a servo motor with a static torque of 0.9 Nm, to which a planetary gear unit with a reduction ratio of 1:12 is flanged (model No. 23S21, Jenaer Antriebstechnik, Jena, Germany). The 100 and 200 L scale is powered by model No. 34S80, static torque of 5.8 Nm, same gear reduction ratio.
The servo motor is actuated via an associated control cabinet and the ECO2WIN software loaded on a PC. The drive allows the simple variation of all the motion parameters, including the oscillation amplitude, frequency, acceleration, deceleration, holding time at reversal point etc. For all results presented here, acceleration and deceleration had the same time constant absolute value. A holding time at reversal point was not applied. The oscillation amplitude was, if not specified otherwise, 90° (which corresponds to a swept angle of 180° or, in other words, to a rotor movement of 180° in one direction and a subsequent movement of 180° back). So, if the oscillation frequency and swept angle are given, the acceleration value has not to be listed since it is already determined by oscillation frequency and swept angle.
The membrane tubing used for the 100 and 200 L model systems (200 L model system not shown in Fig. 2) consisted of silicone tubes having an outer diameter of 3 mm and an inner diameter of 2 mm (Laboflex tubing, SI2.0 NA3.0, Kronlab Chromatographie & Labortechnik, Sinsheim, Germany). The specification selected for the cell culture bioreactors was silastic RX 50 Medical Grade Tubing Special, 0.078 in. (1.98 mm) inner diameter × 0.125 in. (3.18 mm) outer diameter (500 ft roll, Dow Corning, USA).
Determination of the (volume-specific) mass transfer coefficient for oxygen
The volume-specific mass transfer coefficient for oxygen was determined by a transient method. All the oxygen was initially chemically expelled from the water-filled reactor by adding sodium metabisulfite. When the oxygen concentration fell below 5% air saturation, gassing with air took place by way of the membrane tubing (membrane inlet pressure 1.5 bar gauge). The change in oxygen concentration was observed with an oxygen probe (WTW-Sauerstoffsonde EOT 196, Wissenschaftlich-Technische Werkstätten GmbH, Weilheim, Germany) and the measurements were recorded. The maximum dissolved oxygen concentration was in the range 128–133% air saturation (pressure inside membrane >1 bar). The volume-specific mass transfer coefficient was obtained by analyzing the course of dissolved oxygen concentration.
The water is also oxygenated by the oxygen partial pressure of the ambient air and the surface of the reactor. The volume-specific mass transfer coefficient measured thus far is therefore the total mass transfer coefficient (surface + membrane). In order to obtain the volume-specific mass transfer coefficient of the actual membrane aeration system, therefore, the volume-specific mass transfer coefficient of the surface aeration must be subtracted from the volume-specific total mass transfer coefficient. When the surface aeration is measured, the membrane tubing is filled with water.
Determination of the volume-specific power input
The power introduced into the liquid by the oscillating DMA rotor is determined from the drive torque at the shaft and the angular velocity. The torque is composed of the load torque and the no-load torque. In other words, measurements are taken with the reactor filled with water (load torque) and empty (no-load torque). For each measuring point, the no-load torque is subtracted from the load torque, and the result is multiplied by the angular velocity. This gives the power transmitted by the shaft as a function of time. Averaging over time yields the mean power input, and dividing by the reactor capacity gives the volume-specific power input. The load and no-load torque are measured with a torque sensor (DRFL-II-20, ETH-Messtechnik GmbH, Gschwend, Germany). The angular velocity of the rotor is determined by recording the drive control outputs.
Determination of the shear stress
The particle shear stress created in the hydrodynamic environment established in a bioreactor was experimentally evaluated using a method termed floc destruction testing. Floc destruction testing assesses shear stress or forces, respectively, by measuring the rate by which the average diameter of aggregated (flocculated) clay beads in suspension is reduced by agitation within the bioreactor vessel. Bead aggregate (floc) size is related to shear stress with smaller size observed under higher shear forces and large aggregates indicating lower shear forces. Presented are equivalent floc diameters resulting from measurement evaluation based on the method described in (Henzler 2000). This allows a comparison of various reactors, impellers, reactor configurations etc. concerning overall shear stress imposed to suspended particles, i.e., cells.
Determination of the mixing number
The mixing number is the product of the stirrer’s rotational speed and the measured macro-mixing time. For the DMA concept, the stirrer’s rotational speed is substituted accordingly by the oscillation frequency. The macro-mixing time is determined by a visual observation of the decolorization of iodine by thiosulfate using an iodine–starch complex as the colorant. The iodine–starch complex colors the water dark-blue. The macro-mixing time is the lapsed time between the addition of sodium thiosulfate to the free liquid surface and the complete decolorization of the liquid (Henzler 1978).
Cells and media
The CHO cell line, producing subclass IgG monoclonal antibody conjugates against cancer (not further specified due to confidentiality reasons) was used in the cultivations. The cells were routinely maintained in shaker flasks in an incubator at 37 °C in an atmosphere of 7.5% CO2. For reactor experiments they were passaged to shake-flasks (1 L) to reach a preculture volume of ~0.8 L at ~3 × 106 viable cells mL−1 for 20 L reactor inoculation at 4–6 × 105 viable cells mL−1. The 200 L reactor was inoculated from a prior running 20 L bioreactor equipped with a conventional anchor stirrer and membrane stator aeration system.
Flask cultures as well as the reactor cultivations were carried out in Bayer HealthCare AG proprietary medium. Ammonia or lactate inhibition was not observed with this cell line. The cell line and media used for the 12 L continuous cultivations are not described due to secrecy reasons.
Analytical methods
Samples (e.g., 30 mL at 20 L scale) were taken once a day and analyzed for viable and total cell concentration, glucose, lactate, glutamate, ammonia, antibody concentration, and cell diameter distribution. Viable and total cell concentrations as well as cell diameter distribution were measured with a Cedex analyzer (Innovatis, Bielefeld, Germany). Glucose, lactate, glutamate, and ammonia concentrations were determined by an YSI Analyzer 2700 and 7100MBS (Yellow Springs Instruments, USA). Antibody concentration was measured via ELISA. Antibody was purified and checked for correct properties (completely synthesized etc.).
Twenty liter fed-batch bioreactor cultivation
Fed-batch experiments were carried out in a 20 L glass reactor including a stainless steel head plate (manufactured by Tectrion, former Bayer Industry Services, Leverkusen, Germany) using a 3.2 Nm Applikon magnetic coupling and Applikon fermenter accessories (Applikon, Knüllwald, Germany).
In case of the cultivations using DMA (manufactured by Bayer Technology Services), the rotor oscillated at a frequency of 0.25 s−1 (corresponding to a rotor movement of 180° in one direction and a subsequent movement of 180° back in 4 s). Within one oscillation cycle (forth- and back-movement) the rotor thus sweeps an overall angle of 360°, as does a conventional stirrer in one revolution. The average power input for the oscillation frequency of 0.25 s−1 constitutes 11 W m−3. The rotor carried 53 m of vertically wrapped silicone tubing on four rotor arms (50% of the maximum length). The tubing length was divided into four segments (one per rotor arm) into which the gas flow was split. By mounting eight rotor arms, 106 m tubing can be applied.
In case of the cultivations using a membrane stator and an anchor stirrer (conventional/reference system), the cell suspension was agitated at 35 rpm. The membrane stator carried 75 m of vertically wrapped silicone tubing (maximum length). The tubing length was divided into four segments into which the gas flow was split.
Process temperature was kept at 37 °C by a double jacket of the glass fermenter connected to a water bath.
Culture volume started at 9–10 L and reached final volumes around 22 L (conventional/reference system) and 22–25.5 L (DMA), respectively. The increase in culture volume substantially resulted from manual addition of medium mainly during the growth phase but also during the stationary phase. Culture volume also increased due to the manual addition of nutrient concentrate during fed-batch mode. Feedback of added volumes was given by Panther balances with a resolution of 10 g (Mettler Toledo, Gießen, Germany).
Two-hundred liter fed-batch bioreactor cultivation
The 200 L reactor (Applikon) was equipped with a 6.5 Nm Applikon magnetic coupling and Applikon fermenter accessories.
In case of the cultivations using DMA (manufactured by Bayer Technology Services), the rotor oscillated at a frequency of 0.125 s−1 (corresponding to a rotor movement of 180° in one direction and a subsequent movement of 180° back in 8 s). The rotor carried 460 m of vertically wrapped silicone tubing on eight rotor arms (61% of the maximum length). The tubing length was divided into eight segments (one segment per rotor arm) into which the gas flow was split. By mounting 16 rotor arms, 755 m tubing can be applied.
In case of the cultivations using a membrane stator and an anchor stirrer (conventional/reference system), the cell suspension was agitated at 20 rpm. The membrane stator carried 300 m of vertically wrapped silicone tubing (maximum length). The tubing length was divided into four segments into which the gas flow was split.
Process temperature was kept at 37 °C by a double jacket of the fermenter connected to a water bath.
Culture volume started at ~90 L and reached final volumes around 200 L. The increase in culture volume was performed in an analogue way to the 20 L scale.
Twelve liter continuous bioreactor cultivation
Materials and methods for the 12 L continuous bioreactor cultivations is condensed, since only a summary but no courses of data is given.
The 12 L reactor (Applikon) was equipped with a 3.2 Nm Applikon magnetic coupling and Applikon fermenter accessories.
In case of the cultivations using DMA (manufactured by Bayer Technology Services), the rotor oscillated at a frequency of 0.33 s−1, but in this case, the swept angle was only 90°. The oscillation frequency corresponds to a rotor movement of 90° in one direction and a subsequent movement of 90° back in 1.5 s and two rotor movements in 3 s leading to an overall swept angle of the two forth- and back-movements of 360° in 3 s. The rotor carried 59 m of vertically wrapped silicone tubing on eight rotor arms. The tubing length was divided into eight segments (one per rotor arm) into which the gas flow was split.
In case of the cultivations using a membrane stator and an anchor stirrer (conventional/reference system), the cell suspension was agitated at 150 rpm. The membrane stator carried 50 m of vertically wrapped silicone tubing (maximum length, not segmented).
Cell retention was achieved by an inclined plate settler (not further specified due to secrecy reasons).
Process control system
For the 20 L scale, the process control system (FairMenTec, now belonging to Bioengineering, Wald, Switzerland) maintained dissolved oxygen of the bioreactor at 50% air saturation via the flow rate of oxygen in the silicone membrane tubing (0–5 L/min). At highest cell density, the silicone membrane tubing backpressure was increased from atmospheric pressure to 0.16 bar gauge.
In addition, the flow rate of air in the silicone membrane tubing was manually adjusted (0.3 L/min during the growth phase, no air flow during the stationary phase at high cell density and 0.1 L/min during the decline phase of the cultivation).
The headspace of the bioreactor was flushed by a manually set air flow of 0.2 L/min during the early growth phase and of 0.4 L/min during the remaining cultivation. The bioreactor headspace pressure was 0.05–0.12 bar gauge.
The process control system also controlled the pH-value by addition of carbon dioxide into the membrane tubing gas flow (0–0.5 L/h) for pH-values above the setpoint of 7.2. pH-values below 7.05 were avoided by direct metering of 1 M NaOH to the cell suspension via a peristaltic pump that was the actuator of a closed-loop pH control system. No cell damage was observed as a result of direct NaOH addition to the fermenter. About 200 L cultivations were performed in an analogous way with the volume fluxes adapted correspondingly.
Results and discussion
Mass transfer and hydrodynamic performance of the DMA system
Four criteria were applied to evaluate the efficiency of the DMA concept, compared with a conventional membrane stator:
The oxygen mass transfer coefficient—in order to evaluate the extent of increase in the coefficient achieved by the improved flow around the membrane tubing in the case of DMA → evaluation of the efficiency.
The volume-specific oxygen mass transfer coefficient—in order to compare the extent of the overall increase in mass transfer. The maximum useful membrane area was installed in each system, and the comparison was made on the basis of the product of the mass transfer coefficient and the volume-specific membrane area → evaluation of the effectiveness.
The volume-specific power input into the liquid.
The hydrodynamic shear stress imposed on suspended particles. As a comparative criterion, the shear stress in the cell culture is more relevant than the mean power input because cell damage is expected to be controlled by local shear stress peaks rather than the average power dissipation rate. Different reactor configurations with the same mean power input can cause different local shear stress patterns and therefore create different environments for the cells.
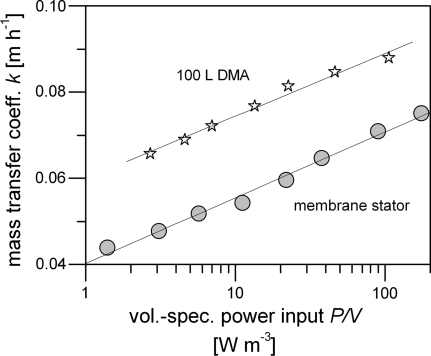
Figure 3 shows the mass transfer coefficient k for oxygen as a function of the volume-specific power input for DMA in the 100 L scale compared with a conventional membrane stator.
Fig. 3.
Mass transfer coefficient k for oxygen as a function of the volume-specific power input P/V for DMA in the 100 L scale compared with a conventional membrane stator. A comprehensive legend of all Figs. 3, 4, 5, and 6 can be found below Fig. 6
With a power input of 6 W m−3, typical for cell culture applications, the mass transfer coefficient is 35% higher with DMA. This underscores the benefit achieved by the simultaneous permanent movement of all membrane tubing through the liquid rather than generating an intermittent liquid flow at the tubing by passing stirrer blades.
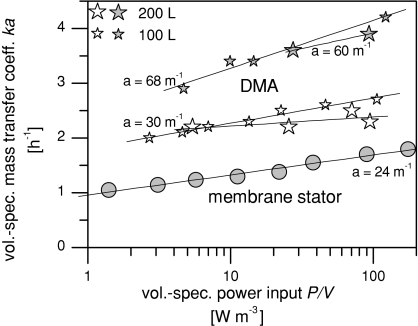
A further benefit of DMA, namely that the installed membrane area can be enlarged, is now illustrated. With membrane stators, increasing the membrane area is generally accompanied by an equivalent decrease in the mass transfer coefficient, as explained in the introduction. The reduction in the mass transfer coefficient practically cancels out the gain in membrane area. Figure 4 shows the volume-specific mass transfer coefficient for oxygen as a function of the volume-specific power input for the configurations presented in Fig. 3 (DMA in the 100 L scale compared with a conventional membrane stator). In addition, Fig. 4 shows a second DMA configuration with a larger membrane area on bifurcated rotor arms. Finally, Fig. 4 contains results of the 200 L DMA scale, also for two membrane area configurations.
Fig. 4.
Volume-specific mass transfer coefficient ka for oxygen as a function of the volume-specific power input P/V for DMA in the 100 and 200 L scale compared with a conventional membrane stator. For each scale, two DMA configurations are plotted: First, membrane tubing resulting in a surface area of 30 m2/m3 reactor capacity. Second, more membrane tubing wrapped onto bifurcated rotor arms resulting in a volume-specific mass transfer area of 68 m2/m3 (100 L scale) and 60 m2/m3 (200 L scale), respectively. A comprehensive legend of all Figs. 3, 4, 5, and 6 can be found below Fig. 6
With a power input of 6 W m−3, typical for cell culture applications, the volume-specific mass transfer coefficient is 140% higher with the DMA system in the configuration with larger membrane area, compared with the membrane stator.
Looking at the volume-specific mass transfer coefficient ka of the DMA configurations with larger and smaller membrane area (volume-specific mass transfer area a = 60 or 68 m−1 and a = 30 m−1), a decrease in the mass transfer coefficient k is observable. This can be seen by the fact that the volume-specific mass transfer coefficient ka does not double when the volume-specific mass transfer area a is doubled. Since there is still a gain in ka despite the decrease in k, increasing the membrane area makes good sense. This does not hold true for the membrane stator. With a membrane stator, an increase in membrane area (e.g., by nesting one membrane stator inside another) is usually ruled out by a decrease in the mass transfer coefficient.
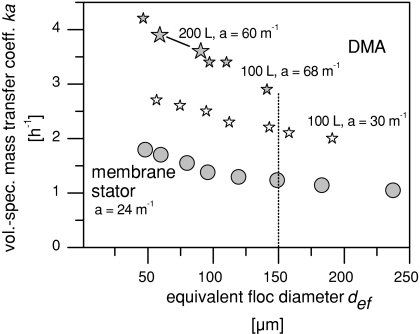
Comparing the mass transfer as a function of the shear stress on suspended particles (rather than as a function of power input) in Fig. 5 shows that DMA in the configuration with larger membrane area offers still 125% more mass transfer capacity than the membrane stator.
Fig. 5.
Volume-specific mass transfer coefficient ka for oxygen as a function of the equivalent floc diameter def as a measure of shear stress for DMA in the 100 and 200 L scale compared with a conventional membrane stator. Large floc diameters correspond to low shear stress and small floc diameters to high shear stress. For the DMA, the following configurations are plotted: First, membrane tubing resulting in a surface area of 30 m2/m3 reactor capacity (100 L scale). Second, more membrane tubing wrapped onto bifurcated rotor arms resulting in a volume-specific mass transfer area of 68 m2/m3 (100 L scale) and 60 m2/m3 (200 L scale), respectively. A comprehensive legend of all Figs. 3, 4, 5, and 6 can be found below Fig. 6
In Fig. 5, an equivalent floc diameter of 150 μm is emphasized by a dotted line. This value served to select the power input for the cultivation of a shear sensitive cell line at a scale of 200 L (results not presented) and corresponds to a power input of around 6 W m−1 in case of the membrane stator. The difference of +125% increase when comparing via shear stress to +140% increase when comparing via power input emphasizes that different reactor configurations with the same mean power input can cause different shear stress levels. However, the volume-specific mass transfer coefficient and therefore the overall mass transfer rate in fermentation can be more than doubled by DMA.
A further favorable characteristic of DMA is that the forenamed benefits are retained if the rotor motion is varied (acceleration/deceleration, amplitude); in other words, the system behavior is robust (results not presented). In addition, a mathematical model simulates the characteristics of torque, power input, fluid velocity, mass transfer etc. in various scales as a function of time.
Mixing characteristics as well as possible variations of the DMA setup that specifically enhance certain mixing aspects are described in (Frahm et al. 2007). For example, the mixing number in the 100 L model system ranged from 30 to 80 depending on the DMA setup variation, widely independent from power input. As another example, the mixing number in the 12 L DMA cell culture bioreactor was around 14, also widely independent from power input.
Cell culture performance of the DMA bioreactor
The following chapter presents and discusses cell culture results employing DMA at 20 and 200 L scale in fed-batch mode and at 12 L scale in continuous mode. The 20 and 200 L scales are discussed more elaborately whereas for the 12 L scale results are only summarized. All cultivations were performed during preclinical research and development runs for antibody production (fed-batch culture) and protein production (continuous culture).
The 20 L scale as well as the 12 L scale discussed later on were chosen for process development/preclinical testing due to their advantage of combining bioreactor conditions with low level media costs. Testing at smaller scales, especially in systems differing from a typical bioreactor, may result in scale-up problems at a later stage.
The 200 L scale represents the next scale-up step on the way to larger scales while remarkable amounts of antibody can yet be produced.
When testing a new type of aeration, the courses of viable cell density are of interest with regard to the bioreactor capability of creating good growth conditions. Furthermore, the final antibody titer is of interest with respect to the aspect of cells utilizing their good growth conditions for antibody production. From the engineering point of view, attention is also given to the degree of utilization of the membrane aeration capacity—how much aeration capacity is left at the maximum fed-batch viable cell density?
Fed batch culture—20 and 200 L scale
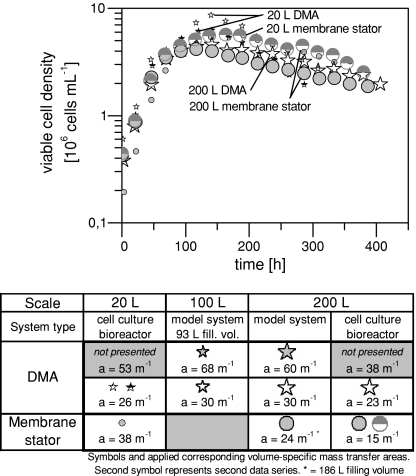
Figure 6 shows the courses of viable cell density for six cultivations—DMA fed-batches versus conventional membrane stator fed-batches. Only three of the seven performed DMA cultivations are presented since only cultivations employing the same medium have been compared.
Fig. 6.
Fed-batch cultivations of a CHO cell line in 20 and 200 L bioreactors. Viable cell density for aeration via dynamic membrane aeration (DMA) and via conventional membrane stator (conventional membrane aeration setup consisting of membrane stator and anchor stirrer)
All courses of viable cell density in Fig. 6 lie in the same area and are similar with some common non-significant differences always occurring from cultivation to cultivation and due to dissimilar inoculation cell densities. Therefore, the DMA technology and its oscillating rotor movement create a comfortable environment for the cells. Another confirmation is the same growth rate of the cultures which can be deducted from the courses of viable cell density during log-phase. An increase in viable cell density of the fed-batch cultivations can not be achieved by any aeration device since these cultivations are not gas transfer limited but the maximum cell density is limited by typical fed-batch circumstances. In contrast to these fed-batch cultivations, the maximum cell density in continuous culture could be increased by the DMA which is outlined in the next section.
No foaming occurred with the DMA and cell sedimentation did not take place. As a test, the 20 L DMA was turned off for 3 h before harvesting in order to force cell sedimentation. The DMA was able to resuspend the cells at standard oscillation frequency, i.e., standard power input, within 1 min.
Final antibody titers are not listed due to secrecy reasons but the DMA achieved similar titers proving that it does not only create good conditions for cell growth but also for antibody production.
As a third criterion mentioned above, the required utilized capacity of the membrane aeration has been analyzed. In case of the 20 L conventional membrane stator, the system is at its maximum oxygen transport capacity—oxygen content in the gas flow through the membrane has been increased to almost 100% by the controller at maximum cell density (still achieving 50% air saturation in the medium and avoiding limitation). The DMA is in a similar situation with only 53 m membrane tubing length contrary to the membrane stator with 75 m. This underlines the previously measured gain in the mass transfer coefficient presented in Fig. 3. The important aspect is that the DMA only carried four rotor arms out of eight in these trials. So it can still double the tubing length at the current configuration by just mounting the remaining four rotor arms and can therefore still provide approximately a doubling of gas mass transfer.
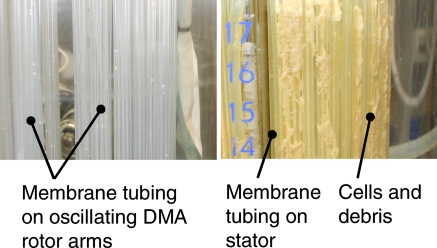
Moreover, the DMA features additional advantages like its property to prevent cell growth on membranes. Figure 7 shows a photo of the silicone membrane tubing after one fed-batch cultivation with DMA in contrast to a membrane stator.
Fig. 7.
Photo of the silicone membrane tubing after one fed-batch cultivation with dynamic membrane aeration (DMA, left) in contrast to conventional membrane aeration (right). The DMA tubing is perfectly clean and no fouling is visible. On the tubing of the stator of the conventional aeration setup, cells and debris can be clearly seen. This leads to decreased mass transfer characteristics, indicates insufficient mixing and shows undesired cultivation conditions for the cells accumulating to such plaques
The DMA tubing is clean and no fouling is visible after ~400 h of cultivation. The root cause for this finding is the good flow around the membrane tubing which is also accounting for the good mass transfer coefficient presented in Fig. 3. On the tubing of the stator of the conventional aeration setup, cells and debris can be clearly seen. This leads to decreased mass transfer characteristics in addition to the already lower mass transfer coefficient. Cells and debris on the membrane stator indicate insufficient mixing and show undesired cultivation conditions for the cells accumulating to such plaques.
Continuous culture—12 L scale
Besides the key advantages of the DMA to increase gas mass transfer capacity at comfortable growth and product formation conditions and to avoid cell and debris deposition, it is a main objective to use the DMA for an oxygen limited high cell density process where it can play off its high gas mass transfer capacity.
A 12 L DMA bioreactor was applied for a high cell-density perfusion process with a cell line difficult to cultivate in suspension, due to its tendency to form large aggregates and to foul bioreactor surfaces. The DMA was operated successfully in comparative runs with a conventional system. A gain in oxygenation efficiency was demonstrated, the sustainable cell density increasing from 15 to 20 million viable cells per mL compared to the conventional system. No adverse effects on the cell biology (apoptosis and cell cycle analysis) were detected. The debris and protein build-up on the aeration tubing was significantly reduced, enabling a much longer operation at target cell density. Debris build-up was found to be a major cause for early run termination in the conventional system. Agitation conditions needed to keep cell aggregate sizes in a given desired range were considerably less vigorous for the DMA system: The same aggregate size could be achieved with only one third of the power input needed in the conventional system, thus confirming the particularly gentle mode of agitation and aeration established in the DMA bioreactor.
Conclusions
This paper described the bioprocess requirements leading to cultivations in membrane aerated bioreactors and their current inherent drawbacks which were the main driver for the invention of the then presented DMA system. Four scales of 12, 20, 100, and 200 L have yet been developed in order to eliminate the oxygen limitation, mainly at high cell densities during continuous or fed-batch cultivations.
Important advantages of the DMA were subsequently demonstrated via investigations concerning mass transfer, power input, shear stress and mixing number. It has been shown that not only the mass transfer coefficient (for oxygen) could be increased (→ increased efficiency), but also the volume-specific mass transfer coefficient (→ increased effectiveness). Compared with the conventional rotor-stator-system, the presented DMA concept offers more than twice the oxygen mass transfer capacity at the same shear stress in the 20, 100, and 200 L scales.
The advantages identified in mass transfer and hydrodynamic investigations were confirmed in a series of cell culture runs. For example, the 20 L DMA was running at 50% of its maximum gas transfer capacity during the fed-batch peak while the conventional system was at its maximum. In continuous culture, the 12 L DMA enabled the sustainable increase of cell density from 15 to 20 million cells per mL compared to the conventional system for a cell line difficult to cultivate. The DMA always created comfortable growth and product formation conditions. Hydrodynamic advantages consist in the avoidance of detrimental deposition of cells and debris on the tubing which the conventional systems could not achieve.
The fields of application of the new DMA system include, in particular
the cultivation of shear-sensitive cell lines that do not tolerate bubble aeration and/or
processes that are oxygen transfer limited (or generally gas transfer limited, e.g., as regards carbon dioxide). This concerns perfusion processes (with cell retention) and fed-batch processes in particular, in which accordingly high cell densities are attained.
Acknowledgments
This development of the DMA was funded by Bayer Technology Services (BTS) within two consecutive research projects. We would like to thank to Messrs. Rose, Gießelmann, and Grodotzki for carrying out the reactor design drawings and to colleagues form Tectrion for manufacturing the reactors (Messrs Commer, Schiffczyk, Drinhausen, Kamphusmann, and Letzner). We wish to thank our colleagues in the Enzyme & Fermentation Technology group of BTS for their support. We express our gratitude to the students Andreas Sinthern, Juliane Schröter, and Maike Rampe for their contributions made in the context of their diploma theses. The described fed-batch cell cultivations with the 20 and 200 L bioreactors took place on the premises of Bayer Health Care AG in Wuppertal. For this, special thanks are due to Mr. Wischniewski and his colleagues. The continuous cell cultivations with the 12 L bioreactors were performed at Bayer Health Care in Berkeley, USA. Special thanks for this to Klaus Jöris and his colleagues.
List of symbols
- a
Fermenter liquid volume-specific membrane surface area (m2 m−3)
- def
Equivalent floc diameter (μm)
- k
(Oxygen) mass transfer coefficient (m h−1)
- ka
Volume-specific (oxygen) mass transfer coefficient (h−1)
- P
Power input (W)
- P/V
Volume-specific power input (W m−3)
- V
Fermenter (liquid) volume (m3)
References
- Ahmed T, Semmens MJ (1992a) The use of independently sealed microporous hollow fiber membranes for oxygenation of water: model development. J Membr Sci 69:11–20 [DOI]
- Ahmed T, Semmens MJ (1992b) Use of sealed end hollow fibres for bubbleless membrane aeration: experimental studies. J Membr Sci 69:1–10 [DOI]
- Ahmed T, Semmens MJ (1996) Use of transverse flow hollow fibers for bubbleless membrane aeration. Water Res 30:440–446 [DOI]
- Ahmed T, Semmens MJ, Voss MA (2004) Oxygen transfer characteristics of hollow-fiber, composite membranes. Adv Environ Res 8:637–646 [DOI]
- Aunins JG, Croughan MS, Wang DIC (1986) Engineering developments in homogeneous culture of animal cells: oxygenation of reactors and scale-up. Biotechnol Bioeng Symp 17:699–723
- Aunins JG, Henzler HJ (1993) Aeration in Cell Culture Bioreactors. In: Biotechnology, Second, Completely Revised Edition, Volume 3: Bioprocessing: 219–281, VCH Wiley
- Birch JR, Thompson PW, Boraston R, Oliver S, Lambert K (1987) The large-scale production of monoclonal antibodies in airlift fermenters. In: Webb C, Mavituna F (eds) Plant and animal cells: process possibilities. Ellis Horwood, Chichester, pp 162–171
- Catapano C, Hornscheidt R, Wodetzki A, Baurmeister U (2004) Turbulent flow technique for the estimation of oxygen diffusive permeability of membranes fort the oxygenation of blood and other cell suspensions. J Membr Sci 230:131–139 [DOI]
- Chisti Y (2000) Animal-cell damage in sparged bioreactors. TIBTECH 18:420–432 [DOI] [PubMed]
- Chu L, Robinson DK (2001) Industrial choices for protein production by large-scale cell culture. Curr Opin Biotechnol 12:180–187 [DOI] [PubMed]
- Côté P, Bersillon JL, Huyard A, Faup G (1988) Bubble-free aeration using membranes: process analysis. J Water Pollut Control Fed 60:1986–1992
- Côté P, Bersillon JL, Huyard A (1989) Bubble-free aeration using membranes: mass transfer analysis. J Membr Sci 47:91–108 [DOI]
- Engasser JM (1988) Bioreactor engineering: the design and optimization of reactors with living cells. Chem Eng Sci 43:1739–1748 [DOI]
- Farid SS (2006) Cell established bioprocesses for producing antibodies as a basis for future planning. Adv Biochem Engin/Biotechnol 101:1–42 [DOI] [PubMed]
- Fenge C, Lüllau E (2006) Cell culture bioreactors. In: Ozturk SS, Hu WS (eds) Cell culture technology for pharmaceutical and cell-based therapies. Taylor & Francis, New York, pp 155–224
- Frahm B, Kirchner S, Kauling J, Brod H, Langer U, Bödeker B (2007) Dynamische Membranbegasung im Bioreaktor zur Intensivierung der Sauerstoffversorgung empfindlicher Zelllinien. Chem Ing Tech 79:1052–1058 [DOI]
- Henzler HJ (1978) Untersuchungen zum Homogenisieren von Flüssigkeiten und Gasen. VDI-Forschungsheft 587:7
- Henzler HJ (2000) Particle stress in bioreactors. Adv Biochem Eng/Biotechnol 67:35–82 [DOI] [PubMed]
- Henzler HJ, Kauling J (1993) Oxygenation of cell cultures. Bioprocess Eng 9:61–75 [DOI]
- Henzler HJ, Obernosterer G (1991) Effect of mixing behaviour on gas–liquid mass transfer in highly viscous, stirred non-newtonian liquids. Chem Eng Technol 14:1–10 [DOI]
- Katinger HWD, Schreier W, Krömer E (1997) Bubble column reactor for mass propagation of animal cells in suspension culture. Ger Chem Eng 2:31
- Kim HK, Ham MS, Hong JS, Lee JH, Park KY, Lee HY (1996) Use of moving aeration membrane bioreactor for the efficient production of tissue type plasminogen activator in serum free medium. Biotechnol Bioprocess Eng 1:32–35 [DOI]
- Kompala DS, Ozturk SS (2006) Optimization of high cell density perfusion bioreactors. In: Ozturk SS, Hu WS (eds) Cell culture technology for pharmaceutical and cell-based therapies. Taylor & Francis, New York, pp 387–416 (2005)
- Lehmann J (1989) European patent 0172478
- Lehmann J, Piehl GW, Schulz R (1987) Bubble-free cell culture aeration with porous moving membranes. Develop Biol Standard 66:227–240 [PubMed]
- Ma N, Chalmers JJ, Aunins JG, Zhou W, Xie L (2004) Quantitative studies of cell-bubble interactions and cell damage at different pluronic F-68 and cell concentrations. Biotechnol Progr 20:1183–1191 [DOI] [PubMed]
- Mahnke EU, Büscher K, Hempel DC (2000) A novel approach for the determination of mechanical stresses in gas-liquid reactors. Chem Eng Technol 23:509–513 [DOI]
- Mayr B (1992) Engineering approach to mixing quantification in bioreactors. Bioprocess Eng 8:137–143 [DOI]
- Murhammer DW, Goochee CF (1990) Sparged animal cell bioreactors: mechanism of cell damage and pluronic F-68 protection. Biotechnol Progr 6:391–397 [DOI] [PubMed]
- Nehring D, Czermak P, Vorlop J, Lübben H (2004) Experimental study of a ceramic microsparging aeration system in a pilot-scale. Biotechnol Progr 20:1710–1717 [DOI] [PubMed]
- Oldshue Y (1996) Fermentation mixing scale up techniques. Biotechnol Bioeng 8:3–24 [DOI]
- Qi H, Jovanovic GN, Michaels JD, Konstantinov KB (2001) The art and science of micro-sparging in high-density perfusion cultures of animal cells. In: Linder-Olsson E, Chatzissavidou N, Lüllau E (eds) Animal cell technology: from target to market. Kluwer Academic Publishers, Dordrecht, pp 412–415
- Qi HN, Goudar CG, Michaels JD, Henzler HJ, Jovanovic GN, Konstantinov KB (2003) Experimental and theoretical analysis of tubular membrane aeration for mammalian cell bioreactors. Biotechnol Progr 19:1183–1189 [DOI] [PubMed]
- Schneider M, Reymond F, Marison IW, von Stockar U (1995) Bubble-free oxygenation by means of hydrophobic porous membranes. Enzyme Microb Technol 17:839–847 [DOI]
- Su WW, Caram HS, Humphrey AE (1992) Optimal design of the tubular microporous membrane aerator for shear sensitive cell cultures. Biotechnol Progr 8:19–24 [DOI] [PubMed]
- Sucker HG, Jordan M, Eppenberger HM, Widmer F (1994) Bubble bed reactor: a reactor design to minimize the damage of bubble aeration on animal cells. Biotechnol Bioeng 44:1246–1254 [DOI] [PubMed]
- Vorlop J, Lehmann J (1988) Scale-up of bioreactors for fermentation of mammalian cell cultures, with special reference to oxygen supply and microcarrier mixing. Chem Eng Technol 11:171–178 [DOI]