Abstract
Cluster Differentiation 90 (CD90) is a cell surface glycoprotein originally identified on mouse thymocytes. Although CD90 has been identified on a variety of stem cells and at varying levels in non-lymphoid tissues such as on fibroblasts, brain cells, and activated endothelial cells, the knowledge about the levels of CD90 expression on different cell types, including human primary cells, is limited. The goal of this study was to identify CD90 as a human primary cell biomarker and to develop an efficient and reliable method for eliminating unwanted or contaminating fibroblasts from human primary cell cultures suitable for research pursuant to cell based therapy technologies.
Keywords: CD90 expression, Human primary cell cultures, Elimination of contaminating fibroblasts, Magnetic bead cell separation
Introduction
Developing cell therapies requires maintenance of specifically differentiated cultures. The production of pure human primary cells for cell therapy cell banks is crucial if they are used in patient treatments. Fibroblast contamination is a frequent problem when culturing human primary cells since fibroblasts have faster generation times and can over populate more highly differentiated cells in vitro (Pal and Grover 1983). The hypothesis of this study is that staining cells with anti-Cluster Differentiation 90 Monoclonal Antibody (α-CD90 MAb) is a specific way to reveal mixed populations containing fibroblasts. Whether other differentiated human cells express CD90 or not is the focus of this paper.
Cluster Differentiation 90 (CD90) is a cell adhesion molecule and the smallest member of the immunoglobulin superfamily with a molecular weight of 25–35 KDa (Clark and Springer 1999). CD90 is also known as thymocyte differentiation antigen-1 (Thy-1) based on its presence on mouse thymocytes. CD90 is a glycoprotein anchored to the cell surface via a glycosylphosphatidylinositol (GPI) motif (Zucchini et al. 2001). Expression of CD90 differs among species. In humans, CD90 is expressed on stem cells including Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs) and Keratinocytic Stem Cells (KSCs) and at varying levels on non-lymphoid tissues such as fibroblasts, neurons and activated endothelial cells (Haack et al. 2007; Araki et al. 2007; Nakamura et al. 2006; Clark and Springer 1999).
CD90 functions in inflammation and wound healing by synthesizing and releasing growth factors, cytokines and extracellular matrix components to assist in repairing damaged tissue. CD90 plays a role in cell–matrix and cell–cell adhesion between inflammatory mediators of the immune response. For instance, monocyte CD90 ligand site binds to CD90 on activated endothelial cells during inflammation, focusing the immune cells migration to the sites of inflammation, tissue injury and infection (Saalbach et al. 2000). CD90 also inhibits neurite outgrowth on mature astrocytes (non-neuronal cells) and may play a role in growth and differentiation of stem cells (Rege and Hagood 2006).
This study assayed a panel of human primary cells for expression of CD90 using a fluorescent labeled mouse anti-human CD90 MAb (α-CD90) by direct immunofluorescence. The quantitation of CD90 expression on the cells was performed by flow cytometry. The primary cell types examined included Human Corneal Epithelial Cells (CEC), Human Retinal Pigmented Epithelial (RPE) cells, Normal Human Epidermal Keratinocytes (NHEK), Normal Human Bronchial Epithelial (NHBE) cells, Human Umbilical Vein Endothelial Cells (HUVEC), Uterine Smooth Muscle Cells (UtSMC), and Pulmonary Artery Smooth Muscle Cells (PASMC). Normal Human Dermal Fibroblasts (NHDF) were used as a positive cell control for CD90 expression (Ko et al. 2001), while K562 cells, a human chronic myelogenous leukemia cell line, were used as a negative cell control for CD90 expression (Lozzio and Lozzio 1975).
CD90 expression or absence on human CECs, RPE cells, NHBE cells, UtSMCs and PASMCs has not been previously reported. However, a study on mouse RPE cells showed that CD90 was barely detectable on RPE extracts (Peirson et al. 2004). NHEKs do not express CD90 (Wittmann et al. 2005). Activated HUVECs express CD90, while non-activated HUVECs do not (Wetzel et al. 2006; Saalbach et al. 2000). In this study, non-activated HUVECs were analyzed for CD90 expression.
In summary, this study consisted of two parts: (1) the identification of CD90 as a biomarker on human primary cells; (2) the development of an efficient method for removing contaminating fibroblasts from human primary cell cultures. The results of this study may impact future cell therapy development efforts. Several novel cell-based therapy projects are currently focused on treating Parkinson’s disease, congestive heart failure, and trophic ulcers of the lower limbs, to name a few (Polgar and Ng 2007; Tao and Li 2007; Sedov et al. 2007).
Materials and methods
Cell culture
Cell preparation
Following a proprietary protocol at Lonza, all normal human cells were isolated by enzymatic digestions, grown in culture and passaged or cryopreserved between passages 1–3. Quality Control (QC) routine testing of the cells included morphological observation through several passages, with the SMC receiving alpha smooth actin staining in addition to the viability, seeding efficiency, and population doublings specifications required for each cell type. All cells also tested negative for HIV-1, Mycoplasma, Hepatitis B and Hepatitis C, and sterility. HUVECs with fibroblast contamination were obtained from Lonza and used for the purpose of researching a method of fibroblast removal. Cryopreserved K562 cells were purchased from American Type Culture Collection (ATCC).
Cell culture medium
Cell culture medium was used per Lonza recommendations. Clonetics® Media systems were specifically developed and optimized to support the growth of human primary derived cells and consist of basal medium combined with the corresponding growth factors, cytokines, and supplements, referred to as a SingleQuots® (SQ) kit (Table 1). Table 2 lists the components of each SQ kit used.
Table 1.
Cell types and culture medium
| Cell type | Cell type abbreviation | Basal medium | Basal medium abbreviation | SingleQuots® kit (SQ)a | SQ abbreviation | FBS (%)c |
|---|---|---|---|---|---|---|
| Human corneal epithelial cells | CEC | Corneal epithelial model basal medium | CEMBM | Keratinocyte growth medium | KGM | 0 |
| Human umbilical vein endothelial cells | HUVEC | Endothelial basal medium | EBM-2 | Endothelial growth medium | EGM-2 | 2 |
| Uterine smooth muscle cells | UtSMC | Smooth muscle basal medium | SmBM | Smooth muscle growth medium | SmGM | 5 |
| Normal human epidermal keratinocyte cells | NHEK | Keratinocyte basal medium | KBM-2 | Keratinocyte growth medium | KGM-2 | 0 |
| Normal human bronchial epithelial cells | NHBE | Bronchial epithelial basal medium | BEBM | Bronchial epithelial growth medium | BEGM | 0 |
| Normal human dermal fibroblast cells | NHDF | Fibroblast basal medium | FBM | Fibroblast growth medium | FGM | 2 |
| Retinal pigmented epithelial cells | RPE | RPMI 1640 | RPMI | 10% FBS + 2 mM l-Glutamineb | NA | 10 |
| Pulmonary artery smooth muscle cells | PASMC | Smooth muscle basal medium | SmBM | Smooth muscle growth medium | SmGM | 5 |
| K562 cells | K562 | RPMI 1640 | RPMI | 10% FBS + 2 mM l-Glutamineb | NA | 10 |
Table 1 lists the cell type and the corresponding cell culture basal medium and supplements tested in this study
aSingleQuots® kits consist of growth factors and supplements. The culture medium was prepared by adding the SingleQuot kit to the basal medium
bThere was not a specific basal medium and SingleQuots® kit for RPE or K562 cells, therefore RPMI 1640 (445 mL), a general purpose medium, was supplemented with 10% Fetal Bovine Serum (FBS) (50 mL) and 2 mM L-Glutamine (5 mL) of 200 mM L-Glutamine (referred to as “RPMI-10% FBS”) and was used as the culture medium for RPE and K562 cells
cFinal FBS concentration in complete growth medium
Table 2.
Basal medium and SingleQuots® kit (SQ) components for cell cultures
| Basal medium 500 mL | SingleQuots® kit (SQ) | SingleQuots® kit (SQ) contents |
|---|---|---|
| Corneal epithelial model basal medium Cat# CC-3251 |
Keratinocyte growth medium Cat# CC-4131 |
BPE, 2 mL = Bovine Pituitary Extract-Cat# CC-4002E rhEGF, 0.5 mL = Epidermal Growth Factor- Human, Recombinant-Cat# CC-4015E GA-1000, 0.5 mL = Gentamicin Sulfate Amphotericin-B-Cat# CC-4081E Insulin, 0.5 mL = Insulin, Bovine-Cat# CC-4021E Hydrocortisone, 0.5 mL = Cat# CC-4031E |
| Endothelial basal medium Cat# CC-3156 |
Endothelial growth medium Cat# CC-4176 |
FBS, 10 mL = Fetal Bovine Serum-Cat# CC-4101A Hydrocortisone, 0.2 mL = Cat# CC-4031E r3-IGF-1, 0.5 mL = Recombinant long R Insulin-like Growth Factor-1 in aqueous solution-Cat# CC-4115A Heparin, 0.5 mL = Cat# CC-4396A |
| Smooth muscle basal medium Cat# CC-3181 |
Smooth muscle growth medium Cat# CC-4149 |
FBS, 25 mL = Fetal Bovine Serum-Cat# CC-4102D rhFGF-B, 1 mL = Recombinant Human Fibroblast Growth Factor-B-Cat# CC-4068D Insulin, 0.5 mL = Insulin, Bovine-Cat# CC-4021D GA-1000, 0.5 mL = Gentamicin Sulfate Amphotericin-B-Cat# CC-4081D rhEGF, 0.5 mL = Epidermal Growth Factor Human Recombinant in a buffered BSA saline solution-Cat# CC-4230D |
| Keratinocyte basal medium Cat# CC-3103 |
Keratinocyte growth medium Cat# CC-4152 |
BPE, 2 mL = Bovine Pituitary Extract-Cat# CC-4304H GA-1000, 0.5 mL = Gentamicin Sulfate Amphotericin-B-Cat# CC-4381H Transferrin, 0.5 mL = Cat# CC-4345H Insulin, 0.5 mL = Insulin, Bovine-Cat# CC-4321H Epinephrine, 0.5 mL = Cat# CC-4346H rhEGF, 0.5 mL = Epidermal Growth Factor. Human. Recombinant in a buffered BSA saline solution-Cat# CC-4315H Hydrocortisone, 0.5 mL = Cat# CC-4331E |
| Bronchial epithelial basal medium Cat# CC-3171 |
Bronchial epithelial growth medium Cat# CC-4175 |
Triiodothyronine (T3), 0.5 mL = Cat# CC-4211F Hydrocortisone, 0.5 mL = Cat# CC-4031F rhEGF, 0.5 mL = Epidermal Growth Factor. Human. Recombinant in a buffered BSA saline solution-Cat# CC-4230F Epinephrine, 0.5 mL = Cat# CC-4221F GA-1000, 0.5 mL = Gentamicin Sulfate Amphotericin-B-Cat# CC-4081F BPE, 2 mL = Bovine Pituitary Extract-Cat# CC-4009F Retinoic Acid, 0.5 mL = Prepared in HBSS and DMSO-Cat# CC-4085F Transferrin, 0.5 mL = Cat# CC-4205F Insulin, 0.5 mL = Insulin, Bovine-Cat# CC-F |
| Fibroblast basal medium Cat# CC-3131 |
Fibroblast growth medium Cat# CC-4126 |
FBS, 10 mL = Fetal Bovine Serum-Cat# CC-4101 J rhFGF-B, 0.5 mL = Recombinant Human Fibroblast Growth Factor-B-Cat# CC-4065 J GA-1000, 0.5 mL = Gentamicin Sulfate Amphotericin-B-Cat# CC-4081 J Insulin, 0.5 mL = Insulin, Bovine-Cat# CC-4021 J |
| RPMI 1640 Cat# 12-167F |
Not applicable | FBS, 50 mL = Fetal Bovine Serum-Cat# 14-501F L-Glutamine, 5 mL of 200 mM = Cat# 17-605E |
Table 2 lists the basal medium and associated SQ kit along with the components of each SQ kit used and the corresponding Lonza catalog numbers
Cell thaw and seed
One frozen ampoule per cell type was thawed in a 37 °C water bath and seeded at 2,500 cells/cm2 in a T-75 flask (Corning, Corning, NY), containing 15 mL of the appropriate culture medium (Table 1). CECs were freshly isolated from neonatal eyes in Lonza’s Cell Customs Laboratory and cultured for one passage. Those cells were seeded at 2,500 cells per cm2 in a T-175 flask and incubated at 37 °C with 5% CO2.
Upon harvesting all of the aforementioned cells from the first thaw, a Master Cell Bank (MCB) was created for each cell type by cryopreservation of the cells (refer to cell cryopreservation method). Cells that were thawed from the MCBs were seeded at 5,000 cells per cm2, or greater, in T-75 flasks.
Cell culture maintenance
The human primary cell culture’s growth medium was completely exchanged every 2–3 days. The primary cultures were incubated at 37 °C with 5% CO2 until the adherent cells reached 80–100% confluence. Once the primary cells were at or near confluence they were harvested, subcultured, cryopreserved and/or stained.
Fresh growth medium was added every 2–3 days to the K562 suspension cells. Alternatively, the K562 cell suspension was centrifuged at 210×g for 5 min at room temperature, the supernatant was aspirated and the cell pellet was resuspended in RPMI-10% FBS, 15 mL per T-75 flask. The K562 cell suspension was then transferred back to the culture flask and incubated at 37 °C with 5% CO2.
Cell harvest
During cell culture incubations and prior to cell harvests, photographs were taken of the cultures using an inverted fluorescence microscope equipped with SPOT Advanced Software (version 4.0) for image documentation.
For cell harvests, spent medium was aspirated from each flask and the flask was washed. NHDF, UtSMC, PASMC, HUVEC and RPE cell cultures were washed with 15 mL of Phosphate Buffered Saline (PBS) (Lonza, Walkersville, MD) per T-75 flask. CEC, NHEK, and NHBE cell cultures were washed with 5 mL of Trypsin-ethylenediamine tetraacetic acid (EDTA), 0.25% (Lonza, Walkersville, MD) per T-75 flask. The wash was then aspirated and Trypsin-EDTA was added to each flask: 3 mL per T-75 flask for NHDF, UtSMC, PASMC, HUVEC and RPE cell cultures, or 5 mL per T-75 flask for CEC, NHEK, and NHBE cell cultures. The flasks were incubated at 37 °C with 5% CO2 for 5 min or until the cell monolayer was detached from the flask. The cell cultures were viewed microscopically to ensure cell dissociation. The Trypsin-EDTA was then neutralized with 9–10 mL of Trypsin Neutralizing Solution (TNS) (Lonza, Walkersville, MD). The cell suspensions were transferred into 50 mL conical tubes. Each flask was rinsed with 15 mL of PBS and the rinse was transferred to the corresponding conical tube. K562 suspension cells were transferred directly to a 50 mL conical tube. The culture flask was rinsed with 15 mL of PBS per T-75 flask and the rinse was transferred to the same conical tube. The conical tube(s) containing the cell suspensions were centrifuged at 210×g for 5 min at room temperature. The supernatant was then aspirated and the cell pellet was resuspended in the appropriate growth medium, approximately 1–5 mL depending on the size of the cell pellet. The cells were counted using trypan blue exclusion. The counted cells were then processed for additional culture, cryopreservation, or staining.
Cell cryopreservation
All cells were frozen at concentrations of 5×105–1×106 cells per mL. Cells were frozen either in an automated Cryogenics freezer or overnight in a closed vessel containing 70% Isopropyl Alcohol (IPA) at −80 °C. All of the cells were then transferred to a liquid nitrogen freezer for storage (Table 3).
Table 3.
Freeze medium formulations
| Cell type | Culture medium (%) | FBS (%) | DMSOa (%) |
|---|---|---|---|
| CEC | 80 | 10 | 10 |
| NHEK | 80 | 10 | 10 |
| NHBE | 80 | 10 | 10 |
| HUVEC | 80 | 10 | 10 |
| RPE | 0 | 90 | 10 |
| UtSMC | 0 | 90 | 10 |
| PASMC | 0 | 90 | 10 |
| NHDF | 0 | 90 | 10 |
| K562 | 0 | 90 | 10 |
Table 3 lists the freeze medium formulation for the cell types
aDimethyl Sulphoxide (DMSO) (Sigma–Aldrich, St Louis, MO)
Reagent and buffer preparation
25% Sodium azide
25% Sodium azide (NaN3) was prepared by dissolving 25 mg of NaN3 (Sigma–Aldrich, St Louis, MO) in 100 mL of Water for Cell Culture (Lonza, Walkersville, MD).
1% Paraformaldehyde
1% Paraformaldehyde was prepared by adding 2 mL of 16% Paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) to 30 mL of PBS.
Staining buffer preparation
Staining buffer (PBS + 3% FBS + 0.1% NaN3) was prepared by combining 966 mL of PBS, 30 mL of FBS and 4 mL of 25% NaN3.
Blocking buffer preparation
Blocking buffer (1% human serum in staining buffer) was prepared by adding 0.3 mL of human serum (Lonza, Walkersville, MD) to 30 mL of staining buffer.
Preparation of 50 mg/mL bovine serum albumin (BSA)
A 50 mg/mL stock of BSA was prepared by dissolving 5 g of BSA (Sigma Aldrich, St Louis, MO) in 100 mL of PBS.
Preparation of magnetic bead kit buffer 1
Buffer 1 (0.1% BSA in PBS) was prepared by adding 5 mL of 50 mg/mL BSA stock to 245 mL of PBS in accordance with the kit’s instructions.
Preparation of magnetic bead kit buffer 2
Buffer 2 (0.1% BSA in PBS plus 0.6% Sodium Citrate) was prepared by adding 5 mL of 50 mg/mL BSA stock to 245 mL of PBS plus 0.15 mL of 4% Sodium Citrate (Lonza, Walkersville, MD) in accordance with the kit instructions.
Cell staining by direct immunofluorescence
Polystyrene sample tubes were labeled with the appropriate cell type and MAb name at the beginning of each cell staining experiment. The labeled sample tubes were then placed on ice and 1×105 cells from the cell suspension were transferred to the appropriate labeled sample tube. Staining buffer was then added, 2 mL per tube, to wash the cells. The sample tubes were mixed by rapping the rack containing the tubes with the open palm. They were then centrifuged at 500×g for 5 min at 4 °C.
After centrifugation the sample tubes were inverted to remove the supernatant, leaving approximately 0.1 mL of liquid above the cell pellet in each tube. Blocking buffer was prepared and 1.0 mL was added to each sample tube. The sample tubes were then covered with parafilm and inverted two to three times to suspend the cell pellet in the blocking buffer. The cells were blocked for 15 min at 4 °C. After the blocking incubation the sample tubes were centrifuged at 500×g for 5 min at 4 °C. After centrifugation the sample tubes were inverted again, leaving approximately 0.1 mL of blocking buffer above the cell pellet in each tube.
In every flow cytometry experiment, mouse anti-human MAbs were used to detect CD90, CD44 or CD31 on the cell surfaces. Positive and negative antibody controls were used with all cells tested (Table 4). The following MAbs were from BD Biosciences (San Jose, CA): Phycoerythrin (PE) Mouse IgG1 κ isotype control (IgG1), PE-Mouse Anti-Human CD90 (α-CD90), PE-Mouse IgG2b κ isotype control (IgG2), and PE-Mouse Anti-Human CD44 (α-CD44). The following MAbs were from Caltag (Invitrogen, Carlsbad, CA): PE-Mouse IgG1 isotype control (IgG1) and PE-Mouse Anti-Human CD31 (α-CD31).
Table 4.
Controls for cell staining by direct immunofluorescence
| Cell type | Positive Ab control | Negative Ab control |
|---|---|---|
| CEC | α-CD44 | IgG2 |
| UtSMC | α-CD44 | IgG2 |
| NHEK | α-CD44 | IgG2 |
| NHBE | α-CD44 | IgG2 |
| RPE | α-CD44 | IgG2 |
| PASMC | α-CD44 | IgG2 |
| HUVEC | α-CD31 | IgG1 |
| NHDFa | α-CD90 | IgG1 |
| K562b | NA | IgG1 |
Table 4 shows the positive antibody control for NHDF (α-CD90 MAb); CEC, NHEK, NHBE, UtSMC, PASMC and RPE cells (α-CD44 MAb); and HUVECs (α-CD31 MAb)
Each MAb had a corresponding negative isotype control, IgG1 or IgG2
aPositive cell control
bNegative cell control
The optimal concentration of each MAb (α-CD90: 20 μg/mL; α-CD31: 2.5 μg/mL; α-CD44: 1.25 μg/mL) was used in all assays, determined by titration experiments. After 1 h incubation at 4 °C, the stained cells were washed with 2.0 mL of staining buffer and centrifuged at 500×g for 5 min at 4 °C. After centrifugation the sample tubes were inverted to remove the supernatant, leaving ~0.1 mL of staining buffer above the cell pellet in each tube. The wash was repeated two more times for a total of three washes. After the final wash the stained cells were resuspended in 0.5 mL of staining buffer (or 1% paraformaldehyde in PBS if storing overnight or longer) and analyzed on the flow cytometer.
Flow cytometric analysis
Each stained cell sample was measured for expression of the specific biomarkers using a FACSort™ flow cytometer and analyzed using CellQuest Pro™ software (version 4.0.2). A flow cytometer has the capability of measuring thousands of fluorescent-labeled suspension cells within seconds based on their size, density (number of cytoplasmic granules, membrane size) and relative fluorescence intensity. There were a total of 10,000 cells measured for each antibody and isotype control. In all cases, less than 5% (<500 cells) of cells stained positive for the isotype control and were subtracted from the percentage of positive cells from the cell sample and this result was reported as the percentage of positive cells expressing the biomarker. In the titration experiments the cumulative fluorescence was reported, which was calculated by multiplying the mean fluorescence intensity by the percentage of positive cells.
Fibroblast elimination
Three methods for removing contaminating fibroblasts from human primary cell cultures were analyzed:
Cold Trypsin-EDTA was used to selectively detach fibroblasts from a CEC culture with contaminating fibroblasts (selective detachment).
A HUVEC culture contaminated with fibroblasts was cultured in medium without FBS verses medium with FBS (medium ± serum).
Fibroblasts were separated from the mixed HUVEC culture using α-CD90 MAb bound to magnetic beads for cell separation (magnetic bead cell separation).
Selective detachment
To remove fibroblasts from the CEC culture, the medium was aspirated from the flask and the cells were washed with 25 mL PBS (per T-175 flask). The PBS was aspirated and 7 mL of cold Trypsin-EDTA (0.25%) was added to the flask. After incubating for 2 min at room temperature the flask was rapped with the open palm to release the fibroblasts and the Trypsin-EDTA was aspirated. The cells were washed with 25 mL PBS, the wash aspirated, and 35 mL of fresh medium was added. The culture was then viewed under the microscope to determine if the fibroblasts were removed. The new CEC culture was then incubated at 37 °C with 5% CO2. The treatment was repeated if fibroblasts proliferated again in the CEC culture.
Medium ± serum
A MCB of a HUVEC culture with fibroblast contamination (referred to as mixed HUVECs) was created for further studies. Two ampoules from the mixed HUVEC MCB, along with the cell controls NHDF, HUVEC, and K562, were thawed and seeded into T-75 flasks. One ampoule of the mixed HUVECs was seeded with the appropriate growth medium (Table 1), which contained 2% FBS. The other ampoule of the mixed HUVECs was seeded with the appropriate growth medium, but without the FBS. The cultures were incubated until they reached 80–100% confluence. When they were at or near confluence, the cells were harvested, stained with α-CD90 and α-CD31 MAbs and analyzed via flow cytometry at both passages.
Magnetic bead cell separation
Cell separation was performed using CELLection™ Pan Mouse IgG Dynabeads® (Invitrogen, Carlsbad, CA) with α-CD90 MAb. Two separation methods were performed: the direct technique and the indirect technique. Using the direct technique, the α-CD90 MAb was attached to the magnetic beads at the human-α-mouse-IgG. (Hu-α-Ms-IgG beads) site and then the cell suspension was incubated with the α-CD90 + Ms-α-Hu-IgG beads complex [(α-CD90 + Hu-α-Ms-IgG beads) + Cells]. Using the indirect technique, the cell suspension was incubated with α-CD90 MAb and then the α-CD90 labeled cells were incubated with the magnetic beads [(α-CD90 + Cells) + Hu-α-Ms-IgG beads]. The α-CD90 bound cells then were isolated from the unbound cells via a magnet.
Three experiments were performed using magnetic bead cell separation on the mixed HUVECs, along with the cell controls. The cell suspensions from the magnetic separation (bound versus unbound) were collected into two 50 mL conical tubes for staining and further culture. All cells were stained with α-CD90 and α-CD31 before and after the magnetic bead separations and analyzed via flow cytometry.
Modifications of the kit protocol for the direct technique included: using Buffer 1 in place of Buffer 2 and using growth medium instead of Buffer 3. Modifications from the kit protocol for the indirect technique included: increasing the amount of α-CD90 from 1 μg/mL (recommended) to 2 μg/mL to attempt to capture more fibroblasts and using growth medium instead of Buffer 3. Buffer 2 was used to avoid cell clumping. A second magnetic separation step was performed after which microscopic inspection confirmed there were no beads present in the remaining cell suspension. A 10-fold increase in the amount of magnetic beads was incorporated to attempt to capture more α-CD90 MAb bound fibroblasts.
Once the appropriate conditions were determined, only one magnetic bead separation was performed on a fresh culture of mixed HUVECs. The cells were grown for an additional three passages and stained at each passage with α-CD90 and α-CD31 MAbs to ensure fibroblasts were not growing back in the purified HUVEC culture.
Results
CD90 Expression on the human primary cell panel
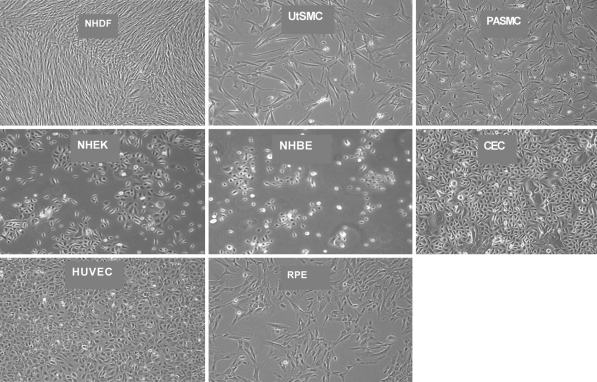
Seven human primary cell types (Table 1) were tested for CD90 expression to identify CD90 as a biomarker. Human primary fibroblasts were used as a positive (CD90-expressing) cell control and K562 cells as a negative (CD90-non-expressing) cell control. Representative photographs of each of the cell culture types are illustrated in Fig. 1. Since the density of the various cells ranged from 50 to 100% confluence (Fig. 1), it was not enough to rely on visual observation for cell morphology. Spindle-shaped cells are typical of fibroblasts. However, fibroblasts entering mitosis or apoptosis have a circular shape.
Fig. 1.
Human primary cell panel. The morphology of the NHDF, UtSMC and PASMC is spindle-shaped. The morphology of the CEC, NHEK, HUVEC and NHBE cells is cuboidal. The RPE cells exhibit both the spindle-shaped and the cuboidal morphologies
The panel of human primary cells was stained with a fluorescent labeled mouse anti-human CD90 MAb (α-CD90) and analyzed by flow cytometry to measure CD90 expression. CD90 expression may be affected by cell density, therefore the cells were routinely harvested and stained at 80–100% confluence to eliminate this as a variable between experiments. The positive and negative cell and antibody control results were as expected. UtSMCs were ≥90% CD90 and therefore were positive for CD90 expression. NHEK, HUVEC and NHBE cells were ≤10% CD90, thus were negative for CD90 expression. PASMCs expressed an average of 12% CD90. Expression of CD90 on RPE cells was variable from 70 to >95% CD90 (Fig. 2). CECs were 50% positive for CD90, which was expected based on the fibroblast contamination that was visualized via microscopy (Figs. 2, 3a, c). Based on this data, cuboidal cell (non-activated) morphology may be loosely correlated with negative CD90 expression, thus possibly providing a useful selection system for cell sorting.
Fig. 2.
Representative of CD90 expression on the human primary cell panel. CD90 was positively expressed on UtSMCs. RPE cells were variable for CD90 expression. CECs were 50% positive for CD90, due to fibroblast contamination. NHEK, PASMC, HUVEC and NHBE cells were negative for CD90 expression. The positive (NHDF) and negative (K562) cell control results were as expected
Fig. 3.
CEC culture before and after fibroblast removal (selective detachment). a CEC culture with contaminating fibroblasts, prior to the first Trypsin-EDTA treatment; b immediately after the Trypsin-EDTA treatment all of the fibroblasts appeared to be gone; c fibroblasts proliferated again after 5 days of incubation; d 9 days after the second Trypsin-EDTA treatment all of the fibroblasts appeared to be gone, but the remaining CECs senesced. The black arrows (→) point to a contaminating fibroblast
Elimination of contaminating fibroblasts from cell cultures
Three methods for removing contaminating fibroblasts from human primary cell cultures were tested: selective detachment of fibroblasts using cold Trypsin-EDTA, culturing mixed HUVECs in medium with FBS versus without FBS (medium ± serum), and separating mixed HUVECs using α-CD90 MAb with magnetic beads for cell separation.
Using the selective detachment method, the cold Trypsin-EDTA treatment on the CEC culture resulted in removal of the unwanted fibroblasts (Fig. 3b), however, after 5 days in culture the fibroblasts proliferated again (Fig. 3c). A second treatment once again cleared the fibroblasts from the CEC culture. After 9 days in incubation, the CECs senesced (Fig. 3d). Since the cells senesced, a second measurement of CD90 expression was not obtained. Overall, the cold Trypsin-EDTA culture treatment was simple, yet inefficient. This method required multiple treatments over which time the cells senesced.
Using the medium ± serum method, experiments were performed to remove contaminating fibroblasts from a HUVEC culture (mixed HUVECs) since fibroblasts need serum to proliferate. The mixed HUVECs were incubated in serum-free growth medium, while a duplicate control culture was incubated in the appropriate growth medium with 2% serum (Table 1). The mixed HUVEC culture incubated in serum-free growth medium proliferated slower than the mixed HUVEC culture incubated in growth medium with serum. This was confirmed visually by the lower percentage confluence (at passage 2 harvest) and by the lower cell counts obtained (data not shown). Fibroblasts were observed in both cultures at passage 2 and 3, but an accurate number of contaminating fibroblasts could not be determined visually. At passage 3, the mixed HUVEC culture in serum-free growth medium senesced.
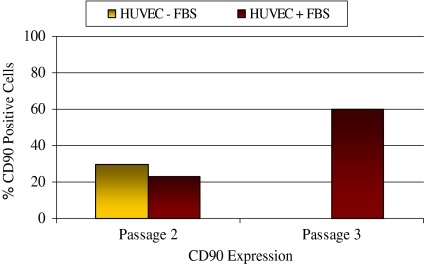
Since an accurate number of fibroblasts in the mixed HUVEC cultures could not be determined visually, the cells were stained with α-CD90 MAb and analyzed by flow cytometry. Theoretically the mixed HUVECs incubated without serum should include fewer fibroblasts and the expression of CD90 was expected to be lower than the mixed HUVECs incubated with serum. Surprisingly, there were more cells expressing CD90 in the culture incubated without serum than from the culture incubated with serum, 30% CD90 and 23%, respectively. There was an increase in CD90 expression from passage 2 (23%) to passage 3 (60%) on the mixed HUVECs from the culture with serum showing fibroblast numbers increased as a percentage of the total population in the presence of serum (Fig. 4). The medium ± serum method was not completely successful in removing contaminating fibroblasts from the mixed HUVECs. One reason was that both HUVECs and fibroblasts require serum for proliferation. However, the lack of serum may work in removing contaminating fibroblasts from cells that are non-serum dependent.
Fig. 4.
Expression of CD90 on HUVEC cultures with fibroblast contamination (medium ± serum). At passage 2, CD90 expression on the mixed HUVECs cultured without serum (30% CD90 positive) was slightly higher than on the mixed HUVECs cultured with serum (23% CD90 positive). At passage 3 the mixed HUVECs cultured without serum senesced and CD90 expression on the mixed HUVECs cultured with serum increased to 60% CD90 positivity, indicating overall fibroblast numbers increased in the presence of serum
Using the magnetic bead cell separation method, magnetic beads were bound to CD90 positive cells to remove contaminating fibroblasts from the mixed HUVEC culture via magnetic separation. The FACS analysis of the unbound cells separated via the direct technique revealed a false positive population of cells (<20% of 10,000) on the isotype controls, within the second to third decade of the histogram. The beads contain a Pan Mouse IgG site where the α-CD90 binds to the bead. The presence of the Pan Mouse IgG in the unbound cell culture would explain the false positive expression represented. Too many beads, not enough α-CD90 antibody or not enough magnetic separation washes could all be contributing factors to the false positive expression of the isotype control.
Three experiments were performed to optimize the conditions for pure separation including the use of the indirect technique, doubling the amount of recommended antibody, increasing the amount of magnetic beads. Tenfold, and performing two magnetic separations to ensure the bead-bound cells were completely isolated from the non-bead-bound cells, which was confirmed microscopically.
With the appropriate conditions established, one magnetic bead separation step was sufficient to successfully remove the contaminating fibroblasts from the mixed HUVEC culture. The HUVECs remained free of fibroblasts after several passages since the cells remained ≥90% CD31 positive and ≤10% CD90 positive. Based on these results, it was concluded that the CD90 magnetic bead cell separation method was an efficient method for eliminating contaminating fibroblasts from human primary cell cultures. Table 5 summarizes the results from the magnetic bead cell separation method.
Table 5.
Summary of results from the magnetic bead cell separation method
| Passage # | Experiment 1 direct → indirect technique |
Experiment 2 indirect technique |
Experiment 3 indirect technique |
|||
|---|---|---|---|---|---|---|
| Pre-beads | Post-beads | Pre-beads | Post-beads | Pre-beads | Post-beads | |
| 2 | 49/51 | 27/56 | 48/30 | 4/ND | 15/82 | <1/ND |
| 3 | 62/24 | ND | 5/91 | <1/ND | 3/93 | ND |
| 4 | ND | 31/84 | 4/93 | ND | 4/90 | ND |
| 5 | 77/13 | ND | ND | ND | 5/95 | ND |
Table 5 switching from the direct technique to the indirect technique, establishing the appropriate amount of α-CD90 MAb (experiment 1) and the appropriate amount of magnetic beads (experiment 2), resulted in fibroblast removal from the mixed HUVEC culture (experiment 2 and 3)
Results expressed as % positive cells. Underlined and non-underlined values (direct versus indirect, respectively) represent % CD90 and italic values represent % CD31 ND not done
Discussion
This study identified CD90 as a biomarker for a panel of human primary cell types. The cells were analyzed for CD90 expression using a fluorescent labeled mouse-α-human CD90 MAb and flow cytometry. An efficient and reliable method for elimination of unwanted or contaminating fibroblasts from human primary cell cultures is also reported.
The general consensus in the field of flow cytometry, in well controlled experiments, is that cells expressing a biomarker ≥90% are considered positive, while cells expressing a biomarker ≤10% are considered negative for the particular biomarker. These cutoff values are based on several parameters including variability of results (differences in parameter or gate settings), background noise (machine sensitivity), cell stickiness (MAb non-specifically sticking to cells), and machine errors (variances in the scatter and fluorescence signals) (Nardone et al. 2005).
Already known to be positively expressed on fibroblasts (positive cell control) (Ko et al. 2001), CD90 was also positively expressed on UtSMCs. Non-activated HUVEC, NHEK, and NHBE cells were found to be negative for CD90 expression. The average expression of CD90 on PASMC was 12% which is just above the 10% cutoff used to define negative expression. CEC and RPE cells displayed varying levels of CD90 expression. The cell culture medium used in this study was in accordance with Lonza recommendations; however, a medium comparison may reveal differential expression of CD90 and would be an interesting study for future experiments. This is the first report of CD90 expression on human UtSMC, RPE, CEC, PASMC and NHBE cells.
Of the cell panel tested, only NHEK and non-activated HUVECs have previously been reported to be negative for CD90 expression, which is in agreement with the results (Saalbach et al. 2000; Wittman et al. 2005). Leik et al. have shown that placental arterial SMCs were negative for CD90 expression; however, there are no previously published studies of CD90 expression on UtSMCs or PASMCs (2004). The role of CD90 on UtSMCs is unclear, and remains to be investigated. The expression of CD90 on CECs was likely due to fibroblast contamination in the culture. The RPE cells expressed variable levels of CD90, thus CD90 was determined to be neither a positive nor negative biomarker on RPE cells. As there is not a good explanation for the variability of CD90 expression on RPE cells, perhaps it is due to the differentiation state of the cells, changes related to re-entry of the cells into the cell cycle, or the presence of two different cell populations (McKay and Burke 1994). Future studies on RPE cells should test different levels of confluence versus CD90 expression over multiple passages of RPE cells. Information from that study could shed light on whether or not there is a correlation between cell density and passage number on CD90 expressing cells in the RPE culture. Also, another antibody control against a cell marker not expected to be expressed on fibroblasts, such as beta III tubulin, should be incorporated into future studies on RPE cells.
Hypothetically, non-activated cuboidal shaped cells are negative for CD90 and activated cuboidal shaped cells are positive for CD90. Activated HUVECs express CD90 and represent a repair phenotype found as part of an inflammatory response or wound healing situation (Zhu et al. 2001). This study showed a loose correlation between cuboidal cell morphology (non-activated) and negative CD90 expression. Additional testing of activated versus non-activated cuboidal cells, starting with HUVECs as an example for CD90 expression could provide useful information for cell separation from mixed cultures.
Three methods for removal of contaminating fibroblasts from mixed cell cultures were tested: Trypsin-EDTA selective cell detachment, cell proliferation in growth medium with and without serum, and cell separation with magnetic beads.
The Trypsin-EDTA selective detachment method is an inexpensive and simple method. While the contaminating fibroblasts were removed from the CEC culture, the fibroblasts grew back after several days of incubation, and after a second treatment the CECs senesced. Thus, overall, the method proved to be not well controlled and inefficient due to a requirement for multiple treatments.
Fibroblast removal from a mixed HUVEC culture was attempted using serum free medium. CD90 positivity, when measured, was due to fibroblast contamination of the HUVEC culture, which was also confirmed visually. Fibroblasts overtook the mixed HUVEC culture incubated with serum. Also, serum removal to rid the mixed HUVEC culture of fibroblasts was unsuccessful because HUVEC proliferation ceased. This happened because, although fibroblasts require serum for proliferation, so did the HUVECs. This method of fibroblast removal could potentially work for cultures that do not require serum for proliferation, such as NHEK, CEC and NHBE cells. Pure endothelial cells (ECs) have been incubated in medium containing 20% serum (Mason et al. 1996). Thus, higher levels of serum in the growth medium does not encourage fibroblast growth, or encourage fibroblast de-differentiation in pure EC cultures over multiple passages (Iacoviello et al. 1995; Mason et al. 1996). These results also showed that pure HUVECs (≥90% CD31 positive and ≤10% CD90 positive) stayed that way over multiple passages without de-differentiating into fibroblasts.
Magnetic beads coupled to α-CD90 MAb separated contaminating fibroblasts from the mixed HUVEC culture. Switching from the direct technique to the indirect technique, along with establishing the appropriate concentration of α-CD90 MAb and magnetic beads improved the efficiency of this method, resulting in only one treatment which remained effective over multiple HUVEC passages. Pure HUVEC cultures with ≥90% CD31 and ≤10% CD90 expression on the purified cells were the final result. The successful separation of CD90 positive and negative cells using the magnetic bead cell separation method was previously performed (Saalbach et al. 2000). The CD90 positive fibroblasts can be detached from the magnetic beads for further applications. This is possible because the α-CD90 MAb attaches to the surface of the magnetic bead via a DNA linker, providing a cleavable site from the magnetic bead after isolation. By adding an endonuclease (DNAse I) to the α-CD90 MAb + cells + magnetic beads complex, the DNA linker region is cleaved and the α-CD90 MAb bound cells are separated from the magnetic beads. Also, biomarkers not expressed on fibroblasts could be used for separating fibroblasts from mixed cell cultures. For example, CD31-magnetic beads could be used to isolate ECs from fibroblasts. The side effect might be activation of the ECs.
The methodology comparison results for fibroblast removal are shown in Table 6.
Table 6.
Methodology comparison of fibroblast removal
| Cell passage number | Trypsin-EDTA | Medium ± serum | CD90-magnetic beads | |
|---|---|---|---|---|
| (−) | (+) | |||
| 2 | 50 → 50 | 29/69 | 23/83 | 15/83 → <1/ND |
| 3 | ND | ND | 60/43 | 3/93 |
| 4 | ND | ND | ND | 4/90 |
Table 6 results expressed as % positive cells. Black values represent % CD90 expressing cells and italic values represent % CD31 expressing cells ND not done
The magnetic bead fibroblast-elimination technique using HUVEC cells was performed as a proof of principle experiment and was chosen as the best and most useful method in general. With the optimal removal conditions established (i.e., antibody amount, bead amount and washes) the method could be used with other cell types that do not express CD90 and that are contaminated with fibroblasts.
The magnetic bead method was very efficient relative to the Trypsin-EDTA method. However, the results show that as the efficiency greatly increased in going from the Trypsin-EDTA method to the magnetic bead method, the cost also increased somewhat due to more time, materials and equipment required.
HUVECs are an easy cell type to obtain, thus the cost of performing the magnetic bead cell separation method on this cell type may not be financially reasonable. However, the ability to purify cell cultures from contaminants is very important, especially when working with more difficult to obtain or costly cells of value, such as cell banks created for cell therapy treatments or patient specific cells used in autologous cell therapy.
While the bead separation method was costly on a research level, the cost of removing contaminating cell types would be a minor component of total cellular therapy manufacturing costs when looking at the big picture of cell based therapies starting from discovery and proceeding to patient treatments. There is a 1,000-fold difference in cost when dealing with cells for research and development ($100 s per vial) versus creating a Master Cell Bank (MCB) for cell therapy ($100,000 s per MCB). When looking at the magnetic bead separation method relative to valuable, high cost cells, magnetic bead fibroblast removal as a model for culture purification becomes a very effective tool. Thus, as the efficiency increases for valuable, high priced cells, the relative cost of performing this method becomes minimal.
Autologous therapies would also benefit from the availability of this affordable, efficient method for purification of cells. The magnetic bead cell separation method could provide a way to salvage cells from a patient dependant on them for therapeutic use. Therefore, as the efficiency of magnetic bead purification of cells increases the relative cost significantly decreases and should be considered during method development.
This study showed that different cell types could be identified based upon CD90 expression. There was a loose correlation between CD90 expression and cuboidal morphology on non-activated cells. Fibroblast contamination could be measured using α-CD90 MAb. Flow cytometry proved an objective, controlled, and quantitative method for identifying contaminating cells in human primary cell cultures. Mixed HUVEC and CEC cell cultures were purified of unwanted cell types by multiple methods using fibroblast removal as a model. Magnetic bead cell separation was the most efficient method found for removing unwanted CD90-expressing fibroblasts from human primary cell cultures, using mixed HUVECs for proof of principle. Future studies could focus on using the cell sorting function of flow cytometry for separating or purifying mixed cell cultures.
In summary, this study showed that staining cells with α-CD90 MAb is a specific way to reveal mixed populations containing contaminating fibroblasts in human primary cell cultures. Also, this study found an effective, efficient method to purify mixed cell cultures for potential use in the production of cell therapy MCBs used for patient treatments.
Acknowledgments
This work was supported and by Lonza Walkersville, Inc. and Hood College; all support is greatly appreciated. We thank Kim Warren, Ali Mohamed, Kim Roberts, Joseph Finny and Chris Elias for all of their help and support throughout this study.
References
- Araki H, Yoshinaga K, Boccuni P, Zhao Y, Hoffman R, Mahmud N (2007) Chromatin-modifying agents permit human hematopoietic stem cells to undergo multiple cell divisions while retaining their repopulation potential. Blood 109:3570–3580. doi:10.1182/blood-2006-07-035287 [DOI] [PubMed]
- Clark RA, Springer TA (1999) Protein reviews on the Web: CD90
- Haack-Sorensen M, Friis T, Bindslev L, Mortensen S, Johnsen HE, Kastrup J (2007) Comparison of different culture conditions for human mesenchymal stromal cells for clinical stem cell therapy. Scan J Clin Lab Invest Sep 12:1–17 [DOI] [PubMed]
- Iacoviello L, Kolpakov V, Salvatore L, Amore C, Pintucci G, Gaetano Gd, Donati MB (1995) Human endothelial cell damage by neutrophil-derived cathepsin g role of cytoskeleton rearrangement and matrix-bound plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol 15:2037–2046 [DOI] [PubMed]
- Ko KS, Arora PD, McCulloch AG (2001) Cadherins mediate intercellular mechanical signaling in fibroblasts by activation of stretch-sensitive calcium-permeable channels. J Biol Chem 276:35967–35977 [DOI] [PubMed]
- Leik CE, Willey A, Graham MF, Walsh SW (2004) Isolation and culture of arterial smooth muscle cells from human placenta. Hypertension 43:837–840. doi:10.1161/01.HYP.0000119191.33112.9c [DOI] [PubMed]
- Lonza (2007) Cambrex bioproducts catalog, Lonza Inc
- Lozzio CB, Lozzio BB (1975) Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 45:321–334 [PubMed]
- Mason JC, Yarwood H, Tarnok A, Sugars K, Harrison A, Robinson PJ, Haskard DO (1996) Human Thy-1 is cytokine-inducible on vascular endothelial cells and is a signaling molecule regulated by protein kinase c. J Immunol 157:874–883 [PubMed]
- McKay BS, Burke JM (1994) Separation of phenotypically distinct subpopulations of cultured human retinal pigment epithelial cells. Exp Cell Res 213:85–92. doi:10.1006/excr.1994.1176 [DOI] [PubMed]
- Michiels C (2003) Endothelial cell functions. J Cell Physiol 196:430–443. doi:10.1002/jcp.10333 [DOI] [PubMed]
- Nakamura Y, Muguruma Y, Yahata T, Miyatake H, Sakai D, Mochida J, Hotta T, Ando K (2006) Expression of CD90 on keratinocyte stem/progenitor cells. Br J Dermatol 154:1062–1070. doi:10.1111/j.1365-2133.2006.07209.x [DOI] [PubMed]
- Nardone M, Bergmann E, Cook L, Leitner W, Leppert G, Mostbock S, Brown F (2005) Flow cytometry: principles and methods. BIO-TRAC Foundation for advanced education in the sciences, Inc
- Pal K, Grover P (1983) A simple method for the removal of contaminating fibroblasts from cultures of rat mammary epithelial cells. Cell Biol Int Rep 7:779–783. doi:10.1016/0309-1651(83)90181-9 [DOI] [PubMed]
- Peirson SN, Bovee-Geurts PHM, Lupi D, Jeffrey G, DeGrip WJ, Foster RG (2004) Expression of the candidate circadian photopigment melanopsin (Opn4) in the mouse retinal pigment epithelium. Brain Res Mol Brain Res 123:132–135. doi:10.1016/j.molbrainres.2004.01.007 [DOI] [PubMed]
- Polgar S, Ng J (2007) A critical analysis of evidence for using sham surgery in Parkinson’s disease: implications for public health. Aust N Z J Public Health 31:270–274. doi:10.1111/j.1467-842X.2007.00060.x [DOI] [PubMed]
- Rege TA, Hagood JS (2006) Thy-1 as a regulator of cell–cell and cell–matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J 20:1045–1054. doi:10.1096/fj.05-5460rev [DOI] [PubMed]
- Saalbach A, Haustein U, Anderegg U (2000) A ligand of human Thy-1 is localized on polymorphonuclear leukocytes and monocytes and mediates the binding to activated Thy-1-positive microvascular endothelial cells and fibroblasts. J Invest Dermatol 115:882–888. doi:10.1046/j.1523-1747.2000.00104.x [DOI] [PubMed]
- Sedov VM, Andreev DY, Smirnova TD, Paramonov BA, Enkina TN, Sominina AA, Kiselev OI, Suissi YY, Lebedev LV (2007) The efficacy of cell therapy in the treatment of patients with trophic venous ulcers of the lower limbs. Angiol Sosud Khir 13(1):65–75. doi:10.1159/000103598 [PubMed]
- Tao Z, Li L (2007) Cell therapy in congestive heart failure. J Zhejiang Univ Sci B 8:647–660. doi:10.1631/jzus.2007.B0647 [DOI] [PMC free article] [PubMed]
- Wetzel A, Wetzig T, Haustein UF, Sticherling M, Anderegg U, Simon JC, Saalbach A (2006) Increased neutrophil adherence in psoriasis: role of the human endothelial cell receptor Thy-1 (CD90). J Invest Dermatol 126:441–452. doi:10.1038/sj.jid.5700072 [DOI] [PubMed]
- Wittmann M, Purwar R, Hartmann C, Gutzmer R, Werfel T (2005) Human keratinocytes respond to Interleukin-18: implication for the course of chronic inflammatory skin diseases. J Invest Dermatol 124:1225–1233. doi:10.1111/j.0022-202X.2005.23715.x [DOI] [PubMed]
- Zhu SN, Nolle B, Duncker G (2001) Coordinating cell proliferation and differentiation. Curr Opin Genet Dev 10:91–97. doi:10.1016/S0959-437X(00)00162-3 [DOI] [PubMed]
- Zucchini A, Del Zotto G, Brando B, Canonico B (2001) CD90. J Biol Regul Homeost Agents 15:82–85 [PubMed]