Abstract
Olive oil intake has been shown to induce significant levels of apoptosis in various cancer cells. These anti-cancer properties are thought to be mediated by phenolic compounds present in olive. These beneficial health effects of olive have been attributed, at least in part, to the presence of oleuropein and hydroxytyrosol. In this study, oleuropein and hydroxytyrosol, major phenolic compound of olive oil, was studied for its effects on growth in MCF-7 human breast cancer cells using assays for proliferation (MTT assay), cell viability (Guava ViaCount assay), cell apoptosis, cellcycle (flow cytometry). Oleuropein or hydroxytyrosol decreased cell viability, inhibited cell proliferation, and induced cell apoptosis in MCF-7 cells. Result of MTT assay showed that 200 μg/mL of oleuropein or 50 μg/mL of hydroxytyrosol remarkably reduced cell viability of MCF-7 cells. Oleuropein or hydroxytyrosol decrease of the number of MCF-7 cells by inhibiting the rate of cell proliferation and inducing cell apoptosis. Also hydroxytyrosol and oleuropein exhibited statistically significant block of G1 to S phase transition manifested by the increase of cell number in G0/G1 phase.
Keywords: MCF-7 cells, Oleuropein, Hydroxytyrosol, Apoptosis
Introduction
The incidence of cancer in Mediterranean countries is lower than in the rest of European countries and the United States (Keys et al. 1981). This is mostly described by the lower rate of the large bowel, breast, endometrial, and prostate cancers by a number of epidemiological studies, and the major reason for this, apart from possible genetic factors, is attributed to the dietary practices. The traditional Mediterranean diet is characterized by high consumption of foods of plant origin, relatively low consumption of red meat, and high consumption of olive oil and its products. There are a number of studies on health beneficial effects of olive oil. Several studies have been reported that olive oil is more favorable against cancer than other forms of added lipids due to its high content of monounsaturated fatty acids (Owen et al. 2000a, b; Visioli and Galli 2001). In recent studies, diet containing 15% olive oil could significantly reduce pre-cancerous lesions in rat breast and colon (Martin-Moreno et al. 1994; Corona et al. 2007; Paula et al. 2007). However, similar amount of soy oil did not have such a protective effect as was expected (La Vecchia et al. 1998). Furthermore, the incidence of breast cancer was 70% less in the rats group fed olive oil than in the rats group fed safflower oil (Owen et al. 2000a, b). These data suggest that cancer preventive effect of olive oil is not only attributed to the “good” fat content. Recently, growing evidence show that minor compounds in olive oil may take a part in cancer protection as well, and more attention is paid to its phenolic compounds.
The phenolic compounds of olive oil and leaf are a complex mixture of compounds that include 3,4-dihydroxyphenylethanol (hydroxytyrosol), 4-hydroxyphenylethanol (tyrosol), 4-hydroxyphenylacetic acid, protocatechuic acid, caffeic acid and p-coumaric acid, among others (Litridou et al. 1997; Caponio et al. 1999). The concentration of the phenolic fraction is several times higher in olive leaf than in oil and varies depending on the cultivar and climate (Tables 1, 2; Servili et al. 1999; Ryan et al. 2002; Abaza et al 2005). In vivo and in vitro studies suggest that these bioactive compounds exhibit powerful antioxidant activity (Visioli et al. 2000; Le Tutour and Guedon 1992). One of various phenolic compounds hydroxytyrosol seems to be among the most important ones. It is present in free form and as a constituent of complex molecules such as oleuropein in leaves and fruits. Both hydroxytyrosol and oleuropein have been shown to possess anti-inflammatory, bactericidal and bacteriostatic activities (Yang et al. 2007). Some in vivo studies on olive leaf have shown that its extract can decrease blood pressure and dilate the coronary arteries surrounding the heart (Tuck and Hayball 2002). Moreover, hydroxytyrosol has been shown to have anti-cancer effect on human colon adenocarcinoma HT-29 cells and human promyelocytic leukemia HL-60 cells (Fabiani et al. 2002, 2006), have anti-melanogenesis activity, whereas oleuropein inhibited cell growth of LN-18, poorly differentiated glioblastoma; TF-1a, erythroleukemia; 786-O, renal cell adenocarcinoma; T-47D, infiltrating ductal carcinoma of the breastpleural effusion; RPMI-7951, malignant melanoma of the skin-lymph node metastasis; and LoVo, colorectal adenocarcinoma cells (Hamdi and Hamdi 2005). Even though anticancer properties of oleuropein and hydroxytyrosol were confirmed in vitro with different cell lines, studies of their protective effect from breast cancer have not been demonstrated. Since many epidemiological studies suggest the possible correlation between olive products consumption and incidence of breast cancer here we tried to elucidate the possible effect of the main phenolic compounds hydroxytyrosol and oleuropein of olive leaf on breast cancer using human breast cancer cell line MCF-7.
Table 1.
The concentrations of major phenolic compounds in extra-virgin olive oil (Servili et al. 1999; Ryan et al. 2002; Abaza et al 2005)
| Phenolic compound | Concentration (mg/kg) |
|---|---|
| Hydroxytyrosol | 14.42 ± 3.01 |
| Oleuropein | 2.04 ± 0.78 |
| Oleuropein agycone | 14.42 ± 3.01 |
| Tyrosol | 27.45 ± 4.05 |
| Apigenin | 15.80 ± 4.51 |
Data expressed in mg/kg SEM
Table 2.
The concentrations of major phenolic compounds in olive leaf (Servili et al. 1999; Ryan et al. 2002; Abaza et al 2005)
| Phenolic compound | Concentration (mg/kg) |
|---|---|
| Hydroxytyrosol | 219 ± 3 |
| Oleuropein | 2,231 ± 52 |
| Demethyloleuropein | 984 ± 47 |
| Verbascoside | 27.45 ± 4.05 |
| Rutin | 15.80 ± 4.51 |
| Luteolin 7-o-glucoside | 15.80 ± 4.51 |
Data expressed in mg/kg SEM
Materials and methods
Chemicals
Dulbeco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS) and trypsin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 3-(4, 5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Wako (Osaka, Japan). Oleuropein and hydroxytyrosol were purchased from Funakoshi (Tokyo, Japan). ViaCount Assay reagent, propidium iodide, sulforhodamine-valyl-alanyl-aspartyl-fluoromethyl-ketone and 7-amino-actinomycin D were purchased from GE Healthcare (WI, USA).
Olive leaf extract preparation
Fresh green olive leaves were collected from the olive tree grown in Tunisia. Dry olive leaves (10 g) were extracted with 70% ethanol (100 mL) for 2 weeks. Extracted solution [10% (w/v)] was filtrated with a filter unit (Millipore, pore size; 0.22 μm).
Cell culture
MCF-7 cells were obtained from American Type Culture Collection (ATCC), and maintained in DMEM, supplemented with 10% FBS (Sigma), 1% 5,000 units/mL penicillin, 5,000 μg/mL streptomycin (Sigma). The cells were incubated in an atmosphere of 5% CO2 at 37 °C. All cells used in this study were between passages 5 and 15.
Growth inhibition assay
MCF-7 cells were seeded in 96-well plates at 2 × 103 cells/well in 100 μL of DMEM with 10% FBS. After 24 h the cells were treated with olive leaf extract [0.001, 0.01, 0.02, 0.1% (w/v)] for 12 h. Also MCF-7 cells were seeded in 96-well plates at 2 × 103, 2 × 104, 2 × 105 cells/well in 100 μL of DMEM with 10% FBS. After 24 h the cells were treated with oleuropein (25, 50, 100, 200 μg/mL) or hydroxytyrosol (6.25, 12.5, 25, 50 μg/mL) for 12 h. The treated cells were washed with PBS and 10 μL of MTT solution (5 mg/mL MTT in PBS) was added to each well. Cells were incubated for 12 h at 37 °C at the end of which 100 μL 10% SDS was added and incubated further for 6 h at 37 °C. Optical densities at 570 nm were measured using a plate reader (Powerscan HT; Dainippon Pharmaceutical, Osaka, Japan). The relative cell viability in percentage was calculated as (A570 of treated samples/A570 of untreated samples) × 100.
Cell proliferation assay
Cells were routinely seeded into six-well plates at 2 × 104 cells/well and incubated at 37 °C for 24 h. Cells were treated in fresh medium and cells were incubated with oleuropein (200 μg/mL) or hydroxytyrosol (50 μg/mL) for 3, 6, and 12 h. At the end of each time period, the cells were trypsinized to produce a single cell suspension, and the viable cell number in each well was counted using the ViaCount Assay (Guava Technologies, Hayward, CA).
Multicaspase assay by flow cytometry
Apoptosis was assessed using a flow cytometry based multicaspase assay kit (Guava Technologies, Burlingame, CA). The multicaspase assay is based on measurement of caspase enzymes activated during apoptosis. MCF-7 cells (2 × 105 cells/plate) were plated on tissue culture dishes (100 mm) in 10% FBS medium. Cells were incubated with oleuropein (200 μg/mL) or hydroxytyrosol (50 μg/mL) for 3, 6 and 12 h. Cells were trypsinized, washed in PBS and stained with sulforhodamine-valyl-alanyl-aspartyl-fluoromethyl-ketone (SR-VAD-FMK), and 7-amino-actinomycin D (7-AAD) according to the manufactures instructions. Cell populations were quantified using Guava personal cytometer (Guava Technologies, Hayward, CA).
Cell cycle analysis
MCF-7 cells (2 × 105 cells/plate) were exposed to concentrations of oleuropein (100 μg/mL) or hydroxytyrosol (25 μg/mL) for 24 h and the treated cells were collected by trypsinisation. The cells were fixed with 70% ethanol and stored at −20 °C. On the day of analysis, the cells were washed with PBS and suspended in 250 μL of PBS. One mL of phosphate–citrate buffer was added to the cell suspension and the suspension was incubated at room temperature for 5 min to facilitate the extraction of low molecular weight DNA. Following centrifugation the cells were resuspended in 500 μL DNA staining solution (20 μg/mL propidium iodide, 200 μg/mL DNase free RNase and 0.1% Triton X-100) and incubated in the dark at room temperature for 30 min. Cell cycle distribution was determined by fluorescence-activated cell sorting analysis of propidium iodide-stained ethanol-fixed cells using a Guava EasyCyte (Guava Technologies, Hayward, CA).
Results
Olive leaf extract has cytotoxic effect
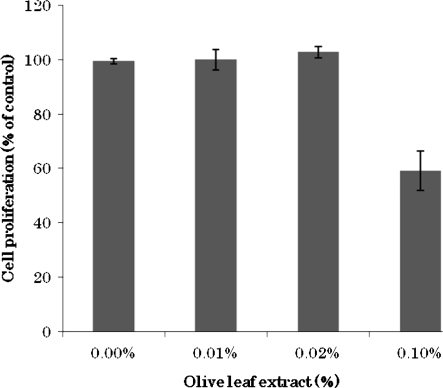
For the screening of anticancer effect of olive leaf we used olive leaf extract to treat breast cancer MCF-7 cells. MTT assay results have shown that after 48 h treatment with the extract at the dosage of 0.1% cancer cell proliferation was inhibited to 60% compared to the vehicle treated control cells (Fig. 1). The other treatment concentrations did not show cytotoxic effect. Since the major phenolic compounds of olive leaf are oleuropein and hydroxytyrosol, in subsequent anticancer assays we assumed that this cytotoxic effect could be mainly due to presence of these compounds and tried to elucidate their tumor suppressive effects.
Fig. 1.
Cytotoxic effect of olive leaf extract. Influence of initial cell number of human breast cancer MCF-7 cells incubated with olive leaf extract [0.1, 0.02, 0.01, 0.001% (w/v)]. Cell viability was determined with the MTT assay. Results (±SD) represent the average of three independent experiments
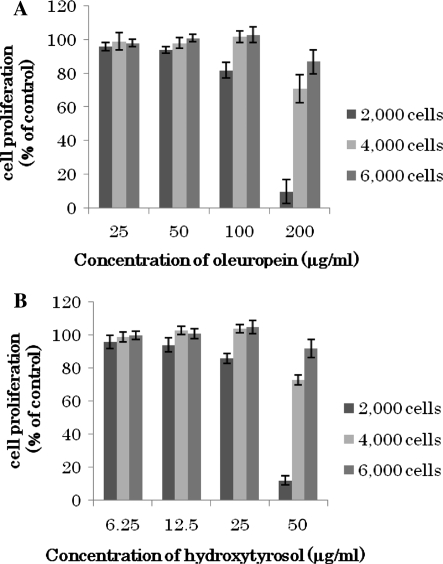
Hydroxytyrosol and oleuropein inhibit cell proliferation
The time-dependent change in MCF-7 cell viability was determined using the MTT assay. As shown in Fig. 2, there was a reduction in cell viability in a time- and concentration-dependant manner after an exposure to hydroxytyrosol and oleuropein. Initial cell number had a great influence on cytotoxic effects of these compounds. 200 μg/mL of oleuropein and 50 μg/mL of hydroxytyrosol treatment had the greatest cytotoxic effects on 2 × 103 cells/well including cell growth arrest in almost 90% of treated cells after 12 h of incubation in comparison to the controls. Whereas in doubled and tripled cell number treatment the effect has diminished but the compounds still could induce cell growth arrest. The results of flow cytometry analysis showed the same trend as with MTT assay. As shown in Fig. 3, the percentage of dead cells increased significantly after 12 h of treatment, and the inhibition rate of cell proliferation was similar with MTT assay results for both compounds. However, the following concentrations of oleuropein and hydroxytyrosol had been shown to be non-toxic for primary cells, such as normal human gingival GN61 fibroblasts and normal human neutrophils. Oleuropein did not show cytotoxicity at a 100 mM concentration. Also, hydroxytyrosol did not show cytotoxicity at a 200 mM concentration (Babich and Visioli 2003).
Fig. 2.
Inhibition of MCF-7 cell proliferation. Influence of initial cell number on the concentration-dependent decrease in viability of human breast cancer MCF-7 cells incubated with oleuropein a and hydroxytyrosol b. Cell viability was determined with the MTT assay. Results (±SD) represent the average of three independent experiments
Fig. 3.
Effect of oleuropein and hydroxytyrosol on the viability of MCF-7 cells. Viability of MCF-7 cells (2 × 104 cells/well) incubated with oleuropein (200 μg/mL) and hydroxytyrosol (50 μg/mL) for 3, 6, and 12 h as determined by the Guava ViaCount Assay. Results represent the average of two independent determinations
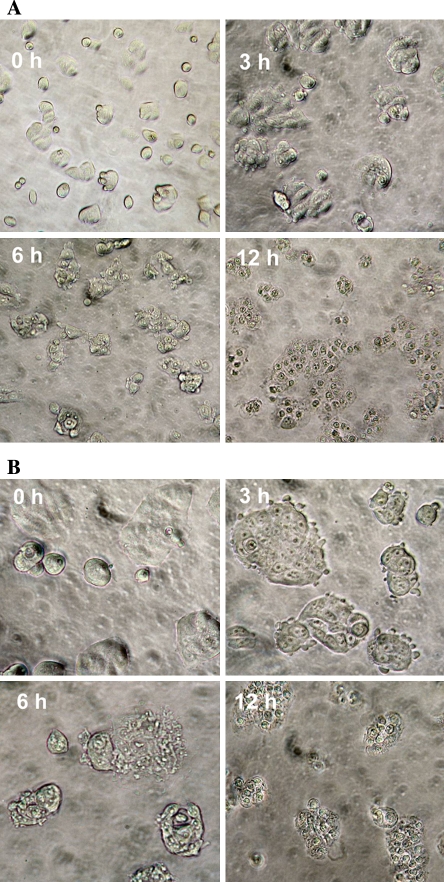
Morphology change of MCF-7 cells
During our trials with cell cytotoxicity assays we observed significant morphological changes in the cells (2 × 103 cells/well) incubated with 200 μg/mL oleuropein. Cell shrinkage, chromatin condensation and formation of apoptotic bodies were clearly defined. The same pattern was also observed in cells incubated with 50 μg/mL hydroxytyrosol for 12 h, suggesting apoptotic cell death of MCF-7 cells (Fig. 4). Since morphological features of apoptosis were well noticed during above mentioned concentration ranges we tried to use these ranges throughout the study, unless stated.
Fig. 4.
Effect of oleuropein and hydroxytyrosol on MCF-7 cells morphology. MCF-7 cells (2 × 103 cells/well) incubated with 200 μg/mL oleuropein a or with 50 μg/mL hydroxytyrosol b for 3, 6, 12 h
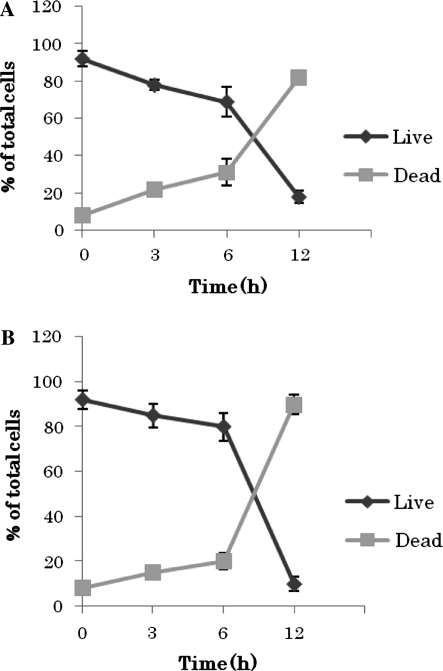
Hydroxytyrosol and oleuropein induce cell apoptosis
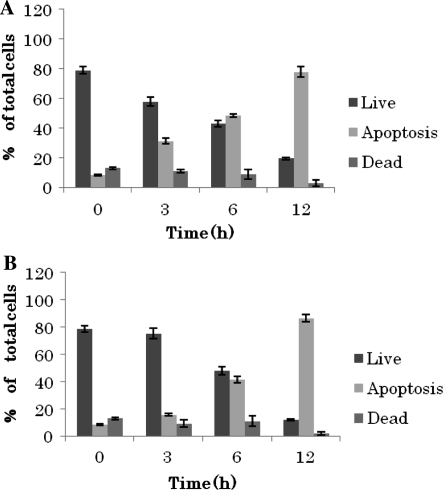
Since caspase activation is the classic feature of apoptosis we performed multicaspase assay using fluorochrome conjugated caspase inhibitor sulforhodamine-valyl-alanyl-aspartyl-fluoromethylketone (SR-VAD-FMK). This inhibitor is cell permeable and non-cytotoxic and binds to multiple caspases activated during apoptosis. Also the cell impermeable dye 7-aminoactinomycin D (7-ADD) was included in the assay to distinguish dead cells from apoptotic ones (Georg et al. 2007). From the multicaspase assay we could observe that caspases were activated soon after treatment with both compounds, besides, induction of the enzymes’ activation was greater in oleuropein treated cells (29.11%) compare to the hydroxytyrosol treated cells (11.64%) at the beginning of the treatment. Nevertheless, both compounds induced significant time dependant caspases activation to compared to the control cells. After 12 h the majority of the cells were apoptotic for both compound treated cells (Fig. 5).
Fig. 5.
Effect of hydroxytyrosol and oleuropein on induction of cell apoptosis. Multicaspase assay of human breast cancer MCF-7 cells incubated for 3, 6 and 12 h with 200 μg/mL oleuropein or 50 μg/mL hydroxytyrosol. Results represent the average of two independent determinations
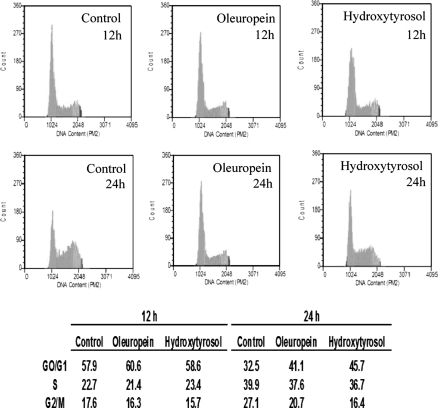
Hydroxytyrosol and oleuropein induce G1 cell cycle arrest in MCF-7 cells
Because it has been reported that the cells undergoing apoptosis are arrested in G1/G0 phase of cell division phase we could verify the induction of apoptosis of breast cancer cells by hydroxytyrosol and oleuropein using cell cycle analysis. In this assay we treated MCF-7 cells with 100 μg/mL of oleuropein and 25 μg/mL of hydroxytyrosol. When cells were treated for 24 h with changed concentration ranges, the cell cycle arrest at G1 phase was evident (41.1 and 45.7% in oleuropein and hydroxytyrosol treated cells, respectively), accompanying a decrease in G2/M phase when compared with the untreated control cells (27.1% in untreated cells; Fig. 6). From this result we suggest that apoptotic cell death of MCF-7 cells after hydroxytyrosol and oleuropein treatment was accompanied with the block of cell cycle in the G1 phase.
Fig. 6.
Effect of hydroxytyrosol and oleuropein on MCF-7 cell cycle. Cell cycle kinetics of human breast cancer MCF-7 cells incubated for 12, 24 and 48 h with 100 μg/mL oleuropein or 25 μg/mL hydroxytyrosol. Results represent the average (%) of two independent determinations
Discussion
Many vegetable foods in Mediterranean diet contain substances possessing anticancer properties (Visioli et al. 1998). Among them olive products such as oil are obviously the substantially different characteristics from western diet. Unlike refined oils, olive oil has a significant content of polyphenolic compounds. Even though recent studies have demonstrated that phenolic compounds of olive oil may play a role in cancer protection the main source of these compounds is olive leaf rather than olive oil. And it is not surprising that olive leaf has a long history for its medicinal remedies. Hydroxytyrosol and the secoiridoid oleuropein are studied well for their biological activities among olive phenolics. It should be noted that while tocopherols, phenolic acids, phenolic alcohols and flavonoids are present in many fruits and vegetables belonging to several botanical families, secoiridoids are present exclusively in plants of the family of Olearacea. Anticancer properties hydroxytyrosol and oleuropein were confirmed in vitro on different cell lines studies. But, breast cancer cell lines have not been demonstrated.
In this study we have demonstrated that hydroxytyrosol and oleuropein of olive leave inhibit the cell proliferation of breast cancer cells and induce apoptotic cell death. The results of MTT assay have shown that both hydroxytyrosol and oleuropein have cytotoxic effect on MCF-7 cells in a dose dependant manner. Also there was not much difference in cell growth inhibition effect between both compounds. Since MTT assay is based on the metabolic activity of the cells we tried to confirm the cytotoxic effect by flow cytometry analysis. Results of flow cytometric analysis also confirmed that the phenolic compounds reduced cell viability with the same trend as MTT assay results. Interestingly, the cell growth arrest was more effective when cells were seeded at a low cell number (Fig. 2). It is probably caused by the contact surface with hydroxytyrosol or oleuropein. The contact surface with hydroxytyrosol or oleuropein at small number of initial cell seeding is wider than the large number of initial cell seeding. These data may suggest that phenolic compounds of olive leaf have health protective effects rather than healing effects. Constant consumption of olive products such as leaf tea and oil may enhance these protective effects thus, suggesting their daily consumption is more effective than the infrequent intake. Also in vivo studies have shown that soon after oral administration the compounds appeared in the plasma suggesting their good absorption from the intestines, but the entire quantity administered was not found in the urine which may suggest accumulation in organs such as breast or erythrocytes (Elisa et al. 2005).
During our trials using cell cytotoxicity assays we observed significant morphological changes in the cells (Fig. 4). Cell shrinkage, chromatin condensation and formation of apoptotic bodies were clearly observed suggesting the cell growth arrest could be due to apoptosis. Apoptosis has been well characterized and is thought to proceed through a series of conserved and well studied steps.
The caspases, cysteine proteases, are of central importance in the apoptotic signaling network which is activated in most cases of apoptotic cell death. Actually, strictly defined, cell death only can be classified to follow a classical apoptotic mode if execution of cell death is dependent on caspase activity. Our multicaspase activity assay revealed that with compounds’ treatment the proportion of caspase enzymes activated cells increased time-dependently, indicating the activation of these enzymes in the cells (Fig. 5).
To further elucidate our hypothesis of apoptotic cell death of breast cancer cells by hydroxytyrosol and oleuropein we tried to use other methods for its detection. Since the G1/G0 cell cycle arrest has been shown to correlates well with apoptosis determined by other means we examined sub-G1 DNA content by flow cytometry analysis following treatment with two phenolic compounds. Our data confirmed that the cells treated with hydroxytyrosol and oleuropein exhibited statistically significant block of G1 to S phase transition manifested by the increase of cell number in G0/G1 phase. Although the oleuropein notably induced less cell cycle arrest than hydroxytyrosol it was still capable to significantly promote breast cancer apoptotic cell death.
The molecular mechanism of hydroxytyrosol and oleuropein induced apoptotic cell death is not clear. Yet, these compounds have high antioxidant activities. In a recent study, the antioxidant activity of α-tocopherol and phenolic extracts from olives and olive oil was compared over time. It was demonstrated that after initiation of radical formation the scavenger activity of α-tocopherol was higher but soon terminated whereas the extract from olives and oil contained compounds continued to reduce the concentration of these radicals and with time the extracts of the olives were much more active than α-tocopherol (Keceli and Gordon 2001). On the other hand, some investigators observed that phenolic compounds with high reducing ability can be not only antioxidants but also pro-oxidants, thus generating reactive oxygen species (ROS) (Wlodek and Steven 2001). It is interesting to note that many human cancer cell types exist in a highly oxidative state due to decreased antioxidant protective enzyme levels compared to their normal tissue counterparts. Thus, cancer cells may be more sensitive to any generated ROS within the cells. Growing evidence exist that ROS may even activate apoptotic death pathways. Zhao et al. (1997) observed that vitamin E induced DNA synthesis arrest and stimulated apoptosis in MCF-7 cells (Wlodek and Steven 2001). Also most chemotherapeutic agents such as radiation, alkylating agents, anthracyclines induce apoptotic death in cancer cells through generating ROS. This could be the possible mechanism of action of antioxidants for tumor suppressive effect.
In conclusion, our results have shown that main phenolic compounds of olive leaf hydroxytyrosol and oleuropein induced apoptotic cell death of human breast cancer MCF-7 cells. Our findings may support the breast cancer protective results of epidemiological studies of Mediterranean diet in relation to olive products’ consumption. Even though the cancer protective effect of the main olive oil component oleic acid in gene regulation was demonstrated further studies are needed to explore molecular mechanisms of tumor suppressive effect of other olive tree products such as olive leaf because they also could be potent inducers of such effect if consumed frequently.
Contributor Information
Junkyu Han, Email: jhan@sakura.cc.tsukuba.ac.jp.
Hiroko Isoda, Phone: +81-29-8535775, FAX: +81-29-8535776, Email: isoda@sakura.cc.tsukuba.ac.jp.
References
- Abaza L, Taamalli W, Zarrouk M et al (2005) Natural antioxidant composition as correlated to stability of some Tunisian virgin olive oils. Rivista Italiano Delle Sostanze Gresse 132:12–18
- Babich H, Visioli F (2003) In vitro cytotoxicity to human cells in culture of some phenolics from olive oil. Farmaco 58:403–407 [DOI] [PubMed]
- Caponio F, Alloggio V, Gomes T (1999) Phenolic compounds of virgin olive oil: influence of paste preparation techniques. Food Chem 64:203–209 [DOI]
- Corona G, Deiana M, Incani A et al (2007) Inhibition of p38/CREB phosphorylation and COX-2 expression by olive oil polyphenols underlies their anti-proliferative effects. Biochem Biophys Res Commun 362:606–611 [DOI] [PubMed]
- Elisa T, Marco G, Garden T et al (2005) The phenolic compounds of olive oil: structure, biological activity and beneficial effects on human health. Nutr Res Rev 18:98–112 [DOI] [PubMed]
- Fabiani R, De Bartolomeo A, Rosignoli P et al (2002) Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur J Cancer Prev 11:351–358 [DOI] [PubMed]
- Fabiani R, Bartolomeo AD, Rosignoli P et al (2006) Virgin olive oil phenols inhibit proliferation of human promyelocytic leukemia cells (HL60) by inducing apoptosis and differentiation. J Nutr 136:614–619 [DOI] [PubMed]
- Georg F, Surajit D, Volker F et al (2007) Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res 67:2187–2196 [DOI] [PMC free article] [PubMed]
- Hamdi K, Hamdi RC (2005) Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochem Biophys Res Commun 334:769–778 [DOI] [PubMed]
- Keceli T, Gordon HM (2001) The antioxidant activity and stability of the phenolic fraction of green olives and extra virgin olive oil. J Sci Food Agric 81:1391–1396 [DOI]
- Keys A, Aravanis C, Van Buchem FSP et al (1981) The diet and all-causes death rate in the seven countries study. Lancet 8237:58–61 [PubMed]
- La Vecchia C, Negri E, Franceschi S et al (1998) Olive oil, other dietary fats, and the risk of breast cancer (Italy). Cancer Causes Control 6:545–550 [DOI] [PubMed]
- Le Tutour B, Guedon D (1992) Antioxidative activities of Olea europaea leaves and related phenolic compounds. Phytochemistry 31:1173–1178 [DOI]
- Litridou M, Linssen J, Schols H et al (1997) Phenolic compounds in virgin olive oils: fractionation by solid phase extraction and antioxidant activity assessment. J Sci Food Agric 74:169–174 [DOI]
- Martin-Moreno JM, Willett WC, Gorgojo L et al (1994) Dietary fat, olive oil intake and breast cancer risk. Int J Cancer 58:774–780 [DOI] [PubMed]
- O’Dowd Y, Driss F, Dang PM et al (2004) Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem Pharmacol 68:2003–2008 [DOI] [PubMed]
- Owen RW, Mier W, Giacosa A et al (2000a) Phenolic compounds and squalene in olive oils: the concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignans and squalene. Food Chem Toxicol 38:647–659 [DOI] [PubMed]
- Owen RW, Giacosa A, Hull WE et al (2000b) The antioxidant anticancer potential of phenolic compounds isolated from olive oil. Eur J Cancer 36:1235–1247 [DOI] [PubMed]
- Paula PC, Pereira AM, Silvério C et al (2007) Fiber, beans, olive oil and breast cancer-toxic or beneficial. Toxicol Lett 172:S199
- Ryan D, Antolovich M, Prenzler P et al (2002) Biotransformations of phenolic compounds in Olea europaea L. Sci Hortic 92:147–176 [DOI]
- Servili M, Baldioli M, Selvaggini R et al (1999) Phenolic compounds of olive fruit: one- and two-dimensional nuclear magnetic resonance characterization of nuzhenide and distribution in the constitutive parts of fruit. J Agric Food Chem 47:12–18 [DOI] [PubMed]
- Tuck LK, Hayball JP (2002) Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem 13:636–644 [DOI] [PubMed]
- Visioli F, Galli C (2001) Phenolics from olive oil and its waste products. Biological activities in vitro and in vivo studies. World Rev Nutr Diet 88:233–237 [DOI] [PubMed]
- Visioli F, Bellomo G, Galli C (1998) Free radical-scavenging properties of olive oil polyphenols. Biochem Biophys Res Commun 247:60–64 [DOI] [PubMed]
- Visioli F, Galli C, Plasmati E et al (2000) Olive phenol hydroxytyrosol prevents passive smokinginduced oxidative stress. Circulation 102:2169–2171 [DOI] [PubMed]
- Wlodek L, Steven HZ (2001) Antioxidants, programmed cell death, and cancer. Nutr Res 21:295–307 [DOI]
- Yang DP, Kong DX, Zhang HY (2007) Multiple pharmacological effects of olive oil phenols. Food Chem 104:1269–1271 [DOI]
- Zhao B, Yu W, Qian M et al (1997) Involvement of activator protein-1 (AP-1) in induction of apoptosis by vitamin E succinate in human breast cancer cells. Mol Carcinog 19:180–190 [DOI] [PubMed]