Abstract
In this study, the authors explored the effect of human mesenchymal stem cell (MSC) implantation on the restoration of degenerative intervertebral discs (IVDs) in the rat. A unique rat coccygeal model was used to investigate the effects of transplanting human MSCs and to examine MSC survival in degenerative discs. MSC implantations into rat coccygeal IVDs were performed at 2 weeks post-injury. Radiologic and histologic evaluations were performed at 2, 4, 6, and 8 weeks post-injury. MSC-injected segments (TS) retained disc height and signal intensity, but injured non-injected segment (IS) progressively lost disc height. Pathological results revealed that the TS group showed relative restoration of the inner annulus structure; however, the IS group showed destruction of the inner annulus structure. Immunohistochemical staining using Anti-Human Nucleic Antibody (#MAB1281 Chemicon) revealed positive staining in the TS group at 2 weeks post-transplantation (4 weeks post-injury). This study shows that human MSCs survive for 2 weeks after transplantation into the IVDs of rats, and that MSCs increased the heights and signal intensities of intervertebral disc.
Keywords: Mesenchymal stem cell, Animal model, Intervertebral Disc, Degeneration
Introduction
Histologically, intervertebral discs (IVDs) are composed of a chondrocytic end plate, a nucleus pulposus containing notochord cell and chondrocyte-analogous cells, and an annulus fibrosus composed of fibroblasts (Cheung et al. 2005; Martin et al. 2002). Furthermore, the annulus fibrosus and nucleus pulposus contain extracellular collagen and proteoglycan matrices. The changes that occur in degenerative IVDs include decreases in chondrocytes, extracellular matrix components, proteoglycan, and Type II collagen, and increases in inflammatory substances and enzymes, such as, the metalloproteinases. Therefore, IVD degeneration is presumed to commence with a decline in the extracellular matrix of the nucleus pulposus, and to culminate in reduced matrix and water content in this tissue, which results in cracks or microfractures in the annulus fibrosus (Leung et al. 2006). Consequently, dynamic imbalance reduces shock-absorbing capacity, which is required to attenuate the effects of external impacts or loads. The detailed pathophysiology of IVD degeneration has not been elucidated, but both mechanical overloading of IVDs and disruption of the mechanical balance afforded by adjacent structures (e.g., facets, ligaments, and muscles) have been suggested to contribute substantially to the degenerative condition (Martin et al. 2002).
Eventually IVD degeneration causes clinical disease. Sometimes, disc degeneration is the direct cause of discogenic back pain, regardless of uncertainties concerning the details of the pathologic mechanism involved. Furthermore, IVD degeneration can develop into serious conditions, such as, IVD prolapse, spondylolisthesis, spinal canal stenosis, or facet joint syndrome (Martin et al. 2002; Antoniou et al. 1996).
Treatments for degenerative IVD disease involve medication to alleviate back pain, physical treatment, and the surgical removal of herniated disc material and spinal fusion. However, these approaches cannot be viewed as fundamental treatments for IVD degeneration because they do not address its cause. Recently, the molecular biological characteristics of IVD degeneration were identified, and treatment strategies are being sought that regenerate IVDs by restoring extracellular matrix or cellular components in animal models (Crevensten et al. 2004; Le Visage et al. 2006; Sakai et al. 2006, 2005, 2003).
In this context, the merits of gene therapy and the transfusion of growth factors, such as, cytokines, are being investigated (Steck et al. 2005; Rousseau et al. 2007). It has been reported that collagen and proteoglycan levels improve after gene therapy with transfected adenovirus strains expressing SOX9, TGF-β1, TIMP1, and BMP2 (Wallach et al. 2003; Paul et al. 2003; Nishida et al. 1999), and that proteoglycan levels increase after injecting OP-1 (BMP-7), GDF-5, and LMP-1 directly into an IVD (An et al. 2005; Kawakami et al. 2005; Li et al. 2002; Takegami et al. 2005; Yoon et al. 2004). However, this approach is unreliable because it does not directly increase the numbers of IVDs cells, and thus, is problematic as a potential therapy for degenerative IVD conditions (Leung et al. 2006).
When cell therapy was hailed as the next generation treatment, another approach to regenerative therapy, autologous nucleus pulposus cell transplantation, also became a major research topic in the context of the regeneration of IVDs (Okuma et al. 2000; Ganey et al. 2003; Gruber et al. 2002; Nishimura and Mochida 1998; Nomura et al. 2001). However, because the supply of autologous IVD cells is problematic (Okano 2002), the notion of using stem cells in this context has increased in importance, and trials are currently in progress (Crevensten et al. 2004; Leung et al. 2006; Sakai et al. 2006, 2005, 2003).
Recently, transplantation therapy for IVD degeneration using mesenchymal stem cells (MSCs) has been attempted. MSCs can be extracted from several organs, such as fetal liver, umbilical cord blood, bone marrow, placenta, adipose tissue, muscle, and dermis (Kraemer 1995), and can be induced to differentiate into cells of the articular cartilage (Deans and Moseley 2000; Liechty et al. 2000; Toma et al. 2002) or chondrocyte lineages (Im et al. 2001; Quintavalla et al. 2002; Wakitani et al. 1994). Furthermore, as IVD cells have phenotypes similar to those of chondrocytes, it would appear that IVD cells are differentiated from MSCs (Risbud et al. 2004).
Hence, we sought to examine the regenerative effects of transplanted human MSCs in a rat degenerative IVD model by using a magnetic resonance imaging and a histologic approach.
Materials and methods
Experimental animals
We used eight female Sprague-Dawley rats in total. The animals were free of infection and weighed 270–300 g. Experiments were performed using coccygeal IVDs; first segments (Co2–3) were used as normal control segments (CS), second segments (Co3–4), the MSC-transplantation segments (TS) were injected with MSCs 2 weeks after blade injury, and third segments (Co4–5), the injured segments (IS) were injected with saline 2 weeks after blade injury. Radiological and histological evaluations were performed at 2, 4, 6, and 8 weeks post-injury.
Bone marrow aspiration, isolation of mesenchymal stem cells, and cell culture
Bone marrow (10 mL) was aspirated, under local anesthesia, from the posterior iliac crests of one human donor (28 years old), and bone marrow mononuclear cells were isolated by Ficoll (Sigma) density gradient centrifugation. Mononuclear cells (1 × 106/mL) were placed in a 175 cm2 flask (Nunc, Invitrogen) and cultivated in low-glucose Dulbecco’s modified Eagle’s medium (Gibco, Invitrogen) containing 10% fetal bovine serum (Gibco, Invitrogen) and 1% penicillin/streptomycin (Gibco, Invitrogen) in a humidified incubator at 37 °C for 5 days under 5% CO2. Non-adherent cells were then removed by replacing the medium (colonies formed within 5–7 days). During monolayer expansion, cells were plated at a density of 1–3 × 104 cells/cm2, and medium was replaced every 3 days. Cells were harvested using 0.25% trypsin when these primary cultures of MSCs reached 80% confluence. We used GMP (Good Manufacturing Practice) conditions (FCB-Pharmicell Co Ltd, Sungnam, South Korea) and clinical grade reagents to prepare the cells (Bang et al. 2005; Li et al. 2008).
Cell preparation for injection
On the day of injection, cells were harvested using trypsin and washed in 10 mL phosphate-buffered saline. Cell viability was determined by trypan blue staining after harvesting and before infusion. Freshly harvested MSCs were placed into a capped 10 mL syringe prior to injection.
IVD degeneration and the transplantation of human mesenchymal stem cells
We used the degenerative IVD model devised by Rousseau et al. (2007). Under 5% isoflurane inhalation anesthesia, we located the IVDs at Co2–3, Co3–4, and Co4–5 by fluoroscopy, and placed a 1-inch longitudinal hemisection using a No. 10 blade along the tail to expose the lateral portions of tail discs. Human MSCs (three passages, 1 × 106 cells/15 µL/segment) (Sakai et al. 2006, 2005, 2003) were then placed into TS using a stereotactic microinjector (Harvard Apparatus; Holliston, MA, USA) and a 26 G needle at 2 weeks post injury. The same amount of saline was injected into IS. CS were not injected.
Methods of evaluation
Radiological method
Radiological evaluations were performed at 2, 4, 6, and 8 weeks post injury, by magnetic resonance imaging and by using a computerized imaging analyzer.
Disc heights: Disc heights were measured using the method devised by Lu et al. (1997), according to which disc height is defined as the distance between the vertebral bodies of upper and lower segments in the center of the disc space. Disk heights were calculated using Paravision software (Paravision version 3.0.2; Bruker Biospin AG, Karlsruhe, Germany). The results shown are the averages of two measurements.
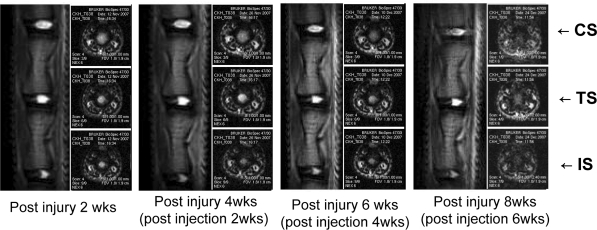
Magnetic resonance imaging of IVDs: Magnetic resonance images were obtained using a 4.7 T Bruker Biospin imager. Rats were positioned prone on a quadrature surface coil and sagittal images were obtained through the lumbar spine (spin echo; repetition time 500–4,000 s; echo time 65–123 s; number of excitations 8; field of view 4 cm; slice thickness 1.0–1.5 mm; no phase wrap). Imaging was performed at 2, 4, 6, and 8 weeks post injury and T2-weighted midsagittal and axial images of each segment were obtained (Fig. 1). Paravision software was used to measure signal intensities. CS served as controls and intensities are quoted as percent ratios versus CS signal intensities.
Fig. 1.
MRI images of a normal control segment (CS), a MSC transplantation segment (TS), and an only injured segment (IS) taken every 2 weeks after disc injury. MCSs were transplanted at 2 weeks post-injury. TS showed much higher T2-weighted signal intensities than IS
Histological evaluations
Rats were anesthetized and transcardinally perfused with 125 mL of normal saline (containing 10 U of heparin sodium per 1 mL), followed by 250 mL of ice-cold 4% paraformaldehyde. After excising coccygeal vertebrae there were fixed in 10% formalin solution for 48 h. They were then decalcified in 5% nitric acid for 3 days (the acid solution was changed every 24 h). Finally, they were washed in ammonia solution for 30 min to neutralize residual acid.
The vertebrae samples so obtained were then embedded in paraffin wax, and paraffin blocks were sectioned longitudinally using a microtome into 20 μm sections. Sections were stained with hematoxylin and eosin (H–E), and degenerative disc changes were histologically graded using the criteria of Nishimura and Mochida (1998) (Table 1).
Table 1.
Nishimura-Mochida histological grading system of disc degeneration
| Grade 0: normal structure |
| Grade 1: mildly serpentine appearance of the annulus fibrosus (AF) with rupture |
| Grade 2: moderately serpentine appearance of the AF with rupture |
| Grade 3: severely serpentine appearance of the AF with mildly reversed contour |
| Grade 4: severely reversed contour |
| Grade 5: indistinct |
Immunohistochemical staining with Anti-human Nucleic Antibody (ANA Ab)
For immunofluorescence experiments, the paraffin blocks were sectioned using a microtome into 4 μm sections. These sections were then rehydrated using an ethanol series and xylene for 40 min, and boiled in 100 mM sodium citrate buffer (pH 6.0) for 1 h. Sections were then incubated in 0.2% Triton X-100 for 40 min at room temperature, in blocking solution [5% rabbit serum in 1× PBS (pH 7.2)] for 30 min at room temperature, and finally with primary antibody against human nuclei (mouse anti-human nuclei monoclonal antibody 1:100, # MAB1281, Chemicon, Temecula, USA) for 24 h at 4 °C. Alexa 488-conjugated secondary antibody (1:100, Invitrogen) and alexa 546-conjugated secondary antibody (1:100, Invitrogen) were then added to sections for 1 h at room temperature. The sections were washed with PBS, mounted in Vectashield with DAPI (Vector laboratories, Burlingame, CA, USA). We only evaluated existence of positive findings in Anti-human nucleic antibody (ANA Ab) staining.
Statistics
Statistical analysis was performed on disc height and signal intensity data using analysis of variance (ANOVA) and Bonferroni post hoc-tests for multiple comparisons. The chi-square test was used to test for histological scores differences between the three groups. All results are presented as means ± SE. A level of significance of p < 0.05 was used and error bars represent standard errors of means (SEM). Analysis was performed using the SPSS for Windows release 13.0 and the Prism (version 4.0) software packages.
Results
Radiological evaluation
Disc heights
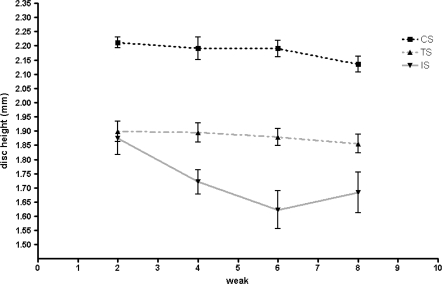
In the CS group, disc height was 2.2 ± 0.1 mm at 2 weeks post-injury, 2.2 ± 0.1 mm at 4 weeks, 2.2 ± 0.1 mm at 6 weeks, and 2.1 ± 0.1 mm at 8 weeks. In the TS group, disc height was 1.9 ± 0.1 mm at 2 weeks, 1.9 ± 0.1 mm at 4 weeks, 1.9 ± 0.1 mm at 6 weeks, and 1.9 ± 0.1 mm at 8 weeks. In the IS, disc height was 1.9 ± 0.2 mm at 2 weeks, 1.7 ± 0.1 mm at 4 weeks, 1.6 ± 0.1 mm at 6 weeks, and 1.7 ± 0.2 mm at 8 weeks. While conducting experiments, disc heights in the TS and IS groups were less than in the CS group, and these differences were statistically significant (p < 0.001). Compared to changes in disc height in the CS group, progressive reductions in height post injury were found in the IS group, whereas no reductions were found in the TS group (p < 0.05) (Fig. 2).
Fig. 2.
Graphical representation of disc height changes. Only injured segment (IS) showed progressive decreases in disc height, whereas MSC transplantation segment (TS) did not
Magnetic resonance imaging of IVDs
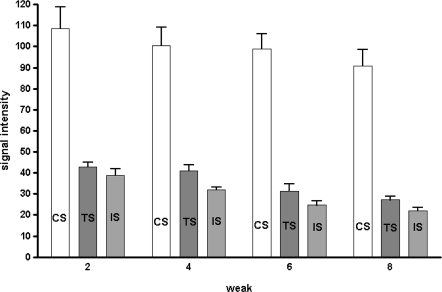
MRI signal intensities were measured in T2 axial images with respect to the CS group as standard control. In the TS group, mean signal intensities reduced to 40.6 ± 6.8% at 2 weeks post-injury, 44.4 ± 18.7% at 4 weeks, 33.6 ± 13.6% at 6 weeks, and 31.2 ± 8.3% at 8 weeks. In the IS group, mean signal intensities reduced to 37.3 ± 10.5% at 2 weeks, 33.4 ± 8.6% at 4 weeks, 25.9 ± 8.5% at 6 weeks, and 24.9 ± 6.2% at 8 weeks (Fig. 3). The signal intensities of the TS and IS groups were less than in the CS group and these differences were statistically significant (p < 0.01). Furthermore, signal intensities in the TS group were higher than in the IS group (p < 0.05). However, no significant recovery of signal intensity to CS level was observed in the TS or IS groups (p = 0.99).
Fig. 3.
Signal intensities of discs in T2 weighted images: the signal intensities of MSC transplantation segment (TS) were greater than those of only injured segment (IS)
Histological examinations
H–E staining
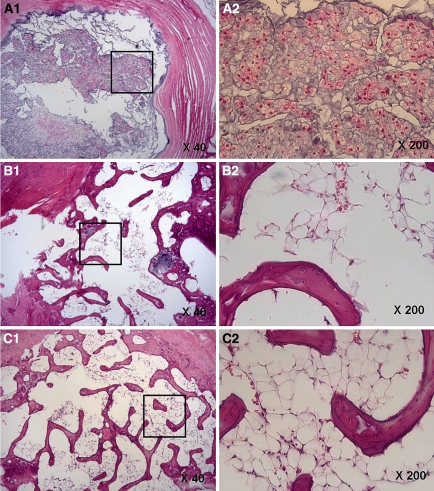
In the CS group, no degeneration or disruption of the internal structure of the annulus fibrosus was observed by H–E staining, and its oval shape was well maintained (Fig. 4A). However, in the IS group severe disruption and excavation of internal annulus fibrosus structures were observed (Fig. 4B) from 8 weeks post-injury. Milder degeneration and a smaller degree of internal structure fibrosis were observed in the TS group than in the IS group (Fig. 4C). When we explored changes in cells comprising the internal structure of the annulus fibrosus, it was found that these cells consisted of large oval or spindle-shaped cells with abundant cytoplasm in both the CS and TS groups, whereas numbers of cells were greatly reduced in the IS group. According to the Nishimura-Mochida grading system, the CS group scored 0 in all cases, IS 4 or 5, and the TS group 1 or 2, and this difference was statistically significant (p < 0.001) (Table 2).
Fig. 4.
A-1, 2 Control segments (CS) showed an oval nucleus with no collapse of the inner annular structure (40×, 200×). B-1, 2 Only injured segments (IS) showed collapse of the inner annulus morphology at 8 weeks post-injury (40×, 200×). C-1, 2 MSC transplantation segments (TS) showed relatively good preservation of the inner annulus structure with minimal fibrosis in the nuclear region (40×, 200×)
Table 2.
Nishimura-Mochida histological grading amongst three disc levels
| Grade | CS | IS | TS |
|---|---|---|---|
| 0 | 6 | 0 | 0 |
| 1 | 2 | 0 | 5 |
| 2 | 0 | 0 | 2 |
| 3 | 0 | 2 | 1 |
| 4 | 0 | 5 | 0 |
| 5 | 0 | 1 | 0 |
| Total | 8 | 8 | 8 |
CS control segment, TS transplantation segment, IS injured segment
Immunohistochemistry
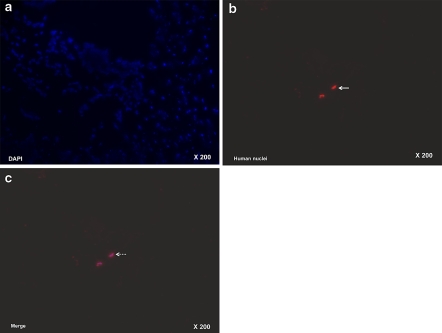
At 2 weeks post-transplantation (4 weeks post-injury) the TS group stained positively for primary antibody against human nuclei (mouse anti-human nuclei monoclonal antibody 1:100, # MAB1281, Chemicon, Temecula, USA) whereas staining in the IS group was only slight, but at 4 and 6 weeks post-transplantation no positive results were observed in either of these two groups (Fig. 5a–c).
Fig. 5.
a Staining with DAPI (4′,6-diamidino-2-phenylindole), b immunohistochemical staining with Anti-human Nucleic Antibody (ANA Ab), c a merge of (a) and (b) in the discs of MSC transplantation segments (TS) at 4 weeks post injury (2 weeks post transplantation) revealed positive staining (arrow), which confirmed MSC survival (dotted arrow) (200×)
Discussion
MSCs (1) are easily harvested; (2) are free of ethical issues; (3) are not immunoreactive; (4) can be used for allogenic and xenogeneic transplantation (because of the lack of immune reaction with HLA class II antigen) (Barry 2003; Cizkova et al. 2006; Rousseau et al. 2007); (5) are not tumorigenic (because they are not immortal, unlike embryonic stem cells); (6) have advantages for clinical applications (because they can be segregated and incubated relatively easily) (Chopp and Li 2002; Preston et al. 2003; Prockop 1997; Sakai et al. 2005); and (7) have been proven to be a valuable treatment option for full-thickness articular cartilage defects, osteogenesis imperfecta, and myocardial infarction (Wakitani et al. 1994; Horwitz et al. 1999). Therefore, research on MSCs has recently intensified (Jiang et al. 2002). However, few studies on MSC-based treatments for IVD degeneration have been undertaken (Leung et al. 2006; Preston et al. 2003; Prockop 1997). In the present study, we explored the effects of human MSC implantation on the restoration of degenerative intervertebral discs (IVDs) in the rat. It was found that human MSCs survived for 2 weeks after transplantation into the IVDs of rats, and that MSC treatment increased heights and signal intensities of intervertebral disc.
In previous studies, animals have been allocated to experimental and control groups for analysis. However, in this study, we adopted an experimental model, in which control and experimental segments were present in the same animals, to avoid confusions arising from individual differences, such as, weight, activity level, infection status, and immune competency. Furthermore, our study model was in the same status in human, and thus, our findings can be applied to human studies.
Previously, to evaluate the effects of MSC transplantation, histological changes, which are clearest parameter, have been quantified. Furthermore, tissue collection harms animals and findings cannot be applied to clinical research on humans. In addition, IVD height reductions and signal intensity changes could be considered aspects of normal aging processes (Kraemer 1995; Antoniou et al. 1996) but are critical diagnostic parameters of spinal diseases related to IVD degeneration (Sakai et al. 2003). Therefore, IVD disc height and MRI signal intensity restorations (the latter of which imply increases of, for e.g., proteoglycan or water, in the cellular and stromal compartments), and changes in IVD height and signal intensity on T2 axial images are reliable, useful parameters. Our study shows that mean IVD height reductions in the TS group were slight, whereas progressive reductions were observed in the IS group (p < 0.05). Contrary to that found in previous studies (Sakai et al. 2006, 2005, 2003), no recovery of height to the CS group level was observed in the TS or IS group. Furthermore, signal intensities in the TS and IS group were less than in the CS group (p < 0.01), though signal intensity in the TS group was greater than in the IS group. However, no recovery of signal intensity to the CS level was observed in the TS and IS group (p = 0.99), which contradicts the results of previous studies (Sakai et al. 2006). Nevertheless, our findings do show that IVD degeneration can be restrained by MSC injection.
The functions of transplanted MSCs remain uncertain. A previous study reported that these cells penetrate damaged tissues and directly secrete or indirectly cause normal cells to generate (via a paracrine mechanism) cytokines, such as, nerve growth factor, neurotrophic factor, and vascular endothelial cell growth factor (Bjorklund and Lindvall 2000; Chen et al. 2002; Chopp and Li 2002; Jendelova et al. 2004; Mahmood et al. 2004), which promote tissue regeneration. Furthermore, MSCs stimulate tissue-associated stem cells to differentiate to target tissues (Li et al. 2002). Le Visage et al. (2006) found that proteoglycan synthesis increased when MSCs were co-cultured with annulus fibrosus cells, and Sakai et al. (2006) reported increased proteoglycan and extracellular annulus fibrosus matrix synthesis, and the restoration of water disc height after transplanting MSCs into IVDs in an rabbit model, which was similar to that found by Sakai et al. (2006).
We evaluated degrees of degeneration of inner annular structures after H–E staining (Sakai et al. 2006) by using the grading system of Nishimura-Mochida (1998) at 8 weeks post-injury. The TS group achieved grades 1–2, whereas IS group only achieved grades 4–5. The CS group maintained grade 0 throughout the study (p < 0.001) (Table 1).
Several studies have shown that stem cells differentiate directly into nucleus pulposus cells. Crevensten et al. (2004) reported that the majority of transplanted stem cells disappeared, but that transplantation increased the level of extracellular matrix and supported the differentiation and regeneration of intrinsic nucleus pulposus-like stem cells. Sakai et al. (2006) in a histologic examination of IVDs transplanted with stem cells reported that the phenotype of differentiated cells was nucleus pulposus-like and found no evidence of osteogenesis. Furthermore, a preliminary study, Gimble and Guilak (2003) found that transplanted MSCs could differentiate into chondrocyte-like cells, namely, nucleus pulposus cells, which expressed collagen II, keratan sulfate, and chondroitin-4-sulfate, and that these cells were capable of further differentiation. However, our immunohistochemical findings differ from those reported by Gimble and Guilak (2003), because we found, by immunostaining with human ANA Ab, that transplanted MSCs survived at 2 weeks post-transplantation (4 weeks pos-injury), but not at 4 and 6 weeks post-transplantation. This observed reduction may have been due to; the fading of the fluorescent membrane stain, the death and/or degradation of MSCs in degenerated discs, or the expulsion of cells through the injection tract. Nevertheless, remaining stem cells or in situ activated stem cells had proliferated, matrix synthesis had increased, and IVD degeneration had been prevented.
Conclusion
Our findings suggest that human MSCs injected into intervertebral discs in the rat survive for 2 weeks and that mesenchymal stem cells increase extracellular matrix levels and disc heights and signal intensities.
Acknowledgments
Financial support was provided by a grant from the Asan institude for life science
References
- An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, Andersson GB, Masuda K (2005) Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine 30:25–31 (discussion 31–22) [DOI] [PubMed]
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M (1996) The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98:996–1003. doi:10.1172/JCI118884 [DOI] [PMC free article] [PubMed]
- Bang OY, Lee JS, Lee PH, Lee G (2005) Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 57:874–882. doi:10.1002/ana.20501 [DOI] [PubMed]
- Barry FP (2003) Biology and clinical applications of mesenchymal stem cells. Birth Defects Res C Embryo Today 69:250–256. doi:10.1002/bdrc.10021 [DOI] [PubMed]
- Bjorklund A, Lindvall O (2000) Cell replacement therapies for central nervous system disorders. Nat Neurosci 3:537–544. doi:10.1038/75705 [DOI] [PubMed]
- Chen X, Katakowski M, Li Y et al (2002) Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res 69:687–691. doi:10.1002/jnr.10334 [DOI] [PubMed]
- Cheung KMC, Ho G, Leung VYL, Chan D (2005) The effect of severity of disc degeneration on mesenchymal stem cells’ ability to regenerate the intervertebral disc: a rabbit model. Eur Cell Mater (Suppl 3): 45
- Chopp M, Li Y (2002) Treatment of neural injury with marrow stromal cells. Lancet Neurol 1:92–100. doi:10.1016/S1474-4422(02)00040-6 [DOI] [PubMed]
- Cizkova D, Rosocha J, Vanicky I, Jergova S, Cizek M (2006) Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol Neurobiol 26:1167–1180. doi:10.1007/s10571-006-9093-1 [DOI] [PubMed]
- Crevensten G, Walsh AJ, Ananthakrishnan D, Page P, Wahba GM, Lotz JC, Berven S (2004) Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng 32:430–434. doi:10.1023/B:ABME.0000017545.84833.7c [DOI] [PubMed]
- Deans RJ, Moseley AB (2000) Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 28:875–884. doi:10.1016/S0301-472X(00)00482-3 [DOI] [PubMed]
- Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, Hutton WC (2003) Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine 28:2609–2620. doi:10.1097/01.BRS.0000097891.63063.78 [DOI] [PubMed]
- Gimble J, Guilak F (2003) Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5:362–369. doi:10.1080/14653240310003026 [DOI] [PubMed]
- Gruber HE, Johnson TL, Leslie K et al (2002) Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine 27:1626–1633. doi:10.1097/00007632-200208010-00007 [DOI] [PubMed]
- Horwitz EM, Prockop DJ, Fitzpatrick LA et al (1999) Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 5:309–313. doi:10.1038/6529 [DOI] [PubMed]
- Im GI, Kim DY, Shin JH, Hyun C, Cho WH (2001) Repair of cartilage defect in the rabbit with cultured mesenchymal stem cells from bone marrow. J Bone Jt Surg Br 83:289–294. doi:10.1302/0301-620X.83B2.10495 [DOI] [PubMed]
- Jendelova P, Herynek V, Urdzikova L et al (2004) Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res 76:232–243. doi:10.1002/jnr.20041 [DOI] [PubMed]
- Jiang Y, Jahagirdar BN, Reinhardt RL et al (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49. doi:10.1038/nature00870 [DOI] [PubMed]
- Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Chubinskaya S, Yoshida M (2005) Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine 30:1933–1939. doi:10.1097/01.brs.0000176319.78887.64 [DOI] [PubMed]
- Kraemer J (1995) Natural course and prognosis of intervertebral disc diseases. International society for the study of the lumbar spine Seattle, Washington, June 1994. Spine 20:635–639. doi:10.1097/00007632-199503150-00001 [DOI] [PubMed]
- Le Visage C, Kim SW, Tateno K, Sieber AN, Kostuik JP, Leong KW (2006) Interaction of human mesenchymal stem cells with disc cells: changes in extracellular matrix biosynthesis. Spine 31:2036–2042. doi:10.1097/01.brs.0000231442.05245.87 [DOI] [PubMed]
- Leung VY, Chan D, Cheung KM (2006) Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J 15(Suppl 3):S406–S413. doi:10.1007/s00586-006-0183-z [DOI] [PMC free article] [PubMed]
- Li Y, Chen J, Chen XG et al (2002) Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59:514–523 [DOI] [PubMed]
- Li WY, Choi YJ, Lee PH et al (2008) Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant 17:1045–1059. doi:10.3727/096368908786991551 [DOI] [PubMed]
- Liechty KW, Mackenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW (2000) Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med 6:1282–1286. doi:10.1038/81395 [DOI] [PubMed]
- Lu DS, Shono Y, Oda I, Abumi K, Kaneda K (1997) Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study. Spine 22:1828–1834. doi:10.1097/00007632-199708150-00006 (discussion 1834–1825) [DOI] [PubMed]
- Mahmood A, Lu D, Chopp M (2004) Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma 21:33–39. doi:10.1089/089771504772695922 [DOI] [PubMed]
- Martin MD, Boxell CM, Malone DG (2002) Pathophysiology of lumbar disc degeneration: a review of the literature. Neurosurg Focus 13:E1. doi:10.3171/foc.2002.13.2.2 [DOI] [PubMed]
- Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, Robbins PD, Evans CH (1999) Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine 24:2419–2425. doi:10.1097/00007632-199912010-00002 [DOI] [PubMed]
- Nishimura K, Mochida J (1998) Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine 23:1531–1538. doi:10.1097/00007632-199807150-00006 (discussion 1539) [DOI] [PubMed]
- Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K (2001) Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Relat Res 389:94–101. doi:10.1097/00003086-200108000-00015 [DOI] [PubMed]
- Okano H (2002) Stem cell biology of the central nervous system. J Neurosci Res 69:698–707. doi:10.1002/jnr.10343 [DOI] [PubMed]
- Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K (2000) Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res 18:988–997. doi:10.1002/jor.1100180620 [DOI] [PubMed]
- Paul R, Haydon RC, Cheng H et al (2003) Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine 28:755–763. doi:10.1097/00007632-200304150-00006 [DOI] [PMC free article] [PubMed]
- Preston SL, Alison MR, Forbes SJ, Direkze NC, Poulsom R, Wright NA (2003) The new stem cell biology: something for everyone. Mol Pathol 56:86–96. doi:10.1136/mp.56.2.86 [DOI] [PMC free article] [PubMed]
- Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71–74. doi:10.1126/science.276.5309.71 [DOI] [PubMed]
- Quintavalla J, Uziel-Fusi S, Yin J et al (2002) Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials 23:109–119. doi:10.1016/S0142-9612(01)00086-2 [DOI] [PubMed]
- Risbud MV, Albert TJ, Guttapalli A, Vresilovic EJ, Hillibrand AS, Vaccaro AR, Shapiro IM (2004) Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine 29:2627–2632. doi:10.1097/01.brs.0000146462.92171.7f [DOI] [PubMed]
- Rousseau MA, Ulrich JA, Bass EC, Rodriguez AG, Liu JJ, Lotz JC (2007) Stab incision for inducing intervertebral disc degeneration in the rat. Spine 32:17–24. doi:10.1097/01.brs.0000251013.07656.45 [DOI] [PubMed]
- Sakai D, Mochida J, Yamamoto Y et al (2003) Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials 24:3531–3541. doi:10.1016/S0142-9612(03)00222-9 [DOI] [PubMed]
- Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, Hotta T (2005) Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine 30:2379–2387. doi:10.1097/01.brs.0000184365.28481.e3 [DOI] [PubMed]
- Sakai D, Mochida J, Iwashina T et al (2006) Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials 27:335–345. doi:10.1016/j.biomaterials.2005.06.038 [DOI] [PubMed]
- Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W (2005) Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells 23:403–411. doi:10.1634/stemcells.2004-0107 [DOI] [PubMed]
- Takegami K, An HS, Kumano F, Chiba K, Thonar EJ, Singh K, Masuda K (2005) Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J 5:231–238. doi:10.1016/j.spinee.2004.11.001 [DOI] [PubMed]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105:93–98. doi:10.1161/hc0102.101442 [DOI] [PubMed]
- Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM (1994) Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Jt Surg Am 76:579–592 [DOI] [PubMed]
- Wallach CJ, Sobajima S, Watanabe Y, Kim JS, Georgescu HI, Robbins P, Gilbertson LG, Kang JD (2003) Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine 28:2331–2337. doi:10.1097/01.BRS.0000085303.67942.94 [DOI] [PubMed]
- Yoon ST, Park JS, Kim KS, Li J, Attallah-Wasif ES, Hutton WC, Boden SD (2004) ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine 29:2603–2611. doi:10.1097/01.brs.0000146103.94600.85 [DOI] [PubMed]