Abstract
The subgroup and serotype specificities of human, bovine, and porcine group A rotaviruses in stool specimens collected in Thailand were examined by an enzyme-linked immunosorbent assay by using subgroup- and serotype-specific monoclonal antibodies. A clear yearly change was observed in the serotype distribution of human rotavirus. Between 1983 and 1984, serotype 4 was the most prevalent, while the highest frequency of serotype 2 was found between 1987 and 1988. All the bovine and porcine rotaviruses examined showed subgroup I specificities and long RNA patterns. It was of note that serotype 3 porcine rotaviruses were found at a high frequency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell L. M., Clark H. F., O'Brien E. A., Kornstein M. J., Plotkin S. A., Offit P. A. Gastroenteritis caused by human rotaviruses (serotype three) in a suckling mouse model. Proc Soc Exp Biol Med. 1987 Jan;184(1):127–132. doi: 10.3181/00379727-184-rc2. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Theil K. W., Saif L. J. Isolation and serotyping of porcine rotaviruses and antigenic comparison with other rotaviruses. J Clin Microbiol. 1984 Feb;19(2):105–111. doi: 10.1128/jcm.19.2.105-111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Mathan M. M., Mathew M., Martin R., Beards G. M., Mathan V. I. Rotavirus epidemiology in Vellore, south India: group, subgroup, serotype, and electrophoretype. J Clin Microbiol. 1988 Nov;26(11):2410–2414. doi: 10.1128/jcm.26.11.2410-2414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Yokoyama T., Nakata S., Morita Y., Urasawa T., Taniguchi K., Urasawa S., Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986 Aug 23;2(8504):417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Borian F. E., Bell L. M., Modesto K., Gouvea V., Plotkin S. A. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis. 1988 Sep;158(3):570–587. doi: 10.1093/infdis/158.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Hoshino Y., Bell L. M., Groff J., Hess G., Bachman P., Offit P. A. Rotavirus isolate WI61 representing a presumptive new human serotype. J Clin Microbiol. 1987 Sep;25(9):1757–1762. doi: 10.1128/jcm.25.9.1757-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S. Variation in neutralization epitopes of human rotaviruses in relation to genomic RNA polymorphism. Virology. 1987 Aug;159(2):209–216. doi: 10.1016/0042-6822(87)90457-0. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Flores J., Taniguchi K., Green K., Perez-Schael I., Garcia D., Sears J., Urasawa S., Kapikian A. Z. Relative frequencies of rotavirus serotypes 1, 2, 3, and 4 in Venezuelan infants with gastroenteritis. J Clin Microbiol. 1988 Oct;26(10):2092–2095. doi: 10.1128/jcm.26.10.2092-2095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Courbot M. C., Beraud A. M., Beards G. M., Campbell A. D., Gonzalez J. P., Georges A. J., Flewett T. H. Subgroups, serotypes, and electrophoretypes of rotavirus isolated from children in Bangui, Central African Republic. J Clin Microbiol. 1988 Apr;26(4):668–671. doi: 10.1128/jcm.26.4.668-671.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Gorziglia M., Valdesuso J., Askaa J., Glass R. I., Kapikian A. Z. An equine rotavirus (FI-14 strain) which bears both subgroup I and subgroup II specificities on its VP6. Virology. 1987 Apr;157(2):488–496. doi: 10.1016/0042-6822(87)90291-1. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Lambert J. P., Marbehant P., Marissens D., Zissis G. Monoclonal antibodies directed against different antigenic determinants of rotavirus. J Virol. 1984 Jul;51(1):47–51. doi: 10.1128/jvi.51.1.47-51.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Offit P. A., Estes M. K. Identification of the simian rotavirus SA11 genome segment 3 product. Virology. 1988 Mar;163(1):26–32. doi: 10.1016/0042-6822(88)90230-9. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesha H. S., Holmes I. H. New porcine rotavirus serotype antigenically related to human rotavirus serotype 3. J Clin Microbiol. 1988 Feb;26(2):171–174. doi: 10.1128/jcm.26.2.171-174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Hoshino Y., Flores J., Kapikian A. Z. Genetic analysis of a human rotavirus that belongs to subgroup I but has an RNA pattern typical of subgroup II human rotaviruses. J Clin Microbiol. 1987 Jul;25(7):1159–1164. doi: 10.1128/jcm.25.7.1159-1164.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley S., Hundley F., Chrystie I., McCrae M. A., Desselberger U. The genomes of rotaviruses isolated from chronically infected immunodeficient children. J Gen Virol. 1984 Jul;65(Pt 7):1141–1150. doi: 10.1099/0022-1317-65-7-1141. [DOI] [PubMed] [Google Scholar]
- Ruiz A. M., López I. V., López S., Espejo R. T., Arias C. F. Molecular and antigenic characterization of porcine rotavirus YM, a possible new rotavirus serotype. J Virol. 1988 Nov;62(11):4331–4336. doi: 10.1128/jvi.62.11.4331-4336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L., Grahnquist L., Pettersson C. A., Grandien M., Stintzing G., Greenberg H. B. Detection of human rotaviruses which do not react with subgroup I- and II-specific monoclonal antibodies. J Clin Microbiol. 1988 Jun;26(6):1238–1240. doi: 10.1128/jcm.26.6.1238-1240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez A., De B. P., Banerjee A. K. In vitro phosphorylation of NS protein by the L protein of vesicular stomatitis virus. J Gen Virol. 1985 May;66(Pt 5):1025–1036. doi: 10.1099/0022-1317-66-5-1025. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K., Chiba S. Serotype determination of human rotavirus isolates and antibody prevalence in pediatric population in Hokkaido, Japan. Arch Virol. 1984;81(1-2):1–12. doi: 10.1007/BF01309292. [DOI] [PubMed] [Google Scholar]
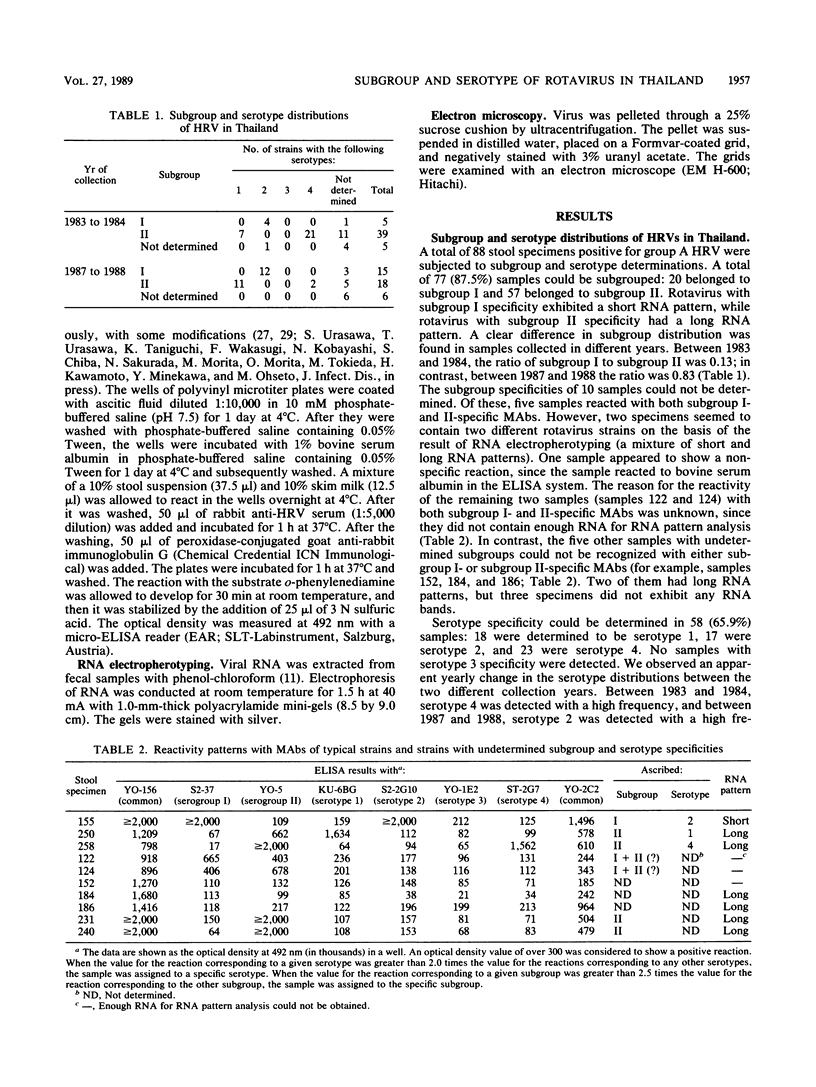
- Urasawa S., Urasawa T., Taniguchi K., Morita Y., Sakurada N., Saeki Y., Morita O., Hasegawa S. Validity of an enzyme-linked immunosorbent assay with serotype-specific monoclonal antibodies for serotyping human rotavirus in stool specimens. Microbiol Immunol. 1988;32(7):699–708. doi: 10.1111/j.1348-0421.1988.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Urasawa T., Urasawa S., Chiba Y., Taniguchi K., Kobayashi N., Mutanda L. N., Tukei P. M. Antigenic characterization of rotaviruses isolated in Kenya from 1982 to 1983. J Clin Microbiol. 1987 Oct;25(10):1891–1896. doi: 10.1128/jcm.25.10.1891-1896.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Zheng S. L., Rosen B. I., Knight N., Gourley N. E., Ramig R. F. Protection between different serotypes of bovine rotavirus in gnotobiotic calves: specificity of serum antibody and coproantibody responses. J Clin Microbiol. 1987 Jun;25(6):1052–1058. doi: 10.1128/jcm.25.6.1052-1058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]