Abstract
Objective
Carotid distensibility (CD) is a measure of carotid artery elasticity that has been introduced as a risk factor for cardiovascular disease. Information regarding reproducibility of sonographic CD measures is limited. The objective of this study was to evaluate the inter-reader reliability of sonographic measurements of common carotid artery (CCA) diameters and derived metrics of CD.
Methods
Two independent readers (R1 and R2) measured the systolic diameter (SD) and diastolic diameter (DD) for the right CCA from the B/M-mode sonographic registrations among 118 subjects. The derived CD metrics (strain, elastic modulus [E], stiffness [β], and CD) were calculated. The inter-reader type 3 intraclass correlation coefficients (ICC3,1) for carotid diameters were calculated.
Results
The mean SDs ± standard deviation were 7.15 ± 1.43 mm for R1 and 7.24 ± 1.43 mm for R2. The mean DDs were 6.71 ± 1.36 mm for R1 and 6.68 ± 1.41 mm for R2. The mean differences of SD and DD between R1 and R2 were 0.08 ± 0.40 mm (paired t test, P = .04) and 0.03 ± 0.43 mm (paired t test, P = .46), respectively. Inter-reader type 3 intraclass correlation coefficients were 0.96 for SD and 0.95 for DD. We observed a significant association of demographics with carotid diameters but not with derived CD metrics or risk factors.
Conclusions
Our results suggest good reproducibility of CCA diameters measured with B/M-mode sonography. However, very small changes in linear measurements of carotid diameters can have big effects on estimates of arterial mechanical properties such as strain and Young’s modulus. The standard boundary identification methods may not be precise and reproducible enough for use in a clinical setting.
Keywords: atherosclerosis, carotid distensibility, carotid sonography, reliability
Arterial distensibility is a measure of the arterial ability to expand and contract with cardiac pulsation and relaxation.1 A decrease of arterial distensibility (increased artery wall stiffness) seems to be a common pathologic mechanism for many factors that lead to the occurrence and progression of the vascular changes associated with cardiovascular disease (CVD).2,3 Functional impairment of the arterial wall may occur in an early stage of the atherosclerotic process before structural wall changes become detectible as well as before the occurrence of clinical symptoms of CVD.3 Early detection of this impairment can lead to more effective strategies for the prevention of CVD. This concept that early changes in functional properties of the arterial wall precede the clinical stage of atherosclerosis has been investigated in peripheral arteries (femoral and brachial) and in the aorta for many years. Recent development of high-resolution and high-definition sonography has focused new investigations on the carotid arteries. With these techniques, arterial wall and vessel diameters can be assessed in a dynamic fashion through-out the cardiac cycle as the artery expands and contracts with each cardiac pulsation and relaxation.2,3 Arterial distensibility is, however, only an estimate of the mean strain and modulus at best because the entire soft tissue surrounding the vessel is responding to the change in the volume of the vessel, and the standard boundary identification methods of the vessel wall may not be as reliable as speckle-tracking methods.4
Carotid distensibility (CD) has been introduced as a novel risk factor for CVD in cross-sectional study designs from population-based cohorts, including Atherosclerosis Risk in Communities (ARIC), Second Manifestations of Arterial Disease (SMART), the Rotterdam Study, the Baltimore Longitudinal Study of Aging (BLSA), and the Multiethnic Study of Atherosclerosis (MESA).5–9 Nevertheless, the value of CD in predicting future stroke is currently under debate.10,11 The discrepancy over CD and its relationship to atherosclerosis seems to arise from several plausible factors, such as small sample sizes, different clinical characteristics of the study populations, analyses of CD restricted to different vascular beds, and measurement variability. Additionally, there is a lack of information regarding the reliability of CD measures. Therefore, we sought to evaluate the inter-reader reproducibility of the common carotid artery (CCA) diameters and distensibility measurements in a sample of 118 stroke-free subjects derived from a multiethnic population of northern Manhattan.
Materials and Methods
This reliability study was performed among 118 individuals who were enrolled in the Northern Manhattan Study (NOMAS), a prospective cohort study of stroke risk factors in a multiethnic, urban population. The NOMAS cohort has been described elsewhere.12,13 In brief, participants were enrolled into the NOMAS if they were free of previous stroke, 40 years or older, and residents of northern Manhattan for 3 months or longer in a household with a telephone. To build the cohort, approximately 23,000 households were contacted by random-digit dialing. The telephone response rate was 91% with 5314 eligible persons identified, and 75% of these agreed to attend Columbia University Medical Center for a standardized in-person health evaluation. A total NOMAS cohort of 3298 was thus amassed. Since January of 1997, 1895 participants underwent carotid sonography. During the first year of enrollment, 118 consecutive individuals were examined twice by 2 sonographers. The study was approved by the local governing Institutional Review Board, and written consent was obtained from all participants.
Cardiovascular Disease Risk Factors
At baseline, subjects completed a comprehensive in-person assessment of sociodemographic data, risk factors, and medical history. Race-ethnicity was defined by self-identification. Medical history was assessed by questions adapted from the Behavioral Risk Factor Surveillance System by the Centers for Disease Control and Prevention.14 Hypertension was defined as a systolic blood pressure (SBP) recording of 140 mm Hg or greater, a diastolic blood pressure (DBP) recording of 90 mm Hg or greater, or a patient’s self-report of a history of hypertension or antihypertensive medication use. Diabetes mellitus was defined as a fasting blood glucose level of greater than 126 mg/dL or a patient’s self-report of such a history or insulin or hypoglycemic medication use. Cigarette smoking was categorized as current (smoking within a current year), former, or nonsmoker.
Carotid Sonography
Carotid sonography was performed on a GE LOGIQ 700 system (GE Healthcare, Milwaukee, WI) with a multifrequency 9- to 13-MHz linear array transducer with the subject in a supine position. Both internal and common carotid arteries as well as bifurcations were imaged in transverse (short axis) and longitudinal planes (anterior, lateral, and posterior views) using standardized carotid sonographic scanning and reading protocols as previously described.15 The 2 independent study sonographers (who were also the readers) performed the scans on all subjects under standardized conditions. Blood pressure (BP) was obtained for each subject from the right brachial artery after a minimum of 10 minutes’ rest in a supine position with a semiautomated oscillometric BP recorder (Dinamap Pro100; Critikon, Inc, Tampa, FL). Blood pressure was measured twice, before and after each examination, and averaged.
Image Acquisition
Carotid images were divided into 3 segments using the lateral extent of each carotid segment as defined relative to the tip of the flow divider, the most clearly defined anatomic reference where blood flow divides in the carotid bifurcation. The segments were as follows: segment 1, the near and far walls of the arterial segment extending from 10 to 20 mm proximal to the tip of the flow divider into the CCA; segment 2, the near and far walls of the carotid bifurcation beginning at the tip of the flow divider and extending 10 mm proximal to the flow divider tip; and segment 3, the near and far walls of the proximal 10 mm of the internal carotid artery. Carotid intima-media thickness (IMT) was measured according to the IMT protocol as described previously.15,16 The total carotid IMT was calculated as a composite measure of the near and far walls IMT of the CCA, bifurcation, and internal carotid artery from both sides of the neck.
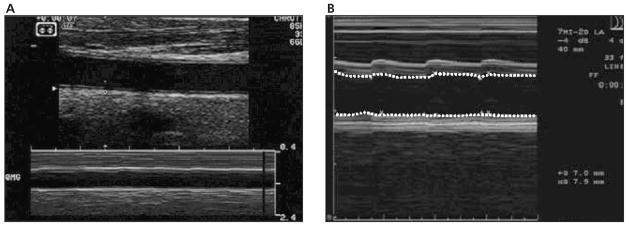
For the CD analyses, 10 mm of the right CCA below the origin of the carotid bulb (segment 1) was analyzed. The transducer was placed on the neck with the least possible pressure that did not compress the overlying jugular vein and allowed expansion of the CCA in all directions. Both near and far wall interfaces defining the blood-intima boundaries were maximized and clearly depicted on B-mode images by change of transducer angulations (Figure 1A). M-mode images were obtained in orientations perpendicular to the arterial walls and were adjusted for the clearest representation of the CCA walls throughout the cardiac cycle (Figure 1B). Two wall interfaces were tracked in up to 10 consecutive cardiac cycles.
Figure 1.
A, B and M-mode image of the CCA diameter change during the cardiac cycles. B, M-mode tracing of the CCA diameter during the cardiac cycles.
Image Processing and Reading Protocol
The offline measurement of CD was performed by Image Pro image analysis software (Microsoft Corporation, Redmond, WA) on a specially designed reading station. Two independent readers (R1 and R2) measured the systolic diameter (SD) and diastolic diameter (DD) of the right CCA. The best visualized blood-intima boundaries from up to 10 M-mode cardiac cycles were manually traced with a computer mouse-controlled tracer, and the SD and DD were automatically computed and averaged by Image Pro and stored in a data file.
Carotid Distensibility Metrics
Distensibility of an artery segment is a reflection of the mechanical stress affecting the arterial wall during the cardiac cycle.1 The stress was defined as the difference in SBP and DBP and strain as the artery system’s response. The CD metrics were calculated using the following algorithms1,2,17:
Strain as the amount of deformation relative to the unstressed state and expressed as percent change in the arterial diameter: strain = (SD – DD)/DD, where SD was the systolic and DD the diastolic CCA diameter;
Stiffness (β) as stress (SBP – DBP)-to-strain ratio: β = ln(SBP/DBP)/strain, where SBP and DBP were brachial BPs measured in the systolic and diastolic cardiac cycles, respectively;
Distensibility as 1/β and adjusted to IMT: 1/β, = 1/[ln(SBP/DBP)/strain × IMT]; and
Pressure-strain Young’s elastic modulus (E): E = K (SBP – DBP)/strain, where K = 133.3 was the conversion factor for mm Hg to Nm−2.
Statistical Analyses
Reliability indices are traditionally expressed as different versions of the intraclass correlation. There are 3 basic forms of the intraclass correlation coefficient (ICC).18,19 Each ICC can produce quite different results when applied to the same data set. The appropriate form of ICC is defined by the experimental study design and objectives. For the purpose of this study, ICC type 3 (ICC3,1) was appropriate because each of a random sample of n targets (subjects) was rated independently by k judges (readers). The ICC type 3 assumes that each subject was assessed by the same raters, but the raters represent the only raters of interest (fixed set of k raters). This is represented by the following algorithm: (ICC3,1) = (BMS − EMS)/[BMS + (k − 1) × EMS], where BMS is the between-subjects mean square (between-subjects variance of diameter measurements), and EMS is the within-subjects mean square (within-subjects variance of diameter measurements). In our study, k = 2, as we had 2 independent readers (R1 and R2), who were the only readers of interest from a single center.
The paired t test was used to analyze differences of the means of SD and DD read between the 2 readers. The Pearson correlation coefficient r was used to express the association between CD metrics between the 2 readers. Multiple linear regression models were used to examine the associations between CD metrics (outcome) and demographics using measurements obtained by the 2 independent readers. For the same outcome (SD or DD), 2 regression models were constructed with the measurements obtained from R1 or R2. The first regression model included SD as a dependent variable and age, sex, race-ethnicity, and vascular risk factors (hypertension, total cholesterol level, diabetes, and smoking) as covariates. The second regression model included DD as a dependent variable and the same covariates.
Results
The CD reliability study was performed among 118 subjects. The mean age ± standard deviation was 66.2 ± 8.8 years; 59% were women; 58% were Caribbean-Hispanic; 21% were black; and 16% were white. The mean systolic BP was 142 ± 18 mm Hg, and the mean diastolic BP was 83 ± 11 mm Hg.
The mean SD and DD, including the mean differences and inter-reader differences between SD and DD, are presented in Table 1. The mean SDs were 7.15 ± 1.43 mm for R1 and 7.24 ± 1.43 mm for R2. The mean DDs were 6.71 ± 1.36 mm (R1) and 6.68 ± 1.41 mm (R2). The mean differences of SD and DD between R1 and R2 were 0.08 ± 0.40 (paired t test, P = .04) and 0.03 ± 0.43 (paired t test, P = .46), respectively. Inter-reader reliability correlation coefficients (ICC3,1) were 0.96 for SD and 0.95 for DD.
Table 1.
Measurements of CCA Diameters Between the 2 Readers
| Measurement | R1* | R2* | Δ, Paired t Test* (P) | ICC3,1 |
|---|---|---|---|---|
| SD, mm | 7.15 ± 1.43 | 7.24 ± 1.43 | 0.08 ± 0.40 (.04) | 0.96 |
| DD, mm | 6.71 ± 1.36 | 6.68 ± 1.41 | −0.03 ± 0.43 (.46) | 0.95 |
| D (SD – DD) | 0.45 ± 0.23 | 0.56 ± 0.24 | −0.11 ± 0.22 (<.01) | 0.64 |
Values are mean ± standard deviation.
Of 2 separate multiple regression models (for R1 and R2) using the SD and subsequently the DD as dependent variables, a significant association was found for older age and men (Table 2). The adjusted parameter estimate for the association between SD and age was 0.04 (P = .01) for both readers; the estimates were 0.74 (P = .01) for the association between SD and men for R1 and 0.78 (P = .01) for R2. The adjusted parameter estimates for the association between DD and age were 0.03 (P = .02) for R1 and 0.04 (P = .01) for R2; the estimates for the association between DD and sex were 0.70 (P = .01) for R1 and 0.69 (P = .01) for R2. The associations with vascular risk factors were not significant (data not shown). No significant associations between demographics and vascular risk factors with CD metrics were observed for measurements obtained by either R1 or R2.
Table 2.
Multivariate Relationship (Linear Regression Models) Between SD/DD and Demographics Using the Measurements Obtained by the 2 Readers
| R1 |
R1 |
|||
|---|---|---|---|---|
| Demographic | Parameter Estimate | P* | Parameter Estimate | P* |
| SD | ||||
| Age | 0.04 | .01 | 0.04 | .01 |
| Male | 0.74 | .01 | 0.78 | .01 |
| Black vs white | 0.08 | .84 | 0.19 | .63 |
| Hispanic vs white | −0.17 | .62 | −0.04 | .89 |
| DD | ||||
| Age | 0.03 | .02 | 0.04 | .01 |
| Male | 0.70 | .01 | 0.69 | .01 |
| Black vs white | 0.03 | .94 | 0.25 | .53 |
| Hispanic vs white | −0.19 | .57 | 0.04 | .89 |
Adjusted for hypertension, diabetes, total cholesterol, and smoking.
A reliability analysis for the CD metrics is presented in Table 3. The mean values for strain were 6.8 ± 3.5 and 8.6 ± 4.5 for R1 and R2, respectively. The paired t test value for mean change was 1.9 ± 4.2 (P < .01), and the Pearson r value was 0.46 (P < .01). The mean stiffness (β) values were 10.5 ± 7.9 and 8.2 ± 7.2 for R1 and R2. The paired t test value for mean change was −1.8 ± 7.1 (P < .01), and the Pearson r value was 0.56 (P < .01). The mean distensibility (1/β) values for R1 and R2 were 0.1 ± 0.1 and 0.2 ± 0.1. The paired t test value for mean change was 0.04 ± 0.1 (P < .01), and the Pearson r value was 0.53 (P < .01). The mean elastic modulus (E) values were 155 ± 123 and 122 ± 116 for R1 and R2. The paired t test value for mean change was −34 ± 108 (P < .01), and the Pearson r value was 0.59 (P < .01).
Table 3.
Strain, Stiffness, Distensibility, and Elastic Modulus for Both Readers, Mean Differences of Carotid Distensibility Metrics Between Readers, and Correlation Coefficients
| Parameter | R1* | R2* | Δ, Paired t Test* (P) | Pearson r (P) |
|---|---|---|---|---|
| Strain, % | 6.8 ± 3.5 | 8.6 ± 4.5 | 1.9 ± 4.2 (<.01) | 0.46 (<.01) |
| Stiffness, β | 10.5 ± 7.9 | 8.2 ± 7.2 | −1.8 ± 7.1 (<.01) | 0.56 (<.01) |
| Distensibility, 1/β | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.04 ± 0.1 (<.01) | 0.53 (<.01) |
| Elastic modulus, E | 155 ± 123 | 122 ± 116 | −34 ± 108 (<.01) | 0.59 (<.01) |
Values are mean ± standard deviation.
Discussion
We have shown good inter-rater reproducibility of sonographic measures of the CCA diameters. Intraclass correlation coefficients (ICC3,1) of 0.96 for SD and 0.95 for DD confirm that the use of B/M-mode carotid sonography for the assessment of these 2 measurements is reliable. Our results are comparable with some of the previously published reliability studies (Table 4), although a wide variety of reproducibility results of CD can be found in the literature.3,5,7,10,11,20–30 Most of the studies used the repeatability coefficient and the coefficient of variation to examine repeated measurements either by the same or by a different sonographer. These coefficients are suitable in the reliability analysis of temporal changes of repeated measurements19 but not for single-time repeated measurements by different sonographers.18
Table 4.
Reliability of the SD and DD Measurements and Carotid Distensibility Metrics in the Literature
| Study Design | n | Reliability Study | Variability | Finding/Conclusions |
|---|---|---|---|---|
| Healthy volunteers20 | 10 | Intraobserver | DD: 1.1%, SD – DD: 1.6% | Vessel wall movement detector system has good technical reproducibility |
| Interobserver | CD: 1.3%, | |||
| Intra/intersession | DD: 1.9% | |||
| SD – DD: 2.6% | ||||
| Hypertensive patients21 | 86 | RC in 10 subjects | DD: 0.312 mm | CD↓ with LV thickness independent of RF |
| Intraobserver | SD – DD: 0.025 mm | |||
| CD: ±0.01 kPa−1 ·10−3 | ||||
| CC: ±0.65 m2 ·kPa−1 ·10−3 | ||||
| End-stage renal disease22,23 | 70 | RC | DD: ±0.273 mm | Carotid diameter correlated with LV diameter independent of RF |
| 79 | Intraobserver | SD – DD: ±0.025 mm | ||
| CD: ±1 kPa−1 ·10−3 | ↑ E predicts CVD and all-cause mortality | |||
| CC: ±0.52 m2 ·kPa−1 ·10−3 | ||||
| Population cohort24 | 10 | CV in 10 cases | DD: 3.3% | Reproducibility of IMT and CD is acceptable when used in large studies |
| Interobserver | CD: 12.3% | |||
| Intraobserver | Stiffness: 19% | |||
| BLSA25 | 110 | ICC and CV in 41 subjects | SD: 0.83, 3.7% | E↑ with age, men, and E of aorta |
| DD: 0.74, 5% | ||||
| SMART Study7,11 | 570 | CV in 10 patients | DD: 2.1%, CD: 6.2% | CD is a marker of CVD risk in patients who already have CVD or RF |
| 474 | Intraobserver | DD: 3.5%, CD: 7.3% | ||
| Interobserver | In patients with carotid stenosis, carotid stiffness is associated with prior ischemic stroke or TIA | |||
| ARIC Study26 | 15,800 | RC and CV in 36 subjects | DD: 0.65, 8% | Excellent reproducibility of sonographically based measures of arterial stiffness |
| Interobserver | SD – DD: 0.76, 29% | |||
| CD: 0.67, 32% | ||||
| E: 0.66, 35% | ||||
| VEAPS3 | 24 | ICC and CV in 24 subjects | SD: 0.97, 1.28% | B-mode sonographic images of carotid artery lumen are highly reproducible and directly applicable to noninvasive imaging of atherosclerosis |
| DD: 0.99, 1.18% | ||||
| Stiffness: | ||||
| ICC: 0.84–0.89 | ||||
| CV: 10%–14% | ||||
| Rotterdam Study5,29 | 3098 | ICC in 47 subjects | CD: 0.80 | Carotid stiffness is associated with CVD along the vascular tree5 |
| 7983 | ||||
| CD is not independently associated with CVD and mortality27 | ||||
| Healthy volunteers27 | 41 | CV | DD: 5.9% | Inverse relationship between CD and age |
| Intraobserver | CD: 8.5% | |||
| Case-control study10 | 299 | ICC in 30 subjects | DD: 0.88, CD: 0.84 | Increased carotid stiffness is associated with ischemic stroke independent of RF |
| Intraobserver | DD: 0.85, CD: 0.82 | |||
| Interobserver | ||||
| Healthy male BLSA30 | 206 | ICC in 10 subjects | Stiffness ICC: 0.96 | Lower levels of testosterone predict carotid stiffness independent of RF |
| Intraobserver | ||||
| NOMAS population-based | 3298 | ICC in 118 subjects | SD: 0.96 | Good reproducibility of SD and DD |
| stroke-free cohort12 | Intraobserver | DD: 0.95 | Small variance in SD and DD caused a considerable variance in derived metrics of CD and produced different results of the associations with RF |
CV indicates coefficient of variation; E, elastic modulus; LV, left ventricle; RC, repeatability coefficient; RF, risk factor; TIA, transient ischemic attack; and VEAPS, Vitamin E Atherosclerosis Prevention Study.
We found a strong association between increased SD and DD with aging as well as among men. These data are in accordance with previous studies.6,25,28 Carotid distensibility metrics derived from these measurements were, however, less reliable. In our study, there were significant differences in these metrics between the 2 readers. The correlation coefficients were moderate, ranging from 0.46 to 0.59. It has been shown that the associations of arterial stiffness and outcomes are biased toward the null if reliability coefficients for CD range from 0.6 to 0.8.25 The high variability of the CD metrics in our study most likely underestimated the associations of CD metrics with the vascular risk factors.
In our study, a small but significant difference in the measurement of SD of the CCA was found between the 2 readers, which may have affected the variability of CD metrics. The variation in SD, although small, most likely accounted for the difference in metric strain (percent change of diameter during the cardiac cycle) between the 2 readers and underestimated the associations of other CD metrics with demographics and risk factors. Similarly, several studies have found a greater variability in composite measures of CD, especially for measures that required 2 or more measured variables for the calculation.3,6,26 This increased variability in CD metrics may be a significant confounder for longitudinal studies aimed to detect changes in CD over time or to predict vascular outcomes. A highly variable measure as the independent variable (predictive variable) in a standard linear or logistic regression analysis would cause biased estimates of association, with the bias being toward the null. Similarly, if a highly variable measure is used as an outcome measure (dependent variable), the strength of its association with other predictors would be decreased, underestimating the true associations.26 Because a measurement error can seriously affect statistical analyses and interpretation, it is important to assess the amount of such an error by examination of the reliability indices of CD metrics.
The variability of the CD metrics is most likely responsible for different, sometimes conflicting, results on the association between CD and the vascular risk factors and vascular outcomes. In the MESA, CD was associated with a variety of risk factors, including hypertension, diabetes, and cigarette smoking, as well as with common carotid IMT.31 Young’s elastic modulus (E, one of the CD metrics), however, was not significantly associated with carotid IMT. In the ARIC Study, carotid stiffness metrics were associated with hypertension, diabetes, trait anger, physical activity, and ethnicity32–36 and also with the highest quartile of carotid IMT.6 In the BLSA, an independent association between suppressed anger and carotid stiffness was reported,37 as well as an increase in stiffness with the clustering of components of metabolic syndrome and decreasing levels of testosterone.28,38 In the SMART Study, decreased CD was a marker of increased CVD risk but in patients who already had vascular disease.7 Recently, an independent association between increased carotid stiffness and a first-ever acute ischemic stroke has been reported,10 although other studies did not find the same relationship.29 The initial results from the Rotterdam Study that showed a significant association between CD and the risk of CVD5 were not confirmed in the recent study.29 Large variance in the measurements of CD metrics, BP, and pulse wave velocities were most likely responsible for these conflicting data and underestimated associations. In addition, arterial distensibility measured by the standard boundary identification methods is only an estimate of the mean strain and modulus at best because the entire soft tissue surrounding the vessel is responding to the change in the volume of the vessel. Speckle tracking within the vessel wall itself may be a better estimate of the true vessel wall modulus.4 Well-performed speckle tracking is more reliable and robust than the boundary detection methods.
In conclusion, the SD and DD of the CCA can be measured reliably by sonography. However, even a small variance in these measurements may cause a considerable variance in derived metrics of CD. The small error of diameter measurements introduced by a semiautomatic technique in our study considerably underestimated the associations between CD metrics and the vascular risk factors, indicating that boundary detection methods may not be precise and reproducible enough for use in a clinical setting. To improve the accuracy of CD assessments, investigators are encouraged to develop methods to reduce variance by improving sonographic scanning protocols and measurement technique, including automated measurement algorithms and speckle tracking, ensure quality control, and conduct periodic reliability studies to reduce measurement errors in their sonography laboratories.
Acknowledgments
This work was supported by the Gilbert Baum Memorial Grant (T.R.), the Goddess Fund for Stroke Research in Women (T.R.), and National Institute of Neurological Disorders and Stroke grant R01 NS 29993 (R.M., S.T., R.R., D.C., R.L.S.).
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- BLSA
Baltimore Longitudinal Study of Aging
- BP
blood pressure
- CCA
common carotid artery
- CD
carotid distensibility
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- DD
diastolic diameter
- ICC
intraclass correlation coefficient
- IMT
intima-media thickness
- MESA
Multiethnic Study of Atherosclerosis
- NOMAS
Northern Manhattan Study
- SBP
systolic blood pressure
- SD
systolic diameter
- SMART
Second Manifestations of Arterial Disease
References
- 1.Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Non-invasive assessment of age related changes in stiffness of major branches of the human arteries. Cardiovasc Res. 1987;21:678–687. doi: 10.1093/cvr/21.9.678. [DOI] [PubMed] [Google Scholar]
- 2.Hoeks AP, Brands PJ, Smeets FA, Reneman RS. Assessment of the distensibility of superficial arteries. Ultrasound Med Biol. 1990;16:121–128. doi: 10.1016/0301-5629(90)90139-4. [DOI] [PubMed] [Google Scholar]
- 3.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 4.Golemati S, Sassano A, Lever MJ, Bharath AA, Dhanjil S, Nicolaides AN. Carotid artery wall motion estimated from B-mode ultrasound using region tracking and block matching. Ultrasound Med Biol. 2003;29:387–399. doi: 10.1016/s0301-5629(02)00760-3. [DOI] [PubMed] [Google Scholar]
- 5.Van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 6.Riley WA, Evans GW, Sharrett AR, Burke GL, Barnes RW. Variation of common carotid artery elasticity with intimal-medial thickness: the ARIC study. Atherosclerosis Risk in Communities Ultrasound Med Biol. 1997;23:157–164. doi: 10.1016/s0301-5629(96)00211-6. [DOI] [PubMed] [Google Scholar]
- 7.Simons PCG, Algra A, Bots ML, Grobbee DE, van der Graaf Y. Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients. The SMART Study (Second Manifestations of Arterial Disease) Circulation. 1999;100:951–957. doi: 10.1161/01.cir.100.9.951. [DOI] [PubMed] [Google Scholar]
- 8.Shock NWGR, Andres RA, Arenberg D, Costa PT, Jr, Lakatta EG, Tobin JD. Design and Operation of the Baltimore Longitudinal Study of Aging. Bethesda, MD: National Institutes of Health; 1984. [Google Scholar]
- 9.Bild DE, Blemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Tsivgoulis G, Vemmos K, Papamichael C, et al. Common carotid arterial stiffness and the risk of ischemic stroke. Eur J Neurol. 2006;13:475–481. doi: 10.1111/j.1468-1331.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 11.Dijk JM, van der Graaf Y, Grobbee DE, Bots ML. Carotid stiffness indicates risk of ischemic stroke and TIA in patients with internal carotid artery stenosis: the SMART Study. Stroke. 2004;35:2258–2262. doi: 10.1161/01.STR.0000141702.26898.e9. [DOI] [PubMed] [Google Scholar]
- 12.White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 13.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL. Elevated white blood cell count and carotid plaque thickness: the Northern Manhattan Stroke Study. Stroke. 2001;32:842–849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 14.Gentry EM, Kalsbeek WD, Hogelin WG, et al. The Behavioral Risk Factor Surveys, II: design, methods, and estimates from combined state data. Am J Prev Med. 1985;1:9–14. [PubMed] [Google Scholar]
- 15.Rundek T, Elkind MS, Pittman J, et al. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6 and hepatic lipase genes: the Northern Manhattan Prospective Cohort Study. Stroke. 2002;333:1420–1423. doi: 10.1161/01.STR.0000015558.63492.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juo SH, Lin HF, Rundek T, et al. Genetic and environmental contributions to carotid intima-media thickness and obesity phenotypes in the Northern Manhattan Family Study. Stroke. 2004;35:2243–2247. doi: 10.1161/01.STR.0000142132.20442.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi K, Sato M, Handa H, Moritake K. Biomechanical study of the constitutive laws of vascular walls. Exp Mech. 1974;14:440–444. [Google Scholar]
- 18.Fleiss JL, Shrout PE. The effects of measurement errors on some multivariate procedures. Am J Public Health. 1977;67:1188–1191. doi: 10.2105/ajph.67.12.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.British Standards Institution. Precision of Test Methods, 1: Guide for the Determination and Reproducibility for a Standard Test Method. BS 597, Part 1. London, England: British Standards Institution; 1975. [Google Scholar]
- 20.Kool MJF, van Merode T, Reneman RS, Hoeks APG, Struyker Boudier HAJ, Van Bortel LMAB. Evaluation of reproducibility of a vessel wall movement detector system for assessment of large artery properties. Cardiovasc Res. 1994;28:610–614. doi: 10.1093/cvr/28.5.610. [DOI] [PubMed] [Google Scholar]
- 21.Boutouyrie P, Laurent S, Girerd X, et al. Common carotid artery stiffness and patterns of left ventricular hypertrophy in hypertensive patients. Hypertension. 1995;25:651–659. doi: 10.1161/01.hyp.25.4.651. [DOI] [PubMed] [Google Scholar]
- 22.London GM, Guerin AP, Marchais SJ, Pannier B, Day SM, Metivier F. Cardiac and arterial interactions in end-stage renal disease. Kidney Int. 1995;50:600–608. doi: 10.1038/ki.1996.355. [DOI] [PubMed] [Google Scholar]
- 23.Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid artery stiffness as a predictor of cardiovascular and all-cause of mortality in end-stage renal disease. Hypertension. 1998;32:570–574. doi: 10.1161/01.hyp.32.3.570. [DOI] [PubMed] [Google Scholar]
- 24.Kanters SD, Elgesma OE, Banga JD, van Leeuwen MS, Algra A. Reproducibility of measurements of intima-media thickness and distensibility in the common carotid artery. Eur J Vasc Endovasc Surg. 1998;16:28–35. doi: 10.1016/s1078-5884(98)80088-9. [DOI] [PubMed] [Google Scholar]
- 25.Nagai Y, Fleg JL, Kemper MK, Rywik TM, Earley CJ, Metter EJ. Carotid arterial stiffness as a surrogate marker for aortic stiffness: relationship between carotid artery pressure-strain elastic modulus and aortic pulse wave velocity. Ultrasound Med Biol. 1999;2:181–188. doi: 10.1016/s0301-5629(98)00146-x. [DOI] [PubMed] [Google Scholar]
- 26.Arnett DK, Chambless LE, Kim H, Evans GW, Riley W. Variability in ultrasonic measurements of arterial stiffness in the Atherosclerosis Risk in Communities Study (ARIC) Ultrasound Med Biol. 1999;25:175–180. doi: 10.1016/s0301-5629(98)00165-3. [DOI] [PubMed] [Google Scholar]
- 27.Morganti T, Ricci S, Vittone F, Palombo C, Tortoli P. Clinical validation of common carotid artery wall distension assessment based on multigate Doppler processing. Ultrasound Med Biol. 2005;31:937–945. doi: 10.1016/j.ultrasmedbio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Reneman RS, van Merode T, Hick P, Muytjens AMM, Hoeks APG. Age-related changes in carotid artery wall properties in man. Ultrasound Med Biol. 1986;12:465–471. doi: 10.1016/0301-5629(86)90218-8. [DOI] [PubMed] [Google Scholar]
- 29.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 30.Hougaku H, Fleg JL, Najjar SS, et al. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol. 2006;290:E234–E242. doi: 10.1152/ajpendo.00059.2005. [DOI] [PubMed] [Google Scholar]
- 31.Sharrett A, Ding J, Criqui M, et al. Smoking, diabetes, and blood cholesterol differ in their associations with subclinical atherosclerosis: the Multiethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2006;186:441–447. doi: 10.1016/j.atherosclerosis.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes: the ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995;91:1432–1443. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 33.Liao D, Arnett DK, Tyroler HA, et al. Arterial stiffness and the development of hypertension: the ARIC study. Hypertension. 1999;34:201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 34.Schmitz KH, Arnett DK, Bank A, et al. Arterial distensibility and physical activity in the ARIC Study. Med Sci Sports Exerc. 2001;33:2065–2071. doi: 10.1097/00005768-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Williams JE, Din-Dzietham R, Szklo M. Trait anger and arterial stiffness: results from the Atherosclerosis Risk in Communities (ARIC) Study. Prev Cardiol. 2006;9:14–20. doi: 10.1111/j.1520-037x.2006.1610.x. [DOI] [PubMed] [Google Scholar]
- 36.Din-Dzietham R, Couper D, Evans G, Arnett DK, Jones DW. Arterial stiffness is greater in African Americans than in whites: evidence from the Forsyth Count, North Carolina, ARIC cohort. Am J Hypertens. 2004;17:304–313. doi: 10.1016/j.amjhyper.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Anderson DE, Metter EJ, Hougaku H, Najjar SS. Suppressed anger is associated with increased carotid arterial stiffness in older adults. Am J Hypertens. 2006;19:1129–1134. doi: 10.1016/j.amjhyper.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Scuteri A, Najjar SS, Muller DC, et al. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]