Abstract
Purpose
Pluronic block copolymers are potent sensitizers of multi-drug resistant (MDR) cancer cells. The sensitization effect by Pluronics is a result of two processes acting in concert: i) intracellular ATP depletion, and ii) inhibition of ATPase activity of drug efflux proteins. This work characterizes effects of Pluronic P85 on ATPase activities of Pgp, MRP1, and MRP2 drug efflux transport proteins and interaction of these proteins with their substrates, vinblastine, and leucotriene C4.
Methods
Using membranes overexpressing Pgp, MRP1, and MRP2, the current study evaluates effects of Pluronic P85 (P85) on the kinetic parameters (Vmax, Km, Vmax/Km) of ATP hydrolysis by these ATPases.
Results
The decreases in the maximal reaction rates (Vmax) and increases in apparent Michaelis constants (Km) for these transporters in the presence of various concentrations of P85 were observed. The mechanism of these effects may involve i) conformational changes of the transporter due to membrane fluidization and/or ii) nonspecific steric hindrance of the drug-binding sites by P85 chains embedded into cellular membranes. The extent of these alterations was increased in the row MRP1 < MRP2 << Pgp.
Conclusions
These data suggest that there are unifying pathways for the inhibition of Pgp and MRPs by the block copolymer. However, the effect of P85 on Pgp ATPase activity is considerably greater compared with the effects on MRP1 and MRP2 ATPases. This may be a reason for greater inhibitory effects of Pluronic in Pgp- compared with MRP-overexpressing cells.
Keywords: ATPase activity, Michaelis-Menten kinetics, MRP1, MRP2, Pgp, Pluronic
INTRODUCTION
Intrinsic or acquired resistance of malignant tumors to chemotherapeutic agents drastically decreases the outcomes of chemotherapy of cancer (1–3). Therefore, discovery and development of agents that are able to reverse resistance of malignancies is necessary to improve the therapeutic out-comes. One major cause of drug resistance is an increase in the activity or amount of drug efflux transport proteins, which are able to extrude xenobiotics out of the tumor cells. The chemical nature of the substrates handled by these proteins is extremely diverse; from inorganic ions to sugars and large polypeptides. The efflux proteins implicated in drug resistance include P-glycoprotein (Pgp) and multidrug resistant proteins (MRP), which belong to a superfamily of ABC transporters (4,5). Intensive laboratory and clinical studies to over-come drug resistance in cancer use inhibitors or reversal agents for these proteins (6–8).
Pluronic block copolymers is a new class of efficient inhibitors of Pgp that were shown to increase accumulation and cytotoxicity of many drugs in Pgp-overexpressing cell lines (9,10). One Pluronic-based formulation of doxorubicin is currently evaluated in clinical trials for treatment of MDR tumors (11). Laboratory studies have shown that Pluronics sensitize Pgp-overexpressing cells resulting in an increase in the cytotoxic activity of anthracyclines and other anticancer drugs by as much as 2 to 3 orders of magnitude (9,12). Studies of the mechanism of sensitization effects by P85 suggested an essential role of ATP depletion in MDR tumors (10,13). Furthermore, it was shown that P85 molecules rapidly adhere to the cell membranes and incorporate into them. This alters the structure of the lipid bilayers accompanied by significant decreases in Pgp ATPase activity (14,15). The combination of 1) ATP depletion and 2) inhibition of ATPase activity is necessary for effective inhibition of Pgp (13–15). These findings are of importance considering that Pgp and MRPs are members of the “traffic ATPase” superfamily (4,5), which use the energy of ATP hydrolysis for maintaining their membrane transport function. However, several studies suggested that Pluronics have much weaker, if any, effect on MRPs compared with Pgp (15–17). The ATP depletion induced by Pluronic has been reported in MRP-overexpressing cells (15). Therefore, we posit that differences in the effects of Pluronic on Pgp and MRP involve different effects of these copolymers on the ATPase activity of the transporters. To test this hypothesis we characterized the effects of Pluronic P85 (P85) on drug efflux transporters by assessing kinetic parameters for ATP hydrolysis in Pgp-, MRP1-, and MRP2-overexpressing membranes. Along with the protein-ATP interactions, the kinetic studies examined the effects of P85 on binding of the drugs (vinblastine and leucotriene C4) and determined to what extent the block copolymer can interfere with the ability of these drugs to specifically activate the ATPase. Overall, the study provides insight into the mechanism of Pluronic sensitization effects in the MDR cancer cells and the reasons for different effects of the block copolymers on Pgp and MRP drug efflux proteins.
MATERIALS AND METHODS
Materials
Pgp-overexpressing membranes were purchased from Gentest Co. (Woburn, MA, USA). Crude plasma membranes fraction overexpressing MRP1 or MRP2 was isolated from COR-L23/R or MDCKII-MRP2 cells respectively as described earlier (15). Pluronic P85 was a gift from BASF Co. (Parispany, NJ, USA). P85 solution in phosphate buffered saline (PBS), 20% (w/v), was used in these studies. All concentrations of P85 solutions are presented in % (w/v). Acridone carboxamide derivative, GF120918, was kindly provided by Dr. Kenneth Brouwer (Glaxo Wellcome, Inc., Research Triangle Park, NC, USA). Vinblastine (Vin), leucotriene C4 (LTC4), Mg2+ATP, and all other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Cells
The porcine kidney epithelial cell line, LLC-MDR1, derived by transfection with human MDR1 in the laboratory of Dr. Piet Borst (The Netherlands Cancer Institute, Amsterdam, The Netherlands), were obtained from Dr. Erin Schuetz (St. Jude Hospital, Memphis, TN, USA). The cells were cultured in Medium 199 with 10% FBS, 10 mM HEPES, and penicillin/streptomycin. LLC-MDR1 cultures were supplemented with 640 nmol/ml vincristine (SP Pharmaceuticals Inc, Albuquerque NM, USA). The MRP2-transfected Madin Darby Canine Kidney (MDCK) cells, MDCKII-MRP2, were also gift from Dr. Piet Borst. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), containing 10% heat-inactivated fetal bovine serum (FBS) and 50 U/ml penicillin/streptomycin. Human lung carcinoma epithelial cells, COR-L23/R, were purchased from European Collection of Cell Cultures. The cells were maintained in RPMI 1640 medium with 2 mM glutamine, 0.2 μg/ml doxorubicin (for COR-L23/R), and 10% FBS. Tissue culture media were obtained from Gibco Life Technologies, Inc. (Grand Island, NY, USA). Serum and medium supplements were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Western Blot Analysis
Identification of Pgp, MRP1, and MRP2 was done using immunoblot technique described previously (18). To avoid cross-staining of the protein bands, three suspensions of the membranes overexpressing Pgp, MRP1, and MRP2 were loaded in three different gels, and following transfer each blot was stained with one type of antibodies, against Pgp, MRP1, or MRP2. The monoclonal antibodies to Pgp, C219 (Dako Corp., Carpinteria, CA, USA), MRP1, MRPm6 (Kamiya Biomedical Corp., Seattle, WA, USA), and MRP2, M2III-6 (Alexis Corp., San Diego, CA, USA), were used at 1:100 dilutions. The monoclonal antibodies to β-actin, anti-β-1-chicken Integrin (Sigma Corp., St. Louis, MO, USA) were used at 1:200 dilution. The secondary horseradish peroxide anti-mouse Ig antibodies (1:1500 dilution) were purchased from Amersham Life Sciences (Cleveland, OH, USA). To correct for loading differences, the levels of proteins were normalized to constitutively expressed β-actin.
Pgp ATPase Assay
Kinetic effects of P85 on Pgp ATPase activity were evaluated using suspension of Pgp-overexpressing membranes purchased from Gentest Co. (Woburn, MA, USA). The Pgp ATPase activity assay was performed as described earlier (14). Briefly, a mixture of Pgp-overexpressing membranes with or without P85 was added to various concentrations Mg2+ATP solutions and incubated at 37°C for 20 min. An identical reaction mixture containing 100 μM sodium orthovanadate was assayed in parallel. Orthovanadate inhibits Pgp by trapping Mg2+ADP in the nucleotide-binding site. Thus, ATPase activity measured in the presence of orthovanadate represents non-Pgp ATPase activity and can be subtracted from the total activity measured in the various samples to yield Pgp ATPase activity. The reaction was stopped by the addition of 10% SDS and liberation of inorganic phosphate was detected by colorimetric reaction with ascorbic acid in ammonium molybdate solution according to (19). To examine the effects of P85 on the protein-drug interactions various concentrations of Pgp substrate, Vin, were added to the mixture of Pgp membranes and Mg2+ATP followed by Pgp ATPase assay performed as described above.
MRP1 and MRP2 ATPase Assay
Effects of P85 on MRP1 and MRP2 efflux were determined using plasma membrane fractions isolated from MRP1-overexpressing COR-L23/R or MRP2-transfected MDCKII-MRP2 cells. Expression of high amounts of MRP1 or MRP2 in these membranes and low amounts of Pgp was shown previously by Western Blot technique (15). Orthovanadate, known as an inhibitor of MRPs ATPase activity (20), was added to a half of the membranes to determine non-MRPs ATPase activity. Values of non-MRPs ATPase activity were subtracted from total ATPase activity to obtain specific MRPs ATPase activity. Similar to Pgp ATPase activity studies values of MRPs ATPase activity were determined with or without P85.
Calculations
Values of Pgp and MRPs ATPase activity were obtained over the range of ATP concentrations. The data were plotted using double reciprocal plots according to Lineweaver and Burk (21) to determine maximal reaction rates (Vmax), and apparent Michaelis constants (Km) for ATP hydrolysis by Pgp or MRPs. Km and Vmax parameters for the protein-drug binding were determined under the pseudo first-order reaction conditions with fixed concentration of ATP (4 mM or 12 mM), and over the range of Vin or LTC4 concentrations. The entire curve fitting was carried out using GraphPad Prism 3.0 (GraphPad software, San Diego, CA, USA).
3H-P85 Accumulation Studies
A tritium label was incorporated into P85 by treatment of the copolymer film with atomic tritium as previously described (22). The sample of 3H-P85 with specific activity of 0.3 Ci/mmol was obtained. This sample was further diluted in the assay buffer to obtain 0.5 μCi/ml (1.6 nM) 3H-P85. The kinetic of 3H-P85 accumulation was examined in confluent LLC-MDR1, COR-L23/R, or MDCK-MRP2 monolayers at 37°C. To evaluate possible effects of Pgp or MRP efflux on 3H-P85 transport, GF120918 (inhibitor of Pgp efflux system), or indomethacin (inhibitor of MRP efflux system) were used in the experiments with Pgp- or MRPs-overexpressing cells respectively. After 30 min pre-incubation period in assay buffer, or in 1 μM GF120918 (in case of LLC-MDR1), or in 10 μM indomethacin (in case of COR-L23/R and MDCK-MRP2) solutions, the cell monolayers were exposed to 3H-P85 in the presence of corresponded inhibitor solution or without it for various time intervals. Then, the treatment solutions were removed and cells were washed with ice-cold PBS. The cell monolayers were solubilized in 1% Triton X-100 (0.5 ml) and aliquots were taken for subsequent determination of radioactivity (Tricarb 4000, Packard, Meriden, CT, USA). All experiments were conducted in quadruplicate. Values for cellular accumulation of 3H-P85 were normalized for cellular protein content. Protein concentrations were determined using the Pierce (Rockford, IL, USA) bicinchoninic acid method.
3H-P85 Transport Studies
Polycarbonate membrane inserts with confluent LLC-MDR1, or MDCK-MRP2 cell monolayers were placed in Side-Bi-Side diffusion cells from Crown Bio Scientific, Inc. (Somerville, NJ, USA) maintained at 37°C. Trans-epithelial electrical resistance (TEER) values were recorded as indexes of cell viability and monolayer integrity. Under basal conditions, mean resistance was 250 ± 19.8 Ω · cm2 and 185.0 ± 16.7 Ω · cm2 for LLC-MDR1 and MDCK-MRP2, respectively. Cell monolayers were pre-incubated for 30 min at 37°C with the assay buffer added to both, donor and receiver chambers, or 1 μM GF120918 (for LLC-MDR1), or 10 μM indomethacin (for MDCK-MRP2). Transport of 3H-P85 in assay buffer or in 1 μM GF120918 across Pgp-transfected cell monolayers from AP to BL as well as from BL to AP direction was studied as described elsewhere (14). Similarly, transport of 3H-P85 in assay buffer, or in 10 μM indomethacin was performed across MRP2-transfected cell monolayers. The amount of 3H-P85 transported across the monolayers was determined using a Tricarb 4000 (Packard, Meriden, CT, USA). All transport experiments were conducted at 37°C and in triplicate.
Statistical Analysis
All statistical tests were performed by GraphPad Prism 3.0 (GraphPad software, San Diego, CA, USA) using the two-tailed heteroscedastic t tests. A minimum p value of 0.05 was estimated as the significance level for the all tests.
RESULTS
Expression of Efflux Proteins in the Cellular Membranes
The levels of the expression of the drug efflux proteins in the cellular membranes were determined using Western blot analysis (Fig. 1). Significant amounts of the drug efflux proteins were found as follows: Pgp in Pgp membranes (line 1), MRP1 in MRP1 membranes (line 2), and MRP2 in MRP2 membranes (line 3). There was little, if any, expression of other tested proteins in each of the membrane samples.
Fig. 1.
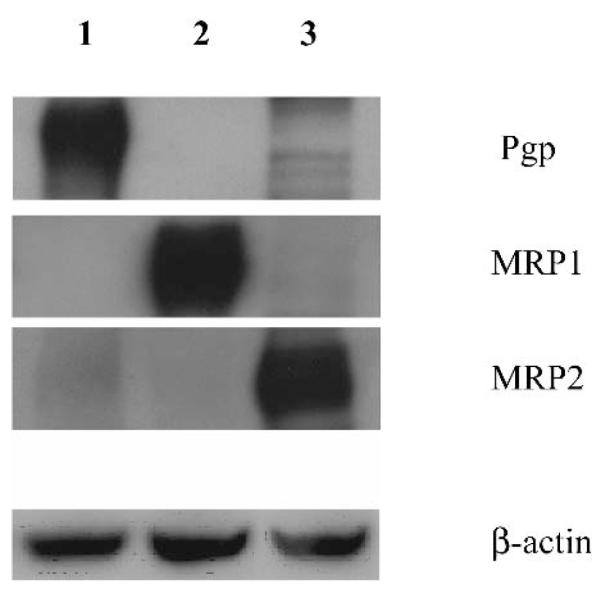
Western blot analysis of the membranes overexpressing drug efflux transporters: Pgp (line 1), MRP1 (line 2), and MRP2 (line 3). A total of 25 μg of protein was loaded onto 7.5% polyacrylamide gels. Primary antibodies for Pgp (C219), MRP1 (MRPm6), and MRP2 (M2III-6) were used at dilutions of 1:100. The monoclonal antibodies to β-actin, anti-β-1-chicken integrin were used at 1:200 dilution. Secondary antibody was used at a 1:1500 dilution.
Effects of P85 on ATP Hydrolysis by Pgp
The rates of ATP hydrolysis in Pgp membranes in the presence of P85 were determined and plotted as a function of a substrate (Mg2+ATP) concentration (Fig. 2A). As is seen in the figure, 0.01% P85 as well as 1% P85 did not affect the maximal rate of ATP hydrolysis (Vmax). In contrast, 0.1% solution of block copolymer significantly decreased Vmax. Using the double reciprocal Lineweaverer-Burk plot (Fig. 2B) we obtained kinetic parameters, Vmax and Km, for this reaction (Table I). Km values were increased in the presence of P85 indicating that the block copolymer decreases affinity of Pgp to ATP. The concentration dependence of Pluronic effect was unusual. Significant changes in Km values were observed at relatively low concentrations of P85. Thus, addition of 0.01% P85 resulted in the increase of Km value about 10-fold. As the concentration of P85 was increased, the magnitude of its effect was diminished. In particular, 1% P85 practically did not affect the kinetic parameters. The Vmax/Km ratio displayed similar pattern as the Km value, suggesting the strongest inhibition effect on Pgp ATPase at a relatively low concentration of P85 (0.01%).
Fig. 2.
(A) Concentration-dependent effects of Mg2+ATP on Pgp ATPase activity of Pgp-expressing membranes in P85-free (filled diamonds), 0.01% P85 (empty squares), 0.1% P85 (empty triangles), and 1% P85 solutions (crosses). Incubation t = 20 min. Each point shows the mean of triplicate experiments ± SD (B) Calculation of kinetic parameters Vmax and Km by double reciprocal Lineweaverer-Burk plot for reaction dephosphorilation of Mg2+ATP by Pgp in P85-free (filled diamonds), 0.01% P85 (empty squares), 0.1% P85 (empty triangles), and 1% P85 (crosses) solutions.
Table I.
Effect of P85 on Kinetic Parameters of ATP-Pgp Interaction
| Treatment |
Vmax (nmol · mg-1 · min-1)a |
Km (mM)a |
Vmax/Km |
|---|---|---|---|
| Assay buffer | 2.4 ± 0.36 | 0.4 ± 0.08 | 6 |
| 0.001% P85 | 2.4 ± 0.41 (NS) | 0.7 ± 0.05* | 3.4 |
| 0.01% P85 | 2.4 ± 0.22 (NS) | 4 ± 0.9* | 0.6 |
| 0.1% P85 | 1.1 ± 0.25* | 0.9 ± 0.17* | 1.2 |
| 1% P85 | 2.4 ± 0.33 (NS) | 0.4 ± 0.07 (NS) | 6 |
Statistical significance of P85 effects compared to the P85-free controls is shown in the brackets: NS, nonsignificant. Each parameter shows the mean of triplicate experiments ± SD.
p < 0.05.
Effects of P85 on Pgp-Drug Interaction
A Pgp substrate, Vin, was used to evaluate the effects of P85 on Pgp-drug specific interactions. Vin is known to increase Pgp ATPase activity (23). Therefore, alterations in Pgp ATPase activity caused by P85 in the presence of Vin should reflect changes in Pgp-Vin interaction induced by the copolymer. Based on the results of the previous experiment, we used 0.01% and 0.1% P85. The experiment was carried out in the presence of 4mM Mg2+ATP, i.e., non-saturating ATP concentration (as follows from the Fig. 2A). The results of the experiment are shown on Fig. 3. As is seen in the figure, P85 inhibited the drug-stimulated ATPase activity in a concentration-depended manner: the more the block copolymer was added, the higher the inhibition effect was. Table II presents kinetic constants for this process. For example, 0.1% P85 decreased Vmax and increased Km by 4.5 and 50 times respectively resulting in overall decrease in the Vmax/Km value by about 230-fold. Moreover, when high concentration of ATP (12 mM) was used in the treatment solutions (ATP-saturated conditions) P85 also inhibited ATPase activity inducing about 120-fold decrease in Vmax/Km ratio (Table II).
Fig. 3.
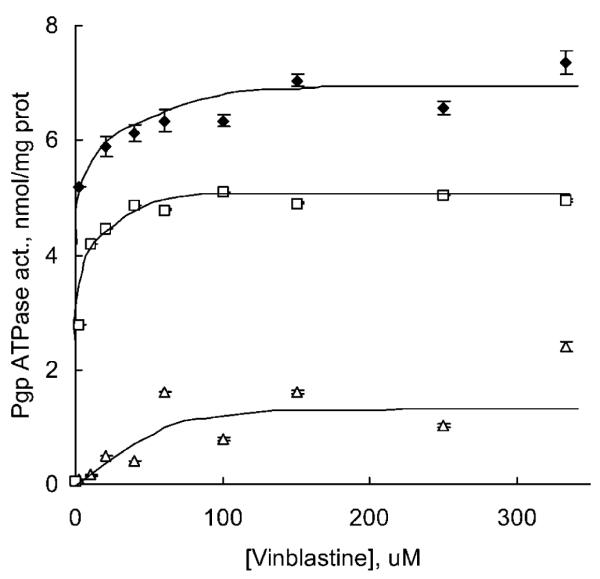
Dose response of Vin-stimulated Pgp ATPase activity of Pgp overexpressing membranes in P85-free (filled diamonds), 0.01% P85 (empty squares), and 0.1% P85 solution (empty triangles). Incubation t = 20 min, [Mg2+ATP] = 4 mM. Each point shows the mean of triplicate experiments ± SD.
Table II.
Effect of P85 on Kinetic Parameters of Vin-Pgp Interaction
| Treatment | ATP (mM) |
Vmax (nmol · mg-1 · min-1)a |
Km (μM)a | Vmax/Km |
|---|---|---|---|---|
| Vin/control | 4 | 7.2 ± 1.2 | 0.63 ± 0.15 | 11.4 |
| Vin/0.01% P85 | 4 | 5.3 ± 0.37* | 11.5 ± 0.98* | 0.46 |
| Vin/0.1% P85 | 4 | 1.6 ± 0.28* | 32.27 ± 5.2** | 0.05 |
| Vin/control | 12 | 9.5 ± 1.48 | 0.79 ± 0.16 | 12.4 |
| Vin/0.1% P85 | 12 | 3.2 ± 0.59* | 31.2 ± 5.5** | 0.1 |
Statistical significance of P85 effects compared to the P85-free controls is shown in the brackets: NS, non-significant. Each parameter shows the mean of triplicate experiments ± SD.
p < 0.05
p < 0.005.
Effects of P85 on ATP Hydrolysis by MRP1 and MRP2
The rates of ATP hydrolysis in MRP1 and MRP2 membranes were obtained and plotted vs. Mg2+ATP concentrations (Figs. 4A and 5A, respectively). As is seen in Fig. 4A, 0.1% P85 caused relatively subtle (but statistically significant) inhibition effect on MRP1. In this case Vmax was decreased by about 1.3 fold, although Km did not change (Table III). The effect of 0.1% P85 on ATP hydrolysis by MRP2 membranes was more pronounced than in the case of MRP1 (Fig. 5A). The Vmax was slightly decreased, and Km was increased, which resulted in 2.4 fold decrease in Vmax/Km ratio (Table III).
Fig. 4.
(A) Effects of Mg2+ATP on MRP1 ATPase activity of COR-L23/R membranes in P85-free (filled diamonds) and 0.1% P85 solutions (empty triangles). Incubation t = 20 min. (B) Dose response of Vin-stimulated MRP1 ATPase activity of COR-L23/R membranes in P85-free (filled diamonds) and 0.1% P85 solution (empty triangles). Incubation t = 20 min, [Mg2+ATP] = 4 mM. Each point shows the mean of triplicate experiments ± SD.
Fig. 5.
(A) Effects of Mg2+ATP on MRP2 ATPase activity of MDCKII-MRP2 membranes in P85-free (filled diamonds) and 0.1% P85 solutions (empty triangles). Incubation t = 20 min. (B) Dose response of Vin-stimulated MRP2 ATPase activity of MDCKII-MRP2 membranes in P85-free (filled diamonds) and 0.1% P85 solution (empty triangles). Incubation t = 20 min, [Mg2+ATP] = 4 mM. Each point shows the mean of triplicate experiments ± SD.
Table III.
Effect of P85 on Kinetic Parameters of MRP1- and MRP2-ATP Interactions
| Treatment |
Vmax (nmol · mg-1 · min-1)a |
Km (mM)a |
Vmax/Km |
|---|---|---|---|
| MRP1 control | 4.2 ± 0.86 | 0.43 ± 0.08 | 9.8 |
| MRP1 in 0.1% P85 | 3.3 ± 0.6* | 0.43 ± 0.07 (NS) | 7.7 |
| MRP2 control | 2.7 ± 0.51 | 0.3 ± 0.05 | 9.2 |
| MRP2 in 0.1% P85 | 1.9 ± 0.37* | 0.49 ± 0.07 (NS) | 3.88 |
Statistical significance of P85 effects compared to the P85-free controls is shown in the brackets: NS, non-significant. Each parameter shows the mean of triplicate experiments ± SD.
p < 0.05.
Effects of P85 on MRP1 and MRP2 Interactions with Drugs
This study initially used a Pgp substrate, Vin, which is also known to interact with MRP1 and MRP2. Figures 4B and 5B show MRP1 and MRP2 ATPase activities in the presence of Vin with and without 0.1% P85, and Table IV summarizes the kinetic parameters. The block copolymer significantly altered the kinetic parameters of MRP-drug interactions. Specifically, Vmax was decreased, and Km was increased resulting in overall decrease of Vmax/Km ratio by about 14 times for MRP1 and 6 times for MRP2-overexpressing membranes (Table IV). Because Vin is not an ideal substrate for MRPs we also evaluated the effect of P85 on interactions of MRP1 and LTC4, a high affinity substrate for MRP1 and MRP2 (24,25). As is seen in Table IV, 0.1% P85 considerably altered the kinetics constants resulting in overall 6-fold decrease in Vmax/Km ratio.
Table IV.
Effect of P85 on Kinetic Parameters of MRP1- and MRP2-Drug Interactions
| Treatment | Drug |
Vmax (nmol · mg-1 · min-1)a |
Km (μM)a | Vmax/Km |
|---|---|---|---|---|
| MRP1 control | Vin | 11 ± 2.5 | 5.2 ± 0.7 | 2.1 |
| MRP1/0.1% P85 | Vin | 6.8 ± 0.88* | 45.9 ± 8.7* | 0.15 |
| MRP1 control | LTC4 | 10.2 ± 0.9 | 0.077 ± 0.12 | 132.5 |
| MRP1/0.1% P85 | LTC4 | 4.2 ± 0.5* | 0.185 ± 0.03* | 22.7 |
| MRP2 control | Vin | 6.0 ± 0.5 | 3.3 ± 0.7 | 1.82 |
| MRP2/0.1% P85 | Vin | 3.75 ± 0.88* | 12.5 ± 8.7* | 0.3 |
Statistical significance of P85 effects compared to the P85-free controls is shown in the brackets. Each parameter shows the mean of triplicate experiments ± SD.
p < 0.05.
3H-P85 Accumulation and Transport Studies
To evaluate whether P85 is a substrate for the efflux proteins the kinetic of 3H-P85 accumulation in cells overexpressing Pgp, MRP1, and MRP2 was examined in the presence of Pgp and MRPs inhibitors, respectively. Specifically, 1 μM GF120918, a Pgp inhibitor (16), and 10 μM indomethacin, a MRPs inhibitor (26), were used in these experiments. Figure 6 shows the kinetics of 3H-P85 accumulation in Pgp- and MRP2-overexpressing cells, LLC-MDR1 and MDCKII-MRP2. As is seen in figure, accumulation levels of 3H-P85 were not altered in the presence of the inhibitors. To reinforce this result we also studied the effect of GF120918 and indomethacin on 3H-P85 transport across polarized LLC-MDR1 and MDCK-MRP2 monolayers. The transport of the block copolymer was performed in both, apical to basolateral and basolateral to apical directions. No differences in the kinetics of 3H-P85 transport in either direction were found (data not shown) indicating that P85 is not a substrate for these transporters.
Fig. 6.

Time-dependent accumulation of 3H-P85 in MDCKII-MRP1 (triangles) and LLC-MDR1 (squares) monolayers using assay buffer (filled symbols), 10 μM indomethacin (filled triangles), and 1 μM GF120918 (filled squares). Values represent the mean ± SD of four cell monolayers.
DISCUSSION
Previous studies demonstrated that P85 1) decreases intracellular ATP levels (energy depletion) and 2) increases membrane fluidity resulting in inhibition of ATPase activity of Pgp in multidrug resistant cells (13,14). These two effects combined are essential for inhibition of Pgp drug efflux system and chemosensitization of these cells. Furthermore, P85 treatment also resulted in ATP depletion in MRP1- and MRP2-overexpressing cells (15). However, the effects of P85 on MRP efflux systems in these cells are substantially less compared to the effects on Pgp. We hypothesized that the difference in the potency of P85 with respect to Pgp and MRPs is due to the different ability of the block copolymer to alter ATPase activity of these transporters. Therefore, we examined the effects of P85 on ATPase activity using isolated Pgp-, MRP1-, and MRP2-overexpressing membranes.
The Km values obtained in this work in Pgp membranes, 0.4 ± 0.08 mM (ATP) and 0.63 ± 0.15 μM (Vin) are in a good agreement with the values reported before: 0.3-0.4 mM (ATP) and 0.5–1.5 μM (Vin) (27–29). Somewhat higher Km (7 μM) was reported for Vin in Pgp-expressing living cells (30). A broad range of Km values for ATP was reported in MRP1 membranes: 3 mM (31), 0.1 mM (32), and 0.023 mM (33). Our measurements for MRP1 membranes suggest Km of 0.43 ± 0.08 mM. The Km for Vin in MRP1 membranes was not available in the literature to compare with our data (5.2 ± 0.7 μM). The Km values for MRP2 membranes obtained in this work, 0.3 ± 0.05 mM (ATP) and 3.3 ± 0.7 μM (Vin), were relatively close to those reported earlier: 0.56 mM (ATP) (34) and 1.5 ± 0.3 μM (Vin) (35). Finally our Km for LTC4 in MRP1 membranes (0.077 ± 0.12 μM) was also in good agreement with earlier reports: 0.07 to 0.12 μM (24,25).
Addition of P85 resulted in substantial decrease in Vmax and increase in Km values in Pgp membranes. The concentration dependence of P85 effects on Pgp was unusual; the block copolymer was most effective at relatively low concentrations. As the concentration of P85 was increased its effect was diminished. Furthermore, the maximal effect of P85 on Vmax was observed at higher concentration of the block copolymer (0.1%) than the effect on Km (0.01%). The Vmax/Km ratio, which characterizes the efficiency of the enzymatic reaction at resting concentrations of ATP within the cell (ca. 1 mM) displayed a minimum at 0.01% P85. Overall, this result is consistent with the earlier study, which demonstrated that low concentrations of P85 (from about 0.001% to 0.1%) increased accumulation of a Pgp substrate, rhodamine 123, in Pgp expressing cells (13). At higher concentrations (about 1%) the effect of P85 was reduced. Most likely these effects relate to the aggregation behavior of P85 that forms micelles in aqueous solutions above the critical micellization concentration (CMC), 0.03% (36). Pluronic molecules can also aggregate into two-dimensional micelles in lipid bilayers (37). While the single chains of P85 are likely to affect Pgp resulting in inhibition of ATPase activity, the interactions of Pgp with the micelles may “salvage” the ATPase activity. The situation, however, may be very complex and involve multiple and different modes of interaction of P85 with Pgp, which is indirectly supported by difference in the copolymer concentrations acting upon Vmax and Km.
It is unlikely that the effects P85 on the ATPase activity involve specific binding of this block copolymer with the transport protein. This study demonstrates that P85 is not a substrate for these proteins and does not bind to them like traditional low molecular mass chemosensitizers. It is more probable, that P85 acts through: 1) changes of the membrane structure, which affect the conformations of the transport proteins, and/or 2) sterically hinders the sites of binding of the drugs with them. The conformational changes may involve the cytoplasmic ATP-binding domains (NBDs) that are present in both Pgp and MRPs (4,5,38–40). It was shown previously that kinetic parameters for binding and hydrolysis of ATP by Pgp depend on the state of the lipid bilayer surrounding the protein. Specifically, the affinity of the protein with respect to ATP diminishes as the fluidity of the membranes increases (upon heating) suggesting tight relationship between NBDs function and the lipid membrane structure (41,42). However, Tween-80 appears to have little effect on the critical Pgp conformations, and it is unclear whether P85 would have such effect (43). Beside the NBDs the efflux proteins contain two membrane-embedded domains, which span the cellular membrane and form high affinity drug-binding sites (4,5,38,39). It was suggested that Pgp (44,45) extract the drugs from the inner leaflet of the lipid bilayer and translocate them directly into the extracellular media. Thus, P85 chains embedded in the membrane may sterically hinder protein-drug interaction in the appropriate binding sites. This mechanism is indirectly supported by the observation that P85 affects the Vmax and Km values for Vin in Pgp membranes under ATP-saturated conditions.
It is noteworthy, that P85 had substantially less effects on MRP1 and MRP2 ATPase activities compared to the pronounced effects on Pgp. These differences were apparent for ATP as well as the drug molecules. Overall, the ATPase inhibition potency of P85 increased in the row MRP1 < MRP2 << Pgp. On the one hand, it cannot be excluded that the structure of MRPs is more robust and less vulnerable to the conformational changes caused by P85 than the structure of Pgp. On the other hand, although it was suggested that MRPs like the Pgp extract the drugs from the lipid bilayer (5,39), the high affinity drug-binding sites of MRPs may be less accessible for the hindrance by the block copolymer than similar sites of Pgp.
The effect of P85 on ATPase activity of the different transport proteins is consistent with the reports that the sensitization effects of the block copolymer in resistant cells also increase in the same order: MRP1 < MRP2 << Pgp (12,15). This reinforces the relationship between the effects of Pluronics on different drug efflux transport systems and ATPase activities of the transport proteins. It is likely that even under the conditions of ATP depletion induced by the block copolymer the efflux proteins retain the ability to transport their substrates if the ATPase function is not affected (14). In other words even if the concentration of ATP in the cells is decreased several fold the amount of ATP available can be still sufficient to maintain the function of the MRP transporters. However, in the case of Pgp there is a simultaneous ATP depletion and strong inhibition of ATPase, which results in strong inhibition of this drug efflux system.
Overall, addition of Pluronic block copolymer to the cells overexpressing Pgp and MRPs efflux proteins reduces affinity and/or accessibility for the substrate-binding sites of the transporter. However, the magnitude of P85 effects is considerably higher for Pgp than for MRP1 and MRP2. This conclusion is in a good agreement with previous reports (15,17,46) showing significant inhibition of drug efflux transporters by nonionic surfactants in Pgp- and much less in MRPs overexpressing cells. These data have provided insight into the mechanism by which P85 affect the Pgp and MRPs efflux transport systems.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grant CA89225. We would like to thank Piet Borst (Netherlands Cancer Institute, Amsterdam, The Netherlands) for kindly providing MRP-transfected cell lines and Dr. Kenneth Brouwer (Glaxo Wellcome, Inc., Research Triangle Park, NC, USA) for kindly providing GF120918.
ABBREVIATIONS
- Km
Michaelis constant
- LTC4
leucotriene C4
- MDR
multidrug resistance
- MRPs
multidrug resistant proteins
- Pgp
P-glycoprotein
- P85
Pluronic P85
- Vin
vinblastine
- Vmax
maximal reaction rate
REFERENCES
- 1.Naito S, Yokomizo A, Koga H. Mechanisms of drug resistance in chemotherapy for urogenital carcinoma. Int. J. Urol. 1999;6:427–439. doi: 10.1046/j.1442-2042.1999.00088.x. [DOI] [PubMed] [Google Scholar]
- 2.Leyland-Jones B, Dalton W, Fisher G, Sikic B. Reversal of multidrug resistance to cancer chemotherapy. Cancer Lett. 1993;72:3484–3488. doi: 10.1002/1097-0142(19931201)72:11+<3484::aid-cncr2820721615>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Chan H, Lu Y, Grogan T, Haddad G, Hipfner D, Cole S, Deeley R, Ling V, Gallie B. Multidrug resistance protein (MRP) expression in retinoblastoma correlates with the rare failure of chemotherapy despite cyclosporine for reversal of P-glycoprotein. Cancer Res. 1997;57:2325–2330. [PubMed] [Google Scholar]
- 4.Ambudkar S, Dey S, Hrycyna C, Ramachandra M, Pastan I, Gottesman M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 5.Hipfner D, Deeley R, Cole S. Structural, mechanistic and clinical aspects of MRP1. Biochim. Biophys. Acta. 1999;1461:359–376. doi: 10.1016/s0005-2736(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 6.Choi C, Sun K, An C, Yoo J, Hahm K, Lee I, Sohng J, Kim Y. Reversal of P-glycoprotein-mediated multidrug resistance by 5,6,7,3′,4′-pentamethoxyflavone (Sinensetin) Biochem. Biophys. Res. Commun. 2002;295:832–840. doi: 10.1016/s0006-291x(02)00755-6. [DOI] [PubMed] [Google Scholar]
- 7.Hwang M, Ahn C, Pine P, Yin J, Hrycyna C, Licht T, Aszalos A. Effect of combination of suboptimal concentrations of P-glycoprotein blockers on the proliferation of MDR1 gene expressing cells. Int. J. Cancer. 1996;65:389–397. doi: 10.1002/(SICI)1097-0215(19960126)65:3<389::AID-IJC19>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Hyafil F, Vergely C, Du Vignaud P, Grand-Perret T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993;53:4595–4602. [PubMed] [Google Scholar]
- 9.Alakhov V, Moskaleva E, Batrakova E, Kabanov A. Hypersensitization of multidrug resistant human ovarian carcinoma cells by Pluronic P85 block copolymer. Bioconjug. Chem. 1996;7:209–216. doi: 10.1021/bc950093n. [DOI] [PubMed] [Google Scholar]
- 10.Batrakova E, Li S, Alakhov V, Miller D, Kabanov A. Optimal structure requirements for pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J. Pharmacol. Exp. Ther. 2003;304:845–854. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]
- 11.Danson S, Ferry D, Alakhov V, Margison J, Kerr D, Jowle D, Brampton M, Halbert G, Ranson M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br. J. Cancer. 2004;90:2085–2091. doi: 10.1038/sj.bjc.6601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venne A, Li S, Mandeville R, Kabanov A, Alakhov V. Hypersensitizing effect of Pluronic L61 on cytotoxic activity, transport, and subcellular distribution of doxorubicin in multiple drug-resistant cells. Cancer Res. 1996;56:3626–3629. [PubMed] [Google Scholar]
- 13.Batrakova E, Li S, Elmquist W, Miller D, Alakhov V, Kabanov A. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br. J. Cancer. 2001;85:1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batrakova E, Li S, Vinogradov S, Alakhov V, Miller D, Kabanov A. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J. Pharmacol. Exp. Ther. 2001;299:483–493. [PubMed] [Google Scholar]
- 15.Batrakova EV, Li S, Alakhov VY, Elmquist WF, Miller DW, Kabanov AV. Sensitization of cells overexpressing multidrug-resistant proteins by pluronic P85. Pharm. Res. 2003;20:1581–1590. doi: 10.1023/a:1026179132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evers R, Kool M, Smith A, van Deemter L, de Haas M, Borst P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br. J. Cancer. 2000;83:366–374. doi: 10.1054/bjoc.2000.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogman K, Erne-Brand F, Alsenz J, Drewe J. The role of surfactants in the reversal of active transport mediated by multidrug resistance proteins. J. Pharm. Sci. 2003;92:1250–1261. doi: 10.1002/jps.10395. [DOI] [PubMed] [Google Scholar]
- 18.Batrakova EV, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br. J. Cancer. 2001;85:1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Druekes P, Schinzel R, Palm D. Photometric microtiter assay of inorganic phosphate in the presence of acid-labile organic phosphates. Anal. Biochem. 1995;230:173–177. doi: 10.1006/abio.1995.1453. [DOI] [PubMed] [Google Scholar]
- 20.Taguchi Y, Yoshida A, Takada Y, Komano T, Ueda K. Anti-cancer drugs and glutathione stimulate vanadate-induced trapping of nucleotide in multidrug resistance-associated protein (MRP) FEBS Lett. 1997;401:11–14. doi: 10.1016/s0014-5793(96)01421-4. [DOI] [PubMed] [Google Scholar]
- 21.Lineweaver H, Burk D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934;56:658–666. [Google Scholar]
- 22.Melik-Nubarov N, Pomaz O, Dorodnych T, Badun G, Ksenofontov A, Schemchukova O, Arzhakov S. Interaction of tumor and normal blood cells with ethylene oxide and propylene oxide block copolymers. FEBS Lett. 1999;446:194–198. doi: 10.1016/s0014-5793(99)00208-2. [DOI] [PubMed] [Google Scholar]
- 23.Sarkadi B, Price E, Boucher R, Germann U, Scarborough G. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J. Biol. Chem. 1992;267:4854–4858. [PubMed] [Google Scholar]
- 24.Loe D, Almquist K, Deeley R, Cole S. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport. J. Biol. Chem. 1996;271:9675–9682. doi: 10.1074/jbc.271.16.9675. [DOI] [PubMed] [Google Scholar]
- 25.Jedlitschky G, Keppler D. Transport of leukotriene C4 and structurally related conjugates. Vitam. Horm. 2002;64:153–184. doi: 10.1016/s0083-6729(02)64005-1. [DOI] [PubMed] [Google Scholar]
- 26.Draper M, Martell R, Levy S. Indomethacin-mediated reversal of multidrug resistance and drug efflux in human and murine cell lines overexpressing MRP, but not P-glycoprotein. Br. J. Cancer. 1997;75:810–815. doi: 10.1038/bjc.1997.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharom F, Liu R, Qu Q, Romsicki Y. Exploring the structure and function of the P-glycoprotein multidrug transporter using fluorescence spectroscopic tools. Semin. Cell Dev. Biol. 2001;12:257–265. doi: 10.1006/scdb.2000.0251. [DOI] [PubMed] [Google Scholar]
- 28.Stein W. Kinetics of the multidrug transporter (P-glycoprotein) and its reversal. Physiol. Rev. 1997;77:545–590. doi: 10.1152/physrev.1997.77.2.545. [DOI] [PubMed] [Google Scholar]
- 29.Litman T, Nielsen D, Skovsgaard T, Zeuthen T, Stein W. ATPase activity of P-glycoprotein related to emergence of drug resistance in Ehrlich ascites tumor cell lines. Biochim. Biophys. Acta. 1997;1361:147–158. doi: 10.1016/s0925-4439(97)00025-2. [DOI] [PubMed] [Google Scholar]
- 30.Landwojtowicz E, Nervi P, Seelig A. Real-time monitoring of P-glycoprotein activation in living cells. Biochemistry. 2002;41:8050–8057. doi: 10.1021/bi025720s. [DOI] [PubMed] [Google Scholar]
- 31.Chang X, Hou Y, Riordan J. ATPase activity of purified multidrug resistance-associated protein. J. Biol. Chem. 1997;272:30962–30968. doi: 10.1074/jbc.272.49.30962. [DOI] [PubMed] [Google Scholar]
- 32.Mao Q, Leslie E, Deeley R, Cole S. ATPase activity of purified and reconstituted multidrug resistance protein MRP1 from drug-selected H69AR cells. Biochim. Biophys. Acta. 1999;1461:69–82. doi: 10.1016/s0005-2736(99)00150-9. [DOI] [PubMed] [Google Scholar]
- 33.Gao M, Cui H, Loe D, Grant C, Almquist K, Cole S, Deeley R. Comparison of the functional characteristics of the nucleotide binding domains of multidrug resistance protein 1. J. Biol. Chem. 2000;275:13098–13108. doi: 10.1074/jbc.275.17.13098. [DOI] [PubMed] [Google Scholar]
- 34.Hagmann W, Schubert J, Konig J, Keppler D. Reconstitution of transport-active multidrug resistance protein 2 (MRP2; ABCC2) in proteoliposomes. Biol. Chem. 2002;383:1001–1009. doi: 10.1515/BC.2002.107. [DOI] [PubMed] [Google Scholar]
- 35.Van Aubel R, Koenderink J, Peters J, Van Os C, Russel F. Mechanisms and interaction of vinblastine and reduced glutathione transport in membrane vesicles by the rabbit multidrug resistance protein Mrp2 expressed in insect cells. Mol. Pharmacol. 1999;56:714–719. [PubMed] [Google Scholar]
- 36.Kozlov M, Melik-Nubarov N, Batrakova E, Kabanov A. Relationship between Pluronic block copolymer structure, critical micellization concentration and partitioning coefficients of low molecular mass solutes. Macromolecules. 2000;33:3305–3313. [Google Scholar]
- 37.Kostarelos K, Tadros T, Luckham P. Physical conjugation of (Tri-) block copolymers to liposomes toward the concentration of sterically stabilized vesicle systems. Langmuir. 1999;15:369–376. [Google Scholar]
- 38.Higgins C. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg M, Mao Q, Holzenburg A, Ford R, Deeley R, Cole S. The structure of the multidrug resistance protein 1 (MRP1/ABCC1). crystallization and single-particle analysis. J. Biol. Chem. 2001;276:16076–16082. doi: 10.1074/jbc.M100176200. [DOI] [PubMed] [Google Scholar]
- 40.Nies A, Konig J, Cui Y, Brom M, Spring H, Keppler D. Structural requirements for the apical sorting of human multi-drug resistance protein 2 (ABCC2) Eur. J. Biochem. 2002;269:1866–1876. doi: 10.1046/j.1432-1033.2002.02832.x. [DOI] [PubMed] [Google Scholar]
- 41.Sharom F, Liu R, Romsicki Y, P L. Insights into the structure and substrate interactions of the P-glycoprotein multi-drug transporter from spectroscopic studies. Biochim. Biophys. Acta. 1999;1461:327–345. doi: 10.1016/s0005-2736(99)00166-2. [DOI] [PubMed] [Google Scholar]
- 42.Lu P, Liu R, Sharom F. Drug transport by reconstituted P-glycoprotein in proteoliposomes. Effect of substrates and modulators, and dependence on bilayer phase state. Eur. J. Biochem. 2001;268:1687–1697. [PubMed] [Google Scholar]
- 43.Nagy H, Goda K, Arceci R, Cianfriglia M, Mechetner E, Szabo G., Jr. P-Glycoprotein conformational changes detected by antibody competition. Eur. J. Biochem. 2001;268:2416–2420. doi: 10.1046/j.1432-1327.2001.02122.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg M, Velarde G, Ford R, Martin C, Berridge G, Kerr I, Callaghan R, Schmidlin A, Wooding C, Linton K, Higgins C. Repacking of the transmembrane domains of P-glycoprotein during the transport ATPase cycle. EMBO J. 2001;20:5615–5625. doi: 10.1093/emboj/20.20.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins C, Linton K. The xyz of ABC transporters. Science. 2001;293:1782–1784. doi: 10.1126/science.1065588. [DOI] [PubMed] [Google Scholar]
- 46.Aszalos A, Thompson K, Yin J-J, D R. Combinations of P-glycoprotein blockers, Verapamil, PSC833, and Cremophor act differently on the multidrug resistance associated protein (MRP) and on p-glycoprotein (Pgp) Anticancer Res. 1999;19:1053–1064. [PubMed] [Google Scholar]





