Abstract
Objective
Although spinal cord injury frequently results in low impact fractures at the distal femur and proximal tibia, there are no standard clinical protocols for assessing bone mineral density at these sites. We evaluated the precision of dual energy x-ray absorptiometry scanning at two skeletal sites at the knee (proximal femur and distal tibia) in individuals with spinal cord injury.
Design
Cross-sectional.
Setting
VA Medical Center.
Participants
20 subjects with chronic SCI.
Interventions
Not Applicable.
Main Outcome Measures
Precision as determined by root mean square coefficient of variation (RMS-CV) and root mean standard deviation (RMS-SD).
Results
At the distal femur the root RMS-CV was 3.01% and the RMS-SD was 0.025 g/cm2. At the proximal tibia the RMS-CV was 5.91% and the RMS-SD was 0.030 g/cm2.
Conclusions
Precision at the distal femur is greater than the proximal tibia and we recommend it as the preferred site for the longitudinal assessment of bone mineral density at the knee in chronic spinal cord injury.
Keywords: Spinal Cord Injury, Osteoporosis, Bone Mineral Density, Precision
Osteoporosis is a potentially devastating complication of spinal cord injury (SCI) that is under-diagnosed and under-treated. Currently, there is no way to predict fracture risk based on bone mineral density (BMD) in the extremities due to a lack of SCI-specific assessment protocols. While dual energy x-ray absorptiometry (DXA) scanning is performed routinely to assign fracture risk in the general population, these scanning protocols have little relevance to the SCI population because the distal femur and proximal tibia, skeletal sites most likely to be fractured following SCI, are not included in these scans. Some studies suggest that as many as 70% of all individuals will sustain a low-impact (i.e., those occurring in the absence of trauma) osteoporotic fracture at some point following their SCI1. These fractures occur in the extremities below the neurological level of injury and often result in prolonged hospitalization. Fracture risk may be greatest following complete injury versus incomplete injury2. However, longitudinal bone loss in the chronic phases of SCI is poorly defined. While the greatest amount of bone is lost in the first 2 years following a SCI 3-7, the mean time to first fracture is 9 years post-injury 8, 9. This suggests that factors determining the rate of bone loss in the chronic phase are clinically relevant and may be important for the prevention of post-SCI fractures. Furthermore, there is no standard of care for the prevention of osteoporotic fractures in this population.
Determination of the efficacy of emerging therapeutic interventions, including antiresorptive medications, is limited without a standardized means to evaluate and monitor BMD following SCI. To our knowledge, there is only one other report in the literature regarding knee DXA scanning in SCI, but precision was not studied 10. Two measures, precision and accuracy, are commonly used together to assess the performance of DXA scanning protocols. The ash weight of the skeletal site of interest is defined as the true “gold standard” of DXA accuracy. However, since this cannot be determined in vivo, accuracy is usually determined by measuring reference phantoms supplied by the densitometer manufacturer. Precision, or the closeness of agreement between independent test results obtained under stipulated conditions, is a reflection of error, both those inherent to the technique as well as those introduced by inconsistent positioning and scan analysis. The least significant change (LSC) refers to the magnitude of change in BMD required to reflect true biological change over time. Precision must be known before LSC can be determined. Precision is an important consideration clinically as change over time in bone mineral density of a skeletal site can not be determined without defining the precision of a scanning technique.
Therefore, the goal of the current study was to evaluate the precision of our DXA scanning protocol for measuring BMD at the knee in SCI. We also compared the precision of measuring BMD at the distal femur to the precision of BMD measurements at the proximal tibia to determine the optimal skeletal site for routine SCI-specific DXA scanning protocols at the knee.
METHODS
Subjects
A convenience sample of 20 participants with motor complete and incomplete SCI over a range of levels were selected from a larger epidemiological study assessing health in chronic SCI. This included veterans with SCI who had previously been treated at our VA facility and from participants in the community 11. The study was approved by our institutional review boards, and all study subjects gave informed consent.
Spinal Cord Injury Classification
Motor level and completeness of injury were assessed by physical exam. Level of injury was classified according to strength preservation in key muscle groups in the upper and lower extremity and reported regionally as tetraplegia (cervical SCI) or paraplegia (thoracic or lumbar SCI). Injury completeness was reported according to guidelines suggested by the American Spinal Injury Association (ASIA) 12. Participants were assigned as motor complete (equivalent to ASIA motor score of A or B, i.e., no motor function below the neurological level of injury), or D (motor incomplete, preservation of motor function below the neurological level and more than half the key muscles below the neurological level are strong enough to overcome gravity).
Assessment of Bone Mineral Density by Dual X-ray Absorptiometry (DXA) Scanning
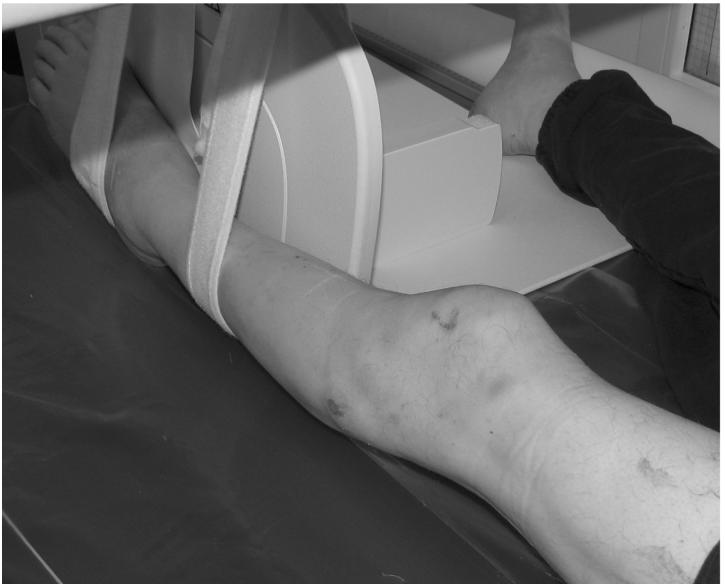
BMD was determined by DXA scan using a 4th generation General Electric Lunar Prodigy Advance densitometer a, with scans performed at both the proximal tibia and distal femur. Unless there was a previous fracture or instrumentation, the non-dominant lower extremity was scanned. After the subject was transferred to the scanning surface, the lower limb was stabilized in full extension and in zero degrees of internal rotation using Velcro straps to immobilize the limb against a 90 degree support (see Figure 1). Three scans of the distal femur and proximal tibia were obtained. For subject comfort we chose not to have the subject transfer on and off the table between scans, but in between each scan the lower limb straps were loosened, the subject moved out of position, and repositioned with the limb stabilized again prior to the next scan. Scanning was performed with no or light clothing covering the examined area. The same trained DXA certified technician positioned and performed all scans.
Figure 1.
Photo illustration of a patient on the GE Lunar Prodigy DXA scanner demonstrating positioning for scanning of the knee.
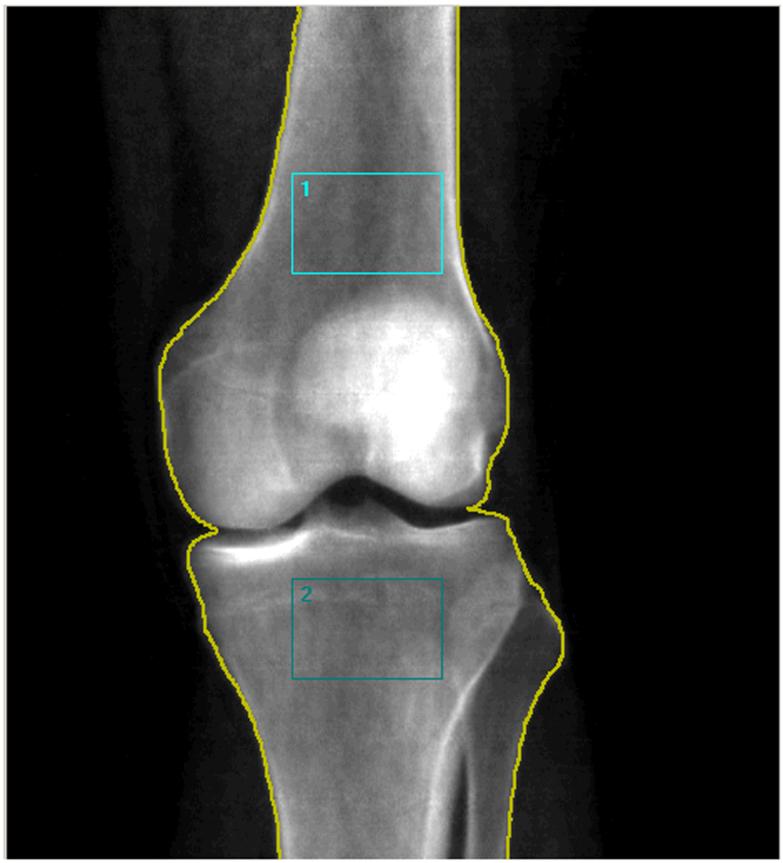
For analysis two regions of interest (ROI) were chosen of equal areas, one at the distal femur and one at the proximal tibia. For the distal femur, the proximal edge of the ROI was set at 20% of the femur length (measured from the lateral femoral condyle) and the distal edge was set at the visible intersection between the patella and the femur, excluding the patella from the ROI (see figure 2). For the femur, the superior edges of the rectangle were defined as the inner borders of the cortical margins. The width of the rectangle varied proportionally to the individual’s geometry. For the proximal tibia, the superior and lateral edge was set at the most proximal point of contact between the tibia and fibular head sites to avoid regions of overlap between the fibula and the tibia. Customized research software (Encore 2006 Software Version #10.20.105) supplied by General Electric was used to determine the BMD. As a standard procedure, a phantom supplied by the manufacturer providing 3 different densities (0.5, 1.0, and 1.5 g/cm2) was measured daily and accuracy was within 0.003 gm/cm2 during the study.
Figure 2.
Region of interest scanned at the distal femur (blue box 1) and the proximal tibia (blue box 2).
Precision Calculations
We followed the short term precision methodology recommended by the International Society for Clinical Densitometry (ISCD). We calculated the average BMD, the standard deviation, and the coefficient of variation for each set of scans. The root mean square coefficient of variation of BMD (RMS-CV) and root mean standard deviation (RMS-SD) for the distal femur and the proximal tibia was calculated 13.
RESULTS
Participant Characteristics
The average age (sd) was 54.9 (±12.7 years) with a mean of 21.2 (±13.0) years since injury (Table 1). The majority of the participants (13 of the 20) had motor complete injuries, and 7 had ASIA D injuries. Mean BMD at the distal femur ranged 0.342 g/cm2 to 1.028 g/cm2 and at the proximal tibia ranged from 0.115 g/cm2 to 1.192 g/cm2.
Table 1. Participant Description.
| Subject | Motor Complete | Age, years | Injury Duration, years |
|---|---|---|---|
| 1 | C07 | 41.6 | 14.8 |
| 2 | C08 | 38.5 | 6.0 |
| 3 | C08 | 49.6 | 32.2 |
| 4 | T02 | 59.2 | 14.3 |
| 5 | T02 | 28.8 | 16.0 |
| 6 | T03 | 43.2 | 25.5 |
| 7 | T06 | 47.1 | 13.3 |
| 8 | T07 | 54.8 | 33.8 |
| 9 | T08 | 46.4 | 9.0 |
| 10 | T10 | 63.9 | 41.6 |
| 11 | T12 | 67.9 | 42.0 |
| 12 | T12 | 53.9 | 24.5 |
| 13 | L04 | 51.3 | 32.0 |
| Motor Incomplete (ASIA D) | |||
| 14 | C06 | 59.5 | 40.7 |
| 15 | C06 | 46.8 | 11.6 |
| 16 | C05 | 56.0 | 3.8 |
| 17 | T12 | 70.7 | 20.8 |
| 18 | T12 | 74.6 | 2.3 |
| 19 | L02 | 75.2 | 30.4 |
| 20 | L02 | 68.9 | 8.7 |
| Mean | 54.9 | 21.2 |
Precision at the Distal Tibia and Proximal Femur
Three scans per subject were attempted in 20 subjects (Tables 2 and 3). Satisfactory data were obtained in all but two scans due to software malfunction. The RMS-CV was 3.01%, and RMS-SD was 0.025 g/cm2 at the distal femur. At the proximal tibia, the RMS-CV was 5.91%, and the RMS-SD was 0.030 g/cm2. Although sample size limited the determination of RMS-CV and RMS-SD within subgroups, our data suggests that persons with complete SCI are more likely to have a greater CV and SD at the proximal tibia than at the distal femur (Tables 2 and 3, subjects 1,2, 4 and 8).
Table 2. Distal femur bone mineral density (g/m2) and coefficient of variation (CV).
| Subject | Motor Complete | Scan 1 | Scan 2 | Scan 3 | Mean | SD | CV, % |
|---|---|---|---|---|---|---|---|
| 1 | C07 | 0.539 | 0.541 | 0.538 | 0.539 | 0.002 | 0.283 |
| 2 | C08 | 0.370 | 0.382 | 0.393 | 0.382 | 0.012 | 3.014 |
| 3 | C08 | 0.828 | 0.842 | 0.841 | 0.837 | 0.008 | 0.933 |
| 4 | T02 | 0.372 | 0.348 | 0.367 | 0.362 | 0.013 | 3.495 |
| 5 | T02 | 0.648 | 0.668 | 0.682 | 0.666 | 0.017 | 2.566 |
| 6 | T03 | 0.343 | 0.341 | ND | 0.342 | 0.001 | 0.414 |
| 7 | T06 | 0.591 | 0.568 | 0.586 | 0.582 | 0.012 | 2.080 |
| 8 | T07 | 0.676 | 0.669 | 0.666 | 0.670 | 0.005 | 0.766 |
| 9 | T08 | 0.679 | 0.600 | 0.659 | 0.646 | 0.041 | 6.358 |
| 10 | T10 | 0.897 | 0.905 | 0.907 | 0.903 | 0.005 | 0.586 |
| 11 | T12 | 0.923 | 0.975 | 0.951 | 0.950 | 0.026 | 2.741 |
| 12 | T12 | 0.719 | 0.721 | 0.718 | 0.719 | 0.002 | 0.212 |
| 13 | L04 | 0.629 | 0.601 | 0.6 | 0.610 | 0.017 | 2.699 |
| Motor Incomplete (ASIA D) | |||||||
| 14 | C06 | 1.043 | 1.111 | 0.929 | 1.028 | 0.092 | 8.949 |
| 15 | C06 | 0.713 | 0.715 | 0.719 | 0.716 | 0.003 | 0.427 |
| 16 | C05 | 0.731 | 0.720 | ND | 0.726 | 0.008 | 1.072 |
| 17 | T12 | 0.556 | 0.556 | 0.563 | 0.558 | 0.004 | 0.724 |
| 18 | T12 | 0.886 | 0.904 | 0.868 | 0.886 | 0.018 | 2.032 |
| 19 | L02 | 0.782 | 0.762 | 0.78 | 0.774 | 0.011 | 1.422 |
| 20 | L02 | 0.881 | 0.859 | 0.847 | 0.862 | 0.017 | 2.000 |
Table 3. Proximal tibia bone mineral density (g/m2) and coefficient of variation (CV).
| Subject | Motor Complete | Scan 1 | Scan 2 | Scan 3 | Mean | SD | CV, % |
|---|---|---|---|---|---|---|---|
| 1 | C07 | 0.379 | 0.456 | 0.455 | 0.430 | 0.044 | 10.272 |
| 2 | C08 | 0.284 | 0.382 | 0.410 | 0.359 | 0.066 | 18.447 |
| 3 | C08 | 0.718 | 0.746 | 0.724 | 0.729 | 0.015 | 2.021 |
| 4 | T02 | 0.303 | 0.328 | 0.364 | 0.332 | 0.031 | 9.246 |
| 5 | T02 | 0.415 | 0.432 | 0.433 | 0.427 | 0.010 | 2.371 |
| 6 | T03 | 0.114 | 0.115 | ND | 0.115 | 0.001 | 0.618 |
| 7 | T06 | 0.598 | 0.583 | 0.619 | 0.600 | 0.018 | 3.014 |
| 8 | T07 | 0.471 | 0.484 | 0.529 | 0.495 | 0.030 | 6.153 |
| 9 | T08 | 0.660 | 0.723 | 0.717 | 0.700 | 0.035 | 4.967 |
| 10 | T10 | 0.884 | 0.893 | 0.874 | 0.884 | 0.010 | 1.076 |
| 11 | T12 | 0.786 | 0.783 | 0.830 | 0.800 | 0.026 | 3.290 |
| 12 | T12 | 0.812 | 0.803 | 0.826 | 0.814 | 0.012 | 1.424 |
| 13 | L04 | 0.775 | 0.773 | 0.773 | 0.774 | 0.001 | 0.149 |
| Motor Incomplete (ASIA D) | |||||||
| 14 | C06 | 1.226 | 1.232 | 1.118 | 1.192 | 0.064 | 5.382 |
| 15 | C06 | 0.815 | 0.825 | 0.836 | 0.825 | 0.011 | 1.273 |
| 16 | C05 | 0.617 | 0.606 | ND | 0.612 | 0.008 | 1.272 |
| 17 | T12 | 0.443 | 0.446 | 0.465 | 0.451 | 0.012 | 2.643 |
| 18 | T12 | 0.979 | 0.88 | 0.922 | 0.927 | 0.050 | 5.360 |
| 19 | L02 | 0.907 | 0.882 | 0.890 | 0.893 | 0.013 | 1.430 |
| 20 | L02 | 1.018 | 0.998 | 0.987 | 1.001 | 0.016 | 1.570 |
DISCUSSION
In this study we report the precision of BMD assessment by DXA scan at the distal femur and the proximal tibia in persons with chronic SCI. It is suggested that at least 30 degrees of freedom be achieved in a DXA scan precision study 13, and in this study there are 38 degrees of freedom at each site. The findings reported for the distal femur are consistent with those reported at traditional DXA scan sites (precision of 1.5-3.0% for the lumbar spine and 1.4-2.3% for the proximal femur) in the able-bodied 14. We found that scanning is more precise at the distal femur than at the proximal tibia and hypothesize that this is due to variability in the spatial geometry of the proximal tibia in contrast to the distal femur and variability in reproducibly repositioning the knee.
An assessment of precision is necessary to define true biological differences in BMD over time. It has been suggested that DXA scan precision studies conducted on the same day may overestimate precision. Leslie et al report less precision in subjects undergoing scanning of the hip and spine on different days as compared to repeat scanning on the same day 15. The authors speculated that the reasons for variation were attributable to small day-to-day calibration shifts and differences in clothing. The largest variation in results was obtained when scanning the spine. Since DXA signal passes through the abdomen, variation was also attributed to day-to-day changes in abdominal contents related to gut peristalsis and meals (called bowel “commotion”). We suggest that scanning the knee is free of many of these sources of variation inherent in scanning the spine and conducting a precision study on the same day as in our report would eliminate the variability due to differences in clothing worn on different days.
We found a wide range of BMD values at the distal femur and proximal tibia, some far below the BMD of 0.6 grams/cm2 suggested by Garland as the bone fracture threshold in SCI 16. Fracture risk based on BMD has not been clearly defined in this population, and the extent of longitudinal bone loss following SCI is not known. However, the mean time to first fracture is reported to be 9 years post-injury 17, 18; therefore, ongoing chronic bone loss years after acute injury is clinically relevant. Standardized DXA scanning protocols for the knee will advance the diagnosis and treatment of osteoporosis in SCI.
Unfortunately, there is currently a wide care gap in the treatment of SCI-induced osteoporosis. Accelerated bone loss triggered by SCI is a well-acknowledged event; however, diagnosis and prevention are limited in part by the lack of readily available SCI-appropriate DXA scanning protocols. Furthermore, limited data exist regarding the efficacy of medications and other interventions aimed at slowing bone loss and preventing fractures. Improvements in the clinical care of SCI-induced osteoporosis will rely on the development of standardized protocols for BMD determination as described in this report.
LIMTATIONS
This is the first report describing efforts to assess the precision of DXA scanning of the knee in SCI using methods suggested by the International Society for Clinical Densitometry (ref: http://www.iscd.org/visitors/pdfs/ISCD2007OfficialPositions.pdf). However, there are several important limitations to consider. The DXA scans were read consecutively and not read blindly, and due to SCI subject repositioning did not include complete dismounting and remounting of the scanning table, factors potentially increasing precision. Internal rotation of the leg was limited by securing the limb at the ankle only, potentially decreasing precision. In future work, we plan to explore additional methods of stabilizing the knee that may result in greater precision, particularly at the proximal tibia, and replicate our findings in additional persons..
CONCLUSIONS
The determination of BMD at the distal femur appears to be more precise than at the proximal tibia and is suggested as a preferred skeletal site for the assessment of BMD in the lower extremity in chronic SCI. However, since rates of BMD loss are unknown in chronic SCI the lower precision at the tibia may be sufficient to detect significant losses in BMD over time. We conclude that the distal femur skeletal site can be used for SCI-specific DXA scanning protocols and the longitudinal assessment of BMD at the knee to determine the risk of fracture in chronic SCI.
ACKNOWLEDGEMENTS
We wish to acknowledge Joda Alian for her kind assistance in manuscript editing and preparation.
The project reported/outlined here was supported by the Office of Research and Development, Health Services R&D Service, Quality Enhancement Research Initiative RRP-07-312, K12 HD001097-08 (Morse), RO1HD42141 (Garshick), and R21HD057030 (Morse).
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
SUPPLIERS
GE Healthcare Inc., Waukesha, WI
All authors have no conflict of interest.
REFERENCES
- (1).Szollar SM, Martin EM, Sartoris DJ, Parthemore JG, Deftos LJ. Bone mineral density and indexes of bone metabolism in spinal cord injury. Am J Phys Med Rehabil. 1998 January;77(1):28–35. doi: 10.1097/00002060-199801000-00005. [DOI] [PubMed] [Google Scholar]
- (2).Morse LR, Battaglino RA, Stolzmann KL, et al. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int. 2008 June 26; doi: 10.1007/s00198-008-0671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dauty M, Perrouin VB, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000 August;27(2):305–9. doi: 10.1016/s8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- (4).Demirel G, Yilmaz H, Paker N, Onel S. Osteoporosis after spinal cord injury. Spinal Cord. 1998 December;36(12):822–5. doi: 10.1038/sj.sc.3100704. [DOI] [PubMed] [Google Scholar]
- (5).Frey-Rindova P, de Bruin ED, Stussi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000 January;38(1):26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]
- (6).Garland DE, Stewart CA, Adkins RH, et al. Osteoporosis after spinal cord injury. J Orthop Res. 1992 May;10(3):371–8. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- (7).Roberts D, Lee W, Cuneo RC, et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab. 1998 February;83(2):415–22. doi: 10.1210/jcem.83.2.4581. [DOI] [PubMed] [Google Scholar]
- (8).Eser P, Frotzler A, Zehnder Y, Schiessl H, Denoth J. Assessment of anthropometric, systemic, and lifestyle factors influencing bone status in the legs of spinal cord injured individuals. Osteoporos Int. 2005 January;16(1):26–34. doi: 10.1007/s00198-004-1638-x. [DOI] [PubMed] [Google Scholar]
- (9).Ragnarsson KT, Sell GH. Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil. 1981 September;62(9):418–23. [PubMed] [Google Scholar]
- (10).Shields RK, Schlechte J, Dudley-Javoroski S, et al. Bone mineral density after spinal cord injury: a reliable method for knee measurement. Arch Phys Med Rehabil. 2005 October;86(10):1969–73. doi: 10.1016/j.apmr.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Garshick E, Kelley A, Cohen SA, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005 July;43(7):408–16. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Marino RJ, Barros T, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26(Suppl 1):S50–S56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- (13).Bonnick SL, Johnston CC, Jr., Kleerekoper M, et al. Importance of precision in bone density measurements. J Clin Densitom. 2001;4(2):105–10. doi: 10.1385/jcd:4:2:105. [DOI] [PubMed] [Google Scholar]
- (14).Tothill P, Hannan WJ. Precision and accuracy of measuring changes in bone mineral density by dual-energy X-ray absorptiometry. Osteoporos Int. 2007 November;18(11):1515–23. doi: 10.1007/s00198-007-0382-4. [DOI] [PubMed] [Google Scholar]
- (15).Leslie WD. Factors affecting short-term bone density precision assessment and the effect on patient monitoring. J Bone Miner Res. 2008 February;23(2):199–204. doi: 10.1359/jbmr.071019. [DOI] [PubMed] [Google Scholar]
- (16).Garland DE, Stewart CA, Adkins RH, et al. Osteoporosis after spinal cord injury. J Orthop Res. 1992 May;10(3):371–8. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- (17).Eser P, Frotzler A, Zehnder Y, Schiessl H, Denoth J. Assessment of anthropometric, systemic, and lifestyle factors influencing bone status in the legs of spinal cord injured individuals. Osteoporos Int. 2005 January;16(1):26–34. doi: 10.1007/s00198-004-1638-x. [DOI] [PubMed] [Google Scholar]
- (18).Frey-Rindova P, de Bruin ED, Stussi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000 January;38(1):26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]