Abstract
We have reported previously that murine bone marrow-derived dendritic cells (DC) pulsed with whole tumor lysates can mediate potent antitumor immune responses both in vitro and in vivo. Because successful therapy was dependent on host immune T cells, we have now evaluated whether the systemic administration of the T cell stimulatory/growth promoting cytokine interleukin-2 (IL-2) could enhance tumor lysate-pulsed DC-based immunizations to further promote protective immunity toward, and therapeutic rejection of, syngeneic murine tumors. In three separate approaches using a weakly immunogenic sarcoma (MCA-207), the systemic administration of nontoxic doses of recombinant IL-2 (20,000 and 40,000 IU/dose) was capable of mediating significant increases in the potency of DC-based immunizations. IL-2 could augment the efficacy of tumor lysate-pulsed DC to induce protective immunity to lethal tumor challenge as well as enhance splenic cytotoxic T lymphocyte activity and interferon-γ production in these treated mice. Moreover, treatment with the combination of tumor lysate-pulsed DC and IL-2 could also mediate regressions of established pulmonary 3-day micrometastases and 7-day macrometastases as well as established 14- and 28-day s.c. tumors, leading to either significant cure rates or prolongation in overall survival. Collectively, these findings show that nontoxic doses of recombinant IL-2 can potentiate the antitumor effects of tumor lysate-pulsed DC in vivo and provide preclinical rationale for the use of IL-2 in DC-based vaccine strategies in patients with advanced cancer.
Dendritic cells (DC) are potent antigen-presenting cells that can elicit primary and boost secondary immune responses to foreign antigens (1, 2). In a variety of settings, these specialized antigen-presenting cells can induce both the generation and proliferation of specific cytotoxic T lymphocyte (CTL) and T helper cells via antigen presentation by major histocompatibility complex (MHC) class I and class II molecules, respectively. We (3–6) and others (7–9) have described the induction of MHC class I and class II specific T cell responses following stimulation with tumor antigen(s)-pulsed DC in vitro and in vivo. Because of these properties, much attention has been directed toward the use of DC in vaccine strategies for the treatment of cancer. In this regard, DC pulsed with tumor-associated antigen(s) in various forms, including as whole cell lysates (3–6), peptides (7, 8), proteins (10), RNA (11), or DNA (12, 13), has been studied for antitumor effects in experimental animals. In these models, immunization with tumor antigen(s) presented by DC has shown much promise in effectively priming the cellular immune response as well as in eliciting tumor regression in vivo. Of importance, host-derived CD8+ and, to a lesser extent, CD4+ T cells have been shown to play the central role in the antitumor effects mediated by DC-based vaccines (6).
We have been studying approaches to enhance the therapeutic activity of murine and human DC, which involve the use of various exogenous cytokines (14). Such enhancement of DC function by cytokines can potentially occur at two levels, namely directly on DC during their generation and maturation (14, 15) and indirectly through actions on the elicited T cell response. Because successful tumor therapy with DC-based vaccines has been shown in vivo to be dependent on host immune T cells, we have now evaluated the systemic administration of interleukin 2 (IL-2) for its capacity to enhance tumor-pulsed DC activity in promoting protective immunity to, and therapeutic rejection of, weakly immunogenic tumors.
IL-2 is a potent stimulator of lymphocyte proliferation and augments the activity of CTL (16) either alone or in combination with other cytokines (17). The systemic administration of IL-2 in vivo also has a broad range of immunologic effects, including the induction of specific T helper cells, natural killer and lymphokine-activated killer (LAK) cells, and autoantibody production in congenitally T cell-deficient mice (16, 18), and the enhancement of the restoration of immune function in irradiated or cyclophosphamide-treated animals (16, 18). Systemic injection of IL-2 is capable of specifically enhancing alloimmune responses in either normal or primed mice (19) and can enhance the antitumor activity of adoptively transferred, LAK cells (20) or specifically immune T lymphocytes (21, 22). Of importance, IL-2 alone can mediate regression of selected, established murine (23, 24) and human (25) tumors by mechanisms that involve the stimulation of in vivo lymphoid proliferation in tissues (26) and by the activation of host-derived T cells (24).
Based on these studies, we have now explored potential therapeutic regimens based on the combined administration of tumor-pulsed DC and IL-2. Collectively, our results show that IL-2 can potentiate the antitumor effects of tumor-pulsed DC in vivo during both primary immunization and treatment of established tumors. These studies are designed to provide rationale for the development of clinical trials in humans by using the administration of IL-2 in conjunction with DC-based vaccine strategies.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old female C57BL/6 (denoted B6) mice were purchased from The Jackson Laboratory and housed at the Animal Maintenance Facility of the University of Michigan Medical Center. The animals were used for experiments between 10 to 14 weeks of age.
Culture Medium.
Complete medium (CM) consisted of RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 0.1 mM nonessential amino acids, 1 μM sodium pyruvate, 2 mM fresh l-glutamine, 100 μg/ml streptomycin, 100 units/ml penicillin, 50 μg/ml gentamicin, 0.5 μg/ml fungizone (all from GIBCO) and 5 × 10−5 M 2-mercaptoethanol (Sigma).
Recombinant Cytokines.
Recombinant cytokines were used at the following concentrations, diluted in CM: recombinant murine granulocyte/macrophage colony-stimulating factor (GM-CSF), 10 ng/ml (specific activity: ≥5 × 106 units/mg; Immunex); and recombinant murine IL-4, 10 ng/ml (specific activity: 2.8 × 108 units/mg; Schering-Plough Research Institute). Recombinant human IL-2 was kindly supplied by Chiron and had a specific activity of 18 × 106 IU/mg protein. In all experiments herein, IL-2 doses are given in IU. One IU is the equivalent of 6 units (U) of IL-2 reported in our earlier studies (23, 24).
Tumors.
The MCA-102 and -207 tumors are weakly immunogenic, 3-methylcholanthrene (MCA)-induced fibrosarcomas of B6 origin (20, 24). Tumors were maintained in vivo by s.c. injection in syngeneic mice and were used within the seventh transplant passage. Tumor cell suspensions were prepared from solid tumors by using a mixture of hyaluronidase, DNase, and collagenase (all from Sigma), as described (20, 23, 24). All tumor cells were subsequently cultured in CM before use, as described (3, 4).
Tumor Lysate Pulsing of DC.
DC were obtained from 7-day cultures of bone marrow cells maintained in GM-CSF and IL-4, as described (6, 27). DC were incubated with freeze-thawed tumor lysates at a ratio of 3 tumor cell equivalents to 1 DC (i.e., 3:1) in CM (3, 4, 6). After an 18-hr incubation, DC were harvested, irradiated with 2,000 rad (Gamma Cell 1000; Nordion, Kanata, Canada), washed twice in Hanks’ balanced salt solution (HBSS; GIBCO), and resuspended in HBSS for further studies.
Primary Immunization.
Normal B6 mice were immunized s.c. in the right flank with 1 × 106 MCA-207 tumor lysate-pulsed DC twice at 7-day intervals. IL-2 was given i.p. twice daily at 20,000 IU in 1 ml HBSS for 5 days consecutively after each immunization. Control groups of mice received either no treatment (HBSS), tumor lysate alone, unpulsed DC alone, tumor lysate-pulsed DC alone, or IL-2 alone. Mice were then rechallenged 12 days after the last immunization with a lethal dose of either 3 × 105 MCA-207 or 2 × 105 of the unrelated MCA-102 viable tumor cells by s.c. injections into the left flank. Injected tumor cells were >95% viable as determined by trypan blue exclusion. The size of the tumors was assessed in a blinded, coded fashion twice weekly and recorded as tumor area (in mm2) by measuring the largest perpendicular diameters with vernier calipers. Data are reported as the average tumor area ±SEM; five or more mice/group.
Treatment of Established Pulmonary Metastases.
B6 mice received 1.5 × 105 MCA-207 viable tumor cells intravenously in the lateral tail vein to establish pulmonary metastases, as described (6, 20, 23, 24). The mice were then immunized s.c. with 1 × 106 MCA-207 tumor lysate-pulsed DC twice on days 3 and 7 (treatment of 3-day pulmonary metastases) or days 7 and 11 (treatment of 7-day pulmonary metastases) after tumor injection and were killed on days 14 or day 15. IL-2 was given i.p. twice daily at 20,000 IU in 1 ml HBSS for treatment of 3-day established pulmonary metastases or at 40,000 IU in 1 ml HBSS for treatment of 7-day established pulmonary metastases from days 3 to 9 or days 7 to 13, respectively. Control groups of mice received either no treatment (HBSS), tumor lysate alone, unpulsed DC alone, tumor lysate-pulsed DC alone, or IL-2 alone. Pulmonary metastases were enumerated in a blinded, coded fashion after insufflation of the lungs with India ink and fixation with Fekette’s solution, as described (20, 23, 24). Data are reported as the mean number of metastases ± SEM; five or more mice/group. In some experiments, the mice receiving combined therapy were immunized three times on days 3, 7, and 11 (for treatment of 3-day pulmonary metastases) or days 7, 10, and 13 (treatment of 7-day pulmonary metastases) after tumor injection, respectively, plus 3 consecutive days of IL-2 after each immunization and were then followed for survival. Control groups of mice received either no treatment, tumor lysate plus IL-2, unpulsed DC alone plus IL-2, or IL-2 alone.
Treatment of Established s.c. Tumor.
A total of 2 × 105 MCA-207 tumor cells were injected s.c. in the right flank of B6 mice. Animals subsequently bearing established day 14 or day 28 MCA-207 tumors were treated with three weekly injections of 1 × 106 MCA-207 tumor lysate-pulsed DC in the left flank. IL-2 was given i.p. twice daily at 20,000 IU in 1 ml HBSS for 5 consecutive days after each immunization. Control groups of mice received either no treatment (HBSS), MCA-207 tumor lysate-pulsed DC alone, or IL-2 alone. The size of the tumors was assessed in a blinded, coded fashion twice weekly and recorded as tumor area (in mm2), as described above. In some experiments, survival was followed and recorded as the percentage of surviving animals over time (in days) after tumor injection.
CTL Induction and Cytotoxicity/Interferon-γ (IFN-γ) Assays.
Spleens were removed from mice receiving either MCA-207 tumor lysate-pulsed DC plus IL-2, MCA-207 tumor lysate-pulsed DC alone, IL-2 alone, or no treatment (HBSS). Splenocytes were harvested 7 days after the final IL-2 (or HBSS) dose and were treated with ammonium chloride-potassium lysing buffer for 1 min to deplete erythrocytes and washed twice with HBSS. These splenocytes (1 × 106 cells/ml) were cultured for 5 days in vitro with irradiated (15,000 rad) MCA-207 tumor cells (1 × 105 cells/ml) in 24-well culture plates (Costar) at a responder-to-stimulator ratio of 10:1. The total volume per well was 2 ml of CM. After 5 days of restimulation, the cells were tested for cytolytic activity against MCA-207 (and the unrelated MCA-102) tumor target cells in a 6-hr 51Cr release assay, as described previously (6, 20, 24). For IFN-γ release, groups of mice were treated with MCA-207 lysate-pulsed DC plus IL-2, MCA-207 lysate-pulsed DC alone, IL-2 alone, or were left untreated. Spleens were removed from mice 12 days after the last immunization. Nylon-wool separated, 1 × 106 T cells were mixed with either 1 × 105 MCA-207 (or unrelated MCA-102) tumor lysate-pulsed DC or unpulsed DC per 2 ml CM in 24-well tissue culture plates (Costar). After 48 hr, culture supernatants were collected for measurement of murine IFN-γ release by standard ELISA (PharMingen), which is reported as mean units (U)/ml ± SEM of triplicate samples.
Statistical Analysis.
Statistical determinations were made by Mann-Whitney U test. Survival analysis used the Breslow modification of the Kaplan–Meier test. Two-sided P values are presented in all experiments, and significance was defined as P < 0.05. No mice were excluded from the statistical evaluations.
RESULTS AND DISCUSSION
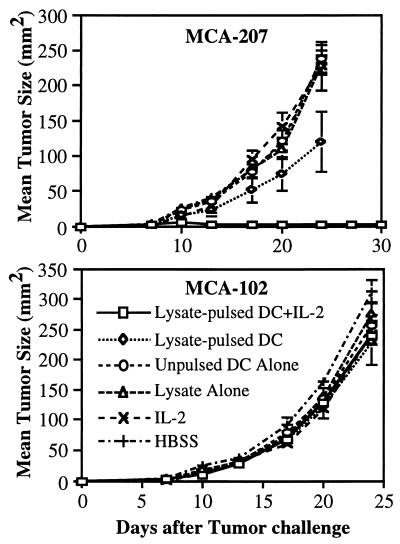
We have recently shown that immunization with MCA-207 tumor lysate-pulsed DC protects B6 mice from a subsequent challenge with viable, syngeneic MCA-207 sarcoma cells (6). To determine whether the systemic administration of IL-2 could enhance immunization by tumor lysate-pulsed DC in this model, B6 mice were subsequently challenged s.c. with a higher tumor cell dose (3-fold) than in our previous study. As shown in Fig. 1, Upper, although immunization with tumor lysate-pulsed DC alone resulted in a significantly slower growth rate compared with control groups (P < 0.01), all mice developed progressively growing tumors upon challenge with the higher dose injection of MCA-207 sarcoma cells. In contrast, all mice treated with the combination of tumor lysate-pulsed DC plus IL-2 were protected from tumor growth and remained disease-free. Immunization of mice with MCA-207 tumor lysate-pulsed DC with or without the systemic administration of IL-2 had no effect on the growth of the unrelated MCA-102 sarcoma on s.c. challenge (Fig. 1, Lower), thus demonstrating the specificity of DC-based immunization.
Figure 1.
Systemic administration of IL-2 can enhance the efficacy of tumor-lysate pulsed DC to induce specific protective immunity to lethal tumor challenge. As described, mice were immunized twice s.c. in the right flank with MCA-207 tumor lysate-pulsed DC and received i.p. injections of IL-2 after each immunization. Control groups of mice received either HBSS, tumor lysate alone, unpulsed DC alone, MCA-207 tumor lysate-pulsed DC alone, or IL-2 alone. All mice were then rechallenged with a lethal dose of either MCA-207 (Upper) or the unrelated MCA-102 (Lower) viable tumor cells by s.c. injection in the left flank. Data are reported as the average tumor area ± SEM of five or more mice/group.
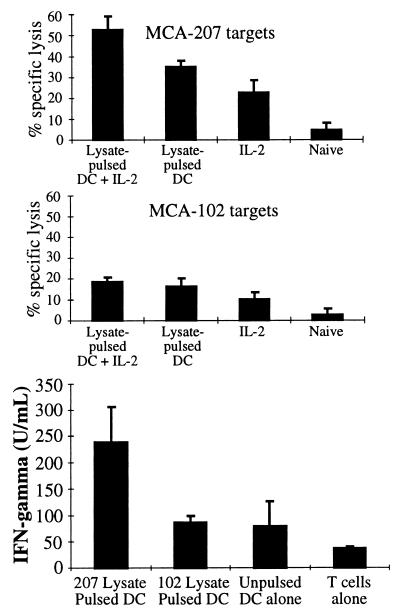
We also examined the level of CTL activity and IFN-γ production in the spleens of these treated and control mice. As shown in Fig. 2, Top, CTL obtained from mice treated with the combination of MCA-207 tumor lysate-pulsed DC plus IL-2 demonstrated a significantly higher level of specific lysis of the MCA-207 tumor target compared with groups receiving either treatment alone (P < 0.05). In contrast, these CTL demonstrated low level lytic activity toward the unrelated MCA-102 tumor target (Fig. 2, Middle). Splenic T cells isolated from mice treated with the combination therapy also produced greater amounts of IFN-γ (>200 units/ml) when specifically stimulated in vitro with MCA-207 tumor lysate-pulsed DC (Fig. 2, Bottom). Splenic T cells obtained from mice treated with MCA-207 lysate-pulsed DC alone, IL-2 alone, or left untreated produced IFN-γ at levels between ≈50 units/ml to undetectable (data not shown). Although GM-CSF was also specifically produced by splenic T cells of mice immunized with MCA-207 tumor lysate-pulsed DC, as reported previously (6), coadministration of IL-2 did not result in enhanced production of this cytokine (data not shown). Collectively, these findings demonstrated that the systemic administration of IL-2 could enhance the efficacy of tumor lysate-pulsed DC immunization in vivo to induce a higher level of CTL activity, IFN-γ production, and protective immunity to lethal tumor cell challenge.
Figure 2.
Systemic administration of IL-2 can enhance the capacity of MCA-207 tumor lysate-pulsed DC to induce tumor-specific CTL (Top and Middle) and IFN-γ production (Bottom) in vivo. Mice were immunized twice weekly following the schedule described in Fig. 1. Spleens were harvested 7 days after the final IL-2 (or HBSS) injection from mice treated with either HBSS, MCA-207 tumor lysate-pulsed DC plus IL-2, MCA-207 tumor lysate-pulsed DC alone, or IL-2 alone. Details of the generation and testing of these CTL are provided in Materials and Methods. Values are the mean ± SEM of triplicate wells at a 100:1 effector-to-target ratio. For measurement of IFN-γ production (Bottom), splenic T cells from the treated mice were stimulated in vitro as described. Culture supernatants were harvested 48 hr later and evaluated for IFN-γ levels by ELISA (in units/ml; mean ± SEM of triplicate samples).
To determine the therapeutic potential of immunization with the combination of tumor lysate-pulsed DC plus IL-2, we attempted to induce tumor rejection in mice with established tumors. In a 3-day treatment model, B6 mice harboring pulmonary micrometastases were treated with the combination of tumor lysate-pulsed DC and nontoxic doses of IL-2; comparisons were made to control groups of mice receiving either no treatment or treatment with single agents alone. As shown in Table 1, mice receiving tumor lysate-pulsed DC alone demonstrated ≈90% reduction in the number of established 3-day pulmonary metastases compared with the control groups (P < 0.01), as previously reported (6). Nevertheless, treatment with the combination of tumor lysate-pulsed DC and IL-2 resulted in a further reduction in the number of lung nodules. In this regard, three of five (60%) mice treated with the combination had no detectable pulmonary metastases, and the remaining two (40%) mice had fewer than 10 nodules (P < 0.01). The addition of a similar course of IL-2 injections to unpulsed DC did not significantly alter the very modest antitumor effect of either alone (data not shown).
Table 1.
Systemic administration of IL-2 can enhance the therapeutic efficacy of tumor lysate-pulsed DC against established pulmonary metastases
| Pulmonary metastases | Treatment* | No. of metastases (range)† | Mean |
|---|---|---|---|
| 3 day | HBSS | ≥250 | 250 |
| IL-2 | 143–167 | 155‡ | |
| Lysate | 243–250 | 249 | |
| Unpulsed DC | 217–250 | 249 | |
| TP-DC | 16–42 | 28‡ | |
| TP-DC + IL-2 | 0–4 | 1‡ | |
| 7 day | HBSS | ≥250 | 250 |
| IL-2 | 141–186 | 159‡ | |
| Lysate | ≥250 | 250 | |
| Lysate + IL-2 | 143–192 | 163‡ | |
| Unpulsed DC | ≥250 | 250 | |
| TP-DC | 138–184 | 155‡ | |
| TP-DC + IL-2 | 4–21 | 10‡ |
Number of MCA-207 tumor cells injected i.v. was 1.5 × 105 A total of 1 × 106 MCA-207 tumor lysate-pulsed DC were injected twice on days 3 and 7 or days 7 and 11 after tumor injection. IL-2 was administered i.p. twice daily at 20,000 IU/dose from days 3–9 or 40,000 IU/dose from days 7 to 13.
Mice were euthanized at day 14 (3-day model) or day 15 (7-day model).
Mann-Whitney U test; P < 0.01 (compared to group receiving HBSS).
We also evaluated the therapeutic efficacy of the combination of tumor lysate-pulsed DC and nontoxic doses of IL-2 on grossly-visible, 7-day pulmonary macrometastases from the MCA-207 sarcoma. Treatment with either tumor lysate-pulsed DC alone, IL-2 alone, or tumor lysate plus IL-2 could mediate a significant, albeit modest, reduction (by ≈25%) in the number of 7-day pulmonary metastases compared with HBSS, tumor lysate alone, or unpulsed DC alone (Table 1). By contrast, the combination of tumor lysate-pulsed DC and IL-2 resulted in a demonstrable antitumor effect. All mice treated with the combination had fewer than 20 nodules (P < 0.01). Similar results were obtained in a second tumor model, namely the MT-901 breast tumor in BALB/c mice (data not shown).
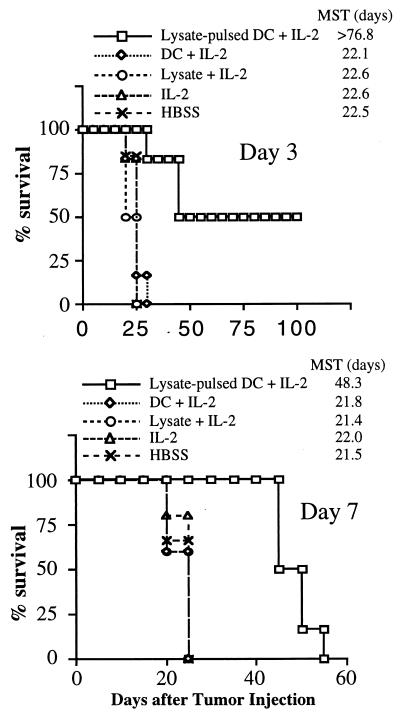
In a separate series of experiments, we sought to determine the therapeutic impact of the combination of tumor lysate-pulsed DC and IL-2 on disease-free survival of mice harboring 3-day or 7-day pulmonary nodules at the time of treatment. The results are shown in Fig. 3. In the 3-day model (Fig. 3, Upper), three of six (50%) mice treated with the combination survived for at least 100 days and had no evidence of tumor when the experiment was terminated [mean survival time (MST) > 77 days, P < 0.01)]. In the 7-day model (Fig. 3, Lower), whereas all control groups of mice survived for <25 days, all mice treated with the combination survived for at least 40 days (MST = 48 days, P < 0.01). Collectively, these findings indicated that treatment with the combination of tumor lysate-pulsed DC and nontoxic doses of IL-2 could mediate antitumor effects on established visceral metastatic disease, leading to increased survival, and, in some cases, to cures.
Figure 3.
Treatment with the combination of tumor lysate-pulsed DC and IL-2 can prolong survival and result in cures of mice bearing established pulmonary metastases. Mice harboring MCA-207 lung metastases were immunized three times on days 3, 7, and 11 (Upper) or days 7, 10, and 13 (Lower) after tumor injection. IL-2 was given i.p. twice daily at 20,000 IU/dose (Upper) or 40,000 IU/dose (Lower) for 3 consecutive days after each immunization. Control groups of mice received HBSS, tumor lysate plus IL-2, unpulsed DC plus IL-2, or IL-2 alone. Survival was monitored over time after tumor injection, and the MST (in days) was determined.
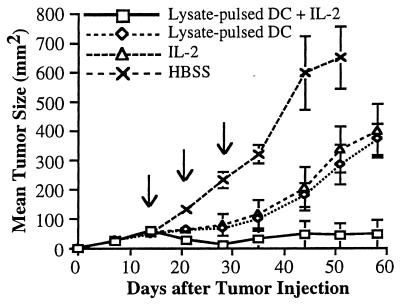
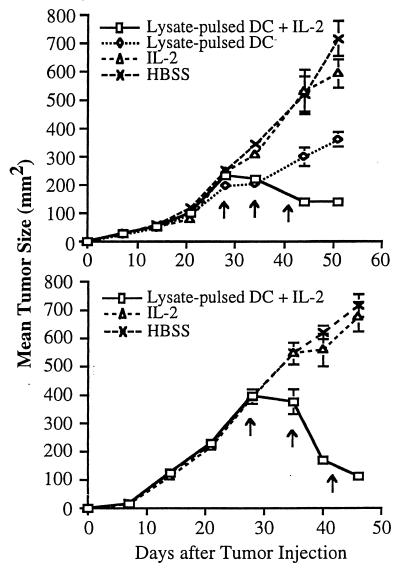
Two treatment models were studied to evaluate the capacity of the combination of tumor lysate-pulsed DC and IL-2 to affect the growth of s.c. MCA-207 tumors. First, 2 × 105 MCA-207 sarcoma cells were injected s.c. in the flank of B6 mice, and 14 days later combination therapy was given as detailed in Materials and Methods. Control groups of mice received HBSS, MCA-207 tumor lysate-pulsed DC alone, or IL-2 alone. As shown in Fig. 4, mice treated with tumor lysate-pulsed DC alone or IL-2 alone experienced significant reductions in tumor growth compared with the control, untreated group; however, no mice was rendered tumor-free. In contrast, mice receiving the combination therapy exhibited a demonstrable antitumor effect, with the majority of tumors undergoing regression. Tumor growth was suppressed by 7 days after the first cycle of combination therapy. Fig. 5 demonstrates that 80% of mice that had received the combination of tumor lysate-pulsed DC and IL-2 survived long-term without evidence of tumor recurrence (MST > 134 days, P < 0.01).
Figure 4.
Treatment of mice with tumor lysate-pulsed DC and the systemic administration of IL-2 results in regression of established, 14-day s.c. tumor. A total of 2 × 105 syngeneic MCA-207 sarcoma cells were injected s.c. in the right flank. Mice bearing 14-day tumors were then treated weekly three times with 1 × 106 MCA-207 tumor lysate-pulsed DC in the left flank. IL-2 was given i.p. twice daily at 20,000 IU/dose for 5 consecutive days after each immunization. Control groups of mice received HBSS, MCA-207 tumor lysate-pulsed DC alone, or IL-2 alone. The size of the tumors was assessed in a blinded, coded fashion twice weekly and recorded as tumor area (in mm2).
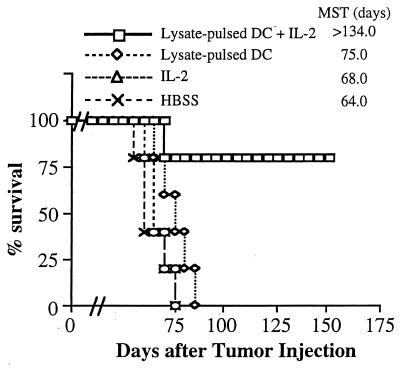
Figure 5.
Combination therapy with tumor lysate-pulsed DC plus IL-2 can prolong survival and result in cures of mice bearing established, 14-day s.c. tumor. Mice were treated 14 days after tumor injection as described in Fig. 4. Survival was monitored over time after tumor injection, and the MST (in days) was determined.
Second, the effectiveness of the combination of tumor lysate-pulsed DC and IL-2 was also evaluated on day 28 MCA-207 tumors. As shown in Fig. 6, combination therapy led to partial regression of these established s.c. tumors (P < 0.01). Although no mice with this tumor burden were rendered completely tumor-free after three cycles of the combined therapy, tumor growth was nevertheless suppressed and the tumor size remained static for >5 weeks after treatment. Mice receiving the combination of tumor lysate-pulsed DC and IL-2 also survived longer (MST = 91 days, P < 0.01) compared with mice receiving IL-2 alone (MST = 65 days), tumor lysate-pulsed DC alone (MST = 75 days), or left untreated (MST = 64 days) (data not shown). Thus, these findings extend those obtained in the pulmonary metastasis model and further demonstrate that the systemic administration of IL-2 can mediate enhancement of the antitumor therapeutic effect of tumor lysate-pulsed DC against established s.c. growing tumors.
Figure 6.
Treatment of mice with tumor lysate-pulsed DC and the systemic administration of IL-2 results in partial regression of established, 28-day s.c. tumor. Mice were treated 28 days after tumor injection as described in Fig. 4. The size of the tumors was assessed in a coded, blinded fashion and recorded as tumor area (in mm2) by measuring the largest perpendicular diameters. Data are reported as the average tumor area ± SEM of five or more mice/group.
In this current study, we demonstrated that the systemic administration of recombinant IL-2, at nontoxic doses, could augment the in vivo antitumor effects of tumor lysate-pulsed DC injections. We first reported that whole tumor lysates could serve effectively as a source of tumor-associated antigen(s) to elicit specific T cell responses in vitro when processed and presented by DC (3, 4). In vivo, s.c. injections of tumor lysate-pulsed DC from the spleen or bone marrow could result in effective immune priming of naive mice to reject subsequent lower dose challenge with viable cells from a syngeneic melanoma (5), sarcoma (6), and breast carcinoma (6). Moreover, immunization of mice with tumor lysate-pulsed DC could mediate regression of established, 3-day pulmonary micrometastases from both a sarcoma and a breast carcinoma (6). Recently, in a pilot study, human DC pulsed with autologous melanoma lysate could induce antigen-specific immunity during DC vaccination of patients and result in objective clinical responses with the apparent absence of physical signs of autoimmunity (28). In the murine models, successful immune priming and tumor regression were dependent on the participation of host-derived T cells (6). Because of this necessity for T cells, it appeared reasonable to hypothesize based on our earlier work (23, 24) that the systemic administration of IL-2 may augment the antitumor effects of tumor lysate-pulsed DC-based vaccine approaches. To measure the potential enhancing effect(s) of IL-2 on tumor lysate-pulsed DC therapy, we either challenged mice with a larger dose of viable tumor cells following immune priming or attempted to treat more advanced disease in mice that were harboring established tumor burdens in the lungs or at a s.c. site.
We and others have shown previously that the systemic administration of recombinant IL-2 alone could mediate profound antitumor effects in mice when administered at high doses (23, 24). Similar results were obtained in certain advanced cancer patients as well (17, 29). However, dose-limiting toxicities of high-dose IL-2 administration resulted in both animals and humans, which have hampered its wider use as a single agent in cancer therapy (29–31). IL-2 toxicity is manifested by a vascular leak syndrome during and following the elicitation of lymphoid cell proliferation and activation of LAK cell activity in situ (30). Given this limitation, attempts have been made to combine IL-2 at lower doses with other cytokines, chemotherapeutic agents, and radiation (17, 32). More recently, IL-2 has been shown to enhance the therapeutic efficacy of a peptide-based vaccine approach in patients with advanced melanoma (33). In our current study, the systemic administration of IL-2 at doses lower than those required for the successful treatment of established tumors in mice as a single agent alone (23, 24), resulted in regression of established tumor by the enhancement of tumor lysate-pulsed DC immunization. Of importance, animals receiving the combination of tumor lysate-pulsed DC and IL-2 showed no gross evidence of treatment-related toxicities (i.e., those manifested as abdominal swelling from fluid retention, ruffled fur, tendency to become isolated). The doses of IL-2 utilized in the current study are relatively low because they are 25- to 50-fold below the maximum tolerated dose (MTD) for the mouse (23, 24, 26, 30). Extrapolating this amount of IL-2 to patients would represent, with certain caveats (e.g., species differences in the injection route, scheduling, biodistribution, and clearance of IL-2), doses between 25- and 50-fold below the established MTD for humans as well. Doses of IL-2 in this range (1–2 × 106 IU/m2) are so-called “T cell reconstituting” doses that have been shown to benefit patients with HIV or undergoing bone marrow transplantation (34–37).
In murine models, the therapeutic efficacy of the systemic administration of high-dose IL-2 against several histologically distinct tumors is mediated predominantly by host-derived CD8+ T cells (24). A similar result was obtained when IL-2 gene-modified syngeneic tumor cells were introduced in vivo and were undergoing rejection in immunocompetent mice (38). In vivo studies have demonstrated that a requirement for CD4+ T cell help in generating an antitumor immune response to such tumors could be bypassed by the provision of IL-2 (38, 39). In our previous studies, we showed that the immune priming and therapeutic activity mediated by tumor lysate-pulsed DC were dependent on host-derived CD8+ and, to a lesser extent, CD4+ T cells. Thus, a commonality between the mechanisms of in vivo antitumor activity of IL-2 and tumor lysate-pulsed DC was shown to involve CD8+ T cells. In the current study, the combination of IL-2 and tumor lysate-pulsed DC was shown to elicit greater CTL activity from the spleens of these animals against the MCA-207 sarcoma target cells. We also observed that splenic T cells obtained from mice treated with the combination therapy produced a greater amount of IFN-γ compared with single agent therapy alone following specific stimulation in vitro by MCA-207 tumor lysate-pulsed DC. This finding is of particular interest because both cytolytic and noncytolytic, tumor-specific TIL were shown previously to mediate their antitumor therapeutic efficacy upon adoptive transfer, in part, by specific secretion of IFN-γ in vivo (40).
Further studies are warranted to examine the therapeutic efficacy of the combination of tumor lysate-pulsed DC and IL-2 on other histologically distinct murine tumors that differ in levels of inherent immunogenicity. It also remains to be determined whether or not escalating the number of DC and/or doses of IL-2, beyond those reported in this study, will result in additional therapeutic benefit. Notwithstanding, the results to date provide preclinical rationale for the use of IL-2 in DC-based vaccine strategies in the treatment of patients with advanced cancer.
Acknowledgments
We thank Dr. Elaine Thomas and Kathleen Picha (Immunex Corporation) and Dr. Satwant Narula (Schering-Plough Research Institute) for providing recombinant murine GM-CSF and recombinant murine IL-4, respectively. This work was supported by grants from the National Cancer Institute/National Institutes of Health (1 R01 CA71669, 5 P01 CA59327, and M01-RR00042), from the Department of Defense/U.S. Army (DAMD17-96-1-6103 and DAAG55-97-1-0239), and by a gift from C. J. & E. C. Aschauer.
ABBREVIATIONS
- DC
dendritic cell(s)
- CTL
cytotoxic T lymphocyte
- IL
interleukin
- LAK cell
lymphokine-activated killer cell
- CM
culture medium
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- MCA
3-methylcholanthrene
- HBSS
Hanks’ balanced salt solution
- IFN-γ
interferon-γ
- MST
mean survival time
References
- 1.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Hart D N C. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- 3.Cohen P J, Cohen P A, Rosenberg S A, Katz S I, Mulé J J. Eur J Immunol. 1994;24:315–319. doi: 10.1002/eji.1830240206. [DOI] [PubMed] [Google Scholar]
- 4.Cohen P A, Cohen P J, Rosenberg S A, Mulé J J. Cancer Res. 1994;54:1055–1058. [PubMed] [Google Scholar]
- 5.Geraghty P J, Fields R C, Mulé J J. Surg Forum. 1996;47:459–461. [Google Scholar]
- 6.Fields R C, Shimizu K, Mulé J J. Proc Natl Acad Sci USA. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porgador A, Snyder D, Gilboa E. J Immunol. 1996;156:2918–2926. [PubMed] [Google Scholar]
- 8.Mayordomo J, Zorina T, Storkus W, Zitvogel L, Celluzzi C, Falo L, Jr, Melief C, Ilstad S, Kast W, Deleo A, Lotze M. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 9.Shuler G, Steinman R M. J Exp Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paglia P, Chiodoni C, Rodolfo M, Colombo M P. J Exp Med. 1996;183:317–324. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boczkowski D, Nair S K, Snyder D, Gilboa E. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D., Jr Nat Med. 1996;2:1122–1126. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 13.Manickan E, Kanangat S, Rouse R J, Yu Z, Rouse B T. J Leukocyte Biol. 1997;61:125–134. doi: 10.1002/jlb.61.2.125. [DOI] [PubMed] [Google Scholar]
- 14.Chen B-G, Shi Y, Smith J D, Choi D, Geiger J D, Mulé J J. Blood. 1998;91:4652–4661. [PubMed] [Google Scholar]
- 15.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1116. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotze M T. In: Biologic Therapy of Cancer. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1995. pp. 207–233. [Google Scholar]
- 17.Atkins M B, Tréhu E G, Mier J W. In: Biologic Therapy of Cancer. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1995. pp. 443–466. [Google Scholar]
- 18.Rosenberg S A, Grimm E A, McGrogan M, Doyle M, Kawasaki E, Koths K, Mark D F. Science. 1984;223:1412–1415. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- 19.Hefneider S H, Conlon P J, Henney C S, Gillis S. J Immunol. 1983;130:222–229. [PubMed] [Google Scholar]
- 20.Mulé J J, Shu S, Schwarz S L, Rosenberg S A. Science. 1984;225:1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg S A, Spiess P, Lafreniere R. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 22.Topalian S L. In: Biologic Therapy of Cancer. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1995. pp. 467–486. [Google Scholar]
- 23.Rosenberg S A, Mulé J J, Spiess P J, Reichert C M, Schwarz S L. J Exp Med. 1985;161:1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulé J J, Yang J C, Lafreniere R, Shu S, Rosenberg S A. J Immunol. 1987;139:285–294. [PubMed] [Google Scholar]
- 25.Schwartzentruber D D, Marincola F, Rosenberg S A, Yang J C, Sznol M. In: Biologic Therapy of Cancer. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1995. pp. 235–279. [Google Scholar]
- 26.Ettinghausen S E, Lipford E H, III, Mulé J J, Rosenberg S A. J Immunol. 1985;135:1488–1497. [PubMed] [Google Scholar]
- 27.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nestle F O, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 29.Lotze M T, Chang A E, Seipp C A, Simpson C, Vetto J T, Rosenberg S A. J Am Med Assoc. 1986;256:3117–3124. [PubMed] [Google Scholar]
- 30.Rosenstein M, Ettinghausen S E, Rosenberg S A. J Immunol. 1986;137:1735–1742. [PubMed] [Google Scholar]
- 31.Urba W J, Steis R G, Longo D L, Kopp W C, Maluish A E, Marcon L, Nelson D L, Stevenson H C, Clark J W. Cancer Res. 1990;50:185–192. [PubMed] [Google Scholar]
- 32.McIntosh J K, Mulé J J, Krosnick J A, Rosenberg S A. Cancer Res. 1989;49:1408–1414. [PubMed] [Google Scholar]
- 33.Rosenberg S A, Yang J C, Schwartzentruber D J, Hwu P, Marincola F M, Topalian S L, Restifo N P, Dudley M E, Schwarz S L, Spiess P J, et al. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khatri V P, Fehniger T A, Baiocchi R A, Yu F, Shah M H, Schiller D S, Gould M, Gazzinelli R T, Bernstein Z P, Caligiuri M A. J Clin Invest. 1998;101:1373–1378. doi: 10.1172/JCI2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khatri V P, Baiocchi R A, Bernstein Z P, Caligiuri M A. Cancer J. 1997;3:129–136. [PubMed] [Google Scholar]
- 36.Massumoto C, Benyunes M C, Sale G, Beauchamp M, York A, Thompson J A, Buckner C D, Fefer A. Bone Marrow Transplant. 1996;17:351–356. [PubMed] [Google Scholar]
- 37.Fefer A, Robinson N, Benyunes M C, Bensinger W I, Press O, Thompson J A, Lindgren C. Cancer J. 1997;3:48–53. [PubMed] [Google Scholar]
- 38.Karp S E, Farber A, Salo J C, Hwu P, Jaffe G, Asher A L, Shiloni E, Restifo N P, Mulé J J, Rosenberg S A. J Immunol. 1993;150:896–908. [PMC free article] [PubMed] [Google Scholar]
- 39.Fearon E R, Pardoll D M, Itaya T, Golumbek P, Levitsky H I, Simons H, Karasuyama H, Vogelstein B, Frost P. Cell. 1990;60:397–404. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- 40.Barth R J, Mulé J J, Spiess P J, Rosenberg S A. J Exp Med. 1991;173:647–658. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]