Abstract
Oxidative stress induced by hyperglycemia is a key factor in the development of cardiovascular diseases in diabetes. Thioredoxin (Trx) system, a major thiol antioxidant system, regulates the reduction of intracellular reactive oxygen species (ROS). In this study, we demonstrated that high glucose significantly increased intracellular ROS levels in human aortic endothelial cells (HAECs). Additionally, high glucose reduced the antioxidant activity of thioredoxin. To investigate the mechanisms involved, we found that glucose enhanced the expression of thioredoxin interacting protein (Txnip), a Trx inhibitory protein, through p38 mitogen-activated protein kinase (MAPK). We also showed that glucose regulated Txnip at transcription level and p38 MAPK and forkhead box O1 transcriptional factor (FOXO1) were involved in the process. Taken together, upregulation of Txnip and subsequent impairment of thioredoxin antioxidative system through p38 MAPK and FOXO1 may represent a novel mechanism for glucose-induced increase in intracellular ROS.
Keywords: p38 MAPK, FOXO1, glucose, ROS, thioredoxin interacting protein
Introduction
Hyperglycemia-induced production of reactive oxygen species (ROS) plays a crucial role in the development of diabetic vascular diseases[1]. Excessive production of ROS can directly oxidizes biological macromolecules and impairs vascular structure and functions[2, 3].
Several antioxidant systems critically regulate cellular reduction/oxidation (redox) balance. The ubiquitously presented thiol-reducing thioredoxin (Trx) system, including Trx, Trx reductase and NADPH, is an important antioxidative mechanism [4]. Trx system reduces oxidized cysteine groups on proteins through an interaction with the redox-active center of Trx (Cys-Gly-Pro-Cys) to form a disulfide bond, which in turn can be reduced by Trx reductase and NADPH[5]. Trx also regulates cell signaling molecules such as ASK-1 and is involved in the regulation of a wide variety of biological processes[4, 7]. Thioredoxin interacting protein (Txnip), also known as vitamin D3 upregulated protein-1 (VDUP-1) or thioredoxin binding protein-2 (TBP-2)[6], is an endogenous inhibitor of Trx. Txnip directly interacts with the catalytic center of reduced Trx and inhibits its reducing activity[7, 8]. Trx-Txnip interaction, therefore, plays an important role in the redox regulation[7].
Trx has been shown to be important in cardiovascular protection and dysregulation of this system has been implicated in the development of cardiovascular diseases. Increasing Trx level attenuates myocardial damage induced by ischemia–reperfusion injury[9], whereas overexpression of Txnip sensitizes the cardiomyocytes to oxidative stress induced apoptosis[10]. Furthermore, significantly increased Txnip expression and reduced Trx activity has been observed in diabetic animals[11], which may play an important role in the development of diabetic vascular diseases.
In the present study, we examined the activation of the Trx system in glucose treated endothelial cells. We observed that high glucose upregulated Txnip expression and subsequently inhibited Trx activity. These effects were mediated by activated p38 MAPK and FOXO1 pathway. Our study reports a novel mechanism for glucose-induced increase in intracellular ROS.
Material and Methods
Cell Culture
Primary human aortic endothelial cells (HAECs, Cell Applications, San Diego, CA) were cultured at 37°C in 5% CO2 in EGM-2 medium (Cambrex, East Rutherford, NJ) containing endothelial cell basic medium (EBM), 2% FBS, hydrocortisone, FGF-2, VEGF, IGF-1, EGF, ascorbic acid, GA-1000 and heparin. The cells were transfected with siRNAs, or treated with the p38 MAPK inhibitor PD169316 (100 nM, Calbiochem), or glucose (Sigma) at various concentrations for the time periods indicated in the text.
siRNA-induced Gene Silencing
Silencing gene expression was achieved using specific siRNAs including Txnip siRNA (Snata Cruz Biotechnology, Santa Cruz, California) and FOXO1 siRNA (Dharmacon; Chicago, Illinois). Transfection of HAECs with siRNAs was carried out using LipofectAMINE™ 2000 (Invitrogen, Carlsbad, California) by following the manufacturer’s instruction. Transfected cells were then treated with glucose and the p38 MAPK inhibitor PD169316 at the designated concentrations for the time periods indicated in the text.
Intracellular ROS Detection
Intracellular ROS was determined using the oxidant-sensitive fluorogenic probe CM-H2DCFDA (5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester) from Invitrogen, Carlsbad, California. HAECs were treated with FFAs for 24 hours and washed with PBS. Treated cells were incubated with 5 μM DCFH-DA in serum free medium for 30 minutes at 37°C. The slides were examined with a Leica DMLS epifluorescence microscope equipped with a Leica DC 100 digital camera.; Data were analyzed with Image-Pro Plus V4.5 software (Media Cybernetics, Inc).
Trx Activity Assay
Trx activity was measured using the insulin disulfide reduction assay as described elsewhere.[12] Total cellular protein was extracted with lysis buffer (20mM HEPES pH 7.9, 100 mM KCl, 300mM NaCl, 10 mM EDTA, 0.1% Triton X-100, 1 mg/ml Protease Inhibitor Cocktail III- Calbiochem). Cellular protein extracts were incubated with buffer (50 mM HEPES pH 7.6, 1 mM EDTA, 1 mg/ml BSA, 2 mM DTT) at 37°C for 15 minutes before incubation with Trx reductase (American Diagnostica Inc., Greenwich, CT) in the reaction buffer (20 mM HEPES pH 7.6, 1 mM EDTA, 200 μM NADPH and 0.3mM insulin) at 37°C for 20 minutes. The reaction was terminated by adding stop mix (6 M guanidine HCl, 1 mM DTNB in 0.2M Tris-HCl pH 8.0) and the absorption at 412 nm was measured. TRX activity was compared with standards and expressed as micrograms TRX per milligram total.
Western Blot Analysis
Cell extracts were prepared with lysis buffer (20mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1mM EGTA, 1.0% Triton 100, 2.5 mM sodium pyrophosphate, 1mM b-glycerophosphate. 2 mM phenylmethylsulfonyl fluoride, 1 mg/ml leupeptin, and 5 mg/ml aprotinin). Protein samples (15 μg per lane) were subjected to SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes. The membranes were blocked with blocking buffer, incubated with primary antibody, washed, and then incubated with the secondary horseradish peroxidase-labeled antibody. Bands were visualized with Enhanced Chemiluminescence (Amersham Biosciences, Piscataway, NJ). The following antibodies were used: Txnip (Santa Cruz Biotechnology; Santa Cruz, California), Trx (Santa Cruz Biotechnology; Santa Cruz, California), FOXO-1 (Cell signaling, USA), phosphor-FOXO1 (Cell signaling, USA), and β-actin (Sigma, St. Louis, MO). The expression of cytokine protein was demonstrated by the ratio of integral optical density (IOD) between specific protein and β-actin.
Real-time Quantitative RT-PCR
Total RNA from treated cells was extracted with Trizol (Invitrogen), according to the manufacturer’s protocol. The mRNAs were reverse-transcribed into cDNAs using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time PCR was performed using iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA). Primers were designed with the use of Beacon Designer 2.0 software. Txnip primers [13]: primer forward 5′-TCATGGTGATGTTCAAGAAGATC-3′; primer reverse5′-ACT TCACACCTCCACTATC-3′. The mRNA levels were acquired from the value of the threshold cycle (Ct) of Txnip normalized against the Ct of β-actin.
Chromatin immunoprecipitation assay
We used the ChIP assay kit (Upstate), according to the Upstate Chromatin Immunoprecipitation Protocol. In brief, treated HAECs were first incubated with formaldehyde to cross-link DNA-protein complexes. Protein-DNA complex was immunoprecipitated with antibody-protein A-agarose slurry. (IgG served as the negative control.) The immunocomplex beads were washed, eluted and reversed the cross-link. The DNA was recovered by extraction with the phenol/chloroform/isoamyl alcohol mixture. The immunoprecipitated DNA was used as a template for PCR. The PCR products were separated by 1.5% agarose gel. The primers used for the FOXO1 binding site in the 5′-flanking region of the human Txnip gene [13] were: forward 5′- AGCACACACCCAAACAACC -3′, reverse 5′- TCTCCCATTGGCTACTGG -3′.
Statistical Analysis
All quantitative variables are presented as means ± SEM from three separate experiments. We compared the differences of three groups or more using one-way ANOVA. To evaluate the interactive effects of two factors (e.g. the siRNA specific inhibition together with the treatment of glucose), we used a two-way ANOVA. Two-tailed p < 0.05 was considered statistically significant.
Results
High Glucose Increases the ROS Production through p38 MAPK Pathway
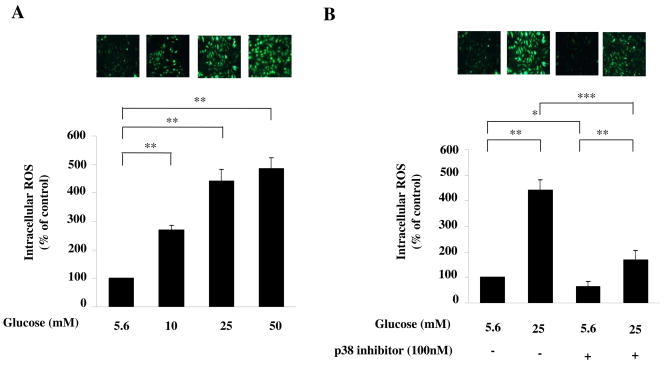
We first tested whether high glucose increased the intracellular ROS production. HAECs were incubated with increasing amounts of glucose. As shown in Fig. 1A, high glucose significantly increased intracellular levels of ROS in a dose-dependent manner (all P<0.01), which is consistent with previous reports[11]. We then determined whether p38 MAPK pathway was involved in high glucose-induced increase in ROS level. HAECs were incubated with glucose in the presence or absence of p38 MAPK inhibitor PD169316. As shown in Fig. 1B, high glucose-induced increase of intracellular ROS could be reduced by PD169316 (all P<0.001), indicating that p38 MAPK pathway is involved in high glucose-induced increase in intracellular ROS level..
Fig. 1. High glucose increased the ROS production through p38 MAPK pathway.
(A) High glucose increased the intracellular ROS level. HAECs were treated with glucose for 24 hours. Treated cells were incubated with oxidant-sensitive fluorogenic probe CM-H2DCFDA. Fluorescence was detected by a fluorescent microscope. Representative microscopic scans from three experiments are shown. Representative microscopic scan from three experiments and quantitative analysis of fluorescent intensity randomly counted in 5 fields per coverslip are shown. Data represent the mean ±SEM (N=3), **P < 0.01, versus control. Glucose significantly increased intracellular levels of ROS in a dose-dependent manner. (B) High glucose increased the ROS production through p38 MAPK pathway. HAECs were treated with glucose in the presence or absence of the p38 MAPK inhibitor PD169316 for 24 hours. Fluorescence was detected by a fluorescent microscope. Data represent the mean ±SEM (N=3), *P < 0.05, **P < 0.01, ***P < 0.001 versus control. Glucose significantly increased intracellular levels of ROS through the p38 MAPK pathway.
High Glucose Increases the Expression of Txnip via p38 MAPK Pathway
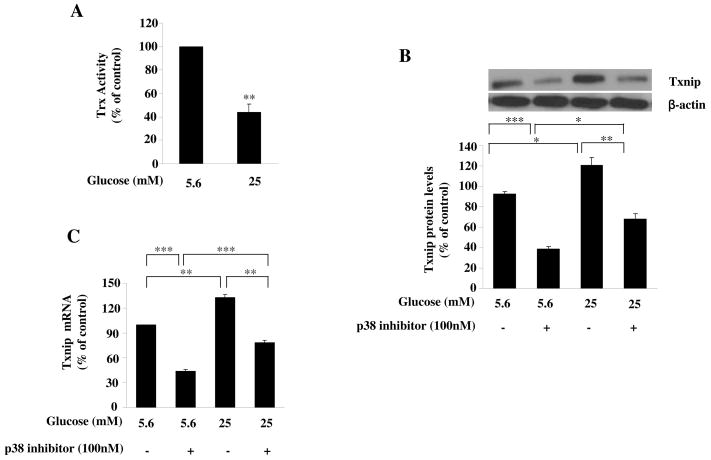
We next examined how p38 MAPK pathway was involved in the increased ROS level induced by high glucose and whether Trx system was affected. As shown in Fig. 2A, high glucose significantly reduced Trx activity, with up to 43% reduction at 25 mM (P<0.001). In investigating the mechanisms for reduced Trx activity in glucose-treated cells, we found that high glucose significantly increased the expression of Txnip protein and p38 pathway was involved in the process (P<0.05, Fig. 2B). RT-PCR revealed that the upregulation of Txnip was at mRNA level (P<0.01, Fig. 2C). Taken together, these data suggest that high glucose can reduce Trx activity by upregulating Txnip, probably through p38 MAPK pathway.
Fig. 2. High glucose increased the expression of Txnip via p38 MAPK pathway.
(A) The effects of glucose on Txnip protein expression. HAECs were treated with glucose in the absence or presence of the p38 MAPK inhibitor PD169316 for 24 hours. Txnip was measured by Western blot. Glucose increased the Txnip expression through p38 MAPK. (B) The effects of glucose on Txnip mRNA level. HAECs were treated with glucose in the absence or presence of the p38 MAPK inhibitor PD169316 for 24 hours, and Txnip mRNA levels were examined by RT-PCR. Glucose upregulated the Txnip mRNA via p38 MAPK. Representative blots from more then 3 independent experiments are shown. Data represent the mean ±SEM (N=3). *P < 0.05, **P < 0.01, ***P < 0.001 versus control.
Txnip Silencing Prevents High Glucose-p38 MAPK-induced Increase in ROS Level
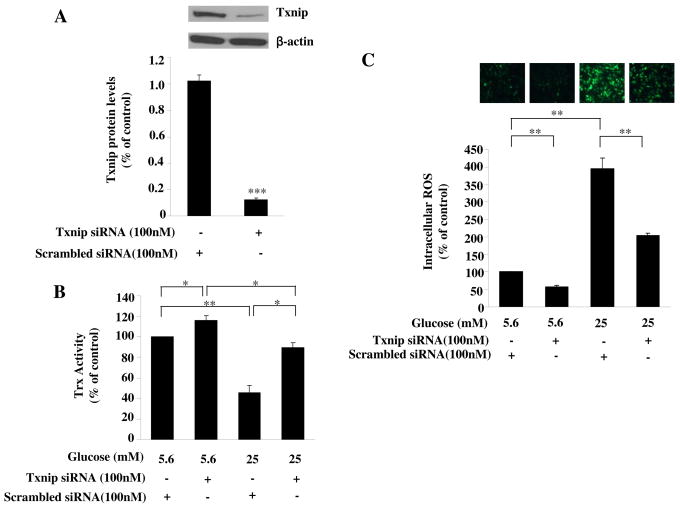
We then determined whether the upregulated Txnip is involved in high glucose induced increase of intracellular ROS levels. Txnip gene knockdown by specific siRNA (P<0.001, Fig. 3A) inhibited the high glucose-induced reduction of thioredoxin activity (P<0.05, Fig. 3B). Furthermore, knockdown Txnip gene prevented the high glucose-induced increase of ROS (all P<0.01, Fig. 3C). These data suggest the involvement of upregulated Txnip in high glucose-induced reduction of Trx activity and increase in intracellular ROS levels.
Fig. 3. Txnip was responsible for glucose induced reduction of trx activity and increase in ROS levels.
(A) The effect of Txnip siRNA on expression of Txnip. HAECs were treated with Txnip siRNA or scrambled siRNA for 24 hours. The expression of Txnip was examined by anti-Txnip antibody. Gene silencing of Txnip reduced Txnip protein. (B) Txnip siRNA prevented the glucose-induced reduction of Trx activity. HAECs were transfected with Txnip siRNA followed by treatment with glucose for 24 hours. Silencing the Txnip gene by siRNA enhanced the activity of Trx. Trx activity was assessed (n=12 per data point). (C) Txnip was involved in the glucose induced increase in ROS level. HAECs were transfected with Txnip siRNA followed by treatment with glucose for 24 hours. Intracellular ROS levels were detected by CM-H2DCFDA. Representative microscopic scans from 3 experiments and the quantitative analysis of fluorescent intensity randomly counted in 5 fields per coverslip are shown. Knockdown of Txnip by siRNA decreased basal and glucose-induced ROS levels. Representative blots from more then 3 independent experiments are shown. Data represent the mean ±SEM (N=3). *P < 0.05, **P < 0.01, ***P < 0.001 versus control.
FOXO 1 is Required for p38 MAPK-induced Upregulation of Txnip
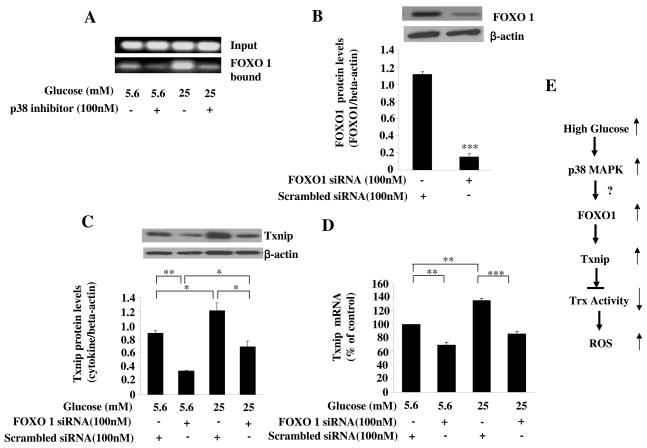
We further explored the mechanisms by which glucose increase the expression of Txnip mRNA levels. The 5′-flanking region of human Txnip gene contains consensus binding sites for many transcription factors and we identified FOXO1 as a potential transcriptional factors which may mediate Txnip upregulation[13]. Using ChIP assay, we observed that FOXO1 can bind to the Txnip promoter (Fig. 4A). Importantly, the binding to this site was significantly increased by glucose treatment that was dramatically decreased in the presence of PD169316 (Fig. 4A). These results indicate that the FOXO1 may be a potential target transcription factor that mediates glucose and p38 MAPK pathway induced induction of Txnip expression. Indeed, silencing FOXO1 with siRNA (P<0.001, Fig 4B) significantly prevented high glucose -induced induction of Txnip expression at protein level (all P<0.01, Fig 4C) and at mRNA level (P<0.01, Fig 4D). These data support a critical role for FOXO1 transcription factor in glucose-induced up-regulation of Txnip transcription.
Fig. 4. FOXO 1 was responsible for glucose-induced induction of Txnip.
(A) Glucose increased the binding of FOXO1 to the Txnip promoter via p38 MAPK pathway. HAECs were treated with glucose in the presence or absence of p38 inhibitor. The binding of FOXO1 to the Txnip promoter was examined by Chip assay as described in the methods. (B) The effect of FOXO1 siRNA on the expression of FOXO1. HAECs were treated with FOXO1 siRNA or scrambled siRNA for 24 hours. The expression of FOXO1 was examined by anti-FOXO1 antibody. Gene silencing of FOXO1 reduces protein levels of FOXO1. (C). FOXO1 was responsible for glucose-induced upregulation of Txnip. HAECs were transfected with FOXO1 siRNA followed by treatment with glucose for 24 hours. Txnip was measured by Western blot. (D) FOXO1 was involved in glucose-induced induction of Txnip mRNA. HAECs were transfected with FOXO1 siRNA for 24 hours. Txnip mRNA was examined by RT-PCR. FOXO1 siRNA decreased basal Txnip and prevented glucose-induced induction of Txnip mRNA. Representative blots from more then 3 independent experiments are shown. Data represent the mean ±SEM (N=3). *P < 0.05, ***P < 0.001 versus glucose with scrambled siRNA. (E) Schematic diagram of possible mechanisms for high glucose induced increase in intracellular ROS. High glucose, by activating p38 MAPK and FOXO1, induces Txnip expression and subsequently reduces Trx activity that leads to increased intracellular ROS levels.
Discussion
In the present study, we have observed that high glucose induced Txnip expression and subsequently reduced the Trx activity, which was partially responsible for increased intracellular ROS. The p38 MAPK and FOXO1 pathways mediated high glucose-induced Txnip up-regulation. Increased ROS level has been consistently reported in diabetes [14, 15] and is involved in vascular dysfunction[15]. Trx is an important antioxidant system and Trx-Txnip interaction is critical in redox regulation[7]. Imbalanced Trx-Txnip interaction and impaired Trx activity may play a role in the increased ROS in diabetes. In this study, we observed that high glucose inhibited Trx activity. Although the expression of Trx may also be affected by glucose, the current study suggests that upregulation of Txnip and subsequent reduction of Trx activity may contribute to the increases in ROS levels in glucose-treated cells[11]. Thus, the induction of Txnip and impairment of Trx system by hyperglycemia may provide an important mechanism for the elevated intracellular oxidative stress and increased activation of stress signaling pathway in the pathogenesis of diabetic vascular diseases.
We also explored the underlying mechanisms that regulate high glucose-induced expression of Txnip. Our results have demonstrated that p38 MAPK mediated the high glucose-induced upregulation of Txnip expression. p38 MAP kinases, a member of family of serine/threonine kinases, is an important stress signaling molecule and is involved in the regulation of many cellular functions. However over activation of this pathway can lead to cellular dysfunction [16]. p38 MAPK pathway can be activated by ROS [17,18]. Our evidence suggests that once p38 MAPK pathway is activated, it is capable of damaging antioxidative system that further increases intracellular ROS level. Thus, activation of the p38 pathway in vascular wall by chronically elevated glucose may play a role in the development of vascular complications in diabetes [19]. To further investigate how the high glucose induced Txnip expression, we found that FOXO1 was involved in the upregulation of Txnip expression [13]. However, further studies are needed to examine whether FOXO1 overexpression can induce Txnip expression and how FOXO1 regulates the Txnip promoter activity in vivo. FOXO transcription factors such as FOXO1, FOXO3, FOXO4 and FOXO6 are important regulators of cellular functions such as cellular metabolism, cell cycle progression and apoptosis[20, 21, 22]. Recently, emerging evidence indicates that FOXOs play critical roles in regulating cellular functions under oxidative stress. It has been shown that FOXOs can be activated by ROS [23, 24]. Once they are activated, FOXOs can either reduce ROS level (our unpublished data) and enhance cell survival in response to physiologic oxidative stress [24, 25], or increase ROS level (from this study) and mediate ROS-induced apoptosis [26]. The findings from this study highlight the importance of FOXO1 in the increase of intracellular ROS levels.
From this pilot study, we hypothesize that p38 MAPK pathway may up-regulate Txnip expression through FOXO1. However, further studies are needed to examine whether p38 can directly bind to and phosphorylate FOXO1, and subsequently activate this transcriptional factor. In addition, p38 MAPK pathway may up-regulate Txnip expression through multiple mechanisms, which include increasing the stability of Txnip mRNA, promoting protein translation, or inhibiting protein degradation. Additional studies will be necessary to define the detailed mechanisms for the regulation of the Txnip by p38 MAPK and FOXO1 pathways in response to metabolic stress. In summary, we observed that high glucose significantly increased intracellular ROS levels in HAECs. This increased oxidative stress is likely caused by the increased expression of Txnip through p38 MAPK and FOXO1, that may lead to impaired Trx activity and hence increased ROS level.
Acknowledgments
This work was supported by AHA-TX 0565134Y(YHS), AHA-0730190N (YHS) R01-HL071608 (XLW), and the National Basic Research Program (also called 973 Program) 2006CB503803, Shandong Province Bureau of Public Health 1020 Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007 Dec;27(12):2627–33. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- 2.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008 Feb;31(Suppl 2):S170–80. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 3.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000 Nov 10;87(10):840–4. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 4.Yamawaki H, Haendeler J, Berk BC. Thioredoxin: a key regulator of cardiovascular homeostasis. Circ Res. 2003;93:1029–1033. doi: 10.1161/01.RES.0000102869.39150.23. [DOI] [PubMed] [Google Scholar]
- 5.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 6.Chen KS, DeLuca HF. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1994;1219:26–32. doi: 10.1016/0167-4781(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 7.Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y, Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 8.Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000 Jun 15;164(12):6287–95. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- 9.Turoczi T, Chang VW, Engelman RM, Maulik N, Ho YS, Das DK. Thioredoxin redox signaling in the ischemic heart: an insight with transgenic mice overexpressing Trx1. J Mol Cell Cardiol. 2003;35:695–704. doi: 10.1016/s0022-2828(03)00117-2. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, DeKeulenaer GW, Lee RT. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem. 2002;277:26496–26500. doi: 10.1074/jbc.M202133200. [DOI] [PubMed] [Google Scholar]
- 11.Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem. 2004 Jul 16;279(29):30369–74. doi: 10.1074/jbc.M400549200. Epub 2004 May 5. [DOI] [PubMed] [Google Scholar]
- 12.Holmgren A, Björnstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- 13.Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008 Apr;11(4):476–87. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RAK, Warnholtz A, Meinertz T, Griendling KK, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:e14–e22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 15.Kim YK, Lee MS, Son SM, Kim IJ, Lee WS, Rhim BY, Hong KW, Kim CD. Vascular NADH oxidase is involved in impaired endothelium-dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes. 2002;51:522–527. doi: 10.2337/diabetes.51.2.522. [DOI] [PubMed] [Google Scholar]
- 16.Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000 Oct;20(10):2175–83. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 17.Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, Feelisch M, Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation. Proc Natl Acad Sci U S A. 2004 Aug 3;101(31):11471–6. doi: 10.1073/pnas.0402941101. Epub 2004 Jul 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh CC, Papaconstantinou J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J. 2006 Feb;20(2):259–68. doi: 10.1096/fj.05-4376com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan S, Bolick DT, Hatley ME, Natarajan R, Reilly KB, Yeh M, Chrestensen C, Sturgill TW, Hedrick CC. Glucose regulates interleukin-8 production in aortic endothelial cells through activation of the p38 mitogen-activated protein kinase pathway in diabetes. J Biol Chem. 2004 Jul 23;279(30):31930–6. doi: 10.1074/jbc.M400753200. Epub 2004 May 15. [DOI] [PubMed] [Google Scholar]
- 20.Hattangadi SM, Lodish HF. Regulation of erythrocyte lifespan: do reactive oxygen species set the clock? J Clin Invest. 2007 Aug;117(8):2075–7. doi: 10.1172/JCI32559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arden KC. FoxOs in tumor suppression and stem cell maintenance. Cell. 2007;128:235–7. doi: 10.1016/j.cell.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Zheng X, Yang Z, Yue Z, Alvarez JD, Sehgal A. FOXO and insulin signaling regulate sensitivity of the circadian clock to oxidative stress. Proc Natl Acad Sci U S A. 2007;104:15899–904. doi: 10.1073/pnas.0701599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 24.Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 25.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007 Jun;8(6):440–50. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T, Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol. 2008;281:47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]