Abstract
Nicotinic acetylcholine receptors (AChR) are ligand-gated cation channels that are present throughout the nervous system. The ganglionic (α3-type) neuronal AChR mediates fast synaptic transmission in sympathetic, parasympathetic and enteric autonomic ganglia. Autonomic ganglia are an important site of neural integration and regulation of autonomic reflexes. Impaired cholinergic ganglionic synaptic transmission is one important cause of autonomic failure.
Ganglionic AChR antibodies are found in many patients with autoimmune autonomic ganglionopathy (AAG). These antibodies recognize the α3 subunit of the ganglionic AChR, and thus do not bind non-specifically to other nicotinic AChR. Patients with high levels of ganglionic AChR antibodies typically present with rapid onset of severe autonomic failure, with orthostatic hypotension, gastrointestinal dysmotility, anhidrosis, bladder dysfunction and sicca symptoms. Impaired pupillary light reflex is often seen. Like myasthenia gravis, AAG is an antibody-mediated neurological disorder. Antibodies from patients with AAG inhibit ganglionic AChR currents and impair transmission in autonomic ganglia. An animal model of AAG in the rabbit recapitulates the important clinical features of the human disease and provides additional evidence that AAG is an antibody-mediated disorder caused by impairment of synaptic transmission in autonomic ganglia.
Keywords: autonomic neuropathy, thymoma, gastrointestinal dysmotility, orthostatic hypotension
Introduction
Anatomy of the peripheral autonomic nervous system
The autonomic nervous system has a unique neuroanatomical structure. Like somatic motor nerves, peripheral autonomic cholinergic motor neurons are found in the brainstem and spinal cord. Unlike the somatic motor and sensory systems, the peripheral autonomic system contains groups of neurons (ganglia) with extensive synaptic connections outside the central nervous system (figure 1A). These project to the periphery and synapse with neurons in autonomic ganglia. Within ganglia, the peripheral autonomic neurons, especially in the intrinsic enteric autonomic nervous system, also synapse extensively with each other. The ganglionic neurons then send axons (postganglionic unmyelinated C fibers) to innervate target organs. Fast synaptic transmission within autonomic ganglia is mediated by acetylcholine acting on nicotinic acetylcholine receptors (AChR). Other neurotransmitters (including neuropeptides and nitric oxide) contribute to modulation of primary synaptic transmission or mediate slow synaptic events.
Figure 1. The autonomic ganglionic synapse.
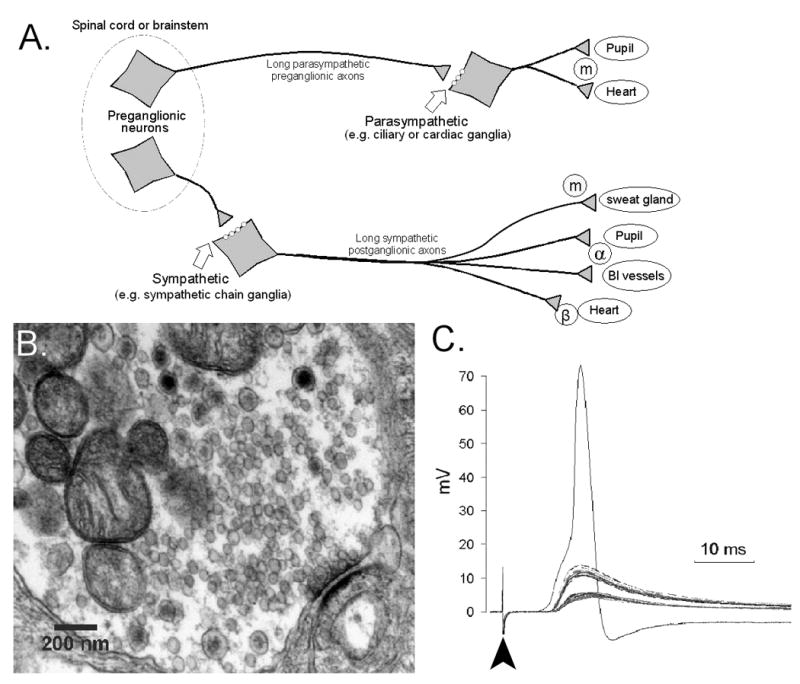
A) A simplified schematic showing the anatomy of the peripheral autonomic nervous system. The autonomic ganglia receive input from cholinergic motor neurons in the brainstem or spinal cord. Fast ganglionic synaptic transmission is mediated by acetylcholine acting on neuronal nicotinic acetylcholine receptors. The postganglionic fibers extend to innervate numerous target organs (a few examples are shown) and release acetycholine acting on muscarinic receptors (m) or norepinephrine acting on alpha and beta adrenergic receptors (α and β).
B) Electron micrograph showing the ultrastructure of a ganglionic synapse in rabbit superior cervical ganglia. The presynaptic terminal contains mitochondria, numerous small clear vesicles containing acetycholine, and larger dense core vesicles presumably containing neuropeptides and other transmitters. The synapse (lower right) is characterized by a short area of close apposition of the nerve terminal and dendrite membranes. Vesicles are poised on the presynaptic side, ready for release. The thickened postsynaptic membrane is the area of synaptic specialization that contains the neurotransmitter receptors.
C) Microelectrode recording of synaptic potentials from a neuron in isolated mouse superior cervical ganglia. Stimulation of the preganglionic nerve (arrowhead) leads to a fast excitatory postsynaptic potential (fEPSP) in the neuron. The y-axis indicates the change in membrane potential from the resting potential (which is usually around -50 to -60mV). Gradually increasing stimulus intensity produces discrete fEPSPs indicating the presence of multiple preganglionic inputs to this single ganglia neuron (a typical single fEPSP causes about a 5mV depolarization in the neuronal soma in this case). The simultaneous activation of several inputs is required to reach threshold and produce an action potential in the neuron. In this example, at least three distinct synaptic inputs combine to reach the action potential threshold.
Neuronal nicotinic acetylcholine receptors
Nicotinic acetylcholine receptors (AChRs) are a family of ligand-gated cation channels found throughout the central and peripheral nervous system. Every nicotinic AChR is formed by the association of five subunits of which at least two are α subunits. The α subunit contains important binding sites for acetylcholine. Muscle-type AChR mediates neuromuscular transmission, and antibodies against the muscle AChR cause the characteristic defect in neuromuscular junction transmission and fatigable weakness in patients with myasthenia gravis (MG) (Drachman, 1994).
Neuronal nicotinic AChRs are formed from a variety of subunits homologous to those in muscle AChRs. These neuronal AChR serve many functions in the nervous system. In the peripheral autonomic nervous system, the ganglionic nicotinic AChR mediates fast synaptic transmission in all peripheral autonomic ganglia (sympathetic, parasympathetic and enteric ganglia). AChRs on autonomic neurons are typically composed of two α3 subunits in combination with three other AChR subunits. Although autonomic ganglia neurons can express numerous neuronal AChR subunits, including α3, α4, α5, α7, β2, and β4, the properties of the AChR at mammalian ganglionic synapses are most similar to AChRs formed by α3 and β4 subunits (Skok et al., 1999). Transgenic mice lacking the α3 subunit have profound autonomic failure with prominent bladder distention, gastrointestinal dymotility and lack of pupillary light reflexes indicating that the α3 subunit is absolutely required for normal autonomic ganglionic neurotransmission (Xu et al., 1999).
Autonomic ganglionic neurotransmission
The vast majority of ganglionic synapses are simple structures located on short dendrites rather than on the cell soma (figure 1B)(Myers, 2000). An action potential in the presynaptic terminal results in the release of neurotransmitter vesicles, predominantly containing acetylcholine. Interaction of acetylcholine with the ganglionic AChR produces a depolarization in the ganglia neuron (fast excitatory post-synaptic potential, fEPSP). If the depolarization is sufficient to reach the threshold for action potential generation, the signal is propagated down the postganglionic axon to the target. The strength of the synapse is dependent on multiple factors including the quantal content (number of vesicles released with each stimuli), the number of postsynaptic AChR, and the geometry of the postsynaptic dendrite.
Autonomic ganglia are more than simple relay centers for autonomic information. There is significant signal integration due to convergence and divergence of synaptic inputs. In most mammalian autonomic ganglia, each preganglionic fiber innervates multiple ganglia neurons (divergence). A minority of “strong” synapses can produce a single fEPSP that is sufficient to produce an action potential. More commonly, multiple preganglionic signals must converge and summate to produce an action potential in the ganglia neuron (figure 1C)(Sacchi et al., 1971). The synaptic strength and the degree of integration vary widely among different autonomic ganglia. Since ganglionic transmission depends on the convergence of multiple subthreshold synaptic events, any process that modulates the strength of ganglionic cholinergic transmission will have profound effects on the function of the autonomic nervous system.
Autoimmune autonomic ganglionopathy
Clinical autonomic disorders can result from diverse causes, including degenerative, inherited, toxic/metabolic, infections and autoimmune/inflammatory conditions. It is particular important to recognize immune-mediated disorders since immunomodulatory therapy may lead to recovery of neurological function. Conceptually, autonomic disorders are often categorized as either central or peripheral. Central disorders affect the autonomic pathways in the central nervous system including the autonomic motor neurons that contribute preganglionic autonomic fibers. Peripheral (postganglionic) disorders affect the neurons of the autonomic ganglia and the small autonomic nerve fibers extending to the target organs. In a third category of autonomic disorders, the pathology lies within the autonomic ganglia itself.
Autoimmune autonomic ganglionopathy (AAG) is an acquired neurological disorder characterized by diffuse autonomic failure. The clinical features of AAG reflect impairment in sympathetic (orthostatic hypotension, anhidrosis), parasympathetic (reduced lacrimation, salivation and pupil constriction) and enteric function (ileus, abdominal colic, diarrhea, and constipation). The constellation of tonic pupils and gastrointestinal dysmotility in the setting of severe orthostatic hypotension is particularly suggestive of AAG (Klein et al., 2003). Full descriptions of the typical features of AAG and of other clinical presentations are presented elsewhere in this edition. Up to 50% of patients with the acute or subacute presentation of AAG have high levels of autoantibodies that bind to ganglionic AChR (Vernino et al., 2000). Ganglionic AChR antibodies are an important serological marker of AAG but also cause the characteristic defect in autonomic ganglionic neurotransmission in this disorder.
Ten years after the first description of ganglionic AChR antibodies, this edition highlights the unique clinical features of AAG, novel observations about the syndrome, reports of effective treatments, and current evidence indicating that AAG is an antibody-mediated disorder. In this introductory article, we discuss how the autonomic ganglia is central to the function of the peripheral autonomic nervous system and how antibodies against the ganglionic AChR produce an autoimmune autonomic disorder.
Methods
Human clinical data and serum samples were collected with approval of the institutional review boards at UT Southwestern Medical Center and at collaborating institutions. Animal experimentation was approved by the UT Southwestern institutional animal care and use committee.
Ganglionic AChR antibodies are detected with a radioimmunoprecipitation assay that has been described in detail previously(Vernino et al., 2008; Vernino et al., 2000). This assay is similar to the method used to detect muscle AChR antibodies in patients with myasthenia gravis. Solubilized membranes from a human neuroblastoma cell line (IMR-32, American Tissue Type Collection, Manassas, VA) are complexed with a high affinity ligand for ganglionic AChR, 125I-labeled epibatidine (Perkin-Elmer, Waltham, MA). Radiolabeled antigen is incubated with serum overnight. The ability of IgG in the serum to precipitate the radiolabeled AChR provides a quantitative measure of specific antibody binding. Based on previous studies, ganglionic AChR precipitating activity greater than 0.05 nmol per liter of serum is considered positive.
Electrophysiological studies on ganglionic AChR expressed by IMR-32 cells were performed using traditional patch clamp techniques as previously described (Wang et al., 2007). Studies on ganglionic synaptic transmission were performed using standard microelectrode recording techniques. After euthanasia, the mouse superior cervical ganglia (with attached preganglionic sympathetic nerve trunk) was excised and placed in an organ bath. Ganglia neurons were visualized with an immersion microscope and impaled with a glass microelectrode to record membrane potential. The preganglionic nerve trunk was stimulated with a bipolar needle electrode to produce a synaptic potential.
Results
Specificity and sensitivity of ganglionic AChR antibodies
Serum levels of ganglionic AChR antibodies correlate with different autoimmune autonomic phenotypes (Table 1). Patients with high antibody levels (> 0.5 nmol/L) typically have a severe phenotype and a rapid onset. Seropositive AAG patients have a mean age around 52 years (22-82) with a slight female predominance (60-65%). As yet, we have not found ganglionic AChR antibodies in children with subacute autonomic syndromes. The demographics of AAG cases in our contemporary experience are very similar to earlier reported series (Vernino et al., 2000).
Table 1.
Ganglionic AChR antibody in patients with dysautonomia and other disorders*
| Diagnostic Group | % seropositive | Antibody levels | Reference |
|---|---|---|---|
| Autonomic disorders | |||
| Subacute AAG | 40 – 50% | 0.5 – 41.0 nM/L | (Vernino et al., 2008; Vernino et al., 2000) |
| Chronic AAG | 30 – 40%** | 0.2 – 5.0 nmol/L | (Klein et al., 2003) |
| Paraneoplastic AAG | 10 – 20% | 0.2 – 20.0 nmol/L | (Vernino et al., 2000) |
| Postural tachycardia syndrome (POTS) | 10 – 15% | < 0.25 nmol/L | (Thieben et al., 2007) |
| Idiopathic gastrointestinal dysmotility | 5 – 10% | < 0.4 nmol/L | (Vernino et al., 2000) |
| Diabetic autonomic neuropathy | < 10% | ||
| Multiple system atrophy (Shy-Drager) | 0 | ||
| Other disorders | |||
| Lambert-Eaton syndrome | 5 – 10% | 0.06 – 0.4 nmol/L | (Vernino et al., 2000) |
| Myasthenia gravis without thymoma | 3% | < 0.25 nmol/L | (Vernino et al., 2008) |
| Paraneoplastic disorders with thymoma | 15 – 20% | 0.06 – 2.0 nmol/L | (Vernino et al., 2004b) |
| Paraneoplastic disorders with SCLC | 3 – 5% |
None of over 200 healthy control subjects were seropositive for ganglionic AChR antibodies.
The exact frequency is not known since antibody status may affect case definition
Lower antibody levels (0.05 - 0.20 nmol/L) may be found in patients with limited forms of dysautonomia, including those with isolated gastrointestinal dysmotility, diabetic autonomic neuropathy, or postural tachycardia syndrome (as discussed in the article by Sandroni and Low). Ganglionic AChR antibodies are also found (at lower levels) in some patients with other paraneoplastic neurological disorders related to thymoma and small-cell lung cancer (including Lambert-Eaton syndrome)(Vernino et al., 2004b). Autonomic failure may occur as a paraneoplastic phenomenon, especially in association with small-cell lung cancer or thymoma. Some of these cases of paraneoplastic AAG are associated with ganglionic AChR antibodies. While it is important to consider a paraneoplastic disorder in a middle-age patient with acute or subacute autonomic dysfunction, the majority of AAG patients with ganglionic AChR antibodies do not have cancer.
A high level of ganglionic AChR antibodies is highly specific for AAG, however, the sensitivity of the antibody for acquired diffuse autonomic failure is only around 50%. Patients with acute or subacute panautonomic failure may be seronegative. One explanation is that the presentation of autonomic disorders is quite varied, and clinical findings, even in sophisticated autonomic testing laboratories, are not entirely specific for AAG. Compared to seronegative cases, patients with ganglionic AChR antibodies are more likely to have impaired pupillary light response, marked gastrointestinal hypomotility and urinary retention (Sandroni et al., 2004). The pathophysiology of seronegative cases is not known, but an autoimmune mechanism is suspected because of the clinical similarity with seropositive AAG and the response of some seronegative patients to immunomodulatory treatment (intravenous immunoglobulin, plasmapheresis or corticosteroids). These cases may be analogous to cases of seronegative myasthenia gravis where an alternate antibody target is implicated. When autonomic failure coexists with sensory or motor neuropathy, ganglionic AChR antibodies are not usually found (ganglionic AChR antibodies would not be expected to affect somatic sensory or motor nerves).
Although muscle and ganglionic AChRs are structurally very similar, patients with AAG typically do not have weakness or other clinical features of MG. Patients with MG do not have prominent autonomic dysfunction. The exceptions are rare patients with an overlap syndrome of myasthenia with subacute autonomic failure often associated with thymoma (Vernino et al., 2001). There is very little cross-reactivity between muscle and ganglionic AChR antibodies in patients with MG or AAG (Table 1) (Vernino et al., 1998; Vernino et al., 2008).
Pathophysiological effects of ganglionic AChR antibodies
Serum ganglionic AChR antibody levels in AAG correlate with the severity of autonomic neuropathy clinically and with the severity on laboratory testing of autonomic function (Klein et al., 2003; Vernino et al., 2000). A decrease in antibody levels is associated with improvement in autonomic function (Vernino et al., 2000). Plasmapheresis to remove autoantibodies can produce a dramatic improvement in autonomic function in some cases (Gibbons et al., 2008; Schroeder et al., 2005) These findings suggest that ganglionic AChR antibodies are direct pathophysiologic effectors of autonomic dysfunction.
Several lines of evidence indicate that AAG is an antibody-mediated disorder. Antibodies (IgG) isolated from the serum of patients with AAG have direct effects on the ganglionic AChR in vitro.(Wang et al., 2007) When neuroblastoma cells are exposed to ganglionic AChR IgG, the amplitudes of neuronal AChR membrane currents are progressively reduced (figure 2A). The characteristics and time course of this AChR inhibition suggests that the antibodies act by binding and cross-linking the receptors leading to active internalization (modulation). A minority of antibodies also produce a more immediate effect, likely due to binding at or near the agonist binding site leading to blocking of the receptor (Vernino et al., 2000; Wang et al., 2007). Ganglionic AChR antibodies also inhibit fast cholinergic synaptic transmission in isolated mouse autonomic ganglia (Figure 2B)(Vernino et al., 2004a).
Figure 2. Effects of ganglionic AChR antibodies on nicotinic AChR responses.
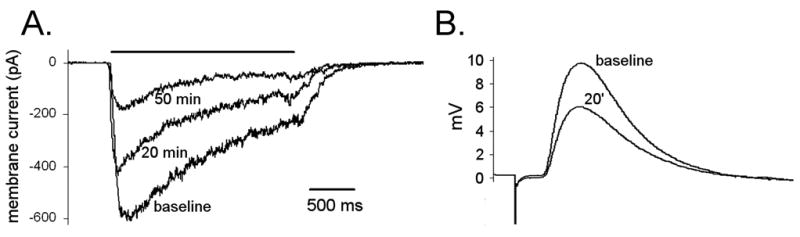
A) Patch-clamp recording of whole-cell membrane current in IMR-32 cells at baseline, 20 minutes and 50 minutes after addition of IgG (1 mg/ml) purified from the plasma of a patient with AAG. Application of agonist (DMPP 50 μM, 4 second horizontal bar) produces an inward current. Exposure to ganglionic AChR IgG results in a progressive reduction of peak AChR current (Wang et al., 2007).
B) Microelectrode recording from ganglia neuron in isolated mouse superior cervical ganglia. The fast excitatory postsynaptic potential (fEPSP) is produced by a fixed stimulus to the preganglionic nerve. The amplitude of the fEPSP progressively decreases after exposure to ganglionic AChR IgG (1 mg/ml added to the bath solution). Each response represents the average of 5 fEPSP recorded at baseline or 20 minutes after bath application of IgG.
Experimental autoimmune autonomic ganglionopathy
Experimental AAG (EAAG) can be induced in animals either by active immunization with peptides derived from the ganglionic AChR or by passive transfer of ganglionic AChR antibodies (Vernino et al., 2004a; Vernino et al., 2003). Passive transfer of IgG from affected rabbits or humans to mice produces reversible autonomic deficits. (Vernino et al., 2004a) Immunization of rabbits with a fusion protein corresponding to the N-terminal (extracellular) domain of the ganglionic AChR α3 subunit leads to persistent production of ganglionic AChR antibodies and a chronic form of experimental AAG (Lennon et al., 2003). Rabbits producing high levels of ganglionic AChR antibody develop gastrointestinal dysmotility and fail to gain weight due to reduced food intake. Severe parasympathetic dysfunction manifests as dilated and poorly responsive pupils, decreased lacrimation, reduced heart rate variability, and dilated bladder. EAAN rabbits also have reduced levels of plasma catecholamines indicating reduced sympathetic tone. As in patients with AAG, the severity of autonomic disturbances is greater in rabbits with higher antibody levels (Vernino et al., 2003).
Neurons in autonomic ganglia from EAAG rabbits are intact but show a selective loss of surface ganglionic AChR (Vernino et al., 2003). This functional deficit can be identified in the animal by examining the physiological responses to autonomic drugs and myocardial imaging to assess the integrity of sympathetic innervation (Figure 3). Both EAAG rabbits and AAG patients (Goldstein et al., 2002) show a unique pattern of baroreflex and sympathetic dysfunction with preserved cardiac postganglionic sympathetic innervation suggesting a disorder of ganglionic neurotransmission. In particular, the response to tyramine (an indirect sympathetic agonist) in EAAG rabbits is similar to the response seen in rabbits after ganglionic blockade (Figure 4).
Figure 3. Preserved cardiac sympathetic innervation in rabbits with EAAG.
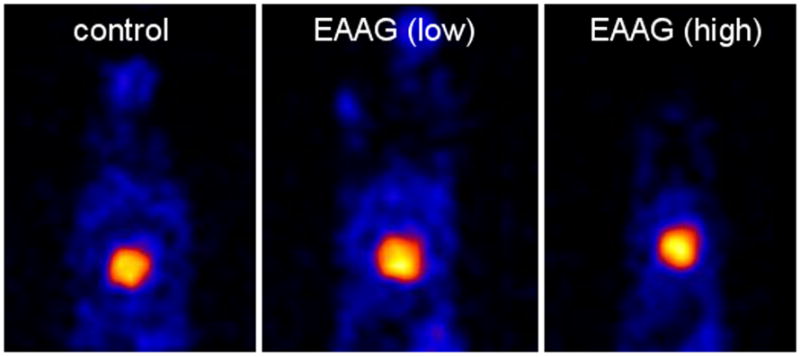
Single-photon computer tomography (SPECT) using 123I-metaiodobenzylguanidine (MIBG) was used to assess cardiac sympathetic innervation in awake, gently restrained rabbits. Images were obtained five hours after intravenous injection with 3mCi of MIBG. 99Tc-MIBI scans (not shown) were performed simultaneously to confirm adequate cardiac perfusion. Cardiac uptake of MIBG (shown as the bright area within the rabbit thorax) indicates the presence of intact sympathetic nerve terminals in the myocardium. There is no difference in the intensity of MIBG uptake in EAAG rabbits (with low or high ganglionic AChR antibody levels) compared to control rabbits. In the setting of low plasma catecholamine levels, this finding suggests pathology at the level of ganglionic neurotransmission (Goldstein et al., 2002; Vernino et al., 2003).
Figure 4. Cardiovascular response to tyramine in rabbits with EAAG.
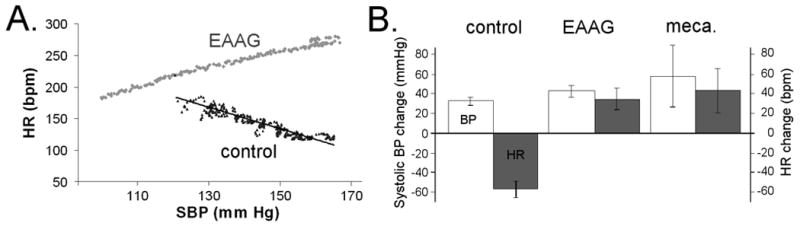
Rabbits were implanted with radiotelemetry catheters to allow continuous recording of arterial blood pressure and heart rate in the awake rabbit. Tyramine, an indirect sympathetic agonist, was infused intravenously over one minute (0.5mg/kg). A) Blood pressure and heart rate response to tyramine infusion. A rise in blood pressure (as seen in both control and EAAG rabbits) indicates intact postganglionic sympathetic nerve terminals. In the control rabbits, the rise in blood pressure leads to a baroreflex-mediated decrease in heart rate. In the EAAG rabbit, both the blood pressure and heart rate increase. This observation indicates failure of the baroreflex as well as direct activation of cardiac beta receptors via intact cardiac sympathetic terminals. B) A summary of the tyramine response in control rabbits (n=9), rabbits with chronic EAAG (n=13), and normal rabbits after treatment with the ganglionic blocker mecamylamine (3 mg/kg, n=3). In all rabbits, tyramine causes a rise in blood pressure (open bars). The magnitude of blood pressure increase is greater in EAAG and mecamylamine-treated rabbits, most likely due to failure of the sympathetic baroreflex to buffer the rise in blood pressure. In control rabbits, there is a marked baroreflex-mediated decrease in heart rate (shaded bar) while the EAAG and mecamylamine-treated rabbits show an increase in heart rate. This unique response to tyramine shows the similarity in autonomic physiology between EAAG and pharmacological blockade of ganglionic transmission.
Discussion
Autoimmune autonomic ganglionopathy is an antibody-mediated neurological disorder. Many patients with AAG have antibodies that specifically recognize the α3 subunit of the ganglionic AChR. Ganglionic AChR autoantibodies reduce membrane current through AChR containing α3 subunits and reduce the strength of fast synaptic transmission in autonomic ganglia. The animal model, experimental autoimmune autonomic ganglionopathy, helps to define AAG as an antibody-mediated disorder and provides an opportunity to dissect the disease mechanisms.
The sensitivity of ganglionic AChR antibodies for the clinical diagnosis of acute or subacute panautonomic failure (typical AAG) remains around 50%. This has been consistent over the 10 years since the first description of the ganglionic AChR antibody. High levels of ganglionic AChR antibodies are quite specific for AAG and are not found in other neurological disorders. Low levels of ganglionic AChR antibodies may be found in mild or restricted forms of autonomic failure or in AAG that has a more indolent course. These antibodies may rarely be found in patients with MG, thymoma or other paraneoplastic disorders.
Acknowledgments
Supported by R01NS48077, P50NS32352 and UT Southwestern Medical Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Drachman D. Myasthenia gravis. N Engl J Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Vernino SA, Freeman R. Combined immunomodulatory therapy in autoimmune autonomic ganglionopathy. Arch Neurol. 2008;65:213–217. doi: 10.1001/archneurol.2007.60. [DOI] [PubMed] [Google Scholar]
- Goldstein D, Holmes C, Dendi R, Li ST, Brentzel S, Vernino S. Pandysautonomia associated with impaired ganglionic neurotransmission and circulating antibody to the neuronal nicotinic receptor. Clin Auton Res. 2002;12:281–285. doi: 10.1007/s10286-002-0020-3. [DOI] [PubMed] [Google Scholar]
- Klein CM, Vernino S, Lennon VA, Sandroni P, Fealey RD, Benrud-Larson L, Sletten D, Low PA. The spectrum of autoimmune autonomic neuropathies. Ann Neurol. 2003;53:752–758. doi: 10.1002/ana.10556. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Ermilov LG, Szurszewski JH, Vernino S. Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease. J Clin Invest. 2003;111:907–913. doi: 10.1172/JCI17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AC. Anatomical characteristics of tonic and phasic postganglionic neurons in guinea pig bronchial parasympathetic ganglia. J Comp Neurol. 2000;419:439–450. [PubMed] [Google Scholar]
- Sacchi O, Perri V. Quantal release of acetylcholine from the nerve endings of the guinea-pig superior cervical ganglion. Pflugers Archiv - European Journal of Physiology. 1971;329:207–219. doi: 10.1007/BF00586615. [DOI] [PubMed] [Google Scholar]
- Sandroni P, Vernino S, Klein CM, Lennon VA, Benrud-Larson L, Sletten D, Low PA. Idiopathic autonomic neuropathy: comparison of cases seropositive and seronegative for ganglionic acetylcholine receptor antibody. Arch Neurol. 2004;61:44–48. doi: 10.1001/archneur.61.1.44. [DOI] [PubMed] [Google Scholar]
- Schroeder C, Vernino S, Birkenfeld AL, Tank J, Heusser K, Lipp A, Benter T, Lindschau C, Kettritz R, Luft FC, Jordan J. Plasma exchange for primary autoimmune autonomic failure. N Engl J Med. 2005;353:1585–1590. doi: 10.1056/NEJMoa051719. [DOI] [PubMed] [Google Scholar]
- Skok MV, Voitenko LP, Voitenko SV, Lykhmus EY, Kalashnik EN, Litvin TI, Tzartos SJ, Skok VI. Alpha subunit composition of nicotinic acetylcholine receptors in the rat autonomic ganglia neurons as determined with subunit-specific anti-alpha(181-192) peptide antibodies. Neuroscience. 1999;93:1427–1436. doi: 10.1016/s0306-4522(99)00160-8. [DOI] [PubMed] [Google Scholar]
- Thieben M, Sandroni P, Benrud-Larson L, Fealey R, Vernino S, Lennon V, Low P. Postural Orthostatic Tachycardia Syndrome - Mayo Clinic Experience. Mayo Clin Proc. 2007 doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- Vernino S, Adamski J, Kryzer TJ, Fealey RD, Lennon VA. Neuronal nicotinic ACh receptor antibody in subacute autonomic neuropathy and cancer-related syndromes. Neurology. 1998;50:1806–1813. doi: 10.1212/wnl.50.6.1806. [DOI] [PubMed] [Google Scholar]
- Vernino S, Cheshire WP, Lennon VA. Myasthenia gravis with autoimmune autonomic neuropathy. Auton Neurosci. 2001;88:187–192. doi: 10.1016/S1566-0702(01)00239-9. [DOI] [PubMed] [Google Scholar]
- Vernino S, Ermilov LG, Sha L, Szurszewski JH, Low PA, Lennon VA. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004a;24:7037–7042. doi: 10.1523/JNEUROSCI.1485-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernino S, Lennon VA. Autoantibody profiles and neurological correlations of thymoma. Clin Cancer Res. 2004b;10:7270–7275. doi: 10.1158/1078-0432.CCR-04-0735. [DOI] [PubMed] [Google Scholar]
- Vernino S, Lindstrom J, Hopkins S, Wang Z, Low PA. Characterization of ganglionic acetylcholine receptor autoantibodies. J Neuroimmunol. 2008;197:63–69. doi: 10.1016/j.jneuroim.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- Vernino S, Low PA, Lennon VA. Experimental autoimmune autonomic neuropathy. J Neurophysiol. 2003;90:2053–2059. doi: 10.1152/jn.00408.2003. [DOI] [PubMed] [Google Scholar]
- Wang Z, Low PA, Jordan J, Freeman R, Gibbons CH, Schroeder C, Sandroni P, Vernino S. Autoimmune autonomic ganglionopathy: IgG effects on ganglionic acetylcholine receptor current. Neurology. 2007;68:1917–1921. doi: 10.1212/01.wnl.0000263185.30294.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou CN, Patrick J, Role L, De Biasi M, Beaudet AL. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci USA. 1999;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]


