Abstract
Previous in vivo and in vitro studies have shown that Akt1 serves as a crucial regulator of vascular maturation, extracellular matrix composition, and angiogenesis in tumors. Hence, we hypothesized that Akt1 may be necessary for other angiogenesis-dependent processes, including wound healing. Using Akt1-/- and Akt2-/- mice, we demonstrate that deficiency of Akt1, but not Akt2, results in impaired assembly of collagen in skin wounds and around the blood vessels. Wounds in Akt1-/- mice, but not in Akt2-/- mice, were characterized by reduced vascular area as well as impaired vascular maturation as evidenced by reduced smooth muscle cell recruitment. Expression level of a major angiogenic growth factor, VEGF, was significantly lower in wound tissues of Akt1-/- mice as compared to WT. However, despite the impaired collagen assembly and reduced angiogenesis in Akt1-/- wounds, no significant difference in migration of fibroblasts into the wound area was observed between WT and Akt1-/- mice. Importantly, the dynamics of wound closure were similar between WT, Akt1-/-, and Akt2-/- mice. Thus, it appears that although the lack of Akt1 impairs VEGF expression, wound angiogenesis, and subsequent maturation of vasculature, it has no effect on the wound closure. These findings may have clinical applications for the improvement of treatment procedures with reported history of wound healing complications.
Keywords: Akt1, Wound healing, Angiogenesis, Endothelial cells, Fibroblasts, VEGF signaling
Introduction
Tissue response to injury is an evolutionally developed defense mechanism consisting of several interdependent stages. It is initiated by blood clotting, followed by recruitment of inflammatory cells, migration of fibroblasts into the affected area, cell proliferation and matrix deposition, sprouting of blood vessels, re-epithelialization, and, finally, wound closure [21, 32]. The concerted efforts of numerous cell types are dramatically influenced by a robust inflammatory response involving neutrophils, macrophages, and mast cells migrating into the wounded area, from nearby tissues as well as from blood [22]. Besides their antibacterial response, macrophages are known to secrete proteases needed for the degradation of matrix and the regulation of cell migration to the affected area [15]. In addition, macrophages are also known to release a number of growth factors and cytokines, thereby initiating activation of endothelial cells leading to the formation of new capillaries [22]. Angiogenesis and the restoration of blood supply is considered to be a time-limiting factor during wound healing [23]. This response is followed by fibroblast invasion leading to wound granulation [12]. Finally, after a brief lag period, keratinocytes migrate into the area undergoing extensive remodeling, thus enhancing closure of wounds [32].
Undoubtedly, the process of wound healing is regulated at multiple levels, including activation of intracellular signaling events. One of the most common pathways activated in numerous cells during inflammation, angiogenesis, and the general response to growth factors is PI3 kinase/Akt signaling [20, 34]. The PI3 kinase/Akt axis is known to be activated by several stimuli [30, 34] and Akt-mediated phosphorylation of its downstream targets influences multiple cellular responses. It is known to modulate angiogenesis and tissue remodeling during embryogenesis, ischemia, tumor growth, and myocardial infarction [34]. About 9,000 substrates have been proposed for Akt by computer modeling studies [11], due to which Akt signaling appears to be quite complicated. To add to this complexity, Akt exists in three different isoforms, Akt1, Akt2, and Akt3, which are shown to be functionally nonredundant [34]. Activation of Akt has been implicated in various malignancies [5]. Considering the possible number of substrates that Akt can phosphorylate [11], signaling pathways that are targeted for cancer treatment may either directly or indirectly involve Akt. Moreover, drugs targeting Akt and its downstream substrates such as mTOR are currently in clinical or preclinical trials for cancer therapies [24].
The vast majority of therapeutics inhibiting angiogenesis or tumor progression causes complications in wound healing, thus endangering patients undergoing surgery [10]. Despite the magnitude of this problem, the underlying mechanisms of wound complications are far from being clear. Thus, a better understanding of the role of Akt signaling in wound angiogenesis, healing, and closure will help to increase the efficacy of therapeutic strategies while minimizing side effects.
At the cellular level, Akt regulates several cell types involved in wound healing, including endothelial cells [5, 35], inflammatory cells [22], fibroblasts [12, 35], and keratinocytes [39]. Our previous study shows that Akt1, the predominant Akt isoform in endothelial cells, is important for the maturation of blood vessels and angiogenesis in tumors [4]. The defect in Akt1-/- mice was associated with a defect in the assembly of extra-cellular matrix (ECM) in skin and vascular basement membrane [4]. While skin of Akt1-/- mice exhibited impaired collagen assembly, blood vessels from tumors developed in Akt1-/- mice were leaky as a result of decreased laminin in the vascular basement membrane [4]. Moreover, in vitro studies showed that fibronectin matrix assembly by Akt1-/- fibroblasts is impaired compared to WT [35]. Thus, a series of skin and vascular abnormalities in Akt1-/- mice suggest a possible role of Akt1 signaling in cutaneous wound healing. At the same time, studies using Akt2-/- mice revealed that Akt2 is necessary for the development of adipose tissue and glucose metabolism [9], but not for hypoxia-induced angiogenesis in vivo [4]. However, impaired collagen secretion and assembly in the absence of Akt1 [4], that results in impaired skin development, is further aggravated in mice deficient in Akt1 and Akt2 [28], thus suggesting that both Akt1 and Akt2 may be important in the process of wound healing.
To carefully dissect the role of Akt1 and Akt2 isoforms in the process of wound healing, we used previously generated Akt1-/- and Akt2-/- mice for a comparative study. Since the process of wound healing is complex, it is possible that Akt signaling might differentially regulate critical components of wound healing such as inflammatory and vascular responses. Thus, several essential steps of wound healing were monitored, including wound angiogenesis and vascular maturation, fibroblast invasion and proliferation, extra-cellular matrix assembly, and expression of growth factor VEGF. Our study showed that blood vessels in Akt1-/-, but not Akt2-/-, wounds were smaller in diameter and immature with decreased vascular area. However, despite impaired collagen matrix organization, reduced VEGF expression, and defective angiogenic response in Akt1-/- wounds, no difference in overall rate of wound closure was observed. Results derived from this study may have implications in therapy for patients with post-surgery wound healing complications during various cancer treatment procedures.
Materials and methods
Animals
Akt1-/- and Akt2-/- mice, generated as previously described [3, 28], were maintained in the 129 R1/C57BL/6 background. We used sex- and age-matched wild-type and Akt1-/- and Akt2-/- littermates for the study. The animals were 8-12 weeks of age at the start of the experiments and all procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Antibodies
Polyclonal rabbit anti-human vWF and monoclonal anti-α-smooth muscle actin/HRP were purchased from DakoCytomation (Glostrup, Denmark). Rat anti-mouse CD31 (PECAM-1) was purchased from BD Biosciences (Franklin Lakes, NJ). Anti-laminin antibody and β-actin antibodies were purchased from Sigma (St. Louis, MO). Rat anti-mouse F4/80 antigen is purchased from Serotec Immunological Excellence (Kidlington, Oxford). Anti-VEGF antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Akt and phosphoS473 Akt antibodies were purchased from Cell Signaling (Danvers, MA).
Wound healing model
Animals were anesthetized under a cocktail of ketamine (80 mg/kg) and xylanine (15 mg/kg). The animal backs were shaved and wiped with 30% isopropyl alcohol, and 15-mm incision skin wounds were made on 20-22 mice/experiment by excising the skin and panniculus carnosus. The wounds were allowed to dry to form a scab. Animals were sacrificed by pentobarbital overdose, and wounds from all animals were harvested at 4 or 7 days after wounding. The wound dimensions were measured using calipers. An area of the wounds, including the complete epithelial margins 2 mm in width, was excised at each time point. A similar amount of skin from the backs of non-wounded sides was used as a control. Half of the wound tissue was fixed in 10% buffer formalin to process for the paraffin sections. The other half was immediately frozen in liquid nitrogen and stored at -80°C until used for tissue lyses for expression of growth factors.
Immunohistochemistry
Immunohistochemical staining was performed as previously described [4]. Paraffin sections were digested with 0.1% trypsin in PBS supplemented with 0.1% calcium chloride for 30 min at 37°C then were exposed to 3% hydrogen peroxide diluted in 0.02 M PBS (pH 7.4) for 5 min at room temperature to block endogenous peroxidase activity. Sections were incubated in a blocking solution containing 10% normal serum and 2% BSA for 1 h at room temperature. Primary antibody diluted with blocking solution was applied and incubated overnight at 4°C. After washing with PBS, the slides were treated with biotinylated secondary antibody and AB complex according to the manufacturer’s protocol (Vector Laboratories, Burlingame, CA). The signal was visualized using 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAKO) in 0.05 M Tris buffer (pH 7.6) containing 0.003% hydrogen peroxide. Sections were counterstained with hematoxylin (Vector). A negative control was performed to ensure the specificity of peroxidase immunostaining by replacing primary antibody with nonimmune IgG.
Immunofluorescence staining was performed as described previously [4]. Briefly, frozen sections were fixed with methanol for 2 min. The nonspecific staining was blocked with 10% normal serum and 2% BSA for 1 h at room temperature. Primary antibody diluted at an appropriate concentration was applied and incubated overnight at 4°C. After washing with PBS, the slides were incubated with Alexa Fluor-labeled secondary antibody for 2 h at room temperature. The slides were mounted with medium (DakoCytomation) and images were taken by confocal microscope.
Western blot analysis
Cell lysates were prepared using lysis buffer (20 mM Tris-HCl, pH 7.4; 1% Triton X-100, 3 mM EGTA, 5 mM EDTA, phosphatase inhibitors (10 mM sodium pyrophosphate, 5 mM sodium orthovanadate, 5 mM sodium fluoride, and 10 μM okadaic acid), protease inhibitor cocktail (Roche Diagnostics, Basel, Switzerland), and 1 mM PMSF. SDS-PAGE and Western blotting were performed as described previously [16].
Image analysis
All stained sections were analyzed using a Leica microscope as previously described [4]. The representative areas were photographed by Image-Pro Plus program. The vascular density was quantified at 20× objective and expressed as vascular area per field based on laminin-positive vessels, SMA-positive vasculature, and vessels revealed by Masson’s Trichrome staining, respectively. The macrophages were counted at 40× objective and expressed as the number of positive cells per field. Four fields were quantified for each section of control skin and wound skin from ten wild type and ten Akt1-/- mice.
Enzyme-linked immunoassay (ELISA)
Assay for VEGF in WT and Akt1-/- wound lysates was performed using ELISA Quantikine kit specific for mouse VEGF (R&D Biosystems, Minneapolis, MN). Assay was performed as per the manufacturer’s protocol.
Statistical analysis
All the data are presented as means ± SD. We performed all the analyses using two-sample t-tests with a two-tailed distribution and the significance was set at 0.05 levels (marked with an asterisk wherever data is statistically significant).
Results
Impaired angiogenesis in wounds of Akt1-/-, but not Akt2-/-, mice
Our previous study showed that Akt1 is the predominant Akt isoform in endothelial cells where it accounts for ∼70% of the total Akt activity [4]. In the absence of Akt1, endothelial cells and fibroblasts exhibit impaired integrin activation, adhesion to extracellular matrix proteins, and cell migration in response to growth factors [35]. These studies demonstrating the importance of Akt1 in endothelial cells and fibroblasts prompted us to further delineate its role in wound angiogenesis. To this end, wounds were created on the backs of Akt1-/- or Akt2-/- mice along with WT controls. Since it generally takes ∼7-9 days to heal this type of wound, tissues were collected at the mid-stage as well as at the end of the healing process, i.e., at days 4 and 7. These tissues were stained for various endothelial/blood vessel-specific markers.
As early as at day 4, staining of WT wounds for the blood vessel marker laminin showed ∼10-fold increase in vascular area compared to normal skin, reflecting a robust angiogenic response to injury (Fig. 1a, b). This response was substantially blunted in Akt1-/- wounds, showing ∼40% decrease in vascular area of injured tissues compared to WT (P = 0.0067) (Fig. 1a, b). Most blood vessels formed in Akt1-/- mice had a smaller diameter than those in WT (Fig. 1a). In contrast to Akt1-/-, no differences in vascular area were found between Akt2-/- and WT mice (P = 0.57) (Fig. 1c), thus showing that the absence of Akt2 does not affect wound angiogenesis.
Fig. 1.
Absence of Akt1, but not Akt2, in mice results in decreased blood vessel area in wounded skin. (a) Micrographs of day 4 wound sections from WT, Akt1-/-, and Akt2-/- mice showing laminin-positive blood vessels. (b) Laminin-positive blood vessel area in WT and Akt1-/- wounded vs. control skin sections as analyzed by Image-Pro Plus. (c) Laminin-positive vessel numbers in WT and Akt2-/- wound sections quantified by manual counting of the photographed sections. Scale bar: 20 μm
Akt1, but not Akt2, deficiency results in matrix abnormalities in wound tissues
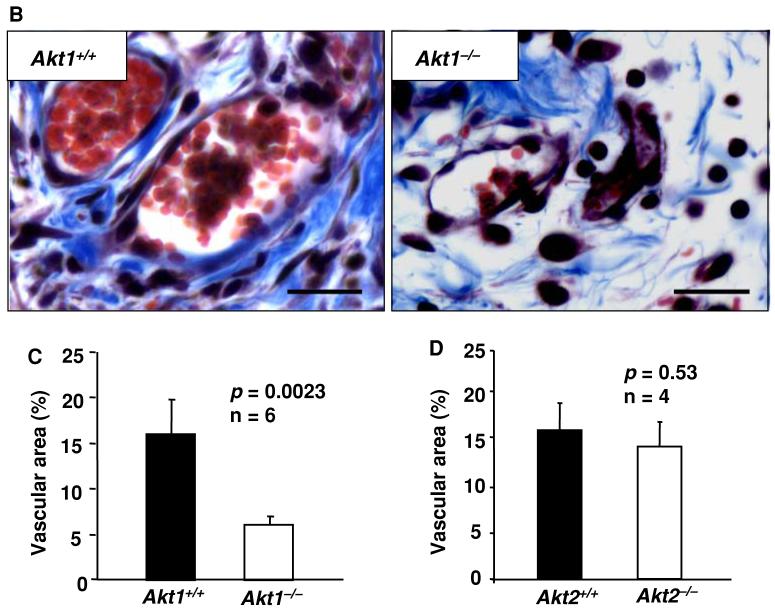
We previously showed that Akt1 is necessary for the secretion and assembly of collagen and fibronectin in skin and laminin within the basement membrane of blood vessels [4, 35]. Hence, we next analyzed the organization of collagen fibrils in WT, Akt1-/-, and Akt2-/- wounds using Masson’s trichrome reagent which specifically stains skin collagen blue. Our analysis showed that while WT skin wound appears dense and tightly packed with well-organized collagen fibers, collagen matrix in Akt1-/- skin wounds was abnormal, resulting in loose and distended skin (Fig. 2a). The defect was apparent not only in wounded skin but also in the blood vessels and the area surrounding the vasculature in Akt1-/- mice compared to WT (Fig. 2b). Similar to the results of laminin staining, vascular area was greatly diminished in Akt1-/- wounds (P < 0.003) (Fig. 2a, c). No abnormalities in collagen amount and organization as well as in vascular area were observed in wounds of Akt2-/- mice, indicating the minimal role for this isoform in wound angiogenesis and healing (Fig. 2d).
Fig. 2.
Absence of Akt1, but not Akt2, in mice results in impaired collagen matrix organization in wounded skin. (a) Micrographs of wound sections from WT, Akt1-/-, and Akt2-/- mice showing collagen matrix assembly stained in blue and blood vessels stained in red. (b) Micrographs of wound sections from WT and Akt1-/- mice showing collagen matrix assembly stained in blue in and around the blood vessels. (c) Blood vessel area in WT and Akt1-/- wound sections as analyzed by Image-Pro Plus software. (d) Blood vessel area in WT and Akt2-/- wound sections as analyzed by Image-Pro Plus software. Scale bar: 20 μm
Impaired maturation is consistent with delayed angiogenesis in Akt1-/- wounds
Our previous studies in the tumor angiogenesis model in Akt1-/- mice showed that Akt1 is necessary for the maturation of blood vessels [4], suggesting that a similar defect might be also present in wound vasculature. Accordingly, we performed double fluorescence staining of wound tissues for α-smooth muscle cell actin (SMA, a marker for mature blood vessels) and CD31 (an endothelial marker).
Figure 3a and b shows that a major portion of CD31-positive blood vessels in Akt1-/- wounds were not stained for SMA. It is evident that an area of CD31-positive blood vessels in Akt1-/- wounds was reduced to ∼65% of that of WT wounds. Likewise, the SMA-positive area in Akt1-/- wounds was also reduced to ∼50% of that of WT (Fig. 3a, b). Overall, ∼30% and ∼20% of blood vessels were SMA positive in WT and Akt1-/- wounds, respectively, and the reduction in the number of mature blood vessels reflected slightly more than the overall reduced vascular area in Akt1-/- wounds.
Fig. 3.
Blood vessel maturation in 4-day wounds is delayed in Akt1-/- mice. (a) Micrographs of frozen wound sections from WT and Akt1-/- mice showing CD31- and SMA-positive blood vessels. (b) Blood vessel area positive for CD31 and SMA as analyzed by Image-Pro Plus software. (c) Micrographs of paraffinized wound sections from WT and Akt1-/- mice showing SMA-positive blood vessels. (d) SMA-positive blood vessel number as analyzed by manual counting of microscopic fields. Results are expressed as the mean ± sd. Scale bar: 20 μm
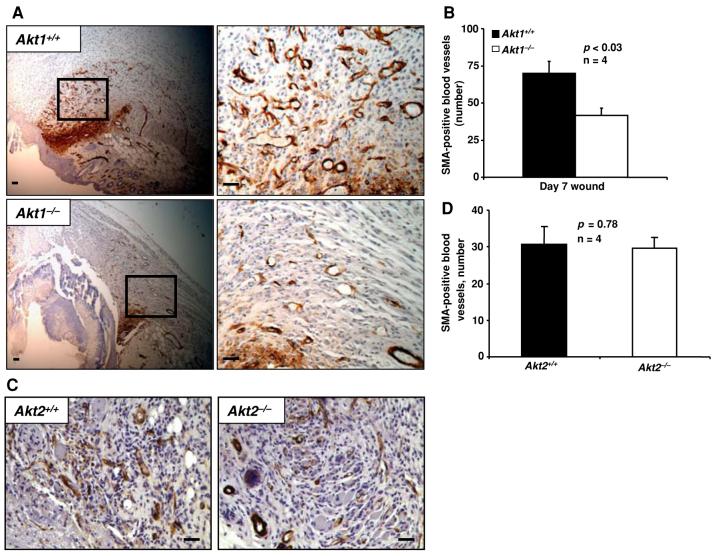
Similar results were observed using SMA-stained paraffin sections of 4-day-old wounds where ∼2-fold decrease in SMA-positive blood vessels was observed in Akt1-/- wounds compared to WT (P < 0.005) (Fig. 3c, d). We further extended our analysis to 7-day-old wounds. On day 7, Akt1-/- wounds still exhibited lower numbers of SMA-positive blood vessels (∼40% decrease) compared to WT (P < 0.03) (Fig. 4a, b). However, we found no difference in the overall SMA-positive blood vessel number in Akt2-/- wounds compared to WT (Fig. 4c, d).
Fig. 4.
Akt1-/- mice, but not Akt2-/-mice, exhibit impaired blood vessel maturation in wounds even on day 7 after wounding. (a) Micrographs of paraffin wound sections from WT and Akt1-/- mice showing SMA-positive blood vessels. (b) SMA-positive blood vessel number as analyzed by manual counting of microscopic fields. (c) Micrographs of paraffin wound sections from WT and Akt2-/- mice showing SMA-positive blood vessels. (d) SMA-positive blood vessel number in WT and Akt2/ wounds as analyzed by manual counting of microscopic fields. Results are expressed as the mean ± sd. Scale bar: 20 μm
Akt1 deficiency results in impaired infiltration of macrophages and reduced expression of VEGF in wound tissues
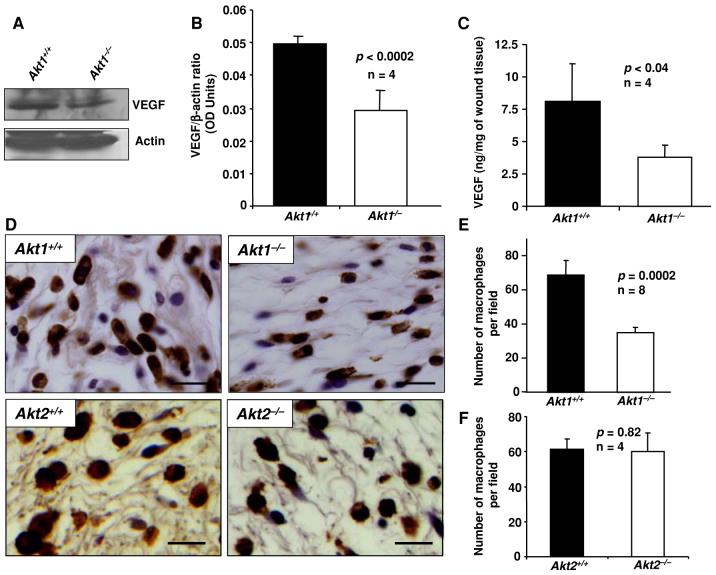
Many cell types including inflammatory cells, endothelial cells, and keratinocytes secrete VEGF under hypoxic conditions [6, 22]. Akt is an important candidate that regulates hypoxia-mediated expression of VEGF by these cells types [8]. Moreover, tumor cells are also known to secrete VEGF during hypoxia involving mammalian target of rapamycin (mTOR) and hypoxia inducible factor-α (HIF1α) pathway, which is directly under the control of Akt [8]. Since Akt1 is the major isoform of Akt in skin [4], we hypothesized that deficiency of Akt1 would result in decreased expression of VEGF in Akt1-/- skin wounds. Hence we sought to analyze the VEGF expression in wound tissues isolated from WT and Akt1-/- mice. Western analysis of 4-day-old wound lysates showed that the expression of VEGF in Akt1-/- wounds were reduced by approximately 40% compared to WT (Fig. 5a, b). To specifically measure the amount of VEGF164 (most predominant VEGF isoform containing 164 amino acids) isoform of mouse VEGF in Akt1-/- mouse skin wounds, we employed ELISA specific for mouse VEGF164. Our data show that deficiency of Akt1 in wounds resulted in >50% decrease in VEGF164 compared to WT (Fig. 5c).
Fig. 5.
Akt1 deficiency results in impaired macrophage infiltration and reduced expression of VEGF in wounds. (a) Western blots of wound lysates from WT and Akt1-/- mice probed with anti-VEGF antibodies. (b) Densitometry analysis of bands showing phosphorylation (activity) and expression levels of VEGF. (c) Bar graph showing expression levels of VEGF in WT and Akt1-/- wounds as measured by ELISA. (d) Micrographs of paraffin-embedded sections of WT, Akt1-/-, and Akt2-/- wounds showing infiltrated macrophages into the wound area. (e) Quantification of the number of macrophages infiltrated into the wound area of WT and Akt1-/- mice by manual counting of the photographed fields. (f) Quantified data showing the number of macrophages infiltrated into the wound area of WT and Akt2-/- mice by manual counting of the photographed fields. Results are expressed as the mean ± sd. Scale bar: 20 μm
While the role of macrophages in angiogenesis and wound healing remains somewhat controversial, recent reports show that macrophages respond rapidly to injury and hypoxia resulting in secretion of growth factors, including VEGF, which, in turn, accelerates wound closure [21, 22]. Accordingly, we determined the number of macrophages infiltrated into wound area of WT and Akt1-/- mice using staining for F4/80 antigen specific to macrophages. Macrophages were mostly distributed in the wound area and at the edges of wound between the layers of the epithelial cells and the muscles. The density of macrophages in Akt1-/- wounds was ∼50% lower compared to that in WT (P = 0.0002) (Fig. 5d, e). In Akt2-/- mice, however, no defects in macrophage infiltration were observed, indicating that Akt1, but not Akt2, is essential for macrophage recruitment (Fig. 5d, f).
Delayed vascularization and impaired collagen assembly in Akt1-/- wounds does not affect fibroblast migration and the rate of wound closure
Based on the above-described defects in the decreased vascularization and impaired collagen assembly in wounds of Akt1-/- mice, it is easy to predict that the overall process of wound healing would be impaired in Akt1-/- mice. However, the measurements of wound size in WT, Akt1-/-, and Akt2-/- mice revealed no differences in wound closure among these groups. This surprising result prompted us to analyze the day-by-day kinetics of wound healing as well as the effect of aging on this process. In all the three groups, WT, Akt1-/-, and Akt2-/- mice, the process of wound healing was similar at the age of 2 months (Fig. 6a-c). Likewise, no differences in wound closure were observed in 4-month-old Akt1-/- mice compared to WT (Fig. 6a). This analysis revealed that the absence of the Akt1 or Akt2 isoform in mice does not affect healing of wounds in vivo. Only a modest delay in wound closure on days 5 and 6 (data not significant) was observed in Akt2-/- mice, which may be due to the previously reported delay in thrombus formation as a result of impaired platelet secretion [40].
Fig. 6.
Deficiency of Akt1 or Akt2 does not affect skin wound healing and migration of fibroblasts in vivo. (a) Bar graph showing the size of wounds in 2- and 4-month-old WT and Akt1-/- mice on day 4. (b) Bar graph showing the size of wounds in 2-month-old WT and Akt1-/- mice on day 7. (c) Bar graph showing the size of wounds in 2-month-old WT and Akt2-/- mice measured on days 1-7. (d) Micrographs of paraffin-embedded sections of WT and Akt1-/- wounds showing vimentin, which specifically stains fibroblasts. (e) Quantification of the number of fibroblasts migrated into the wound area of WT and Akt1-/- mice as analyzed by Image-Pro Plus software. Results are expressed as the mean ± sd. Scale bar: 20 μm
In addition to blood supply, another important factor that determines the course of wound healing is the ability of fibroblasts to migrate to the wound area and perform the necessary tissue repair. Migration of fibroblasts on fibronectin is also impaired in the absence of Akt1 [35]. Since we did not observe a delay in wound closure in Akt1-/- mice despite the impaired blood vessel maturation and macrophage infiltration, we determined whether fibroblast migration to the wound area is affected in Akt1-/- mice. We stained WT and Akt1-/- wound sections for vimentin, a marker for fibroblasts. Our analysis showed that migration of fibroblasts into the wound is not affected in Akt1-/- mice (P = 0.072) (Fig. 6d, e).
Discussion
Recent studies using cell culture-based and animal models conclusively showed that Akt, a major target downstream of PI3 kinase, is important for various angiogenesis-dependent functions such as recovery from ischemia and tumor progression [4, 26, 34, 37]. To determine the role of Akt1 and Akt2 isoforms, two isoforms of Akt necessary for the normal development of skin [28], in hypoxia-induced angiogenesis and cutaneous wound healing, we created wounds in WT, Akt1-/-, and Akt2-/- mice and analyzed the samples for key parameters known to be crucial for the process of wound healing in skin. Our study showed that deficiency of Akt1, but not Akt2, results in substantially diminished vascular area in wound tissues compared to WT. Impaired vascular response seems to be due to the reduced expression of VEGF, an endothelial-specific growth factor, in wounds of Akt1-/- mice. Normal skin and wound areas in Akt1-/-, but not Akt2-/-, mice were characterized by impaired collagen assembly, compared to WT. Additionally, analysis of vascular maturation based on SMA staining indicated that Akt1, but not Akt2, is necessary for the maturation of blood vessels in wounds. Despite observed abnormalities in vascular density, vascular maturation, and assembly of collagen in the wound tissues in Akt1-/- mice, the absence of either Akt1 or Akt2 did not have any effect on the rate of wound closure. Thus, in addition to the regulation of VEGF expression and, as a result, the process of angiogenesis, Akt1 appears to trigger alternative pathways in various cell types.
It is well established that Akt is activated upon stimulation by growth factors and cytokines [30]. Our previous studies have clearly documented that Akt is activated in endothelial cells and fibroblasts downstream of many growth factors such as VEGF, Fibroblast growth factor (FGF), and Insulin-like growth factor-1 (IGF-1) [35]. In addition to growth factors, interactions of integrins with extracellular matrix proteins also activate Akt in vascular cells as a result of outside-in signaling [35]. This activation of Akt is necessary for the cell survival and proliferation of endothelial cells and protection from ‘anoikis’ [30]. In addition, Akt activated by VEGF is involved in outside-in activation of integrins thus facilitating adhesion of endothelial cells and cancer cells to various matrix proteins [32, 35]. Thus, it is clear that Akt is an important signaling molecule activated downstream of VEGF in endothelial cells.
At the same time, ablation of Akt1 leads to diminished VEGF expression and reduced angiogenesis in the setting of ischemia [26]. However, when an exogenous source of VEGF, such as tumor xenografts is provided, angiogenesis is augmented in Akt1-/- mice [4, 34]. Thus, it seems to be important to discriminate the role of Akt upstream of VEGF expression from its function as a signaling molecule downstream of VEGF. In this study, VEGF expression in wounds was triggered by tissue injury and hypoxia and VEGF levels in Akt1-/- mice were substantially lower than that in WT mice. While tumor cells express VEGF necessary for the chemotaxis of endothelial cells into the growing tumor tissue, the process of wound angiogenesis is dependent on endogenously expressed VEGF produced by various cells, including macrophages and keratinocytes [13, 27]. Thus, inactivation of Akt causes inhibition of VEGF expression as has been reported by several groups [29, 33, 38, 41]. In turn, reduced VEGF levels seem to be responsible for defective angiogenesis in wounds of Akt1-/- mice.
Recent developments have implicated that Akt is necessary for the hypoxia-driven expression of VEGF by endothelial cells [18], macrophages [13], and keratinocytes [27] involving an mTOR and HIF1α signaling pathway [19]. Although mTOR does not regulate stabilization of HIF1α, it enhances its transcriptional ability through interaction of HIF1α with its associating protein ‘raptor’, suggesting that HIF1α is a direct substrate of mTOR-Raptor [17]. mTOR signaling is impaired in Akt1 knockout mice (P.R. Somanath and T.V. Byzova, unpublished observation), leading to diminished expression of VEGF in Akt1-/- wound tissues. As a result, overall wound angiogenesis and maturation is diminished in Akt1-/- mice.
Reduced levels of VEGF in Akt1-/- mice might be responsible for the defects in proliferation and integrin-dependent migration of endothelial cells. Importantly, Akt also mediates integrin activation and integrin-dependent adhesion and migration of endothelial cells and fibroblasts on various matrix proteins [4, 35]. Since endothelial cell migration represents an important event in angiogenesis, this mechanism, in addition to reduced VEGF levels, also contributes to diminished angiogenic response in Akt1-/- wounds. Thus, in the settings of tissue injury, Akt pathway plays a triple role in integrin signaling: it controls the levels of integrin activator, VEGF, it determines VEGF-induced integrin activation and ligand binding and, finally, it is involved in post-ligand binding (outside-in) integrin signaling.
Integrin activity is crucial not only for adhesive and migratory properties of cells, but also for assembly of extracellular matrix proteins such as fibronectin, laminin, and collagen [2, 7, 35]. We have previously shown that impaired inside-out activation of α5β1 integrin in mouse fibroblasts due to Akt1 deficiency resulted in impaired fibronectin matrix assembly in vitro [35]. This was corroborated with impaired assembly of collagen in Akt1-/- skin and laminin in tumor blood vessels developed in Akt1-/- mice [4]. Current study showed that collagen assembly is also impaired in Akt1-/- wound tissues as compared to WT. Since laminin and collagen are integral components of the vascular basement membrane, impaired assembly of these matrix proteins might result in impaired vascular maturation. As evidenced by the reduced number of SMA positive blood vessels, maturation of blood vessels is compromised in Akt1-/- wounds on both day 4 and day 7 after creation of wounds.
Extra-cellular matrix assembly is particularly important for the tissue remodeling upon injury [36]. Akt1 deficiency results in decreased heart size [3] and impaired tissue regeneration upon myocardial ischemia [25]. In contrast, constitutive activation of Akt pathway by expression of myrAkt1 in the heart results in cardiac hypertrophy and enhanced tissue regeneration [31]. Akt1 has also been shown to be important for tissue remodeling followed by hind-limb ischemia [1]. All of these pathological events are dependent on extracellular matrix remodeling and assembly [36]. This study also emphasized a crucial role of Akt signaling in regulation of extracellular matrix composition. Since extracellular matrix appears to be defective in Akt1-/- wounds, we concluded that despite the normal wound closure, the process of wound healing is impaired in Akt1-/- mice compared to WT. Due to the abnormal matrix composition, the scar tissue in Akt1-/- mice might have quite different mechanical characteristics, which, in turn, will affect skin responses to further challenges. It is important to recognize that other events during wound healing, i.e., fibroblast and keratinocyte migration into the wound tissues are also dependent on the density or extracellular matrix and its degradation by the matrix metalloproteinases (MMPs) [32]. “Loose” collagen matrix in Akt1-/- wounds effective migration of keratinocytes into the wound area, therefore, contributing to normalization of wound closure in Akt1-/- mice despite defective angiogenesis.
A number of recent reports demonstrate that due to multiple possible effectors, manipulation of Akt activity might lead to unpredictable results in vivo [26, 31, 34]. It was shown that upregulation of Akt activity might lead either to increased or diminished angiogenic responses in tissues [26, 34]. At the same time, inhibition of Akt might also have opposite consequences on the vasculature: depending on the pathophysiological process involved, it might promote vascularization in vivo [4] or diminish it [26, 34]. While manipulating with the activity of multi-task signaling molecules such as Akt, it is essential to consider the diversity of its downstream targets and an array of functions it mediates in various different cell types.
Besides the development of tumor resistance [14], anti-angiogenic therapies suffer a major drawback due to numerous side effects and post-surgery wound healing complications [10]. Our present study suggests that even though Akt1 deficiency results in impaired vascular maturation and angiogenesis in cutaneous wounds, wound closure is normal due to effective migration of keratinocytes and fibroblasts into the Akt1-/- wound area through the loose extracellular matrix. Hence, inhibition of Akt signaling upon use of Akt inhibitors for therapy in cancer patients should not cause serious wound healing complications. Since many procedures in clinical trials for anti-angiogenic therapy for cancer develop tumor resistance and wound healing complications, inhibition of Akt would be an ideal choice to avoid these complications while inhibiting angiogenesis and cancer progression without affecting the normal host tissue. Additionally, this will also overcome wound healing complications on treatments involving surgical procedures. Overall, the resulting effect of Akt ablation is dictated by a complex combination of several responses, often with counter-balancing effects in vivo and hence could be an effective therapeutical approach for various skin-related malignancies as well as in cosmetic surgery.
Acknowledgments
This work was supported by NIH Grant HL071625 to T. V. Byzova. Authors thank Dr. Nissim Hay, University of Illinois at Chicago for providing Akt1-/- and Akt2-/- mice for the study. Authors also thank Alla Gomer and Miroslava Tischenko for their technical assistance.
Footnotes
Payaningal R. Somanath and Juhua Chen have contributed equally to this work
References
- 1.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alavi A, Stupack DG. Cell survival in a three-dimensional matrix. Methods Enzymol. 2007;426:85–101. doi: 10.1016/S0076-6879(07)26005-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YL, Law PY, Loh HH. Inhibition of PI3 K/Akt signaling: an emerging paradigm for targeted cancer therapy. Curr Med Chem Anticancer Agents. 2005;5:575–589. doi: 10.2174/156801105774574649. [DOI] [PubMed] [Google Scholar]
- 6.Detmar M, Yeo KT, Nagy JA, Van De WL, Brown LF, Berse B, Elicker BM, Ledbetter S, Dvorak HF. Keratinocyte-derived vascular permeability factor (vascular endothelial growth factor) is a potent mitogen for dermal microvascular endothelial cells. J Invest Dermatol. 1995;105:44–50. doi: 10.1111/1523-1747.ep12312542. [DOI] [PubMed] [Google Scholar]
- 7.Ekblom P, Lonai P, Talts JF. Expression and biological role of laminin-1. Matrix Biol. 2003;22:35–47. doi: 10.1016/s0945-053x(03)00015-5. [DOI] [PubMed] [Google Scholar]
- 8.Garcia JA. HIFing the brakes: therapeutic opportunities for treatment of human malignancies. Sci STKE. 2006;2006:e25. doi: 10.1126/stke.3372006pe25. [DOI] [PubMed] [Google Scholar]
- 9.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish D, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005;69(Suppl 3):25–33. doi: 10.1159/000088481. [DOI] [PubMed] [Google Scholar]
- 11.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 13.Jeon SH, Chae BC, Kim HA, Seo GY, Seo DW, Chun GT, Kim NS, Yie SW, Byeon WH, Eom SH, Ha KS, Kim YM, Kim PH. Mechanisms underlying TGF-beta1-induced expression of VEGF and Flk-1 in mouse macrophages and their implications for angiogenesis. J Leukoc Biol. 2007;81:557–566. doi: 10.1189/jlb.0806517. [DOI] [PubMed] [Google Scholar]
- 14.Jubb AM, Oates AJ, Holden S, Koeppen H. Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer. 2006;6:626–635. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- 15.Kahari VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Land SC, Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem. 2007;282:20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 20.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin P. Wound healing - aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 22.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, Freyssinet JM. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–2604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- 24.Morgensztern D, McLeod HL. PI3 K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16:797–803. doi: 10.1097/01.cad.0000173476.67239.3b. [DOI] [PubMed] [Google Scholar]
- 25.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill BT, Abel ED. Akt1 in the cardiovascular system: friend or foe? J Clin Invest. 2005;115:2059–2064. doi: 10.1172/JCI25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberley CC, Gourronc F, Hakimi S, Riordan M, Bronner S, Jiao C, Dunnwald M. Murine epidermal side population possesses unique angiogenic properties. Exp Cell Res. 2008;314(4):720–728. doi: 10.1016/j.yexcr.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosano L, Di C, Spinella VF, Tortora G, Nicotra MR, Natali PG, Bagnato A. Combined targeting of endothelin A receptor and epidermal growth factor receptor in ovarian cancer shows enhanced antitumor activity. Cancer Res. 2007;67:6351–6359. doi: 10.1158/0008-5472.CAN-07-0883. [DOI] [PubMed] [Google Scholar]
- 30.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 31.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 32.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 33.Slomiany MG, Rosenzweig SA. Hypoxia-inducible factor-1-dependent and -independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J Pharmacol Exp Ther. 2006;318:666–675. doi: 10.1124/jpet.106.104158. [DOI] [PubMed] [Google Scholar]
- 34.Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somanath PR, Kandel ES, Hay N, Byzova TV. Akt1 signaling regulates integrin activation, matrix recognition, and fibronectin assembly. J Biol Chem. 2007;282:22964–22976. doi: 10.1074/jbc.M700241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 37.Sun JF, Phung T, Shiojima I, Felske T, Upalakalin JN, Feng D, Kornaga T, Dor T, Dvorak AM, Walsh K, Benjamin LE. Microvascular patterning is controlled by fine-tuning the Akt signal. Proc Natl Acad Sci USA. 2005;102:128–133. doi: 10.1073/pnas.0403198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trisciuoglio D, Iervolino A, Zupi G, Del Bufalo D. Involvement of PI3 K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol Biol Cell. 2005;16:4153–4162. doi: 10.1091/mbc.E04-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 40.Woulfe D, Jiang H, Morgans A, Monks R, Birnbaum M, Brass LF. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J Clin Invest. 2004;113:441–450. doi: 10.1172/JCI20267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao D, Li M, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Lew KL, Zeng Y, Marynowski SW, Singh SV. Diallyl trisulfide inhibits angiogenic features of human umbilical vein endothelial cells by causing Akt inactivation and down-regulation of VEGF and VEGF-R2. Nutr Cancer. 2006;55:94–107. doi: 10.1207/s15327914nc5501_12. [DOI] [PubMed] [Google Scholar]