Abstract
Previous functional neuroimaging studies of temporal-order memory have investigated memory for laboratory stimuli that are causally unrelated and poor in sensory detail. In contrast, the present functional MRI (fMRI) study investigated temporal-order memory for autobiographical events that were causally interconnected and rich in sensory detail. Participants took photographs at many campus locations over a period of several hours, and the following day they were scanned while making temporal-order judgments to pairs of photographs from different locations. By manipulating the temporal lag between the two locations in each trial, we compared the neural correlates associated with reconstruction processes, which we hypothesized depended on recollection and contribute mainly to short lags, and distance processes, which we hypothesized to depend on familiarity and contribute mainly to longer lags. Consistent with our hypotheses, parametric fMRI analyses linked shorter lags to activations in regions previously associated with recollection (left prefrontal, parahippocampal, precuneus, and visual cortices) and longer lags with regions previously associated with familiarity (right prefrontal cortex). The hemispheric asymmetry in prefrontal cortex activity fits very well with evidence and theories regarding the contributions of left vs. right prefrontal cortex to memory (recollection vs. familiarity processes) and cognition (systematic vs. heuristic processes). In sum, using a novel photo-paradigm this study provided the first evidence regarding the neural correlates of temporal-order for autobiographical events.
When we remember personally experienced past events, or episodic memory retrieval (Tulving, 1983), we usually retrieve not only what events happened (item memory) but also when they happened (temporal-order memory). Temporal-order memory is an important form of source memory (Johnson et al., 1993) and an integral and a defining characteristic of episodic memory (Wheeler et al., 1997). Indeed, in many situations, episodic memories are useful only to the extent that temporal-order information is also available (e.g. remembering today's vs. yesterday's parking spot). Lesion (Milner, 1971; Milner et al., 1991; Petrides, 1991) and functional neuroimaging (Cabeza et al., 1997; Eyler Zorilla et al., 1996; Nyberg et al., 1996) studies have shown that the prefrontal cortex (PFC) is a critical region for temporal-order memory. There is also evidence of the important role of the medial temporal lobes (MTL) (Eichenbaum & Fortin, 2003; Konishi et al., 2002; Downes et al., 2002). However, the neural correlates of temporal-order memory, especially as they relate to autobiographical events, are not well understood. Addressing this issue was the goal of the present fMRI study.
It has been suggested that temporal-order memory for autobiographical memories involves both reconstruction and distance processes (Friedman, 1993, 2004). Reconstruction processes are effortful operations that include retrieving contextual details and using them to infer the order of past events (Curran & Friedman, 2003; Skowronski, Walker & Betz, 2003). For example, when trying to determine if, during a one-day tour of Paris, the visit to the Louvre occurred before or after lunch, one might remember the pleasant feeling of resting tired legs in a comfortable restaurant chair and conclude that the visit to the Louvre happened before lunch. Moreover, this inference might be confirmed by the image of walking from the Louvre to a nearby restaurant in the Rue de Rivoli. In contrast, distance processes are less effortful operations that rely on feelings associated with the strength of the memory trace. For example, one does not need to use reconstruction processes to conclude that the clearly remembered trip to Paris occurred more recently than a vaguely remembered trip to London. Although reconstruction and distance processes could be used to discern the temporal order of the same set of events, reconstruction processes are generally more effective for events that are relatively close in time whereas distance processes are usually more effective for events that are sufficiently far away (Burt, Kemp, Grady & Conway, 2000; for reviews see Friedman, 1993, 2004). Closeness in time benefits reconstruction processes because it makes causal links more obvious. In the aforementioned example, reconstructing the Louvre-restaurant order is facilitated by the causal relationship between walking and feeling tired, which might not exist if these events occurred farther away in time. In contrast, closeness in time reduces the effectiveness of distance processes because it attenuates differences in memory strength. It is worth noting that distance processes might be used for ordering events that are close in time when the interval between encoding and retrieval is very short, as is the typical case in laboratory studies of temporal-order memory (e.g., comparing the order of words presented two minutes ago vs. five minutes ago in the same list). In contrast, the use of sensory-poor, causally unrelated stimuli in these studies hinders the use of reconstruction processes (e.g., it is difficult to reconstruct what happened between the two words).
In sum, temporal-order memory for autobiographical events is likely to involve both reconstruction processes (increase with closeness) and distance processes (decrease with closeness); in contrast, temporal-order memory for laboratory events often taps mainly distance processes. Thus, although several functional neuroimaging studies have investigated temporal-order memory for laboratory events (Cabeza et al., 1997; Eyler Zorilla et al., 1996; Konishi et al., 2002, 2006; Nyberg et al., 1996; Suzuki et al., 2002), it is critical to also investigate the neural correlates of temporal-order memory for more complex real-world events, such as autobiographical memories.
The reconstruction-distance distinction (Friedman, 1993, 2004) in retrieving temporal order memory for autobiographical events is similar the recollection-familiarity distinction (Mandler, 1980; Yonelinas, 2002) in memory retrieval (see also Bastin, Van der Linden, Michel, & Friedman, 2004; Curran & Friedman, 2003). Like recollection, reconstruction involves the recovery of contextual details, and, similar to familiarity, distance processes rely on the strength of memory traces. There is growing evidence that recollection and familiarity involve distinct neural correlates (Eichenbaum, Yonelinas, & Ranganath, 2007; Rugg & Yonelinas, 2003). Recollection is associated with greater activity in regions including the left PFC (Dobbins et al., 2003; Dobbins et al., 2004; Eldridge et al., 2000; Henson et al., 1999a), hippocampus and posterior parahippocampal cortices (Eldridge et al., 2000; for a review, see Eichenbaum, Yonelinas, & Ranganath, 2007), posterior cingulate/precuneus cortices (for a review, see Wagner et al., 2005) and visual cortex (Kahn et al., 2004; Wheeler et al., 2000; Cabeza et al., 2004). In contrast, familiarity processes have been associated with greater activity in regions such as the right PFC (Dobbins et al., 2003; Dobbins et al., 2004; Henson et al., 1999a) and perirhinal cortices (for a review, see Eichenbaum, Yonelinas, & Ranganath, 2007). In sum, recollection and familiarity processes differ with respect to activation in MTL and posterior brain regions and hemispheric asymmetry in the PFC. Previous fMRI studies have also associated differences in PFC lateralization with retrieval success (hits > misses) and retrieval effort (misses > hits). We and others have associated left PFC with retrieval success (Prince et al., 2005) and right PFC with retrieval effort (e.g. Fleck et al., 2006; Henson et al., 2000), and others have found hemispheric asymmetries in PFC activity that reflects qualitative rather than quantitative differences (Dobbins et al., 2002; Dobbins et al., 2003; Ranganath et al., 2000). For example, in an event-related fMRI study Dobbins et al. (2003) found that source memory judgments activated left more than right PFC, whereas recency judgments activated right more than left PFC, but importantly lateralization differences were independent of retrieval success. Thus, accuracy might also be an important factor in distinguishing the neural correlates of the reconstruction-distance distinction.
Although some fMRI evidence is available regarding the contribution of these regions in temporal-order memory for laboratory stimuli (Konishi et al., 2006; Konishi et al., 2002; Suzuki et al., 2002), their contribution to temporal-order memory for autobiographical stimuli is unknown. The goal of the present study was to investigate the neural correlates underlying temporal-order memory for autobiographical events. To address this goal while maintaining control over critical memory factors, such as accuracy, we adapted a novel photo paradigm we previously applied to recognition memory of autobiographical versus laboratory events (Cabeza et al., 2004) to investigate memory for temporal order. In our previous study (Cabeza et al., 2004), we found that memories elicited via the photo paradigm contained greater self-referential processing, visual/spatial imagery and recollection compared to laboratory memories, therefore validating the use of this paradigm to study the retrieval of complex real-life events or controlled autobiographical memories (for review see Cabeza & St. Jacques, 2007).
Given that reconstruction processes are more effective for discriminating events close in time whereas distance processes are more effective for discriminating events farther away in time, we assumed that brain regions involved in reconstruction processes would show greater activity for shorter than longer lags, whereas regions involved in distance processes would show greater activity for longer than shorter lags (e.g., Suzuki et al., 2002). Our fMRI predictions were based on the similarity between the reconstruction-distance distinction (Friedman, 1993, 2004) and the recollection-familiarity distinction (Mandler, 1980; Yonelinas, 2002). We predicted that reconstruction processes during shorter lags would involve regions previously associated with recollection, such as left PFC, hippocampus and posterior parahippocampal cortices, posterior cingulate/precuneus cortices and visual cortex. In contrast, distance processes during longer lags would involve regions previously associated with familiarity, such as right PFC and perirhinal cortices. In sum, we predicted that temporal-order decisions for shorter lags (reconstruction processes/recollection) would differentially engage left PFC and temporal-order decisions for longer lags (distance processes/familiarity) would differentially engage right PFC. On the basis of Dobbins et al.'s (2003) findings we predicted that this hemispheric asymmetry would not vary with retrieval success (hits vs. misses). Additionally, we predicted that shorter lags would elicit greater activity in other regions associated with recollection, such as the hippocampus and posterior parahipipocampal cortices, posterior cingulate/precuneus cortices, and visual cortex.
Methods
Participants
Seventeen young adults (9 males; Mean Age = 21.6, S.D. = 2.7) participated in the study. Participants were healthy, right-handed, native English speakers, with no history of neurological or psychiatric episodes. They were mostly undergraduate students at Duke University and in all cases were very familiar with the Duke campus. Participants gave written informed consent for a protocol approved by the Duke University Institutional Review Board.
Materials and Procedure
Photo-taking session
The study took place on two consecutive days. On Day 1, participants took 480 photographs at 80 campus locations (6 pictures per location). The locations were well-known places within the Duke West Campus (e.g., the Duke Chapel), both indoors and outdoors. The order of the locations was selected to reduce the correlation between temporal distance and spatial distance, r = .13, p = .07, such that participants visited new locations in the same building/spatial region at different time points during the day and followed a circular route in that they started and ended at the same building (for examples see Figure 1-A). Participants were provided with a digital camera (Kodak Easy Share CX6200), a booklet with 80 locations (one per page), and training on how to use the camera and take photos. Participants were provided with instructions during the encoding task. They were told that the study was interested in how people take photographs and they were asked to consider each photo as a distinct event by paying attention to the particular physical (e.g., viewpoint, body position, etc.) and psychological (e.g., preference, mood, etc.) phenomena associated with each picture to ensure that they were not simply clicking the camera without viewing the scene they were photographing. The photo-taking session was a classic incidental encoding task with instructions designed to draw attention from intentional memory encoding and with no mention of a subsequent memory test. Participants were instructed to complete the photo-taking task without stopping for breaks so that picture taking was continuous (mean time = 5.00 hours, S.D. = 0.40 hours). The camera's LCD screen was blocked to prevent participants from reviewing the photos. At each location participants took six pictures from different positions and/or angles. Participants were instructed to tear off one page of the booklet after each location so that at the end of the photo session the whole booklet would be completed. The cameras were returned the cameras to the lab immediately after the last picture was taken and the photographs were digitally enhanced using a finite impulse response (FIR) filter.
Figure1.
A. The Duke Campus map shows the locations and route of the locations where the photographs were taken prior to scanning. Examples of photographs taken by participants with the average time (in hours and minutes) and the SD to visit the locations are depicted.
Figure1B. During scanning participants saw pairs of photographs taken on the previous day and were asked to make temporal-order judgments and whether the decision was made with low or high confidence. DL = Definitely Left, PL = Probably Left, PR = Probably Right, DR = Definitely Right
Scanned task
On day two, participants were scanned using an event-related fMRI design while making temporal-order judgments on their photographs from the previous day. Across 5 scans of 30 trials each, they were shown 180 photograph pairs from different locations (2 photos side by side) and asked to indicate which picture they took first and whether the decision was made with low or high confidence (i.e., Definitely Left, Probably Left, Probably Right, Definitely Right). We manipulated the lag between pairs of photos from short (1 to 9 locations apart), medium (10 to 39 locations apart), and long (40 to 80 locations apart) so that lags were equally spaced on a logarithmic scale. Photo pairs were selected so that the campus locations were equally represented across time lag. Each photo was presented for 4 sec, followed by a self-paced response screen (up to 6 sec), and then by a fixation cross for a varying interval between 500 msec to 2500 msec plus any additional time from the response screen (total trial length = 10.50 to 12.50 sec; see Figure 1-B). Reaction times were not informative because participants were required to wait until the response screen appeared before making a response. The rationale for this procedure was to allow for equal viewing time in each condition. Post-scanning, participants were asked rate their familiarity with each of the 80 campus locations (1 = low to 4 = high). Debriefing suggested that participants were unaware of the nature of the scanning task during the photo-taking session.
fMRI Methods
Scanning
Scanning was conducted using a 4T GE magnet. Stimuli were presented using liquid crystal display goggles (Resonance Technology, Northridge, CA) and behavioral responses were recorded using a four-button fiber optic response box (Resonance Technology). Head motion was minimized using foam pads and a headband. Anatomical scanning started with a T1-weighted sagittal localizer series. The anterior (AC) and posterior commissures (PC) were identified in the midsagittal slice, and 34 contiguous oblique slices were prescribed parallel to the AC-PC plane. High resolution T1-weighted structural images were acquired with a 12 ms repetition time (TR), a 5 ms echo time (TE), 24 cm field of view (FOV), 68 slices, 1.9 mm slice thickness, and a 2562 matrix. Functional scanning employed an inverse spiral sequence with a 1500 ms TR, 36 ms TE, 24 cm FOV, a 642 image matrix, and a 60° flip angle. Thirty-four contiguous slices were acquired with the same slice prescription as the anatomical images. Slice thickness was 3.75 mm, resulting in cubic 3.75 mm3 isotropic voxels.
fMRI analyses
Image processing and analyses were performed using Statistical Parameter Mapping software implemented in Matlab (SPM2; Wellcome Department of Cognitive Neurology, London, UK). Functional images were corrected for slice acquisition order, realigned to correct for motion artifacts, and then spatially normalized to a standard stereotactic space, using the template implemented in SPM2. Subsequently, the functional images were spatially smoothed using an 8 mm isotropic Gaussian kernel. For each subject, evoked hemodynamic responses to event types were modeled with a delta (stick) function corresponding to two seconds after stimulus presentation (the middle of the photograph presentation time), convolved with a canonical hemodynamic response function (HRF) within the context of the general linear model (GLM). This onset was selected because behavioral pilot data suggested that it took participants a couple of seconds to identify the locations, and we were primarily interested in the decision process.
Short vs. Long contrast
To isolate activity in the brain that was exclusively involved in the short and long time lag conditions we employed the GLM to generate contrasts for the temporal-order memory for correct trials with each of the time lag conditions, as well as, the short versus long contrast. Subsequently, random-effects analysis was performed on the parameter estimates of the conditions (P = .05, uncorrected, with a cluster size > 15 voxels). We used a cluster-size threshold (R = 15) to establish a Type 1 error level of p < .005 for false discovery of voxels within each cluster (Forman et al., 1995). In order to isolate activity exclusive to short versus long conditions, we inclusively masked this contrast with the effect of the time lag condition of interest (short or long) greater than baseline fixations at P = .001. Thus, the resulting activity isolating activity related to short or long conditions also had to be confirmed by real differences observed in each time lag condition in comparison with the implicit baseline (short > baseline, long > baseline).
Parametric contrasts
To examine changes in the neural correlates modulated by temporal distance we employed a parametric approach, which allowed us to examine how activity was modulated as a function of discrete changes in time lag as opposed to examining only the overall activity level averaged within the short and long time lag bins. To identify regions of interest showing temporal-order memory-related activity increases as a function of increasing lag, we created a GLM in which correct trial onsets were modulated by the lag between pairs of photographs (e.g., from lags of 1 to 80) using the first-order parametric modulation option integrated in SPM2 and its reverse (i.e., increasing activity associated with decreasing time lag). The pairs of photographs also differed with respect to spatial distance and we also entered this factor as a covariate in the model. Thus, we examined the unique contribution of temporal lag orthogonalized with respect to spatial distance in the design matrix. We did not further examine spatial distance because it was not the main focus of the study (i.e. participants were only asked to make a temporal-order decision). Confidence responses were combined because they did not produce any additional information. Subsequently, random-effects analysis was performed on the parameter estimates of the parametric regressor for temporal lag (P = .05, uncorrected, with a cluster size > 15 voxels). We used a cluster-size threshold (R = 15) to establish a Type 1 error level of p < .005 for false discovery of voxels within each cluster (Forman et al., 1995). In order to examine positive activations in the condition of interest we inclusively masked with a contrast of the main effect of temporal order trials greater than baseline fixations at P = .001.
Accuracy and Difficulty
Regions identified by the parametric modulation of time lag, which were greater than baseline, were further interrogated to determine if there were effects of accuracy (Hits > Misses). To create these contrasts, we employed the GLM to generate parameter estimates for Hit and Miss responses in a separate design matrix. Statistical Parametric Maps were created for each subject by applying linear contrasts to the parameter estimates for these events of interest, resulting in a t statistic for every voxel. We performed additional random-effects analyses to determine the effects of retrieval accuracy. A liberal threshold was chosen in order to maximize the power to detect the effects of accuracy in regions demonstrating parametric modulation (both at P = .05, uncorrected, with cluster size > 5). Because we were only interested in changes in the neural correlates of temporal-order memory, we used the results from the parametric modulation and its reverse as spatial inclusive masks for the contrasts of retrieval accuracy. Thus, we defined masks in which activity had to be above baseline fixation, demonstrate a parametric modulation by temporal lag and show changes as a function of accuracy. Finally, we examined the effects of task difficulty and familiarity by entering each participant's overall accuracy and familiarity scores as a covariate in the parametric analysis on temporal lag.
Results
Behavioral Results
As expected, there was a significant difference in accuracy (proportion correct) as a function of lag, F (2,16) = 24.05, p < .0001 (see Figure 2). Shorter lags (M = .74, SD = .07) were less accurate than medium lags (M = .82, SD = .07), t (16) = 4.49, p < .0005, and longer time lags (M = .87, SD = .06), t (16) = 6.42, p < .0001, which also differed from one another, t (16) = 2.85, p < .05. The overall familiarity scores suggested that participants were generally familiar with the campus (M = 3.08, SD = 0.51).
Figure 2.
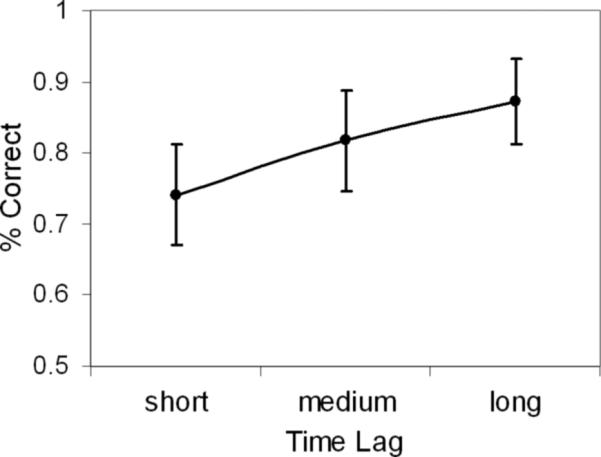
Mean proportion correct for short, medium and long time lags. Error bars indicate standard error of the mean.
fMRI Results
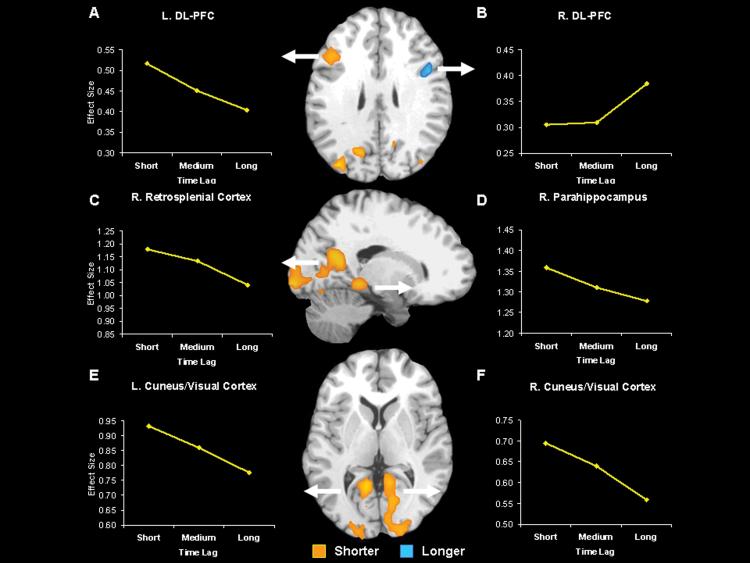
Table 1 lists regions revealed in the direct contrast of the short versus long time lag conditions. Consistent with our predictions, left dorsolateral PFC showed greater activity for the short lag condition, whereas right dorsolateral PFC showed greater activity for the long time lag condition. Given that left vs. right PFC regions have been respectively associated with recollection and familiarity (Dobbins et al., 2003; Henson et al., 1999a), this hemispheric asymmetry finding supports our hypothesis that reconstruction processes depend on recollection whereas distance processes depend on familiarity. Also consistent with our hypotheses, the short time lag condition elicited greater activity in other regions associated with recollection, including MTL (right parahippocampal gyrus), posterior midline cortex (retrosplenial, posterior cingulate, precuneus), and visual cortex/cuneus, extrastriate including bilateral activity in the precuneus, posterior cingulate, and cuneus. Although the long time lag condition did not show activity in perirhinal cortex, we also found activity in right fusiform gyrus, angular gyrus, and superior parietal cortex.
Table 1.
Brain regions showing activity in Short vs. Long time lags
| Talairach Coodinates |
|||||||
|---|---|---|---|---|---|---|---|
| Region | BA | H | x | y | z | voxels | T Score |
| Long > Short | |||||||
| Dorsolateral PFC | 9 | R | 48 | 8 | 24 | 30 | 2.70 |
| Premotor Cortex | 6 | L | −41 | 6 | 49 | 15 | 3.05 |
| Superior Parietal | 7 | R | 26 | −53 | 41 | 27 | 2.55 |
| Angular Gyrus | 39 | R | 52 | −69 | 14 | 19 | 3.43 |
| Fusiform Gyrus | 37 | R | 37 | −44 | −14 | 91 | 3.16 |
| Short > Long | |||||||
| Dorsolateral PFC | 46 | L | −41 | 26 | 20 | 49 | 2.93 |
| Premotor Cortex | 6 | L | −30 | 6 | 56 | 23 | 3.36 |
| Central Cingulate Gyrus | 32 | L | −4 | 17 | 45 | 43 | 3.99 |
| Parahippocampus | R | 19 | −37 | −5 | 60 | 3.46 | |
| Posterior Cingulate | 31 | L | −7 | −65 | 21 | 224 | 3.75 |
| R | 11 | −57 | 20 | 224 | 3.50 | ||
| Retrosplenial Cortex | 30 | L | −7 | −51 | 6 | 224 | 2.85 |
| Precunues | 7 | R | 7 | −63 | 52 | 15 | 3.24 |
| Visual Cortex/Cuneus | 17/18 | L | −15 | −99 | −5 | 91 | 4.60 |
| R | 15 | −98 | −1 | 75 | 2.94 | ||
| 19 | L | −37 | −78 | 35 | 43 | 4.08 | |
Note. BA, Brodmann's Area; H, Hemisphere. All p's < .005.
Furthermore, we also identified regions where activity monotonically increased or decreased as a function of time lag using parametric modulation analyses on the fMRI activity in which we covaried out the effects of spatial distance (see Table 2 and Figure 3). In these parametric analyses, lag was entered as a continuous variable (lag 1 to 80), but, for display purposes, the line graphs in Figure 3 shows fMRI activity averaged across three lag ranges: short (lag 1 to 9), medium (lag 10 to 39), and long (lag 40 to 80). Likewise, to simplify the description of results, activity that parametrically decreased or increased as a function of lag is respectively described as “greater for shorter lags” or “greater for longer lags.”
Table 2.
Brain regions showing activity parametrically modulated by time lag
| Talairach Coodinates |
|||||||
|---|---|---|---|---|---|---|---|
| Region | BA | H | x | y | z | voxels | T Score |
| Longer Time Lags | |||||||
| Dorsolateral PFC | 9 | R | 48 | 8 | 24 | 16 | 3.35 |
| Fusiform Gyrus | 37 | R | 48 | −52 | −13 | 65 | 2.84 |
| Shorter Time Lags | |||||||
| Dorsolateral PFC | 46 | L | −41 | 23 | 20 | 51 | 2.66 |
| Central Cingulate Gyrus | 32 | L | −4 | 17 | 45 | 61 | 3.95 |
| Parahippocampus | R | 19 | −37 | −5 | - | 3.42 | |
| Posterior Cingulate | 31 | L | −15 | −61 | 21 | 123 | 4.28 |
| 31 | R | 11 | −57 | 20 | - | 3.63 | |
| Retrosplenial Cortex | 30 | L | −11 | −54 | 6 | - | 3.75 |
| 29/30 | R | 11 | −51 | 10 | - | 3.81 | |
| Precuneus | 7 | L | −30 | −45 | 41 | 30 | 2.62* |
| Visual Cortex/Cuneus | 17/18/19 | L | −15 | −99 | −5 | 136 | 4.58 |
| 18 | R | 19 | −98 | −1 | 298 | 2.75 | |
| 19 | L | −37 | −79 | 32 | 59 | 4.05 | |
| R | 41 | −75 | 35 | 17 | 3.27 | ||
Note. BA, Brodmann's Area; H, Hemisphere. All p's < .005, unless indicated * p < .01.
Figure 3.
Activity parametrically modulated by increasing time lag (longer) and decreasing time lag (shorter), which was mutually exclusive from spatial distance
As indicated by Table 2 and Figure 3, a subset of the regions found in the direct contrast also showed activity that monotonically increased or decreased as a function time lag, and are consistent with our predictions. We found hemispheric asymmetry in the PFC, with greater activity in left dorsolateral PFC for shorter lags, whereas right dorsolateral PFC showed greater activity for longer lags. Shorter lags also elicited greater activity in other regions associated with recollection, including MTL (right parahippocampal gyrus), posterior midline cortex (retrosplenial, posterior cingulate, precuneus), and visual cortex/cuneus, extrastriate including bilateral activity in the precuneus, posterior cingulate, and cuneus. In contrast, longer lags revealed greater activity in the right fusiform gyrus. Because of our a priori hypothesis about the hippocampus and perirhinal cortices, we further examined activity in the MTL using a less conservative threshold (P = .05, with a cluster size > 5 voxels and inclusively masked with the main effect of temporal order trials greater than baseline fixations at P = .05). Shorter lags revealed greater activity in the left hippocampus, whereas, longer lags did not reveal any activity in the MTL. Thus, the results of the parametric analysis support the prediction that the neural correlates of temporal-order memory decisions differ according to shorter and longer time lags, irrespective of how “short” or “long” conditions are categorized.
To confirm the hemispheric asymmetry in dorsolateral PFC, we extracted the peak responses in each hemisphere and entered these into a hemisphere (left, right) × time lag (short, long) repeated ANOVA (only two levels of time lag were used because we were interested in examining the extremes). This analysis yielded significant 2-way interaction, F(1,16) = 32.23, p < .0001, and follow-up tests revealed that activity was greater for shorter than longer lags in left dorsolateral PFC, t(16) = 3.13, p < .01, whereas the opposite was true in right dorsolateral PFC, t(16) = 2.70, p < .05.
To investigate the relationship between the hemispheric asymmetry in PFC activity and potential differences in retrieval success and task difficulty, we conducted two ancillary analyses on the regions that showed time lag effects. First, we determined if activity in these regions differed between hits and misses. As illustrated by Figure 4, no significant differences between hits and misses were found either in left or right PFC (main effect of item: p = .12, item x hemisphere interaction: p = .36), nor in other brain regions. Second, we determined if activity in the regions showing an effect of time lag varied as a function of task difficulty. Finally, the main findings from the parametric analysis were not affected by entering accuracy or familiarity as covariates.
Figure 4.
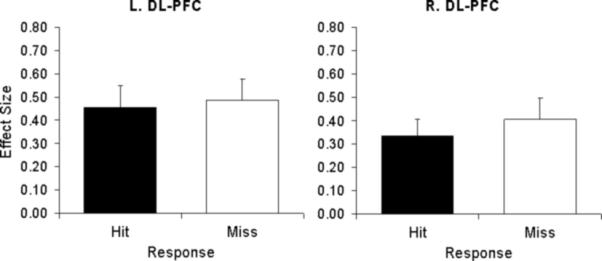
Activity related to hits and misses in left and right dorsolateral PFC for shorter and longer time lags, respectively.
Discussion
The results of the present study suggest that reconstruction and distance are distinct processes involved in temporal-order memory for autobiographical events, and that these processes are differentially recruited depending upon the temporal distance between events. The study yielded three main findings. First, when events occurred closer in time, activity in the left dorsolateral PFC, MTL, left parietal, posterior midline, and visual cortices indicated that temporal-order memory involved the recollection of contextual details. Second, when events occurred further away in time, activity in the right dorsolateral PFC and fusiform gyrus indicated that temporal-order memory involved familiarity processes. Finally, the left lateralization of PFC activity for shorter lags coupled with the right lateralization of PFC activity during longer lags yielded a marked hemispheric asymmetry in PFC activity. We discuss these three findings in separate sections below.
Regions associated with reconstruction processes during shorter time lags
At shorter time lags, a network of brain regions were activated, including left dorsolateral PFC, posterior parahippocampal, parietal and visual cortices, suggesting that recollection of contextual details was utilized to temporally parse the events. Several studies have indicated a greater role for left PFC during tasks involving recollection (Dobbins et al., 2002; Dobbins et al., 2003; Henson et al., 1999a; Kahn et al., 2004; Nolde et al., 1998a; Ranganath et al., 2000; Raye et al., 2000; Rugg et al., 1999), with the majority of studies reporting activity in dorsolateral PFC as in the present study (for exceptions see Kahn et al., 2004; Rugg et al., 1999). The left dorsolateral PFC involvement in recollection memory is thought to reflect an increase in reflective and evaluative demands, and greater episodic specificity during the retrieval of contextual information. Left dorsolateral PFC has also been found in laboratory memory studies that have specifically investigated temporal context memory (Cabeza et al., 1997; Konishi et al., 2002; Suzuki et al., 2002). For example, Suzuki et al. (2002) manipulated the temporal distance between pairs of line drawings by as much as 3 hours, which is more similar to the present time frame. Consistent with the results of the present study, Suzuki et al. (2002) found greater left dorsolateral PFC activity for temporal judgments made for pairs of drawings separated by shorter temporal distances than those separated by longer distances. The results of the present study allow us to further characterize the involvement of left dorsolateral PFC in temporal memory for autobiographical events involving a richer and extensive temporal context.
As predicted, we found greater activity in posterior parahippocampus for shorter lags, but the hippocampus activity was sub threshold. These results suggest that although the hippocampus was recruited, suggesting that the photo-paradigm was eliciting recollection of autobiographical events (e.g., Cabeza et al., 2004), activity in this region did not differentiate the temporal order effects as strongly as the posterior parahippocampus. The finding that the right posterior parahippocampal cortex was associated with reconstruction of temporal context is consistent with three different accounts of parahippocampal function. First, several fMRI studies, including studies using words and objects, have associated the posterior parahippocampal gyrus with recollection and relational memory (e.g., Eldridge et al., 2000; Kahn et al., 2004; Prince et al., 2005; Wais et al., 2006; Yonelinas et al., 2001; Yonelinas et al., 2005). In the present study, the parahippocampal activation could have reflected the recollection of the photo-taking episodes, which is required to reconstruct the order of events close in time. Second, the posterior parahippocampal gyrus has been strongly associated with spatial scene perception. In particular, a region dubbed the “parahippocampal place area” (PPA) has been found to be more activated during the processing of spatial layout than during the processing of objects, faces, and other control stimuli (Epstein and Kanwisher, 1998). The reported parahippocampal activation was near the PPA and might have reflected the processing of the spatial aspects of retrieved mental images or the photos used as cues. However, it is not clear why spatial scene processing would differ as a function of decreasing temporal distance. Third, the parahippocampus has been linked to mental navigation and retrieval of topographical information (e.g., Aguirre, Zarahn, & D'Esposito, 1998; Maguire, Burgess, Donnett, Frackowiak, Frith, & O’Keefe, 1998; Maguire, Frith, Burgess, Donnett, & O'Keefe, 1998; Mellet et al., 2000; for a review see Burgess et al., 2002), which might be more important as spatial distance decreases. However, in the present study, temporal distance was not significantly positively correlated with spatial distance. Furthermore, the reported activity in the parametric analysis reflects the unique contribution of temporal lag after covarying out the effects of spatial distance. Thus, of these accounts, the involvement of the posterior parahippocampus in recollection is the most parsimonious within the framework of the present study, in which both spatial and other information linked to the photo-taking event might contribute to reconstructing the temporal order memory. Future work is needed to clarify the role of the MTL, including the involvement of the hippocampus, in temporal-order memory for autobiographical events relying on reconstructive processes.
In addition to PFC and MTL regions, recollection for temporal judgments involving shorter time lags also engaged intervening posterior parietal, posterior midline (retrosplenial, posterior cingulate, precuneus), and visual cortex regions. Posterior parietal and posterior midline activations are among the most typical findings in functional neuroimaging studies of episodic memory retrieval (for a review, see Cabeza & Nyberg, 2000). Several fMRI studies have linked subregions of parietal and posterior midline cortices with recollection (for review see Wagner et al., 2005). For example, we recently found recollection-related activity in a left parieto-temporal and retrosplenial regions very close to the ones we observed here (Daselaar et al., 2006). Damage to the retrosplenial cortex can cause amnesia (Valenstein et al., 1987), and in the right side it has been found to specifically affect memory for spatial relationships (for review see Maguire, 2001). Thus, in our study, this region might contribute to both recollection and spatial memory. As for the precuneus, some evidence has linked its role in memory retrieval to the processing of visual images (Fletcher et al., 1995), and this function might have contributed to the reconstruction of temporal order. Finally, activations in visual cortex and cuneus regions likely reflect the retrieval of visual details (for review see Buckner & Wheeler, 2001), which is critical for autobiographical memory retrieval (Greenberg et al., 2005; Rubin et al., 2003; Rubin & Greenberg, 1998; Daselaar et al., 2007). In a prior study using the photo-paradigm (Cabeza et al., 2004), we found greater visual cortex and cuneus activity when participants recognized campus photos they took themselves compared to similar photos taken by others.
Regions associated with distance processes during longer time lags
The analysis identifying brain regions where activity increased as a function of lag yielded only two regions: right dorsolateral PFC and right fusiform areas. The finding of right dorsolateral PFC activity in association with long lags is consistent with functional neuroimaging evidence linking this region to familiarity (Dobbins et al., 2004; Eldridge et al., 2000; Henson et al., 1999a). A related interpretation of these right PFC activations is that they reflect an increase in monitoring for items that are close to the response criterion (Henson et al., 2000). This issue is discussed in the next section. The present right dorsolateral PFC activation is also consistent with several studies investigating temporal context memory that have reported activations in this region (Cabeza et al., 1997; Dobbins et al., 2003; Fujii et al., 2004; Konishi et al., 2002; Suzuki et al., 2002). Some of these studies also found activations in left PFC (e.g. Cabeza et al., 1997; Konishi et al., 2002), but these activations might reflect the contributions of recollection to temporal order judgments in some conditions. In fact, when these contributions are controlled by subtracting out activity during source memory from activity during temporal-order judgments, as done by Dobbins et al. (2003), the resulting pattern of PFC activity is strongly right lateralized.
Turning to the right fusiform gyrus, this is a region that some studies have associated with the processing of specific perceptual information, as opposed with the left fusiform activity, which is assumed to mediate more conceptual or abstract processing (Garoff et al., 2005; Koutstaal et al., 2001; Simons et al., 2003). For example, in one fMRI study (Koutstaal et al., 2001), priming-related activity in the right fusiform gyrus (but not in the left fusiform) was reduced when the perceptual properties of the items changed between study (e.g., a yellow umbrella) and test (e.g., a striped umbrella). In another fMRI study (Garoff et al., 2005), encoding activity in the right fusiform gyrus (but not in the left fusiform) predicted memory for specific perceptual properties of stimuli. Thus, the right fusiform gyrus seems to be involved in processing item-specific perceptual information, which might underlie familiarity processes (Jacoby & Dallas, 1981; Mandler, 1980; Whittlesea & Williams, 2000; although see Voss & Paller, 2006). The role of the right fusiform gyrus in temporal-order decisions for longer lags might reflect greater reliance on perceptual aspects of the information during distance judgments. For instance, participants could have ordered the two photos in time by paying attention to their perceptual details. Although we predicted greater activity for longer lags in the perirhinal cortex, a region important for familiarity processes (e.g. Eichenbaum et al., 2007), the results of the present study suggest instead that perceptual fluency via the fusiform gyrus is involved in temporal order memory judgments for autobiographical events separated by longer time lags and that activity in this region might underlie distance processes. Further work is needed to clarify the role of the perirhinal cortex and other regions that might mediate distance processes.
Hemispheric Asymmetry in PFC Activity
The main finding of the present study was a hemispheric asymmetry in dorsolateral PFC during temporal-order memory such that left PFC showed greater activity for shorter than longer time lags and right PFC showed greater activity for longer than shorter lags. The hemispheric asymmetry we observed is consistent with our hypothesis that reconstruction vs. distance processes are similar to recollection vs. familiarity processes, respectively, which have been previously associated with left vs. right PFC activations (Dobbins et al., 2003; Dobbins et al., 2004; Eldridge et al., 2000; Henson et al., 1999a). For example, Henson et al. (1999a) used the “remember-know” procedure (Tulving, 1985) to determine when memory is accompanied by contextual details (“remember” judgment) versus when it is not (“know” judgment), as based on participant introspections. The study yielded greater left dorsolateral PFC activity for remember responses (recollection), but greater right dorsolateral PFC activity for know responses (familiarity). Thus, the hemispheric asymmetry in the present study extends this finding to the temporal-order memory domain, supporting the hypothesis that temporal-order decisions for shorter vs. longer lags are based on reconstruction (recollection) vs. distance (familiarity) processes, respectively.
Importantly, the hemispheric asymmetry in PFC activity cannot be attributed to differences in retrieval success. Although PFC activity varied as a function of time lag, it did not differ as a function of accuracy; in both left and right dorsolateral PFC, activity was similar for hits and misses (see Figure 4). This finding is consistent with several fMRI studies showing that during episodic retrieval some PFC regions are sensitive to retrieval orientation rather than to retrieval success (Dobbins et al., 2002; Dobbins et al., 2003; Kahn et al., 2004; Ranganath et al., 2000; for a review, see Rugg & Wilding, 2000). For example, in Dobbins et al. (2003), recollection-related left PFC activity and familiarity-related left PFC activity did not differ between correct and incorrect memory decisions. The results of the present study are consistent with retrieval orientation accounts of PFC function whereby activity reflects the processes involved during the attempt to retrieve irrespective of retrieval success. Moreover, including accuracy as a covariate did not affect the results of the parametric analysis, which further supports the suggestion that activity in these regions is independent of retrieval effort and task difficulty.
Beyond the recollection-familiarity distinction, another account that explains the hemispheric asymmetry finding is the distinction between systematic and heuristic processes (Nolde et al., 1998b). Systematic processes involve the retrieval of more detailed information that engages greater reflection and evaluation, such as during source memory attributions, and relies on left PFC. In contrast, heuristic processes involve the simple maintenance of information and comparison of that information to a response criterion, such as during simple item-recognition tasks, and relies on right PFC. Like the recollection-familiarity distinction, the systematic-heuristic distinction is supported by a substantial amount of evidence (Dobbins et al., 2002; Nolde et al., 1998a, 1998b; Ranganath et al., 2000; Raye et al., 2000; Rugg et al., 1999). The present hemispheric asymmetry finding can be easily explained in terms of the systematic-heuristic distinction because making temporal-order decisions for shorter lags requires the systematic evaluation of available source information. Conversely, temporal-order decisions for longer lags can be based on a simple heuristic evaluation of memory strength. The systematic/heuristic distinction has been typically investigated by contrasting different memory tasks (e.g., source memory vs. item recognition; although see Dobbins et al., 2002; Dobbins & Wagner, 2005), but in the present study, we found differences consistent with this distinction within the same task (shorter vs. longer lags). This result provides further support for the idea that the critical factor determining PFC activity is not the type of task but the type of memory processes recruited by the task. These results are consistent with the source-monitoring framework (Johnson et al., 1993), which proposes that performance in source memory tasks involves both systematic and heuristic processes (also see Dobbins & Wagner, 2005).
In contrast, our results are less consistent with the post-retrieval monitoring account of lateralization differences in the PFC activity. Post-retrieval monitoring refers to the evaluation of items retrieved from memory in accordance with their accuracy and relevance to the task (Burgess & Shallice, 1996; Koriat & Goldsmith, 1996; Norman & Bobrow, 1979). Evidence from both neuropsychology (Curran et al., 1997; Schacter et al., 1996) and neuroimaging (e.g. Allan et al., 2000; Henson et al., 2000; Henson et al., 1999b; Rugg et al., 1999) have suggested a role for right PFC in post-retrieval monitoring (also see Fleck et al., 2006). In the present study we found that activity in right and left PFC that varied as a function of time lag was not affected by task difficulty. At the same time, the lateralization of PFC varied as a function of recollection vs. familiarity (or systematic vs. heuristic) demands but not as a function of retrieval success, suggesting that retrieval orientation is the critical factor determining PFC lateralization in our task rather than effort or monitoring demands.
Conclusions
In the present study we employed a novel photo paradigm to investigate temporal order memory for autobiographical events, in which we were able to control for the order of when events occurred along with other factors (e.g. Cabeza et al., 2004). Although a controlled way to elicit autobiographical memories, the photo paradigm replicates the common experience of taking photographs during sightseeing. Unlike typical laboratory stimuli, the use of the photo paradigm also allowed us to manipulate the temporal distance between events over longer intervals, which was important for separating the processes involved in temporal context memory. Furthermore, in the photo paradigm participants were also personally familiar with the campus, and thus, recall of these events might have been influenced by the autobiographical salience associated with particular locations (e.g., Westmacott & Moscovitch, 2003). Although we did not find that differences in overall familiarity affected the main results, the interaction between autobiographical significance and temporal context memory will be interesting for future research.
Our results are consistent with behavioral evidence in autobiographical memory suggesting that there are two distinct processes which are involved in temporal context memory: 1) reconstruction, based on the rich recollection of contextual details, and 2) distance, based on differences in the familiarity signal (Friedman, 1993, 2004). Reconstruction and distance processes are similar to recollection and familiarity but not identical. For example, Friedman (1993, 2004) proposed that distance processes are the preferred method for determining temporal context, with reconstruction processes accessed only when accuracy is important (such as in the present study), but not all models of recollection and familiarity make this same assumption (for review see Yonelinas, 2002). While we found parahippocampal activity for shorter lags suggesting the use of reconstruction processes in this condition, activity in the hippocampus was not as strong a predictor of temporal order memory. Furthermore, although we found activity in fusiform gyrus for longer lags consistent with the use of distance processes, we did not observe the predicted perirhinal activity. Future research is needed to better characterize how the distance-reconstruction distinction differs from the recollection-familiarity distinction, such as determining the characteristics and content of the autobiographical memories that are retrieved via self-report measures. In particular, the interaction of distance-reconstruction processes and the retrieval of episodic versus semantic autobiographical events is an important issue (e.g., Cabeza & St. Jacques, 2007). Despite possible differences between the two constructs, by characterizing these temporal processes within the framework of recollection and familiarity we were able to make predictions about lateralization differences in the PFC and activity in posterior regions, which were not directly discernable in the behavioral model of reconstruction and distance (Friedman, 1993, 2004).
The results of the present study are consistent with prior evidence from lesion and neuroimaging suggesting that the PFC is important for temporal information (e.g. Butters et al., 1994; Cabeza et al., 1997; Eyler Zorilla et al., 1996; Kesner et al., 1994; Mangels, 1997; McAndrews & Milner, 1991; Milner, 1971; Milner et al., 1991; Nyberg et al., 1996). However, we extend these findings by showing that the left dorsolateral PFC is particularly implicated in reconstruction-based temporal judgments, whereas the right dorsolateral PFC is involved in distance-based judgments. Furthermore, we found that posterior brain regions were also important in these temporal processes, especially in the case of reconstruction. To our knowledge, this is the first fMRI study to investigate temporal context memory for autobiographical events. In the present study the time lag between the events took place within a single day; future studies should probe longer lags between autobiographical events to determine if the findings generalize to broader temporal distances in memory.
Acknowledgements
We thank Ian Dobbins for helpful comments on an earlier version of the manuscript. This research was supported by NIA grant R01 AG023123.
References
- Aguirre GK, Zarahn E, D'Esposito M. Neural components of topographical representation. Proc Natl Acad Sci U S A. 1998;95(3):839–846. doi: 10.1073/pnas.95.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan K, Dolan RJ, Fletcher PC, Rugg MD. The role of the right anterior prefrontal cortex in episodic retrieval. Neuroimage. 2000;11(3):217–227. doi: 10.1006/nimg.2000.0531. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M, Michel AP, Friedman WJ. The effects of aging on location-based and distance-based processes in memory for time. Acta Psychol (Amst) 2004;116(2):145–171. doi: 10.1016/j.actpsy.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nat Rev Neurosci. 2001;2(9):624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Confabulation and the control of recollection. Memory. 1996;4(4):359–411. doi: 10.1080/096582196388906. [DOI] [PubMed] [Google Scholar]
- Burt CDB, Kemp S, Grady JM, Conway M. Ordering autobiographical experiences. Memory. 2000;8:323–332. doi: 10.1080/09658210050117744. [DOI] [PubMed] [Google Scholar]
- Butters MA, Kaszniak AW, Glisky EL, Eslinger PJ, Schacter DL. Recency discrimination deficits in frontal lobe patients. Neuropsychology. 1994;8:343–353. [Google Scholar]
- Cabeza R, Nyberg L. Neural bases of learning and memory: functional neuroimaging evidence. Curr Opin Neurol. 2000;13(4):415–421. doi: 10.1097/00019052-200008000-00008. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Mangels J, Nyberg L, Habib R, Houle S, McIntosh A, et al. Brain regions differentially involved in remembering what and when: A pet study. NEURON. 1997;19(4):863–870. doi: 10.1016/s0896-6273(00)80967-8. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, et al. Brain activity during episodic retrieval of autobiographical and laboratory events: An fmri study using a novel photo paradigm. Journal of Cognitive Neuroscience. 2004;16(9):1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11(5):219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Curran T, Friedman W. Differentiating location- and distance-based processes in memory for time: An erp study. Psychonomic Bulletin & Review. 2003;10(3):711–717. doi: 10.3758/bf03196536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Schacter DL, Norman KA, Galluccio L. False recognition after a right frontal lobe infarction: Memory for general and specific information. Neuropsychologia. 1997;35(7):1035–1049. doi: 10.1016/s0028-3932(97)00029-8. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza RE. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. J Neurophysiol. 2006 doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. Spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity and reliving. Cerebral Cortex. 2007;18(1):217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35(5):989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: Separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41(3):318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Simons JS, Schacter DL. Fmri evidence for separable and lateralized prefrontal memory monitoring processes. J Cogn Neurosci. 2004;16(6):908–920. doi: 10.1162/0898929041502751. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cerebral Cortex. 2005;15(11):1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Downes JJ, Mayes AR, MacDonald C, Hunkin NM. Temporal order memory in patients with Korsakoff's syndrome and medial temporal amnesia. Neuropsychologia. 2002;40(7):853–861. doi: 10.1016/s0028-3932(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Fortin N. Episodic memory and the hippocampus: It's about time. Current Directions in Psychological Science. 2003;12(2):53–57. [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30(20):123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3(11):1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392(6676):598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Eyler Zorilla LT, Aguirre GK, Zarahn E, Cannon TD, D'Esposito M. Activation of the prefrontal cortex during judgements of recency: A functional mri study. Neuroreport. 1996;7:2803–2806. doi: 10.1097/00001756-199611040-00079. [DOI] [PubMed] [Google Scholar]
- Fleck MS, Daselaar SM, Dobbins IG, Cabeza R. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb Cortex. 2006 doi: 10.1093/cercor/bhj097. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind's eye--precuneus activation in memory-related imagery. Neuroimage. 1995;2(3):195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fmri): Use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friedman W. Memory for the time of past events. Psychological Bulletin. 1993;113(1):44–66. [Google Scholar]
- Friedman W. Time in autobiographical memory. Social Cognition. 2004;22(5):591–605. [Google Scholar]
- Fujii T, Suzuki M, Okuda J, Ohtake H, Tanji K, Yamaguchi K, et al. Neural correlates of context memory with real-world events. Neuroimage. 2004;21(4):1596–1603. doi: 10.1016/j.neuroimage.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Garoff RJ, Slotnick SD, Schacter DL. The neural origins of specific and general memory: The role of the fusiform cortex. Neuropsychologia. 2005;43(6):847–859. doi: 10.1016/j.neuropsychologia.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Eacott MJ, Brechin D, Rubin DC. Visual memory loss and autobiographical amnesia: A case study. Neuropsychologia. 2005;43(10):1493–1502. doi: 10.1016/j.neuropsychologia.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg M, Shallice T, Dolan R. Confidence in recognition memory for words. Journal of Cognitive Neuroscience. 2000:23–23. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. J Neurosci. 1999a;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: A functional MRI test of the monitoring hypothesis. Brain. 1999b;122:1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Dallas M. On the relationship between autobiographical memory and perceptual-learning. Journal of Experimental Psychology-General. 1981;110(3):306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Bull. 1993;114(1):3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: Implications for models of recognition memory. J Neurosci. 2004;24(17):4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Hopkins RO, Fineman B. Item and order dissociation in humans with prefrontal cortex damage. Neuropsychologia. 1994;32(8):881–891. doi: 10.1016/0028-3932(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Konishi S, Asari T, Jimura K, Chikazoe J, Miyashita Y. Activation shift from medial to lateral temporal cortex associated with recency judgements following impoverished encoding. Cerebral Cortex. 2006;16(4):469–474. doi: 10.1093/cercor/bhi126. [DOI] [PubMed] [Google Scholar]
- Konishi S, Uchida I, Okuaki T, Machida T, Shirouzu I, Miyashita Y. Neural correlates of recency judgment. J Neurosci. 2002;22(21):9549–9555. doi: 10.1523/JNEUROSCI.22-21-09549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriat A, Goldsmith M. Monitoring and control processes in the strategic regulation of memory accuracy. Psychological Review. 1996;103(3):490–517. doi: 10.1037/0033-295x.103.3.490. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: Functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39(2):184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Maguire EA. The retrosplenial contribution to human navigation: A review of lesion and neuroimaging findings. Scand J Psychol. 2001;42(3):225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280(5365):921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Burgess N, Donnett JG, O'Keefe J. Knowing where things are parahippocampal involvement in encoding object locations in virtual large-scale space. J Cogn Neurosci. 1998;10(1):61–76. doi: 10.1162/089892998563789. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing - the judgment of previous occurrence. Psychological Review. 1980;87(3):252–271. [Google Scholar]
- Mangels JA. Strategic processing and memory for temporal order in patients with frontal lobe lesions. Neuropsychology. 1997;11(2):207–221. doi: 10.1037//0894-4105.11.2.207. [DOI] [PubMed] [Google Scholar]
- McAndrews M, Milner B. The frontal-cortex and memory for temporal-order. Neuropsychologia. 1991;29(9):849–859. doi: 10.1016/0028-3932(91)90051-9. [DOI] [PubMed] [Google Scholar]
- Mellet E, Briscogne S, Tzourio-Mazoyer N, Ghaem O, Petit L, Zago L, Etard O, Berthoz A, Mazoyer B, Denis M. Neural correlates of topographic mental exploration: the impact of route versus survey perspective learning. Neuroimage. 2000;12(5):588–600. doi: 10.1006/nimg.2000.0648. [DOI] [PubMed] [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. British Medical Bulletin. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Milner B, Corsi P, Leonard G. Frontal-lobe contribution to recency judgments. Neuropsychologia. 1991;29(6):601–618. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, D'Esposito M. Left prefrontal activation during episodic remembering: An event-related fmri study. Neuroreport. 1998a;9(15):3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, Raye CL. The role of prefrontal cortex during tests of episodic memory. Trends in Cognitive Sciences. 1998b;2(10):399–406. doi: 10.1016/s1364-6613(98)01233-9. [DOI] [PubMed] [Google Scholar]
- Norman DA, Bobrow DG. Descriptions - intermediate stage in memory retrieval. Cognitive Psychology. 1979;11(1):107–123. [Google Scholar]
- Nyberg L, McIntosh AR, Cabeza R, Habib R, Houle S, Tulving E. General and specific brain regions involved in encoding and retrieval of events: What, where, and when. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):11280–11285. doi: 10.1073/pnas.93.20.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Functional specialization within the dorsolateral frontal cortex for serial order memory. Proc Biol Sci. 1991;246(1317):299–306. doi: 10.1098/rspb.1991.0158. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. J Neurosci. 2005;25(5):1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D'Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J Neurosci. 2000;20(22):RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Nolde SF, D'Esposito M. Fmri investigations of left and right pfc contributions to episodic remembering. Psychobiology. 2000;28(2):197–206. [Google Scholar]
- Rubin DC, Burt CDB, Fifield SJ. Experimental manipulations of the phenomenology of memory. Memory & Cognition. 2003;31(6):877–886. doi: 10.3758/bf03196442. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Greenberg DL. Visual memory-deficit amnesia: A distinct amnesic presentation and etiology. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(9):5413–5416. doi: 10.1073/pnas.95.9.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Chua PM, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: An fmri study. Neuroimage. 1999;10(5):520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Wilding EL. Retrieval processing and episodic memory. Trends in Cognitive Sciences. 2000;4(3):108–115. doi: 10.1016/s1364-6613(00)01445-5. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Yonelinas AP. Human recognition memory: A cognitive neuroscience perspective. Trends in Cognitive Sciences. 2003;7(7):313–319. doi: 10.1016/s1364-6613(03)00131-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Curran T, Galluccio L, Milberg WP, Bates JF. False recognition and the right frontal lobe: A case study. Neuropsychologia. 1996;34(8):793–808. doi: 10.1016/0028-3932(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL. Neural mechanisms of visual object priming: Evidence for perceptual and semantic distinctions in fusiform cortex. Neuroimage. 2003;19(3):613–626. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Skowronski JJ, Walker WR, Betz AL. Ordering our world: An examination of time in autobiographical memory. Memory. 2003;11:247–260. doi: 10.1080/09658210244000009a. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Fujii T, Tsukiura T, Okuda J, Umetsu A, Nagasaka T, et al. Neural basis of temporal context memory: A functional mri study. Neuroimage. 2002;17(4):1790–1796. doi: 10.1006/nimg.2002.1303. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. Clarendon Press; Oxford: 1983. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26(1):1–12. [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Fluent conceptual processing and explicit memory for faces are electrophysiologically distinct. J Neurosci. 2006;26(3):926–933. doi: 10.1523/JNEUROSCI.3931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49(3):459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmacott R, Moscovitch M. The contribution of autobiographical significance to semantic memory. Mem Cognit. 2003;31(5):761–774. doi: 10.3758/bf03196114. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: The frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121(3):331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory's echo: Vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci U S A. 2000;97(20):11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittlesea BWA, Williams LD. The source of feelings of familiarity: The discrepancy-attribution hypothesis. Journal of Experimental Psychology-Learning Memory and Cognition. 2000;26(3):547–565. doi: 10.1037//0278-7393.26.3.547. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NEA, Baynes K. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: An fmri study. Neuroreport. 2001;12(2):359–363. doi: 10.1097/00001756-200102120-00035. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25(11):3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]