Abstract
Interactions between natural enemies and their victims are a pervasive feature of the natural world. In this paper, we discuss trophic interactions as determinants of geographic range limits. Predators can directly limit ranges, or do so in conjunction with competition. Dispersal can at times permit a specialist predator to constrain the distribution of its prey—and thus itself—along a gradient. Conversely, we suggest that predators can also at times permit prey to have larger ranges than would be seen without predation. We discuss several ecological and evolutionary mechanisms that can lead to this counter-intuitive outcome.
Keywords: natural enemy–victim, range limits, gradient
1. Introduction
All species have limited geographic ranges, and no species lives alone since each interacts with many others in complex food webs. How are these two facts related? In recent years, there has been an explosion of interest in the analysis of species' ranges (Gaston 2003), but most of this literature as yet makes scant connection with the trophic interactions that comprise food web ecology. Experimental studies of species' distributions along gradients in temperature reveal that species' interactions can lead to surprising responses to environmental perturbations (Davis et al. 1998a,b), suggesting that the interplay of food web interactions and range dynamics warrants further study.
From an abstract point of view, a species's realized range reflects how its demography—birth, death and movement—responds to environmental heterogeneity across space and through time. Range limits reflect the imprint of many factors, including variation in the physical environment, dispersal limitation, historical accidents, demographic stochasticity, evolution and spatial variation in the strength of interspecific interactions (Case & Taper 2000; Gaston 2003; Case et al. 2005; Holt et al. 2005; Antonovics et al. 2006; Bahn et al. 2006; Travis et al. 2006; Goldberg & Lande 2007; Ricklefs 2007; Thuiller et al. 2008). A basic determinant of a species's range is the relationship of its niche requirements to a spatially varying template of environmental factors. The ability to cope with natural enemies, either by avoiding mortality or by reproducing copiously enough to offset the extra mortality, is an important aspect of species' niches (Chase & Leibold 2003). Moreover, species' traits, including their niches, are not fixed but evolutionarily labile, so ranges can have evolutionary as well as ecological dynamics (Antonovics et al. 2001; Holt 2003). Owing to the multiple causal forces that determine range limits, natural enemies can in many distinct ways influence both the ecological and evolutionary dynamics of species' ranges. Space precludes full treatment of this theme, so we will focus on a few theoretical studies showing how both generalist and specialist natural enemies can constrain the range limits of their victims. We will also suggest that, at times, predators can facilitate range expansion along gradients. (For simplicity, we refer mainly to ‘predators’ and their ‘prey’, but the basic ideas we present pertain to most natural enemy–victim systems.)
In distributional ecology, a fundamental distinction is between sites where, over some range of densities, average birth rates exceed average death rates, and sites where birth rates are always lower than death rates. The former are potentially source populations—where a species's niche requirements are met—and the latter, potential sink populations—where they are not (Holt 1985; Pulliam 2000; Kawecki 2008). Which sites are actually sources and sinks depend upon dispersal. Some sites may be perfectly suitable but unoccupied and outside the range, simply because dispersers have not colonized them; conversely, some sites may be occupied and thus within the range because dispersal sustains sink populations in habitats with conditions outside the niche. When dispersal occurs at a low trickle and there are no barriers to dispersal (so all sites in a region can be reached occasionally), to a reasonable approximation, the distribution should reflect how the niche requirements of a species map onto spatial variation in the environment (except for trace abundances in sink habitats). Predation can be a key niche axis determining a species's presence or absence in a local community, in effect by creating sinks, and so potentially can strongly influence range limits.
2. The interplay of competition and predation along gradients
It has long been recognized that interspecific competition can produce abrupt range limits along smooth gradients. Models based on the Lotka-Volterra competition model, in the limit when dispersal rates are small, have been explored by MacLean & Holt (1979), Roughgarden (1979) and Holt et al. (2005) to examine patterns of species replacement along gradients. In like manner, range edges of a focal prey species may emerge directly from mortality inflicted by a predator, and indirectly from the availability of alternative prey or resources sustaining that predator. This is the theme of apparent competition (Holt & Lawton 1994) played out over a broad geographic scale.
To illustrate how predation and competition might jointly influence range limits, we add a predator (of density P) to the Lotka-Volterra competition model for two species distributed along an environmental gradient. We assume that dispersal is weak, so to a reasonable approximation, local densities are driven by local interactions, rather than immigration and emigration. The general model is
| (2.1) |
where the competitors/prey have density Ni (i=1, 2; all densities are implicitly functions of position along the gradient, x); ri is the intrinsic growth rate of species i; c is the strength of intraspecific density dependence; and α is the competition coefficient (Nj is the density of the competitor to species i). The attack rate on the prey is a, the quantity b converts consumption into predator births and m is the predator death rate (for simplicity, we have assumed that most parameters are the same for both prey). Each competitor when alone grows logistically, and the predator has uniform linear functional and numerical responses to each prey species. Without the predator, if the growth rates of species 1 and 2 vary linearly along a gradient, but in opposite directions (so that r1=r′−gx, and r2=gx for 0<x<r′/g, where g measures the strength of the gradient in intrinsic growth rates), the two competitors coexist in a zone of width
| (2.2) |
When α is nearly one (so interspecific and intraspecific competition are comparable) there will be little overlap and the species pair will exhibit a nearly parapatric distribution.
Similarly, shared predation alone can lead to reduced geographic overlap in the ranges of two prey species because of apparent competition. In equation (2.1), if α=0 the two prey species do not directly compete. The quantity is the decline in prey density at equilibrium with the predator, relative to prey numbers without the predator, when only a single prey species with growth rate r′ is present (Holt 1984). Prey coexistence requires that the prey not differ too greatly in their intrinsic growth rates (the coexistence condition is ). The overlap zone between the prey species has width
| (2.3) |
The more effective the predator is at limiting its prey (i.e. the higher is Δ), the narrower is the overlap zone. In the limit Δ≈1, there is effectively no overlap, so a parapatric distribution between prey species emerges from shared predation. In the limit Δ≈0, the predator can barely subsist, so shared predation will not cause a reduction in the size of prey ranges (with competition, the predator reaches 0 density at a higher Δ).
If we now assume that the prey both share a predator and directly compete, and make the same symmetry assumptions as before, the width of the overlap zone is
| (2.4) |
Overlap is negligible if either the competition coefficient is near 1, or the strength of predation is near 1. Comparing (2.4) with (2.3), we see that there is a synergistic interaction between competition and predation in determining species' ranges. In this model, direct competition further narrows the range of overlap expected from shared predation alone, and predation likewise reduces the overlap permitted by interspecific competition.
Gradients in mortality imposed by predators with a numerical response to their prey can thus lead to narrowly overlapping or even parapatric distributions for species that do not directly compete but share a predator, and direct competition further shrinks the zone of overlap. Predation acting as a density-independent source of mortality can also cause parapatry when a pair of consumer species competes for a single limiting resource. This can be true even when each prey species experiences the same rate of mortality from the predator. A general model illustrating this point is as follows:
| (2.5) |
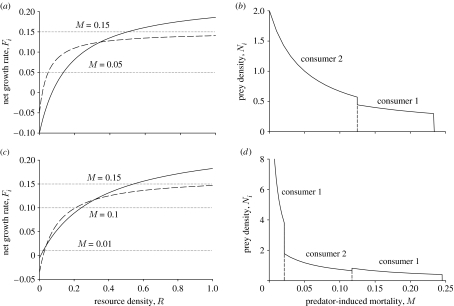
Here, Ci is the per capita rate of consumption by consumer i (of density Ni) of the single resource (of density R). The per capita growth rate of consumer i in the absence of predation is Fi, which is assumed to increase with R (figure 1a). We assume that consumer 2 is better at resource exploitation at low resource levels, but that consumer 1 is better at higher resource levels, so the two F-curves cross as shown in figure 1a. To complete the model, Q(R) is resource recruitment, and M(x) is the rate of predation uniformly imposed on both species at position x on the gradient (this assumes that the predator is a generalist, whose abundance is determined by factors other than the particular prey species in question). Again, we assume that dispersal is over spatial scales that are small relative to the gradient as a whole.
Figure 1.
(a) The per capita growth rate of two consumers in the absence of predation (Fi), as a function of R. Consumer 2 is better at resource exploitation at low resource levels, while consumer 1 is better at higher resource levels. Solid curve, consumer 1; dashed curve, consumer 2. (b) Patterns in abundance along a linear mortality gradient. We assume an abiotic resource with a constant input rate. At low mortality, consumer 2 outcompetes consumer 1, but eventually, as mortality increases, consumer 1 becomes dominant. The resource has a constant input rate of 0.1. The consumption functions are Type II functional responses with attack rates 2 and 4 and handling times 3 and 5 for consumers 1 and 2, respectively. The densities of both consumers are measured in units of the resource, and the intrinsic mortality is 0.1 for consumer 1 and 0.05 for consumer 2. Owing to the Type II functional responses, the maximum growth rate of consumer 1 is 0.2333 (the reciprocal of the handling time minus the intrinsic mortality). As M approaches this level, the equilibrium R needed to sustain the consumer increases without bound, and the consumer density approaches 0.3 (product of the resource input rate and handling time). When M reaches the critical level, the consumer consumption cannot match its mortality, so its density suddenly goes to 0, as shown. (c,d) Same as (a) and (b), but now consumer 1 is better at both low and high resource levels. The attack rates are 0.75 and 2, the handling times 4 and 5, and the intrinsic mortalities 0.005 and 0.035 for consumers 1 and 2, respectively (other parameters the same as (a,b)).
For a given mortality rate, the winning competitor is the one persisting at the lower value of R (its R*). If a competitor is alone, it will be in equilibrium when resources are reduced to the level at which Fi just equals M. Along a linear gradient in mortality (e.g. M(x)=gx), the winning competitor switches from consumer 2 to consumer 1 as mortality increases, with patterns in abundance along the gradient as shown in figure 1b.
There has been a debate in the literature about whether abundances should typically be greater at the centre of a range than near a range limit. The standard view is that peripheral populations are rarer than central populations, but there are many empirical counter-examples (Sagarin & Gaines 2002; Sagarin et al. 2006). Standard resource–consumer models such as that shown in equation (2.5) show that it is easy to generate such counter-examples from plausible trophic interactions along gradients. In figure 1, note that consumer 1 reaches its highest abundance near its border with consumer 2. Moreover, note that at the other range limit for consumer 1, its abundance drops abruptly to 0 from a positive value (0.3) at a point along the mortality gradient. This occurs because it was assumed that the functional response saturates (say at Cmax, which is the reciprocal of handling time for a Type II functional response), so that prey growth rate as a function of resource availability also approaches an asymptotic upper limit (say Fmax). As the mortality approaches this limit from below, the resource concentration rises, the consumer functional response approaches Cmax and the consumer density approaches bQ(R)/Cmax (b converts resources into consumer births). Once the mortality reaches the maximum growth rate Fmax, however, consumer growth cannot keep up with mortality, and so its equilibrium density plummets to 0. Thus, near the range margin, there will be an abrupt break in density, from some moderate value to zero, with increasing mortality along the gradient.
An interesting result of this familiar resource–consumer model is that there can be abrupt shifts in species abundances along a smooth gradient, even for a single species in the absence of competition. Similarly, abrupt changes in abundance can occur along a gradient in mortality due to the shifts in competitive dominance, leading to parapatric distributions. Moreover, for some parameters, the Fi curves can cross twice (figure 1c), and thus the identity of the dominant consumer switches twice along a mortality gradient. Figure 1d shows that this leads to a complex distributional pattern. Were we to include dispersal in the model (e.g. in a reaction-diffusion formulation) and assume that the rate of dispersal is weak, these transitions could be smoothed out, but there could still be dramatic shifts in abundance over short distances (details not shown).
We have emphasized how predation can constrain range limits. Generalist predators acting as sources of density-independent mortality may at times also permit a prey species to occupy a wider distributional range than is possible without predation, because increased mortality rates can counter-intuitively increase abundance. Sih et al. (1985), in a review of predator removal experiments, reported a surprising number of cases in which prey numbers decline following predator removal, suggesting that predators are indirectly benefiting prey. If extinction risk declines with increasing abundance, then predators in these examples may actually reduce prey extinction risk, and so the predator may permit the prey to persist in areas where otherwise it would be absent. One obvious way this can happen is if the predator is a generalist, and attacks other species that more strongly harm the focal prey than does the predator itself (e.g. competitors or intraguild predators; an example is given in figure 2, discussed below). But such indirect effects are not necessary for predators to ‘benefit’ their prey. Abrams (2002) provides a number of examples of standard ecological models in which a species's abundance increases with an increase in the rate of density-independent mortality, which, if sufficiently great, ensures extinction. His discussion focused on temporal changes in the environment, but the same basic message pertains to gradients in space as well. One way this can happen is if there are strong nonlinear feedbacks involving resource populations. In stage-structured models of population dynamics, if there is strong overcompensatory density dependence acting at one stage prior to reproduction, mortality at prior stages may relax density dependence sufficiently to permit an overall higher abundance of reproductive individuals. Finally, if prey behaviourally respond to predators by reducing foraging, the prey may be less likely to overexploit its own resource base. For all these reasons, generalist predators may at times enhance prey abundance and thus facilitate a larger range by that species along an environmental gradient.
Figure 2.
Predator and prey densities as a function of gradient position x for equation (3.1). Above x=0.27, the system is stable, and the lines show the equilibrium values given by the equations above (the two-species equilibrium below x=0.72, where the predator goes extinct, the one-species equilibrium above this x). For x<0.27, the system is unstable, and the solid lines indicate the time-average densities, and the dashed lines the minima and maxima. The predator values are indicated by the thicker lines. Parameters are r=1, c=0.02, a=0.1, b=1, h=0.4, m=0.2 and g=1.
Generalist predators with nonlinear functional and numerical responses to their prey can generate distributional patterns in prey distributions along gradients differing in several respects from the above model results. For example, Holt (1997a) describes a model in which a focal prey species with a low intrinsic growth rate suffers predation from a generalist predator. The predator is assumed to experience direct density dependence (e.g. territoriality), to be sustained by alternative prey, and to have a saturating functional response to its prey. Along a gradient in productivity of the alternative prey, the focal species may be present at the low-productivity end (where few predators are present), as well as at the high-productivity end (where predator numbers are constrained and functional responses are saturated, hence diluting attacks upon the focal prey), but excluded from a zone of intermediate productivity. Equation (2.1) above assumed that the predator was an indiscriminate generalist. A widespread generalist predator with switching behaviour could instead increase the zone of overlap between competing prey species along environmental gradients.
3. Specialist natural enemies and range limits
If the natural enemy is a specialist depending entirely upon the victim species, there are two range limits to consider: the victim's, and that of the natural enemy itself. Again, we take a predator–prey system and, as an illustrative example, assume that the gradient is in density-independent mortality, acting equally upon both the specialist enemy and its victim. For instance, in intraguild predation, a generalist top predator can attack both an intermediate specialist predator and the prey it requires. We assume the prey has logistic growth, and the predator a Type II functional response, as well as a numerical response proportional to its functional response. Along a one-dimensional gradient x, mortality imposed on both species increases linearly with x. The model is
| (3.1) |
where h is the handling time and the other parameters and variables are as in equation (2.1). The parameter g (assumed greater than 0) describes the strength of a gradient in mortality of both predator and prey.
In the absence of the predator, prey density is , so maximal prey density is reached at x=0. Predator persistence requires . The densities of both species at x are
| (3.2) |
As x increases, N* also increases, given that the predator is present. Thus, the presence of the specialist predator reverses the pattern of abundance expected for the prey species along the gradient. For the predator, note that the first term in the expression for P* decreases with increasing x, but the second increases, so equilibrial predator density P* can initially either increase or decrease with x, depending on parameter values. For the increasing case, the equilibrium given by (3.2) is unstable (details not shown). At some x, however, P* will begin to decrease with further increases in x, and there is an x at which P* is 0, defining the predator species's border. Above this x, the prey is too rare to sustain the predator, and so again the prey density follows (r−gx)/c, declining with increasing x until finally its solitary species border is reached at x=r/g.
Figure 2 shows a numerical example. On the right, we see a range limit in the prey imposed by extrinsic mortality, and also a range limit for the predator, imposed by its need for threshold prey abundance. In this part of the gradient, the predator and prey have nested distributions. There are certainly examples of nested distributions for specialist natural enemy–victim distributions (Shenbrot et al. 2007). For instance, Alexander et al. (2007) found that peripheral populations of the forest sedge Carex blanda in Kansas were more likely to lack two pathogens (a smut and a rust) and a seed predator (a chalcid), compared with central populations further east.
At the other end of the gradient, the system shows limit cycles, increasing in amplitude as the mortality factor declines (decreasing x). It is likely that local populations face extinction because of the very low densities reached during each cycle. This simple example shows that a generalist predator, acting as a mortality agent on both an intermediate predator and a basal prey, can stabilize the dynamics of these lower levels. For this part of the gradient to remain within the range of each species, there would need to be at least occasional recolonization events; the distribution would then be a patchwork of empty and occupied sites.
One simple conclusion from this model is that trophic interactions can lead to counter-intuitive patterns in abundance along environmental gradients. In this case, considering just the impact of the density-independent mortality factor, one might expect numbers of prey to decline along the gradient, when in fact, owing to its interaction with a specialized predator, its numbers peak at an intermediate point. It is also possible for predators as well as their prey to show non-intuitive patterns of responses in abundance along gradients. This has been known for a long time in the literature on resource–consumer interactions, but the message has not yet penetrated the field of biogeography. For instance, along a gradient in predator attack rates, predator numbers can peak quite near the range limit for the predator (fig. 2.2 in Holt & Hochberg 2001). The reason is that, when attack rates are high, predators overexploit their prey, so fewer predators will be sustained on a given prey base than at the lower attack rates (and dynamics are probably unstable at high attack rates, as well). We will return to this theme below.
4. When can specialist predators constrain the ranges of their prey?
Compared with generalist natural enemies, it is plausible that specialist natural enemies are unimportant in limiting the distributions of their prey. After all, just beyond the range limit, by definition a species is vanishingly rare. For specialist predators with prey-dependent functional responses or pathogens with density-dependent transmission, there is a minimum prey or host population size below which the predator should decline towards extinction. This in turn means that its role in driving down the number of its victims should become unimportant at the range margin; hence specialist predators seem likely to be less important than generalists in explaining chronic rarity and in particular local extinction in their prey at range margins.
This may typically be true—so that specialist predation, though important for determining local abundance and stability over much of a prey species's range, is not usually causally responsible for that prey's range limits. Contrary to this intuitive expectation, however, theoretical studies have identified several avenues through which specialist predators can influence range limits in their prey, both to constrain limits and to increase them, over ecological and evolutionary time-scales. As noted above, when dispersal rates are low, we might expect nested distributions. At higher dispersal rates, such nesting may break down. Theoretical studies suggest that specialist predators can then impose range limits on their own prey. Holt (1979) conjectured that specialist natural enemies could lead to range limits in prey or hosts given spatial variation in productivity. Predators should be abundant where their preys are productive, and predator spillover from such areas could cause intense predation and prey extinction at unproductive sites, where prey reproduction cannot compensate for heightened predation (Holt 1984). Hochberg & Ives (1999) examined this suggestion for a model of host–parasitoid interactions and found that the scenario works, given three conditions. First, the parasitoid should have a high per capita attack rate, so that moderate parasitoid numbers can effectively limit the host to well below carrying capacity. Increases in parasitoid numbers above these moderate levels can then drive the host to local extinction. Second, there should be spatial variation in host productivity (or other factors that govern predator abundance). Increased production shows up largely as increases in average parasitoid abundance, so spatial variation in host production translates into spatial variation in parasitoid numbers. Finally, the parasitoid should have high dispersal rates leading to an asymmetric flow of parasitoids from ‘hot spots’ of high host production to ‘cool spots’ of low host production. When this flow is sufficiently great, hosts are eliminated in low-productivity areas. Along a gradient in host production, continual spillover of parasitoids can thus constrain host range to a smaller area than is observed in the absence of parasitism.
This effect is magnified given strong Allee effects in the prey, so that prey growth rates are negative at low densities (Keitt et al. 2001). Owen & Lewis (2001) used reaction-diffusion equations to illustrate how Allee effects can permit specialist predators to collapse a prey species's range. The basic idea is that if predators disperse sufficiently from occupied areas, they may impose mortality at the edge sufficient to push prey populations below Allee thresholds. In a homogeneous landscape, once this process starts, there is no countervailing force, and the range retracts. Fagan et al. (2005) applied this idea to colonization of the lava fields of Mt St Helens by a lupine (Lupinus lapidus), where herbivory by several species of moth is inversely density-dependent (in part due to their own satiation), and therefore particularly severe in low-density lupine populations. These authors develop a model suggesting that the moth greatly slows invasion by the lupine. The model predicts that, were the lupine to have a higher intrinsic growth rate than observed, herbivory would not stall the invasion; by contrast, at a substantially lower growth rate, herbivory should cause range retraction. Thus, along a gradient in lupine growth rates that is steep relative to herbivore dispersal distances, one would expect to see a range limit, determined by the combination of herbivore spillover and Allee effects.
Distributional ranges reflect not only ongoing ecological forces, but also the imprint of the past. Even in the absence of persistent limit cycles or more complex dynamics, a general property of natural enemy–victim systems is that introducing an effective natural enemy can lead initially to a large increase in its population to well above its ultimate equilibrium, concurrently driving the victim down to abundances substantially below its long-term equilibrial level (Holt & Hochberg 2001). If numbers are sufficiently low during this trough, this could cause local extinctions; with constraints on re-colonization, this could produce permanent shrinkage of the victim's range. This is particularly probable if the victim and enemy do not share an evolutionary history. For instance, there are dramatic examples of introduced parasites reducing the host species' ranges. The chestnut blight Cryphonectria parasitica (an ascomycete sac fungus) eliminated the American chestnut Castanea dentata as a viable reproductive population from almost the entire eastern United States. Remnant populations (often only a few scattered individuals) are found mainly on the fringe of the former range. (This example may not reflect solely an overshoot of an ultimate equilibrium by a highly effective natural enemy; the fungus can apparently persist to some degree on alternative hosts, such as chinkapin and post oak, which may enhance its effectiveness as a limiting factor.) If the American chestnut survives, it will be as a vastly diminished spatial shadow of its former extensive self; given dispersal limitations, its new range might forever retain the imprint of a severe historical bout of an intense trophic interaction.
5. When can specialist predators Expand the range of their prey?
Are there circumstances in which a specialist natural enemy leads to a larger geographic range for its victim? Two possible ecological mechanisms occur to us, comparable with the points made above for generalist natural enemies.
Consider a prey species with metapopulation dynamics at each point along a gradient (large in spatial scale, compared with the dispersal distance relevant for each metapopulation). Several authors have used metapopulation models to highlight how range limits can arise because (i) suitable habitat patches are too scarce, (ii) extinction rates are too high or (iii) colonization rates from occupied to empty patches are too low (Carter & Prince 1981; Lennon et al. 1997; Holt & Keitt 2000). Normally, it is reasonable to assume that predators depress prey colonization rates, or enhance extinction rates. Along a gradient in the availability of suitable habitat for the prey, this leads to a predator distribution nested inside that of its prey (Holt 1997b).
These assumptions seem reasonable yet may not always hold. Predators could at times enhance prey persistence in metapopulation dynamics and facilitate a greater range for the prey (and thus the predator itself) along the gradient. Empirical studies show that the presence of predators in patches can induce prey to flee those patches, thereby increasing the rate of colonization of empty, habitable patches (Gilliam & Fraser 2001). A theoretical study by Prakash & De Roos (2002) incorporating this mechanism shows that this behavioural effect of predation can strongly enhance prey persistence in a metapopulation. Thus specialized predation may permit a prey species to occupy a broader range along a gradient.
At times predation may reduce prey extinction; for instance, by moderating the amplitude of prey population fluctuations due to unstable dynamics arising from the interaction between the prey and its own resources. A plausible field example of predator-mediated stability comes from islands in the Baltic, where voles on predator-free islands increase to very high numbers and overgraze their plant food base, then crash and risk extinction, whereas, when predators are present, vole numbers are relatively stable and bounded away from low numbers (Banks et al. 2004). Even specialist predators can theoretically prevent prey from overexploiting their own resources and then plummeting to extinction. May (1972) showed that a predator-herbivore–plant interaction could be stable, even though the herbivore–plant interaction alone is strongly unstable, with limit cycles large enough to risk extinction. This effect readily arises if the top predator has direct density dependence (e.g. from interference), whereas the herbivore has weak direct density dependence and a saturating functional response to its required resource.
If predators dampen prey oscillations, prey extinction rates should be lower with the predator than without it. A specialist predator can then extend the range of its prey along a gradient. Holt (2002) presented a metapopulation model for a tritrophic interaction embodying these top–down stabilizing effects of predation along a gradient in habitat availability. A predator attacks an intermediate consumer (e.g. an herbivore), which in turn depends on a basal biotic resource (e.g. a plant). Along a gradient in habitat availability for the resource, because the predator stabilizes the interaction between the resource and the consumer, the range limit for the consumer can extend to lower values of habitat availability with the predator than without it.
6. Specialist predation and evolutionary range limits
As is customary in most discussions of range limits, we have implicitly assumed that species' ecological properties are fixed. Yet the issue of range limit stability over long time spans requires a consideration of evolutionary as well as ecological processes. Mayr (1963) long ago argued that maladaptive gene flow from abundant central populations could restrain adaptation by natural selection at sparser range margins, constraining geographic distributions along gradients. If individuals move randomly, more individuals leave dense populations than enter from sparse populations. This asymmetric movement implies that gene flow into low-density populations will be particularly strong where low- and high-density populations are neighbours. Thus, evolutionary constraints should be more likely along sharp gradients in density. This idea has received considerable attention in recent years (e.g. Kirkpatrick & Barton 1997; Gomulkiewicz et al. 1999; Case & Taper 2000; Antonovics et al. 2001; Holt 2003; Case et al. 2005; Bridle & Vines 2007). How do trophic interactions influence the potential evolutionary limitation of ranges by gene flow? A full answer requires splicing a model for evolutionary dynamics in each interacting species with a model for its population dynamics. We have begun to examine this question by adding a predator to the model of Filin et al. (2008; based on Kirkpatrick & Barton 1997) and will report the results in detail elsewhere. Specialist predators, or generalist predators with switching behaviours, are expected to have stronger relative effects on prey abundance at sites where prey are abundant, than where prey are rare (when a prey species is quite rare, it might be ignored by a switching generalist predator, and any specialist predator depending upon it will tend to go extinct). Thus, predation can flatten spatial gradients in prey abundance, in turn making gene flow weaker relative to local selection, and thus indirectly relax the evolutionary constraint of gene flow on expansion by the prey species along a gradient.
7. Conclusion
The theoretical studies sketched here suggest that predators can have a diverse range of impacts upon the geographic distributions of their prey, and thus indirectly on their own distributions. For instance, the interplay of predation and competition can have a variety of impacts upon prey range limits. Combining direct and apparent competition can lead to a tighter zone of overlap between prey species than for either interaction considered alone. A standard resource–consumer model, where a predator acts as a density-independent mortality agent on two competing consumer species, reveals surprising distributional patterns, such as abrupt edges to ranges and nested distributions of competing species. Considering purely ecological processes, dispersal by specialist predators can at times constrain the distribution of their prey. Conversely, specialist predators might facilitate larger prey ranges via behavioural mechanisms such as prey fleeing patches with predators, and ecological mechanisms such as the moderation by predation of intrinsically unstable plant–herbivore interactions. Generalist predators can also have a diverse effect upon prey range limits. We have suggested that predation can also alter the evolutionary dynamics of species' ranges. This is a theme that will need more attention elsewhere, and one that needs to be pursued to link the geographic mosaic theory of coevolution (Thompson 2005) to the analysis of geographic range limits.
In the literature we have reviewed and the models we have presented, we have assumed that the environment itself is temporally constant, so that geographic ranges can settle into an equilibrium. One reason for the growing interest in range limits, we believe, is the concern with predicting the probable consequences of global climatic change. When environmental gradients themselves are dynamic, species' ranges will also be dynamic (Mustin et al. 2009). The experiments reported by Davis et al. (1998a,b) demonstrated that trophic interactions could lead to surprising effects of shifting climate on species' ranges. For instance, if predation becomes more intense with increasing temperature, even if the predator attacks its prey in a uniform manner, there could be shifts in competitive dominance (as in equation (2.5) above), and shrinking zones of overlap between prey species (as in equation (2.1)). Many generalist predators and pathogens are more mobile than their prey or hosts; if climate change permits them to colonize regions from which they had been previously excluded, there could be large and surprising effects on the geographic distributions of the novel prey or host species they encounter. In conclusion, we suspect that the interaction between natural enemies and their victims plays a larger role in determining species' range limits than is obvious from the extant literature, and this interaction can have surprising, counter-intuitive effects on distributional limits. These surprising effects are probably even more important when one considers temporal change as well as shifting interactions along gradients. This topic warrants much more attention by both theoreticians and empiricists.
Acknowledgments
We thank David Hall for his assistance with figure preparation, and R. Sagarin and two reviewers for useful suggestions. This research was funded by the National Science Foundation (DEB0515598) and supported by the University of Florida Foundation.
Footnotes
One contribution of 17 to a Special Issue ‘Geographic range limits of species’.
References
- Abrams P.A. Will small population sizes warn us of impending extinctions? Am. Nat. 2002;160:293–305. doi: 10.1086/341521. doi:10.1086/341521 [DOI] [PubMed] [Google Scholar]
- Alexander H.M., Price S., Houser R., Finch D., Tourtellot M. Is there reduction in disease and pre-dispersal seed predation at the border of a host plant's range? Field and herbarium studies of Carex blanda. Ecology. 2007;95:446–457. doi:10.1111/j.1365-2745.2007.01228.x [Google Scholar]
- Antonovics J., Newman T.J., Best B.J. Spatially explicit models on the ecology and genetics of population margins. In: Silvertown J., Antonovics J., editors. Integrating ecology and evolution in a spatial context. Blackwell; Oxford, UK: 2001. pp. 97–116. [Google Scholar]
- Antonovics J., Newman T.J., McKane A.J. Spatiotemporal dynamics in marginal populations. Am. Nat. 2006;167:16–27. doi: 10.1086/498539. doi:10.1086/498539 [DOI] [PubMed] [Google Scholar]
- Bahn V., O'Connor R.J., Krohn W.B. Effect of dispersal at range edges on the structure of species ranges. Oikos. 2006;115:89–96. doi:10.1111/j.2006.0030-1299.14825.x [Google Scholar]
- Banks P.B., Norrdahl K., Nordstrom M., Korpimaki E. Dynamic impacts of feral mink predation on vole metapopulations in the outer archipelago of the Baltic Sea. Oikos. 2004;105:79–88. doi:10.1111/j.0030-1299.2004.12855.x [Google Scholar]
- Bridle J.R., Vines T.H. Limits to evolution at range margins: when and why does adaptation fail? Trends Ecol. Evol. 2007;22:140–147. doi: 10.1016/j.tree.2006.11.002. doi:10.1016/j.tree.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Carter R.N., Prince S.D. Epidemic models used to explain biogeographical distribution limits. Nature. 1981;293:644–645. doi:10.1038/293644a0 [Google Scholar]
- Case T.J., Taper M.L. Interspecific competition, gene flow, and the co-evolution of species borders. Am. Nat. 2000;155:583–605. doi: 10.1086/303351. doi:10.1086/303351 [DOI] [PubMed] [Google Scholar]
- Case T.J., Holt R.D., McPeek M.A., Keitt T.H. The community contexts of species' borders: ecological and evolutionary perspectives. Oikos. 2005;108:28–46. doi:10.1111/j.0030-1299.2005.13148.x [Google Scholar]
- Chase J.M., Leibold M.A. University of Chicago Press; Chicago, IL: 2003. Ecological niches: linking classical and contemporary approaches. [Google Scholar]
- Davis A.J., Jenkinson L.S., Lawton J.H., Shorrocks B., Wood S. Making mistakes when predicting shifts in species range in response to global warming. Nature. 1998a;391:783–786. doi: 10.1038/35842. doi:10.1038/35842 [DOI] [PubMed] [Google Scholar]
- Davis A.J., Lawton J.H., Shorrocks B., Jenkinson L.S. Individualistic species responses invalidate simple physiological models of community dynamics under global environmental change. J. Anim. Ecol. 1998b;67:600–612. doi:10.1046/j.1365-2656.1998.00223.x [Google Scholar]
- Fagan W.F., Lewis M., Neubert M.G., Aumann C., Apple J.L., Bishop J.G. When can herbivores slow or reverse the spread of an invading plant? A test case from Mount St. Helens. Am. Nat. 2005;166:669–685. doi: 10.1086/497621. doi:10.1086/497621 [DOI] [PubMed] [Google Scholar]
- Filin I., Holt R.D., Barfield M. The relation of density regulation to habitat specialization, evolution of a species' range, and the dynamics of biological invasions. Am. Nat. 2008;172:233–247. doi: 10.1086/589459. doi:10.1086/589459 [DOI] [PubMed] [Google Scholar]
- Gaston K.J. Oxford University Press; Oxford, UK: 2003. The structure and dynamics of geographic ranges. [Google Scholar]
- Gilliam J.F., Fraser D.F. Movement in corridors: enhancement by predation threat, disturbance, and habitat structure. Ecology. 2001;82:258–273. doi:10.2307/2680101 [Google Scholar]
- Goldberg E.E., Lande R. Species' borders and dispersal barriers. Am. Nat. 2007;170:297–304. doi: 10.1086/518946. doi:10.1086/518946 [DOI] [PubMed] [Google Scholar]
- Gomulkiewicz R., Holt R.D., Barfield M. The effects of density dependence and immigration on local adaptation in a black-hole sink environment. Theor. Popul. Biol. 1999;55:283–296. doi: 10.1006/tpbi.1998.1405. doi:10.1006/tpbi.1998.1405 [DOI] [PubMed] [Google Scholar]
- Hochberg M.E., Ives A.R. Can natural enemies enforce geographical range limits? Ecography. 1999;22:268–276. doi:10.1111/j.1600-0587.1999.tb00502.x [Google Scholar]
- Holt, R. D. 1979 Predation and the structure of prey communities. PhD thesis. Harvard University.
- Holt R.D. Spatial heterogeneity, indirect interactions, and the coexistence of prey species. Am. Nat. 1984;124:377–406. doi: 10.1086/284280. doi:10.1086/284280 [DOI] [PubMed] [Google Scholar]
- Holt R.D. Population dynamics in two-patch environments: some anomalous consequences of an optimal habitat distribution. Theor. Popul. Biol. 1985;28:181–208. doi:10.1016/0040-5809(85)90027-9 [Google Scholar]
- Holt, R. D. 1997a Community modules. In Multitrophic Interactions in Terrestrial Ecosystems, 36th Symposium of the British Ecological Society (eds A. C. Gange & V. K. Brown), pp. 333–349. Oxford, UK: Blackwell Science.
- Holt R.D. From metapopulation dynamics to community structure: some consequences of spatial heterogeneity. In: Hanski I., Gilpin M., editors. Metapopulation biology. Academic Press; New York, NY: 1997b. pp. 149–164. [Google Scholar]
- Holt R.D. Food webs in space: on the interplay of dynamic instability and spatial processes. Ecol. Res. 2002;17:261–273. doi:10.1046/j.1440-1703.2002.00485.x [Google Scholar]
- Holt R.D. On the evolutionary ecology of species ranges. Evol. Ecol. Res. 2003;5:159–178. [Google Scholar]
- Holt R.D., Hochberg M.E. Indirect interactions, community modules, and biological control: a theoretical perspective. In: Waijnberg E., Scott J.K., Quimby P.C., editors. Evaluation of indirect ecological effects of biological control. CAB International; Wallingford, UK: 2001. pp. 13–37. [Google Scholar]
- Holt R.D., Keitt T.H. Alternative causes for range limits: a metapopulation perspective. Ecol. Lett. 2000;3:41–47. doi:10.1046/j.1461-0248.2000.00116.x [Google Scholar]
- Holt R.D., Lawton J.H. The ecological consequences of shared natural enemies. Annu. Rev. Ecol. Syst. 1994;25:495–520. doi:10.1146/annurev.es.25.110194.002431 [Google Scholar]
- Holt R.D., Keitt T.H., Lewis M.A., Maurer B.A., Taper M.L. Theoretical models of species' borders: single species approaches. Oikos. 2005;108:18–27. doi:10.1111/j.0030-1299.2005.13147.x [Google Scholar]
- Kawecki T. Adaptation to marginal habitats. Annu. Rev. Ecol. Evol. Syst. 2008;39:321–342. doi:10.1146/annurev.ecolsys.38.091206.095622 [Google Scholar]
- Keitt T.H., Lewis M.A., Holt R.D. Allee dynamics, critical phenomenon and species borders. Am. Nat. 2001;157:203–216. doi: 10.1086/318633. doi:10.1086/318633 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M., Barton N.H. Evolution of a species' range. Am. Nat. 1997;150:1–23. doi: 10.1086/286054. doi:10.1086/286054 [DOI] [PubMed] [Google Scholar]
- Lennon J.J., Turner J.R.G., Connell D. A metapopulation model of species boundaries. Oikos. 1997;78:486–502. doi:10.2307/3545610 [Google Scholar]
- MacLean W.P., Holt R.D. Distributional patterns in St. Croix Sphaerodactylus lizards: the taxon cycle in action. Biotropica. 1979;11:189–195. doi:10.2307/2388038 [Google Scholar]
- May R.M. Time-delay versus stability in population models with two and three trophic levels. Ecology. 1972;54:315–325. doi:10.2307/1934339 [Google Scholar]
- Mayr E. Harvard University Press; Cambridge, MA: 1963. Animal species and evolution. [Google Scholar]
- Mustin K., Benton T.G., Dytham C., Travis J.M.J. The dynamics of climate-induced range shifting; perspectives from simulation modelling. Oikos. 2009;118:131–137. doi:10.1111/j.1600-0706.2008.17025.x [Google Scholar]
- Owen M.R., Lewis M.A. How predators can slow, stop or reverse a prey invasion. Bull. Math. Biol. 2001;63:655–684. doi: 10.1006/bulm.2001.0239. doi:10.1006/bulm.2001.0239 [DOI] [PubMed] [Google Scholar]
- Prakash S., De Roos A.M. Habitat destruction in a simpler predator–prey patch model: how predators enhance prey persistence and abundance. Theor. Popul. Biol. 2002;62:231–249. doi: 10.1006/tpbi.2002.1611. doi:10.1006/tpbi.2002.1611 [DOI] [PubMed] [Google Scholar]
- Pulliam H.R. On the relationship between niche and distribution. Ecol. Lett. 2000;3:349–361. doi:10.1046/j.1461-0248.2000.00143.x [Google Scholar]
- Ricklefs R.E. History and diversity: explorations at the intersection of ecology and evolution. Am. Nat. 2007;170:S56–S70. doi: 10.1086/519402. doi:10.1086/519402 [DOI] [PubMed] [Google Scholar]
- Roughgarden J. Macmillan; New York, NY: 1979. Theory of population genetics and evolutionary ecology: an introduction. [Google Scholar]
- Sagarin R.D., Gaines S.D. The ‘abundant centre’ distribution: to what extent is it a biogeographical rule? Ecol. Lett. 2002;5:137–147. doi:10.1046/j.1461-0248.2002.00297.x [Google Scholar]
- Sagarin R.D., Gaines S.D., Gaylord B. Moving beyond assumptions to understand abundance distributions across the ranges of species. Trends Ecol. Evol. 2006;21:524–530. doi: 10.1016/j.tree.2006.06.008. doi:10.1016/j.tree.2006.06.008 [DOI] [PubMed] [Google Scholar]
- Shenbrot G., Krasnov B., Lu L. Geographical range size and host specificity in ectoparasites: a case study with Amphipsylla fleas and rodent hosts. J. Biogeogr. 2007;34:1679–1690. doi:10.1111/j.1365-2699.2007.01736.x [Google Scholar]
- Sih A., Crowley P., McPeek M. Predation, competition, and prey communities: a review of field experiments. Annu. Rev. Ecol. Syst. 1985;16:269–311. doi:10.1146/annurev.es.16.110185.001413 [Google Scholar]
- Thompson J.N. University of Chicago Press; Chicago, IL: 2005. The geographic mosaic of coevolution. [Google Scholar]
- Thuiller W., et al. Predicting global change impacts on plant species' distributions: future challenges. Perspect. Plant Ecol. Evol. Syst. 2008;9:137–152. doi:10.1016/j.ppees.2007.09.004 [Google Scholar]
- Travis J.M.J., Brooker R.W., Clark E.J., Dytham C. The distribution of positive and negative species interactions across environmental gradients on a dual-lattice model. J. Theor. Biol. 2006;241:896–902. doi: 10.1016/j.jtbi.2006.01.025. [DOI] [PubMed] [Google Scholar]