Abstract
Understanding the factors that determine the geographic range limits of species is important for many questions in ecology, evolution and conservation biology. These limits arise from complex interactions among ecology and dispersal ability of species and the physical environment, but many of the underlying traits can be conserved among related species and clades. Thus, the range limits of species are likely to be influenced by their macroevolutionary history. Using palaeontological and biogeographic data for marine bivalves, we find that the range limits of genera are significantly related to their constituent species richness, but the effects of age are weak and inconsistent. In addition, we find a significant phylogenetic signal in the range limits at both genus and family levels, although the strength of this effect shows interoceanic variation. This phylogenetic conservatism of range limits gives rise to an evolutionary pattern where wide-ranging lineages have clusters of species within the biogeographic provinces, with a few extending across major boundaries.
Keywords: geographic range limits, macroevolution, biogeography
1. Introduction
Biologists have long sought to understand the factors that determine the northern and the southern range limits of species because the topic is central to many ecological and biogeographic questions (Gaston 1990, 2003; Holt 2003; Parmesan et al. 2005). However, the determinants of range limits of most species remain poorly understood, primarily because such limits arise from complex interactions among a large array of physical, biotic and historical factors (Holt 2003; Parmesan et al. 2005; Goldberg & Lande 2007). Empirical analyses of species range limits have mainly focused on the correlations between aspects of the physical environment (e.g. temperature) and species distributions (Gaston 1990, 2003; Holt 2003; Parmesan et al. 2005), and such relationships are being quantified for an increasing number of species (Jeschke & Strayer 2008). Theoretical studies of range limits, on the other hand, have explored how biotic parameters such as gene flow, local adaptation, species interactions and dispersal can interact with the physical environment to set species range limits (Caughley et al. 1988; Kirkpatrick & Barton 1997; Holt 2003; Case et al. 2005; Goldberg & Lande 2007). These models consistently highlight the importance of microevolutionary processes (Holt 2003; Goldberg & Lande 2007), but empirical tests of their predictions are still rare (Davis et al. 1998; Angert & Schemske 2005; Sanford et al. 2006). How macroevolutionary processes set or constrain range limits has received even less attention, as the majority of existing studies, empirical and theoretical, focus on single species or a few closely related species.
The many factors potentially interacting to set species range limits can be divided into three general categories—species niches, dispersal and spatial variations in the environment (Brown & Lomolino 1998; Holt 2003). Both species niches and dispersal ability have been shown to be phylogenetically conserved in some groups (see Chazdon et al. 2003 and Wiens & Graham 2005) although this effect is certainly not universal (Knouft et al. 2006; Pearman et al. 2007) and may be difficult to detect (Gaston & Chown 1999; Losos & Glor 2003). Dispersal is particularly relevant for marine invertebrates where it may play an important role in constraining species' range limits (Gaylord & Gaines 2000; Byers & Pringle 2006; Sanford et al. 2006), and the variety of larval and other traits that determine species' dispersal abilities tend to be conserved at the level of higher taxa (Hunt et al. 2005; Jablonski et al. 2006b; Bradbury et al. 2008). Local and regional abundances of species, also important determinants of species range limits, can also be influenced by phylogeny (Webb 2000; Webb et al. 2002; Kelly et al. 2008). Similarly, biogeographers have long recognized that regional species assemblages often consist of groups of closely related species (Pielou 1977, 1978), a pattern that also emerges when regional distributions of species are overlain on molecular phylogenies (e.g. Richman & Price 1992; Hellberg 1998; Meyer 2003; Duda & Kohn 2005; Latiolais et al. 2006; but see Fitzpatrick & Turelli 2006). Furthermore, in some cases, distributions of individual species can be predicted from the ecological characteristics of their sister taxa (Peterson et al. 1999). In general, there is a growing recognition that macroevolutionary history can play an important role in determining the spatial distributions of species and structures of communities (McPeek 1996; Webb et al. 2002; Chazdon et al. 2003; Cavender-Bares et al. 2006; Kraft et al. 2007). However, large-scale empirical analyses of how evolutionary history affects species range limits are still lacking.
It is important to recognize that focusing on the macroevolutionary scale does not negate the role of ecological and microevolutionary processes long considered to be the key determinants of species range limits. Those processes are indeed likely to be the proximate causes of species range limits, albeit interacting in complex ways whose strengths probably vary among taxa (Jablonski & Hunt 2006). Ultimately, however, the deeper evolutionary history determines how traits that underlie these proximate processes vary among species and thus needs to be taken into account for a better understanding of the causes of species range limits.
The range limits of any clade are obviously set by the maximum latitudinal and longitudinal limits achieved by its constituent species, and every clade starts with its range limits defined by that of a single species. As the clade ages, its range limits as well as those of the constituent species change as new species evolve and achieve their geographic distributions, existing species go extinct and the range limits of individual species shift in response to changing environments. The range limits of the living clades and species within them are thus a result of all of these historical processes and ultimately reflect how ecological, physiological and life-history traits interact and evolve to determine these limits. In this paper, we explore how evolutionary history influences the range limits of species and clades by evaluating (i) the relationship between the distributional limits of a clade and its age and species richness and (ii) whether the distributional limits of species are phylogenetically conserved (i.e. whether the range limits of closely related species are more similar compared to those that are more distantly related). These relationships address two issues that have previously been explored in the context of range sizes but not for range limits. Palaeontological data suggest that the geographic distributions of the clades (genera), and hence their distributional limits, increase over time (Miller 1997; Jablonski et al. 2006b; Finnegan et al. 2008), but whether such changes primarily result from speciation into new regions as the clade diversifies or from the expansion of ranges of existing species remains poorly known. Similarly, there has been considerable discussion about the extent to which the range sizes of species are phylogenetically conserved (Pielou 1977, 1978; Jablonski 1987, 2008; Ricklefs & Latham 1992; Webb & Gaston 2003, 2005; Hunt et al. 2005; Jones et al. 2005; Fitzpatrick & Turelli 2006; Jablonski & Hunt 2006; Mouillot & Gaston 2007; Waldron 2007), with a substantial phylogenetic signal documented by multiple studies. However, range limits have so far received little attention in this context. Even though these are related questions, it is important to note that a phylogenetic signal in range size does not necessarily imply that range limits are also phylogenetically conserved (two species can have exactly the same range size but different limits).
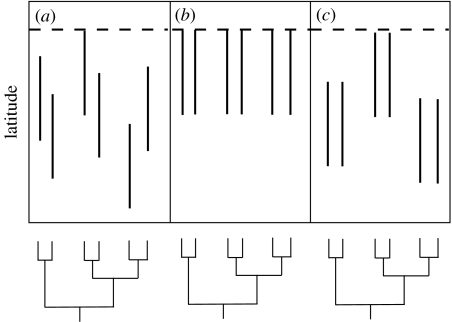
In considering the distributions of species within a given clade, we can define three scenarios: (i) the range limits of closely related species diverge from each other during speciation or subsequently (figure 1a), (ii) the range limits of closely related species are similar to each other (figure 1b), and (iii) the range limits of sister species are similar to each other but diverge between different sister species pairs (figure 1c). Scenario (i) leads to poor correspondence between the range limits of clades and their constituent species as would be expected if ecological niches, dispersal abilities, physiological tolerances and other limit-setting properties are not phylogenetically conserved (i.e. species evolve individualistically). Scenarios (ii) and (iii) are based on different levels of phylogenetic conservatism of limit-setting traits, leading to a perfect correspondence between the species and clade range limits in (ii) and a conservatism of range limits at the level of sister species but not a good correspondence between the species and clade range limits in (iii). Of course, real-world patterns are likely to be more complex than these hypothetical scenarios. Nonetheless, they provide a conceptual framework for examining how diversification of species within lineages constrains the distributions of lineages themselves.
Figure 1.
Conceptual models relating range limits of individual species to those of their clades. These hypothetical scenarios show how the northern limits of each species (the top of each line segment) relate to other species in the clade as well as the northern limit of the clade itself (shown by dashed lines). The size of the ranges is kept constant for simplicity and the figure is not meant to make any statement about the southern limits. See text for more details.
We explore these questions using marine bivalves as a focal group and integrating the estimates of stratigraphic ranges of genera from the fossil record with the distributional data for living species on a global scale (5132 species in 854 genera) and, at higher spatial resolution, along the northeastern (NE) Pacific (921 species in 400 genera) and western (W) Atlantic (883 species in 319 genera) coasts. These two coastal databases share 218 genera but only 183 species. We focus on the northern and the southern distributional limits of species simply because the information for those is currently more reliable than those for longitudinal limits. Molecular phylogenetic analyses are starting to provide a more refined picture of the relationships among the deep nodes of the bivalve tree (Taylor et al. 2007; Giribet 2008), but the relationships at or near the tips of the tree (i.e. among species and genera) have been resolved for only a handful of bivalve clades. Consequently, given the spatial scales of our analyses and the number of taxa involved, analyses using a well-resolved phylogeny are not possible at present. Instead, we use the taxonomic hierarchy (following a standardized taxonomic scheme; see below) as an indicator of phylogenetic relationships, and test for phylogenetic effects by comparing the range limits of species within individual genera with those across different genera. For some questions, we also repeat the analyses at the family level. This approach remains the best choice for groups where well-resolved phylogenies are not available (Harvey & Pagel 1991; Ricklefs & Nealen 1998; McGill 2008) and assumes that the relationships between individual taxa are unresolved (i.e. they represent a polytomy), which should make any phylogenetic signal in our data conservative. We use genera (including subgenera, which we elevate to genus rank here; Jablonski et al. 2006b) as operational phylogenetic units because they are fairly stable units and morphologically defined molluscan genera map well onto molecular phylogenies in most instances (Jablonski et al. 2006a). Finally, some of the analyses presented here take advantage of the excellent fossil record of marine bivalves, which provides direct estimates of the divergence times of genera (Jablonski et al. 2003, 2006b).
2. Material and methods
(a) Databases
Our global, NE Pacific and W Atlantic databases for living bivalves, along with the database on first occurrences of genera in the fossil record, are described in the electronic supplementary material.
(b) Statistical analyses
We explored the relationship between the range limits (in degrees latitude) of a genus and its age and species richness using multiple regressions (the data for age and species richness were natural-log transformed). For the analyses of species range limits within and among genera, we focused on two of the best sampled coasts in the world—the NE Pacific and the W Atlantic (Roy et al. 2000, 1998). In each case, we computed the differences between the species range limits (in absolute degrees of latitude, separately for the northern and the southern limits) within individual genera as well as between the species in different genera, and used the difference in the medians of these two distributions (between-genus differences minus within-genus differences) as a test statistic. If range endpoints are not conserved within the genera, the within- and between-genus distributions should be similar, and the test statistic should be close to zero. Conversely, if endpoints are conserved, we expect large positive values of the test statistic, reflecting systematic differences between the genera. We assessed the significance of this statistic by comparing it to a null expectation generated by randomly assigning species to the genera. Randomizing taxonomic affinities preserved the observed distributions of range sizes as well as range limits, so that the difference between the observed and expected values of the test statistic reflects the phylogenetic component. Because the genera of marine bivalves represent relatively small clades (median species: genus ratio in our global data is 3), we repeated the same analyses at the family level to explore the effects of the taxonomic scale. We also used a nested analysis of variance (Harvey & Pagel 1991; Ricklefs & Nealen 1998; McGill 2008) to explore how the variance in species range limits is partitioned among the taxonomic levels. Species were nested within the genera within families and each level was treated as a random effect. In addition, for the northern range limits along the NE Pacific and the W Atlantic, we also computed two commonly used metrics for phylogenetic signal: λ (Freckleton et al. 2002) and K (Blomberg et al. 2003), using taxonomy to provide a crude proxy for phylogeny. Together, these tests can differentiate between figure 1a and the other two scenarios. To differentiate between the scenarios in figure 1b,c, we calculated the differences between the northern and southern range limits of each species and the corresponding limits for the genus they belong to, and compared the median of these differences to the null distribution derived by the randomizing algorithm described above. All analyses were carried out in R statistical environment (R Development Core Team 2006), and further details are provided in the electronic supplementary material.
3. Results
For the global data, age and species richness of a genus are both significantly and positively related to its northern range limit (table 1; multiple R2=0.18, n=711). Age, however, has a very small effect compared with species richness as revealed by the changes in Akaike weights as each variable is removed from the full model (table 2). By contrast, only species richness is significantly correlated with the southern range limits of genera for the global dataset (table 1; multiple R2 =0.25, n=711). For the NE Pacific, the results for the northern and the southern range limits are qualitatively similar to the global results (tables 1 and 2). However, the total variance explained by richness and age is much smaller along this coast than in the global data (for the northern limits, multiple R2=0.05, and for the southern limits, multiple R2=0.06; n=366). Finally, for the W Atlantic, the age of a genus is not significantly related to either its northern or southern limits but the species richness of a genus is positively related to both (tables 1 and 2). In contrast to the NE Pacific, the W Atlantic variance explained by multiple regression is comparable to the global data (for the northern limits, multiple R2=0.12, and for the southern limits, multiple R2=0.20; n=319). In all cases, the variance inflation factor is less than 2, indicating that the collinearity between age and richness is unlikely to be a problem in these analyses (Bowerman & O'Connell 1990).
Table 1.
Summary of the multiple regression coefficients. (Note that the range limits in the Southern Hemisphere are coded as negative numbers in our data, which lead to the negative slopes for the analyses of the southern range endpoints. Significant relationships are shown in italic.)
| analysis | coefficient | estimate | s.e. | p-value |
|---|---|---|---|---|
| global north limits (n=711) | intercept | 17.69 | 2.3 | 0.000 |
| ln(genus age) | 1.69 | 0.76 | 0.026 | |
| ln(species richness) | 9.80 | 0.95 | 0.000 | |
| global south limits (n=711) | intercept | −5.45 | 2.22 | 0.0142 |
| ln(genus age) | 0.03 | 0.73 | 0.9648 | |
| ln(species richness) | −13.04 | 0.92 | 0.000 | |
| NE Pacific north limits (n=366) | intercept | 31.78 | 2.59 | 0.000 |
| ln(genus age) | 1.50 | 0.72 | 0.0379 | |
| ln(species richness) | 5.36 | 1.34 | 0.000 | |
| NE Pacific south limits (n=366) | intercept | 6.14 | 2.70 | 0.02 |
| ln(genus age) | 1.20 | 0.75 | 0.11 | |
| ln(species richness) | −6.89 | 1.40 | 0.000 | |
| W Atlantic north limits (n=319) | intercept | 17.04 | 5.13 | 0.001 |
| ln(genus age) | 2.37 | 1.47 | 0.109 | |
| ln(species richness) | 11.87 | 2.14 | 0.000 | |
| W Atlantic south limits (n=319) | intercept | −1.80 | 5.42 | 0.739 |
| ln(genus age) | −0.15 | 1.55 | 0.922 | |
| ln(species richness) | −18.33 | 2.26 | 0.000 |
Table 2.
The effects of genus age and species richness on Akaike weights in the multiple regressions. (The full models included both genus age and species richness. Note that the Akaike weights are comparable only within datasets. In every case, removing the genus age has a much smaller effect than removing the species richness (see text for details).)
| comparison | model | Akaike weights |
|---|---|---|
| global north limits (n=711) | full | 0.818 |
| ln(genus age) | 0.182 | |
| ln(species richness) | 0.000 | |
| global south limits (n=711) | full | 0.269 |
| ln(genus age) | 0.731 | |
| ln(species richness) | 0.000 | |
| NE Pacific north limits (n=366) | full | 0.817 |
| ln(genus age) | 0.182 | |
| ln(species richness) | 0.001 | |
| NE Pacific south limits (n=366) | full | 0.622 |
| ln(genus age) | 0.378 | |
| ln(species richness) | 0.000 | |
| W Atlantic north limits (n=319) | full | 0.622 |
| ln(genus age) | 0.378 | |
| ln(species richness) | 0.000 | |
| W Atlantic south limits (n=319) | full | 0.269 |
| ln(genus age) | 0.731 | |
| ln(species richness) | 0.000 |
For the NE Pacific, the northern range limits of species within a genus are significantly closer to one another than expected if species were distributed randomly within the genera (observed test statistic is 8.5°, p<0.001). Similar clustering is also evident for the southern range limits (observed test statistic is 7°, p<0.001). The results are not affected if monotypic genera are excluded from the analyses (p<0.001 in both the cases). The same results also hold for the analyses at the family level (p<0.001 for both the northern and the southern limits). Estimates of the variance components show that most of the variation in range endpoints is accounted for by phylogenetic (taxonomic) grouping. For the northern range limits, 60 per cent of the variance is associated with genus membership and 24 per cent with family membership. Similarly, for the southern limits, 54 per cent of the variance is associated with the genus level and 15 per cent with the family level.
The results for the W Atlantic are qualitatively similar to those for the NE Pacific, with both the northern and the southern range limits of species within genera and within families being significantly closer to each other than would be expected in the absence of phylogenetic effects (p<0.001, in all cases). However, the variance components are partitioned differently for this coast, with much less of the variation accounted for by taxonomy. For the northern limits, 22.7 per cent of the variance is associated with the genus level and 13.8 per cent with the family level. For the southern limits, 21.3 per cent of the variance is associated with the genus level and only 5.7 per cent with the family level.
For the northern range endpoints, metrics of the phylogenetic signal (Freckleton et al. 2002; Blomberg et al. 2003) are moderate to high in the NE Pacific (λ=0.32, K=0.78) and somewhat lower in the W Atlantic (λ=0.11, K=0.53). Non-parametric tests for the phylogenetic signal (Blomberg et al. 2003) are significant (p<0.001) for both the coastlines.
The median difference between the range limits of species and the genus they belong to is significantly smaller than expected in the absence of phylogenetic effects for both the northern (observed test statistic=6°, p<0.0001) and the southern limits (observed test statistic=4°, p<0.0001) for the NE Pacific taxa. For the W Atlantic, the test statistic is highly significant for the northern range limits (observed=11°, p<0.0001) but marginally so (observed=28°, p=0.05) for the southern limits.
4. Discussion
Previous analyses have shown that the genera of marine bivalves tend to originate preferentially in the tropics and over time extend their range limits polewards with the older taxa reaching further towards the poles (Goldberg et al. 2005; Jablonski et al. 2006b; Roy & Goldberg 2007). The analyses presented here are consistent with this ‘out of the tropics’ dynamic (see below) but they also find an interesting asymmetry in the behaviour of the northern versus the southern range limits of genera. Older genera have more poleward northern limits on a global scale, as well as along individual coasts, but age is not a significant correlate of the southern range limits. The reason for this asymmetry is unclear but could result from different processes being responsible for setting the northern versus the southern ranges of taxa (MacArthur 1972; Jablonski & Valentine 1990). We also cannot rule out the possibility that the asymmetry reflects poorer sampling of the southern oceans (see Clarke et al. 2007). However, for the majority of taxa used here the southern range limits are in the comparatively well-sampled Northern Hemisphere and so some of this asymmetry is likely to be real. More importantly, our results show that the correlation between the age and distributional limit, when it exists, is largely due to increased species richness in older clades rather than being a direct effect of age. In fact, for the global data, species richness emerges as a much stronger correlate of distributional limits of the genera than does age (see also Krug et al. 2008).
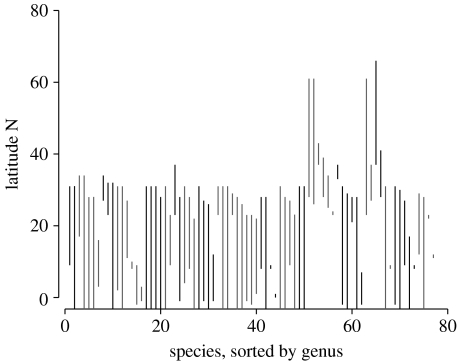
The range limits of marine bivalve species show significant phylogenetic conservatism at both the genus and family levels (figure 2; also figure 3 in the electronic supplementary material). Our estimates of K are similar to those previously documented for morphological, life-history and physiological traits (Blomberg et al. 2003). Estimates of λ for our data are lower but still comparable with those for ecological characters (Freckleton et al. 2002). While these estimates should be interpreted with caution given the crude and largely unresolved phylogeny used here, they do suggest that the phylogenetic signal in range limits may be substantial in marine bivalves. The strength of the effect and how variation in range limits is partitioned among taxonomic levels also clearly differs between the NE Pacific and the W Atlantic coasts. Overall, the nested ANOVA as well as the estimates of λ and K suggest a stronger phylogenetic signal in the NE Pacific than in the W Atlantic. These differences presumably reflect interoceanic differences in compositions of species assemblages as well as the past and present differences in environmental conditions. Furthermore, the range limits of species and the genus they belong to are significantly closer to each other than would be expected by chance, but again the interoceanic differences are present with the concordance much higher in the NE Pacific than in the W Atlantic.
Figure 2.
A plot showing the distribution of the northern range limits of the species of the family Veneridae along the NE Pacific. The top of each line represents the northern limit of that species. Clusters of species with the same line type represent individual genera and only non-monotypic genera are shown. The lines that intersect the x-axis represent the species whose southern limits are south of the equator. In this case, the test for phylogenetic conservatism is marginally significant (p=0.07), although the power of the test is low because of small sample size.
Marine biogeographers have long known that species range limits tend to cluster in certain areas, marking the boundaries between the biotic provinces and subprovinces (Valentine 1966; Roy et al. 1998; Briggs 1999; Spalding et al. 2007; Reaka et al. 2008). Many of these boundaries coincide with the convergences between major water masses or other changes in coastal oceanography (Valentine 1966; Spalding et al. 2007), and both the theoretical models (Gaylord & Gaines 2000; Byers & Pringle 2006) and empirical studies (Doyle 1985; Gilman 2006) suggest that such barriers to dispersal may be important determinants of the range limits of some marine species. Our results indicate that the responses of species to such barriers are, in part, determined by their phylogenetic affinities; if barriers affected all species equally then the analyses above would not have found conservatism of range limits at the level of genera and families. Thus, for marine bivalves, traits that determine the dispersal abilities of species are likely to be conserved at the level of genera and to a lesser extent the families. The specific traits involved remain poorly known, but for broadcast spawning species they should include those that enhance the transport and survival of larvae in the plankton as well as attributes that lead to post-settlement growth and survival (Morgan 2001; Sanford et al. 2006). Rafting of adults may also be significant in some clades, particularly at smaller body sizes, another trait that has a strong phylogenetic component (e.g. Smith et al. 2004). Physiological tolerances have also been shown to be important determinants of the range limits of some marine species (Tomanek & Somero 1999; Stillman & Somero 2000). In some groups of intertidal gastropods and crabs, closely related species have been shown to have similar physiological tolerances (Tomanek & Somero 1999; Stillman & Somero 2000) but such information is still scarce for most species. There is, however, a growing interest in measuring the physiological traits across large spatial scales with the aim of integrating ecological physiology with macroecology. Such macrophysiological studies (sensu Chown et al. 2004) are being undertaken for both marine and terrestrial organisms (Chown & Gaston 1999; Chown et al. 2004; Compton et al. 2007; Osovitz & Hofmann 2007; Deutsch et al. 2008; Tewksbury et al. 2008) and these data, analysed in a phylogenetic context, should be useful for exploring the interactions between the evolutionary history, physiology and range limits.
The finding that the range limits of living bivalve species show a phylogenetic signal strongest at the genus level but detectable even at the family level, supplements and illuminates previous findings on the historical underpinnings of the present-day bivalve biogeography. The Cenozoic Era witnessed a general cooling trend during which tropical climates retreated equatorward, interrupted by local climatic optima as during the Eocene and the mid-Miocene and by warm interglacial episodes during the Pleistocene (Zachos et al. 2001). The fossil record indicates that during the last 11 Myr most bivalve genera originated in the tropics, and their persistence there helps to explain the formation and maintenance of the latitudinal diversity gradient (Jablonski et al. 2006b). Over time, the range limits of many of these genera extended poleward even as high latitudes cooled. In fact, 75 per cent of today's polar bivalve genera also occur in the tropics, and many of these are likely to have originated in tropical waters (Goldberg et al. 2005). These poleward extensions of the genus range limits probably occurred mainly through speciation since less than 1 per cent of bivalve species extend from the tropics to the poles, and the more widely distributed genera tend to have more species in each climate zone (and in each province), including the tropics, than the genera that are more restricted geographically (Krug et al. 2008). These patterns are consistent with the results shown here, with distributional limits of the genera correlating more strongly with species richness than with genus age. The phylogenetic conservatism of range limits leads to wide-ranging lineages having clusters of species within the biogeographic provinces, with a few extending across these major boundaries. This pattern suggests a diversity-dependent dynamic, with the chance of breaking the phylogenetic conservatism (presumably through larger than the usual divergences of the underlying traits during speciation) increasing with the number of speciation events. This probabilistic model is further supported by the fact that the difference between range limits of species and the limits of their genus tends to be positively correlated with the species richness of the genus but not with the age of the genus. For example, for the NE Pacific species in the Northern Hemisphere, the median difference in the northern limits is significantly correlated with species richness (p<0.0001) but not with age (p=0.68). A positive relationship between the richness of a genus and the median difference in range limits is expected from sampling, but taxonomic clustering of range limits should make the slope shallower than expected from sampling alone. We estimated the slope expected in absence of taxonomic clustering by randomizing the taxonomic affinities of individual species and repeating the regression 1000 times. The observed slope (0.74) is indeed significantly shallower than that expected from sampling alone (95% confidence interval of the expected slope is 0.77–1.2). Thus, the general phylogenetic conservatism of range limits holds irrespective of the species richness of the genus (table 3), but as more species are added to a genus, there is evidently more opportunity for niche and dispersal-related traits of the species to diverge and the pattern becomes closer to figure 1c than to figure 1b.
Table 3.
Randomization tests for phylogenetic conservatism of range limits (see text for details) in the NE Pacific bivalve genera with different levels of species richness. (Separate tests were performed for each range of species richness. The results are qualitatively the same for the W. Atlantic genera.)
| species richness | total number of genera | total number of species | p-value (north, south) |
|---|---|---|---|
| 2–4 | 147 | 380 | <0.001,<0.001 |
| 5–7 | 36 | 207 | <0.001,<0.001 |
| 8+ | 8 | 89 | <0.001, 0.001 |
Macroevolutionary history clearly plays a role in determining the range limits of marine bivalve species, but the spatial scale and/or the phylogenetic level at which such factors are most important remains poorly explored. Here, we focused on large spatial scales involving large numbers of species from most of the major bivalve lineages, with the distributional data for individual species resolved at a moderately coarse resolution. We suspect that the phylogenetic conservatism of range limits is likely to be most evident at these macroecological scales with population-level mechanisms becoming more important for setting distributional limits at local scales (e.g. Gross & Price 2000). Similarly, given that speciation and extinction rates differ substantially among taxa, the degree to which range limits are phylogenetically conserved is also likely to vary among the living genera and families and may even be absent in some. Both simulation studies and empirical analyses have already demonstrated such scale dependence (both spatial and taxonomic) of phylogenetic conservatism for community structure (Cavender-Bares et al. 2006; Kraft et al. 2007). Furthermore, detection of phylogenetic conservatism becomes more difficult for clades and/or assemblages with smaller numbers of species since the power of the statistical tests declines with sample size (Kraft et al. 2007). So, for marine bivalves, tests of phylogenetic conservatism of range limits at finer spatial and taxonomic scales than attempted here will require better phylogenetic resolution at the level of individual genera and better spatial sampling, especially in the tropics and the southern oceans.
For the vast majority of marine and terrestrial species, the processes determining distributional limits remain unknown. Given the complex interactions between the organismic traits and the biotic and abiotic environments involved in setting range limits, this situation is unlikely to change in the near future. On the other hand, such knowledge is essential for addressing a number of real-world problems, from the responses of species to climate change to the success and spread of introduced species. The assumption that the traits involved in setting range limits are phylogenetically conserved is common in predictions of how the distributional limits of species will change in response to future climate change (Peterson et al. 1999; Wiens & Graham 2005; Jeschke & Strayer 2008), but without explicit tests it is impossible to know how well the assumption holds for various groups. Finally, we note that it remains an open question whether macroevolutionary history plays a similar role to that seen here in setting the range limits of terrestrial animals, where long-distance larval dispersal and recruitment dynamics, key components of marine population biology, have virtually no analogues (Paine 2005). Comparative analyses using better-resolved phylogenies and across different groups of marine and terrestrial organisms are needed to understand how evolutionary history constrains the distributional limits of species and lineages.
Acknowledgments
We thank K. J. Gaston for the invitation to contribute to this special issue and two anonymous reviewers for their very insightful comments that improved the manuscript. This research was supported by a grant from the NASA.
Footnotes
One contribution of 17 to a Special Issue ‘Geographic range limits of species’.
Supplementary Material
Materials and Methods
Figure 3
References
- Angert A.L., Schemske D.W. The evolution of species' distributions: reciprocal transplants across the elevation ranges of Mimulus cardinalis and M. lewisii. Evolution. 2005;59:1671–1684. doi:10.1554/05-107.1 [PubMed] [Google Scholar]
- Blomberg S.P., Garland T., Jr, Ives A.R. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. doi:10.1554/0014-3820(2003)057[0717:TFPSIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bowerman B.L., O'Connell R.T. PWS-Kent Publishing Company; Boston, MA: 1990. Linear statistical models: an applied approach. [Google Scholar]
- Bradbury I.R., Laurel B., Snelgrove P.V.R., Bentzen P., Campana S.E. Global patterns in marine dispersal estimates: the influence of geography, taxonomic category and life history. Proc. R. Soc. B. 2008;275:1803–1809. doi: 10.1098/rspb.2008.0216. doi:10.1098/rspb.2008.0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs J.C. Coincident biogeographic patterns: Indo-West Pacific ocean. Evolution. 1999;53:326–335. doi: 10.1111/j.1558-5646.1999.tb03769.x. doi:10.2307/2640770 [DOI] [PubMed] [Google Scholar]
- Brown J.H., Lomolino M.V. Sinauer Associates; Sunderland, MA: 1998. Biogeography. [Google Scholar]
- Byers J.E., Pringle J.M. Going against the flow: retention, range limits and invasions in advective environments. Mar. Ecol. Prog. Ser. 2006;313:27–41. doi:10.3354/meps313027 [Google Scholar]
- Case T.J., Holt R.D., McPeek M.A., Keitt T.H. The community context of species' borders: ecological and evolutionary perspectives. Oikos. 2005;108:28–46. doi:10.1111/j.0030-1299.2005.13148.x [Google Scholar]
- Caughley G., Grice D., Barker R., Brown B. The edge of the range. J. Anim. Ecol. 1988;57:771–785. doi:10.2307/5092 [Google Scholar]
- Cavender-Bares J., Keen A., Miles B. Phylogenetic structure of Floridian plant communities depends on taxonomic and spatial scale. Ecology. 2006;87:S109–S122. doi: 10.1890/0012-9658(2006)87[109:psofpc]2.0.co;2. doi:10.1890/0012-9658(2006)87[109:PSOFPC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chazdon R.L., Careaga S., Webb C., Vargas O. Community and phylogenetic structure of reproductive traits of woody species in wet tropical forests. Ecol. Monogr. 2003;73:331–348. doi:10.1890/02-4037 [Google Scholar]
- Chown S.L., Gaston K.J. Exploring links between physiology and ecology at macro-scales: the role of respiratory metabolism in insects. Biol. Rev. 1999;74:87–120. doi:10.1017/S000632319800526X [Google Scholar]
- Chown S.L., Gaston K.J., Robinson D. Macrophysiology: large-scale patterns in physiological traits and their ecological implications. Funct. Ecol. 2004;18:159–167. doi:10.1111/j.0269-8463.2004.00825.x [Google Scholar]
- Clarke A., Griffiths H.J., Linse K., Barnes D.K.A., Crame J.A. How well do we know the Antarctic marine fauna? A preliminary study of macroecological and biogeographical patterns in southern ocean gastropod and bivalve molluscs. Divers. Distrib. 2007;13:620–632. [Google Scholar]
- Compton T.J., Rijkenberg M.J.A., Drent J., Piersma T. Thermal tolerance ranges and climate variability: a comparison between bivalves from differing climates. J. Exp. Mar. Biol. Ecol. 2007;352:200–211. doi:10.1016/j.jembe.2007.07.010 [Google Scholar]
- Davis A.J., Jenkinson L.S., Lawton J.H., Shorrocks B., Wood S. Making mistakes when predicting shifts in species range in response to global warming. Nature. 1998;391:783–786. doi: 10.1038/35842. doi:10.1038/35842 [DOI] [PubMed] [Google Scholar]
- Deutsch C.A., Tewksbury J.J., Huey R.B., Sheldon K.S., Ghalambor C.K., Haak D.C., Martin P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. doi:10.1073/pnas.0709472105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, R. F. 1985 Biogeographical studies of rocky shores near Point Conception, California, Santa Barbara. PhD thesis, University of California, Santa Barbara.
- Duda T.F.J., Kohn A.J. Species-level phylogeography and evolutionary history of the hyperdiverse marine gastropod genus Conus. Mol. Phylogenet. Evol. 2005;34:257–272. doi: 10.1016/j.ympev.2004.09.012. doi:10.1016/j.ympev.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Finnegan S., Payne J.L., Wang S.C. The Red Queen revisited: re-evaluating the age selectivity of Phanerozoic marine genus extinctions. Paleobiology. 2008;34:318–341. doi:10.1666/07008.1 [Google Scholar]
- Fitzpatrick B.M., Turelli M. The geography of mammalian speciation: mixed signals from phylogenies and range maps. Evolution. 2006;60:601–615. doi:10.1554/05-453.1 [PubMed] [Google Scholar]
- Freckleton R.P., Harvey P.H., Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. doi:10.1086/343873 [DOI] [PubMed] [Google Scholar]
- Gaston K.J. Patterns in the geographical ranges of species. Biol. Rev. 1990;65:105–129. doi:10.1111/j.1469-185X.1990.tb01185.x [Google Scholar]
- Gaston K.J. Oxford University Press; Oxford, UK: 2003. The structure and dynamics of geographic ranges. [Google Scholar]
- Gaston K.J., Chown S.L. Geographic range size and speciation. In: Magurran A.E., May R.M., editors. Evolution of biological diversity. Oxford University Press; Oxford, UK: 1999. pp. 236–259. [Google Scholar]
- Gaylord B., Gaines S.D. Temperature or transport? Range limits in marine species mediated solely by flow. Am. Nat. 2000;155:769–789. doi: 10.1086/303357. doi:10.1086/303357 [DOI] [PubMed] [Google Scholar]
- Gilman S.E. The northern geographic range limit of the intertidal limpet Collisella scabra: a test of performance, recruitment, and temperature hypotheses. Ecography. 2006;29:709–720. doi:10.1111/j.0906-7590.2006.04572.x [Google Scholar]
- Giribet G. Bivalvia. In: Ponder W.F., Lindberg D.R., editors. Phylogeny and evolution of the Mollusca. University of California Press; Berkeley, CA: 2008. pp. 105–141. [Google Scholar]
- Goldberg E.E., Lande R. Species' borders and dispersal barriers. Am. Nat. 2007;170:297–304. doi: 10.1086/518946. doi:10.1086/518946 [DOI] [PubMed] [Google Scholar]
- Goldberg E.E., Roy K., Lande R., Jablonski D. Diversity, endemism, and age distributions in macroevolutionary sources and sinks. Am. Nat. 2005;165:623–633. doi: 10.1086/430012. doi:10.1086/430012 [DOI] [PubMed] [Google Scholar]
- Gross S.J., Price T.D. Determinants of the northern and southern range limits of a warbler. J. Biogeogr. 2000;27:869–878. doi:10.1046/j.1365-2699.2000.00440.x [Google Scholar]
- Harvey P.H., Pagel M.D. Oxford University Press; Oxford, UK: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Hellberg M.E. Sympatric sea shells along the sea's shore: the geography of speciation in the marine gastropod Tegula. Evolution. 1998;52:1311–1324. doi: 10.1111/j.1558-5646.1998.tb02013.x. doi:10.2307/2411301 [DOI] [PubMed] [Google Scholar]
- Holt R.D. On the evolutionary ecology of species' ranges. Evol. Ecol. Res. 2003;5:159–178. [Google Scholar]
- Hunt G., Roy K., Jablonski D. Species-level heritability reaffirmed: a comment on ‘on the heritability of geographic range sizes’. Am. Nat. 2005;166:129–135. doi: 10.1086/430722. doi:10.1086/430722 [DOI] [PubMed] [Google Scholar]
- Jablonski D. Heritability at the species level: analysis of geographic ranges of Cretaceous mollusks. Science. 1987;238:360–363. doi: 10.1126/science.238.4825.360. doi:10.1126/science.238.4825.360 [DOI] [PubMed] [Google Scholar]
- Jablonski D. Species selection: theory and data. Annu. Rev. Ecol. Evol. Syst. 2008;39:501–524. doi:10.1146/annurev.ecolsys.39.110707.173510 [Google Scholar]
- Jablonski D., Hunt G. Larval ecology, geographic range, and species survivorship in Cretaceous mollusks: organismic versus species-level explanations. Am. Nat. 2006;168:556–564. doi: 10.1086/507994. doi:10.1086/507994 [DOI] [PubMed] [Google Scholar]
- Jablonski D., Valentine J.W. From regional to total geographic ranges: testing the relationship in Recent bivalves. Paleobiology. 1990;16:126–142. [Google Scholar]
- Jablonski D., Roy K., Valentine J.W., Price R.M., Anderson P.S. The impact of the Pull of the Recent on the history of marine diversity. Science. 2003;300:1133–1135. doi: 10.1126/science.1083246. doi:10.1126/science.1083246 [DOI] [PubMed] [Google Scholar]
- Jablonski D., Finarelli J.A., Roy K. What, if anything, is a genus? Testing the analytical units of paleobiology against molecular data. Geol. Soc. Am. Abstr. 2006a;38:169. [Google Scholar]
- Jablonski D., Roy K., Valentine J.W. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science. 2006b;314:102–106. doi: 10.1126/science.1130880. doi:10.1126/science.1130880 [DOI] [PubMed] [Google Scholar]
- Jeschke J.M., Strayer D.L. Usefulness of bioclimatic models for studying climate change and invasive species. Ann. NY Acad. Sci. 2008;1134:1–24. doi: 10.1196/annals.1439.002. doi:10.1196/annals.1439.002 [DOI] [PubMed] [Google Scholar]
- Jones K.E., Sechrest W., Gittleman J.L. Age and area revisited: identifying global patterns and implications for conservation. In: Purvis A., Gittleman J.L., Brooks T., editors. Phylogeny and conservation. Cambridge University Press; Cambridge, UK: 2005. pp. 141–165. [Google Scholar]
- Kelly C.K., Bowler M.G., Pybus O., Harvey P.H. Phylogeny, niches, and relative abundance in natural communities. Ecology. 2008;89:962–970. doi: 10.1890/07-0322.1. doi:10.1890/07-0322.1 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M., Barton N.H. Evolution of a species' range. Am. Nat. 1997;150:1–23. doi: 10.1086/286054. doi:10.1086/286054 [DOI] [PubMed] [Google Scholar]
- Knouft J.H., Losos J.B., Glor R.E., Kolbe J.J. Phylogenetic analysis of the evolution of the niche in lizards of the Anolis sagrei group. Ecology. 2006;87:S29–S38. doi: 10.1890/0012-9658(2006)87[29:paoteo]2.0.co;2. doi:10.1890/0012-9658(2006)87[29:PAOTEO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kraft N.J.B., Cornwell W.K., Webb C.O., Ackerly D.D. Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am. Nat. 2007;170:271–283. doi: 10.1086/519400. doi:10.1086/519400 [DOI] [PubMed] [Google Scholar]
- Krug A.Z., Jablonski D., Valentine J.W. Species-genus ratios reflect a global history of diversification and range expansion in marine bivalves. Proc. R. Soc. B. 2008;275:1117–1123. doi: 10.1098/rspb.2007.1729. doi:10.1098/rspb.2007.1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latiolais J.M., Taylor M.S., Roy K., Hellberg M.E. A molecular phylogenetic analysis of strombid gastropod morphological diversity. Mol. Phylogenet. Evol. 2006;41:436–444. doi: 10.1016/j.ympev.2006.05.027. doi:10.1016/j.ympev.2006.05.027 [DOI] [PubMed] [Google Scholar]
- Losos J.B., Glor R.E. Phylogenetic comparative methods and the geography of speciation. Trends Ecol. Evol. 2003;18:220–227. doi:10.1016/S0169-5347(03)00037-5 [Google Scholar]
- MacArthur R.H. Harper and Row; New York, NY: 1972. Geographical ecology. [Google Scholar]
- McGill B.J. Exploring predictions of abundance from body mass using hierarchical comparative approaches. Am. Nat. 2008;172:88–101. doi: 10.1086/588044. doi:10.1086/588044 [DOI] [PubMed] [Google Scholar]
- McPeek M.A. Linking local species interactions to rates of speciation in communities. Ecology. 1996;77:1355–1366. doi:10.2307/2265533 [Google Scholar]
- Meyer C.P. Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biol. J. Linn. Soc. 2003;79:401–459. doi:10.1046/j.1095-8312.2003.00197.x [Google Scholar]
- Miller A.I. A new look at age and area: the geographic and environmental expansion of genera during the Ordovician radiation. Paleobiology. 1997;23:410–419. doi: 10.1017/s0094837300019813. [DOI] [PubMed] [Google Scholar]
- Morgan S.G. The larval ecology of marine communities. In: Bertness M.D., Gaines S.D., Hay M.E., editors. Marine community ecology. Sinauer Associates, Inc; Sunderland, MA: 2001. pp. 159–181. [Google Scholar]
- Mouillot D., Gaston K.J. Geographical range size heritability: what do neutral models with different modes of speciation predict? Glob. Ecol. Biogeogr. 2007;16:367–380. doi:10.1111/j.1466-8238.2007.00292.x [Google Scholar]
- Osovitz C.J., Hofmann G.E. Marine macrophysiology: studying physiological variation across large spatial scales in marine systems. Comp. Biochem. Physiol. A. 2007;147:821–827. doi: 10.1016/j.cbpa.2007.02.012. doi:10.1016/j.cbpa.2007.02.012 [DOI] [PubMed] [Google Scholar]
- Paine R.T. Cross environment talk in ecology: fact or fantasy? Mar. Ecol. Prog. Ser. 2005;304:280–283. [Google Scholar]
- Parmesan C., Gaines S.D., Gonzalez L., Kaufman D.M., Kingsolver J.G., Peterson A.T., Sagarin R. Empirical perspectives on species borders: from traditional biogeography to global change. Oikos. 2005;108:58–75. doi:10.1111/j.0030-1299.2005.13150.x [Google Scholar]
- Pearman P.B., Guisan A., Broennimann O., Randin C.F. Niche dynamics in space and time. Trends Ecol. Evol. 2007;23:149–158. doi: 10.1016/j.tree.2007.11.005. doi:10.1016/j.tree.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Peterson A.T., Soberon J., Sanchez-Cordero V. Conservatism of ecological niches in evolutionary time. Science. 1999;285:1265–1267. doi: 10.1126/science.285.5431.1265. doi:10.1126/science.285.5431.1265 [DOI] [PubMed] [Google Scholar]
- Pielou E.C. Latitudinal spans of seaweed species and their patterns of overlap. J. Biogeogr. 1977;4:299–311. doi:10.2307/3038189 [Google Scholar]
- Pielou E.C. Latitudinal overlap of seaweed species:evidence for quasi-sympatric speciation. J. Biogeogr. 1978;5:227–238. doi:10.2307/3038038 [Google Scholar]
- R Development Core Team. The R Foundation for Statistical Computing; Vienna, Austria: 2006. R: a language and environment, v. 2.7.0. [Google Scholar]
- Reaka M.L., Rodgers P.J., Kudla A.U. Patterns of biodiversity and endemism on Indo-West Pacific coral reefs. Proc. Natl Acad. Sci. USA. 2008;105:11474–11481. doi: 10.1073/pnas.0802594105. doi:10.1073/pnas.0802594105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman A.D., Price T. Evolution of ecological differences in the old-world leaf warblers. Nature. 1992;355:817–821. doi: 10.1038/355817a0. doi:10.1038/355817a0 [DOI] [PubMed] [Google Scholar]
- Ricklefs R.E., Latham R.E. Intercontinental correlation of geographical ranges suggests stasis in ecological traits of relict genera of temperate perennial herbs. Am. Nat. 1992;139:1305–1321. doi:10.1086/285388 [Google Scholar]
- Ricklefs R.E., Nealen P. Lineage-dependent rates of evolutionary diversification: analysis of bivariate ellipses. Funct. Ecol. 1998;12:871–885. doi:10.1046/j.1365-2435.1998.00263.x [Google Scholar]
- Roy K., Goldberg E.E. Origination, extinction, and dispersal: integrative models for understanding present-day diversity gradients. Am. Nat. 2007;170:S71–S85. doi: 10.1086/519403. doi:10.1086/519403 [DOI] [PubMed] [Google Scholar]
- Roy K., Jablonski D., Valentine J.W., Rosenberg G. Marine latitudinal diversity gradients: tests of causal hypotheses. Proc. Natl Acad. Sci. USA. 1998;95:3699–3702. doi: 10.1073/pnas.95.7.3699. doi:10.1073/pnas.95.7.3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K., Jablonski D., Valentine J.W. Dissecting latitudinal diversity gradients: functional groups and clades of marine bivalves. Proc. R. Soc. B. 2000;267:293–299. doi: 10.1098/rspb.2000.0999. doi:10.1098/rspb.2000.0999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford E., Holzman S.B., Haney R.A., Rand D.M., Bertness M.D. Larval tolerance, gene flow, and the northern geographic range limit of fiddler crabs. Ecology. 2006;87:2882–2894. doi: 10.1890/0012-9658(2006)87[2882:ltgfat]2.0.co;2. doi:10.1890/0012-9658(2006)87[2882:LTGFAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith F.A., et al. Similarity of mammalian body size across the taxonomic hierarchy and across space and time. Am. Nat. 2004;163:672–691. doi: 10.1086/382898. doi:10.1086/382898 [DOI] [PubMed] [Google Scholar]
- Spalding M.D., et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience. 2007;57:573–583. doi:10.1641/B570707 [Google Scholar]
- Stillman J.H., Somero G.N. A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol. Biochem. Zool. 2000;73:200–208. doi: 10.1086/316738. doi:10.1086/316738 [DOI] [PubMed] [Google Scholar]
- Taylor J.D., Williams S.T., Glover E.A., Dyal P. A molecular phylogeny of heterodont bivalves (Mollusca: Bivalvia: Heterodonta): new analyses of 18S and 28S rRNA genes. Zool. Script. 2007;36:587–606. doi:10.1111/j.1463-6409.2007.00299.x [Google Scholar]
- Tewksbury J.J., Huey R.B., Deutsch C.A. Ecology:putting the heat on tropical animals. Science. 2008;320:1296–1297. doi: 10.1126/science.1159328. doi:10.1126/science.1159328 [DOI] [PubMed] [Google Scholar]
- Tomanek L., Somero G.N. Evolutionary and acclimation-induced variation in the heat–shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J. Exp. Biol. 1999;202:2925–2936. doi: 10.1242/jeb.202.21.2925. [DOI] [PubMed] [Google Scholar]
- Valentine J.W. Numerical analysis of marine molluscan ranges on the extratropical north-eastern Pacific shelf. Limnol. Oceanogr. 1966;11:198–211. [Google Scholar]
- Waldron A. Null models of geographic range size evolution reaffirm its heritability. Am. Nat. 2007;170:221–231. doi: 10.1086/518963. doi:10.1086/518963 [DOI] [PubMed] [Google Scholar]
- Webb C.O. Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am. Nat. 2000;156:145–155. doi: 10.1086/303378. doi:10.1086/303378 [DOI] [PubMed] [Google Scholar]
- Webb C.O., Ackerly D.D., McPeek M.A., Donoghue M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Evol. Syst. 2002;33:475–505. doi:10.1146/annurev.ecolsys.33.010802.150448 [Google Scholar]
- Webb T.J., Gaston K.J. On the heritability of geographic range sizes. Am. Nat. 2003;161:553–566. doi: 10.1086/368296. doi:10.1086/368296 [DOI] [PubMed] [Google Scholar]
- Webb T.J., Gaston K.J. Heritability of geographic range sizes revisited: a reply to Hunt et al. Am. Nat. 2005;166:136–143. doi: 10.1086/430726. doi:10.1086/430726 [DOI] [PubMed] [Google Scholar]
- Wiens J.J., Graham C.H. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 2005;36:519–539. doi:10.1146/annurev.ecolsys.36.102803.095431 [Google Scholar]
- Zachos J., Pagani M., Sloan L., Thomas E., Billups K. Trends, rhythms and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. doi:10.1126/science.1059412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Figure 3