Abstract
Dispersal is a key component of a species's ecology and will be under different selection pressures in different parts of the range. For example, a long-distance dispersal strategy suitable for continuous habitat at the range core might not be favoured at the margin, where the habitat is sparse. Using a spatially explicit, individual-based, evolutionary simulation model, the dispersal strategies of an organism that has only one dispersal event in its lifetime, such as a plant or sessile animal, are considered. Within the model, removing habitat, increasing habitat turnover, increasing the cost of dispersal, reducing habitat quality or altering vital rates imposes range limits. In most cases, there is a clear change in the dispersal strategies across the range, although increasing death rate towards the margin has little impact on evolved dispersal strategy across the range. Habitat turnover, reduced birth rate and reduced habitat quality all increase evolved dispersal distances at the margin, while increased cost of dispersal and reduced habitat density lead to lower evolved dispersal distances at the margins. As climate change shifts suitable habitat poleward, species ranges will also start to shift, and it will be the dispersal capabilities of marginal populations, rather than core populations, that will influence the rate of range shifting.
Keywords: individual-based model, habitat quality, habitat turnover, population dynamics, spatial ecology, evolutionary ecology
1. Introduction
Dispersal, or movement from the site of birth to the site of reproduction, results from a complex set of interacting processes. The dispersal strategy, here defined as the expected frequency distribution of dispersal distances of offspring, evolved is the result of a set of costs and benefits (reviewed for plants in Cousens et al. 2008). The costs of dispersing include (i) the requirement for structures associated with dispersal, such as samaras and pappi for seeds, or wings, fat stores and flight muscles for insects, (ii) the inherent risk of travel, (iii) the possibility of arriving in a poor quality environment and (iv) the movement away from the areas to which the individual is adapted. The benefits include (i) the potential to found new populations, (ii) the avoidance of kin competition, thus increasing inclusive fitness, and (iii) a reduction in the probability of inbreeding. Despite this complexity, the search for an evolved dispersal strategy can be distilled to the simple comparison of inclusive fitness for different dispersal distances, although, in most cases, the optimal dispersal distance will vary with local conditions and between years.
Dispersal clearly has huge benefits for individuals at the margins of expanding ranges as dispersing individuals will be the ones founding new populations beyond the current range. This has been shown in simulation modelling (Travis & Dytham 2002; Hughes et al. 2007), in the seed morphology of lodgepole pines (Cwynar & MacDonald 1987), in a switch investment from abdomen (reproduction) to thorax (movement) in the speckled wood butterfly (Hughes et al. 2003), in an increased proportion of long-winged morphs in a bush cricket (Simmons & Thomas 2004) and in the longer legs of the cane toad (Phillips et al. 2006). However, Travis & Dytham (2002) showed that even a small Allee effect (decreased survival or fecundity at low densities) can have a huge impact on the rate of expansion and the dispersal strategies of individuals at the margins. This can lead to evolution of traits correlated with dispersal (Roff & Fairbairn 2001), such as an increase in self-compatibility or asexual reproduction at range margins to avoid the Allee effect being strongly associated with dispersal morphologies. Duckworth (2008) linked a social behaviour to dispersal, showing how in a cooperatively breeding bird, the western bluebird, it is the more aggressive individuals that succeed at the margins as they are more likely to occupy new habitat, but less aggressive individuals raise more offspring in higher-density, core areas.
In static ranges, the dispersal strategies evolved at the range margins can often have much lower mean distances than those at the core of the range (Gros et al. 2006). In the extreme, an isolated island of static, high-quality habitat results in much lower dispersal distances being favoured around the edge because of the increased mortality risk from ‘falling off’ the edge of the habitat. There is evidence of rapid evolution for reduced dispersal distances in the populations on small islands (Cody & Overton 1996). This can have implications for the success of reserve networks (Baskett et al. 2007). Conversely, if the range edge is characterized by high turnover of populations, then this will lead to increased dispersal at the margins as successful dispersers will be the founders of new populations (McPeek & Holt 1992; Holt 2003). Of course, if the turnover of habitat is too rapid, this will exclude the species whatever its dispersal strategy (Travis & Dytham 1999), although isolated pockets of high-quality habitat at the margin might support high local densities of individuals (Päivinen et al. 2005) with very short-distance dispersal strategies (With et al. 1999; Dytham 2003). One consequence of the influence of habitat availability on dispersal strategy is that the ability of ranges to expand through the zones of sparse habitat may be adversely affected (e.g. With & Crist 1995; McInerny et al. 2007).
In this paper, I use a generic, individual-based modelling approach to show how dispersal strategies at the range margin differ from those at the range core. Margins are created in a variety of ways: by increasing the turnover of habitat towards the margins; increasing death rates; decreasing birth rates; increasing cost of dispersal; and decreasing patch quality. Results show that the cost of dispersal is the biggest determinant of evolved dispersal strategy, and that dispersal varies across the range with evolved dispersal strategies at the margin higher or lower than those at the core, depending on the ecology of the species.
2. Material and methods
(a) Overview
The model is an individual-based simulation model of an organism with a static adult phase (i.e. sessile adult plants and dispersal of seeds away from the adult, a life history that can also describe many sessile animals, such as corals). There are only two types of events: birth (including dispersal) and death. Possible events occur singly and in series with a small amount of time passing after each event according to an adapted version of the Gillespie algorithm (Gillespie 1977; Renshaw 1991; Slepoy et al. 2008). Essentially, at each possible event, an amount of time is drawn from an exponential distribution with a mean of 1/(2n), where n is the current population size, and that amount of time passes.
Each individual carries information on its exact location in continuous space and its dispersal strategy (a single number representing the mean distance its offspring will move). I consider a haploid, asexual organism with dispersal strategy being passed from the parent to the offspring with a small probability of error (mutation). Space is arbitrary and continuous, but for some scenarios of habitat loss, a grid of habitat patches has to be imposed on the landscape. For convenience, cells, or patches of habitat, within the landscape are always squares with a side length of 1 unit. The entire landscape is a square area of side length 100 units. Boundary conditions are absorbing, although this is unimportant as the scenarios considered below always consider a limited range that does not reach the edge. The underlying population dynamics model is one of logistic growth. Birth rate decreases with local density, while death rate increases with density. Birth and death rates balance at the equilibrium density, which is 20 individuals per unit area. Vital rates may also be affected by the properties of the landscape as described in the various scenarios below.
(b) Initialization
At the start of each realization of the model, 100 000 individuals are scattered randomly across the 100×100 unit landscape (each located in continuous space). Each individual is assigned a dispersal strategy drawn from a continuous uniform distribution between 0 and 4: the expected mean distance travelled by that individual's offspring, often termed the dispersal kernel.
(c) Dispersal and mutation
Individuals only disperse at birth. They move a distance from their parent taken from a negative exponential distribution with a mean value taken from the parent's dispersal strategy. In all simulations reported here, a mutation rate of 0.5 per cent is used and mutants have a dispersal strategy drawn from a uniform distribution between −0.1 and +0.1 of the parent's strategy, with a lower limit of zero and no upper limit. The direction of dispersal is chosen randomly from a uniform, circular distribution, and dispersal is assumed to be in a straight line. Dispersal carries risks that rise with the distance travelled. Here, the cost of dispersal is applied as a linearly increasing probability of mortality during the dispersal event (Murrell et al. 2002). An individual will only successfully disperse if it survives those risks and arrives at a suitable habitat. In these simulations, three levels of risk are considered: high (20% mortality per unit distance travelled); medium (2.5% mortality per unit); and low (0.1% mortality per unit).
(d) Scenarios
In each scenario, the core of the range is at the centre of the simulated arena. Parameters change based on the distance from the centre point, producing a circular range. After 10 000 time steps, the dispersal strategies of each living individual are recorded along with their locations and linear distance from the range centre. This endpoint is long after the system has settled to equilibrium as simulations show no change in the variance of dispersal strategies in the population after 1000 time steps.
Parameters for each scenario are selected so that it supports between 50 000 and 80 000 individuals.
A habitat island. All patches with a centroid less than 40 units from the centre of the landscape are permanently active, and all other habitat patches are unavailable. There are three replicates of each of three costs of dispersal.
Increasing habitat turnover. The probability of habitat being destroyed in a time step is zero at the core and increases linearly towards the margin at 2 per cent per unit, so that a habitat patch 50 units from the centre will have a 100 per cent probability of destruction for each time step. The probability of habitat creation is constant across the range at 50 per cent per time step. There are three replicates of each of three costs of dispersal.
Decreasing birth rates. Simulations take place in a world of homogeneous, permanent habitat, but there is an additional, decreasing probability of a birth event being successful, falling linearly from the core of the range. Births have 100 per cent chance of success at the core declining at 2 per cent per unit away from the core. There are three realizations of each of three costs of dispersal.
Increasing death rates. As with birth, there is a continuous, homogeneous, permanent habitat, but an increasing probability of a death event occurring rising linearly from the core of the range. This is implemented as an additional mortality hurdle applied whenever there is a death event. There is no additional mortality at the core and mortality rises 2 per cent per unit away from the core. There are three realizations of each of three costs of dispersal.
Increasing cost of dispersal. The habitat is homogeneous and permanent, but there is an increasing risk to dispersal increasing with the distance from the core. Mortality per unit distance travelled is 1/(s/1.5), where s is the distance from the core. There are three replicates.
Decreasing patch quality. Habitat patches are permanent but the quality of patches, measured as the local density at which the death and birth rates are even, declines linearly with the distance from the core. Equilibrium density at the core is 45 and this declines by 1 per unit distance from the core.
3. Results
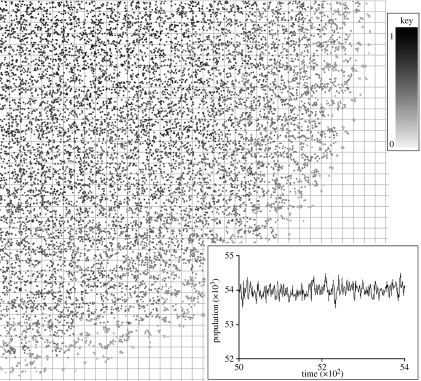
Generally, the cost of dispersal has the biggest influence on the evolved dispersal strategy: low cost of dispersal leads to longer dispersal distances. An example of the distribution of individuals in one-quarter of one realization of the scenario where there is an increasing cost of dispersal towards the range margin is shown in figure 1, along with the global population size for this example, which shows a relatively static population size. Variance in the dispersal strategies of individuals is usually 0.02–0.03, but, as the cost of dispersal has the biggest effect on evolved dispersal strategies, it is unsurprising that the scenario where cost changes across the range has higher variance (approx. 0.045).
Figure 1.
One-quarter of a realization of the increasing cost of dispersal simulation model. Individuals are shown as small squares shaded by their evolved dispersal strategies from light grey (zero) to black (one or more). In this realization, the overall mean evolved dispersal strategy is 0.42 and variance in dispersal strategy is 0.046. Faint lines show the scale of the habitat patch grid relevant to some scenarios. The inset shows population size from time step 5000 through 400 time steps.
(a) A habitat island
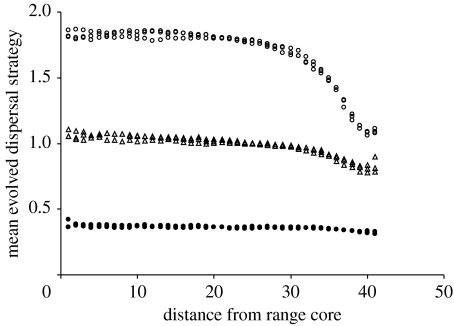
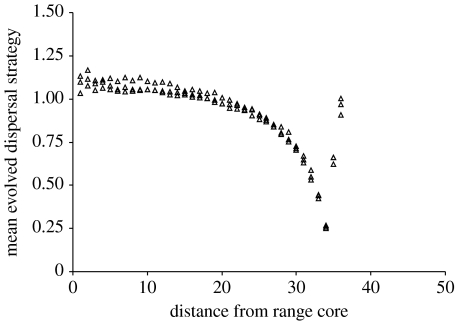
Mean evolved dispersal strategies for habitat islands are shown in figure 2. As expected from the results of Travis & Dytham (1998), at the core of the range the evolved dispersal strategies are shorter when the cost of dispersal is high. For all realizations, there is a clear decline in the dispersal strategy towards the margin, although the decline is much more pronounced in the low-cost realizations where the core strategy is much higher. At the margin, the cost of dispersal has very little impact on the dispersal strategy, as the chance of dispersing into an unsuitable habitat is itself a high cost to dispersal, as described in Gros et al. (2006)
Figure 2.
Mean evolved dispersal strategies on a homogeneous island of habitat. Three replicates of each of three dispersal costs are shown. Cost of dispersal is high (filled circles), moderate (open triangles) or low (open circles). In all cases, the evolved dispersal strategy is lower towards the range margin.
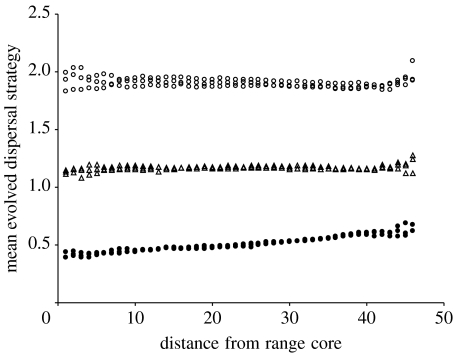
(b) Increasing habitat turnover
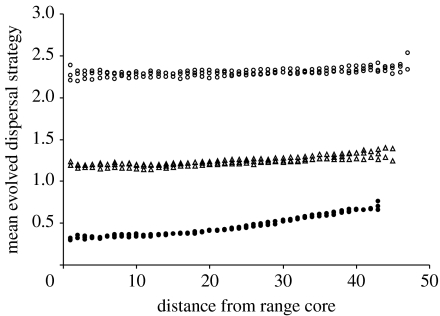
As is clearly shown in figure 3, the evolved dispersal distance rises with increasing patch turnover towards the margins when there is a high cost of dispersal (i.e. evolved dispersal strategies at the core are low). However, despite the turnover of habitat requiring dispersal away from the natal patch, with lower costs of dispersal, there is almost no change in the dispersal strategies with turnover except for a slight upturn very close to the margins. This is because the evolved strategy at the low cost of dispersal is already far enough for the offspring to be very likely to reach new patches, whereas, at the high cost of dispersal, many offspring at the core will be retained in the natal patch, so the evolved response to habitat turnover is increased dispersal distance.
Figure 3.
Mean evolved dispersal strategies with habitat turnover increasing towards the range margins. Three replications of each of five dispersal costs are shown. Cost of dispersal is high (filled circles), moderate (open triangles) or low (open circles).
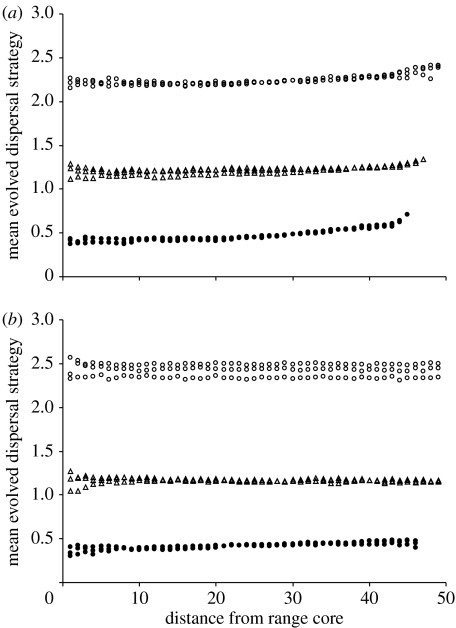
(c) Decreasing birth rate, increasing death rate
As birth rate decreases towards the range margin, there is a slight increase in the evolved dispersal strategy for all costs of dispersal (figure 4a). This effect is most evident quite close to the margin, where populations will be at very low density, but is also apparent right across the range. By contrast, as death rate increases towards the margin, there is very little effect on the evolved dispersal strategy (figure 4b). For high costs of dispersal, there is a general, slight increase in the dispersal strategy towards the margin, while for lower costs of dispersal there is a very slight decrease in the evolved strategy. Restricting birth rates clearly interacts with the cost of dispersal to reduce the overall range size (high cost of dispersal leads to smaller ranges because the range limit is nearer the core when the cost of dispersal is high).
Figure 4.
Mean evolved dispersal strategies with (a) birth rate decreasing with the distance from the core and (b) death rate increasing with the distance from the core. In each case, there are three replicates of each of three costs of dispersal. Cost of dispersal is high (filled circles), moderate (open triangles) or low (open circles). Note that the replicates are more varied close to the core and at the margin; these are the regions where sample sizes are smaller (smaller circles of habitat at the core of a circular range and low density of individuals at the margins).
(d) Increasing cost of dispersal
The cost of dispersal is a very strong determinant of the evolved dispersal strategy. This is shown clearly in figure 5, where the dispersal strategy drops towards the margin as only individuals dispersing a short distance from their parent will be able to survive. There are several outliers beyond the clear margin; these are single individuals that have been cast beyond the margin from within the range. These individuals beyond the margin will not produce any offspring, as the cost of dispersal is so high that any movement would be fatal.
Figure 5.
Mean evolved dispersal strategies with cost of dispersal increasing with the distance from the core. Three replicates are depicted. Here, the interaction of cost of dispersal and range size is particularly apparent: high cost of dispersal reduces the size of the range.
(e) Decreased habitat quality towards the margin
As with the birth rate, there is a decline in the range size at high costs of dispersal and a clear and continuous increase in the evolved dispersal strategy towards the margins for realizations where the cost of dispersal is high (figure 6). However, where the cost of dispersal is low, there is only a slight increase in the dispersal strategy towards the margins.
Figure 6.
Mean evolved dispersal strategies for three replicates of each of three costs of dispersal when the quality of habitat declines away from the core. Cost of dispersal is high (filled circles), moderate (open triangles) or low (open circles).
4. Discussion
The dispersal strategy, here defined as the expected frequency distribution of the distances travelled by offspring, that evolves towards the range margin is profoundly influenced by the way the range limit is generated. In some cases, such as an increase in the cost of dispersal from the core to the margin, there will be a huge impact of position within the range on the evolved dispersal strategy, even when there is little change in the density of individuals.
The evolved dispersal strategy is the result of a tension between the forces selecting for increased dispersal distance (such as the chance to found new populations or the increase in inclusive fitness by the avoidance of kin competition) and those selecting against dispersal (such as the failure to reach suitable habitat or the cost of dispersal). Any change in the evolved dispersal strategy must arise because of a shift in the balance of those costs and benefits. Some results are easy to interpret: the island of habitat will lead to lower dispersal strategies at the edge of the island because offspring suffer an increased cost to dispersal from ‘drowning’ (e.g. Stefan 1984; Gros et al. 2006). The results in figure 2 show that the influence of the margin is greater when the core dispersal strategy is higher, because offspring will be exposed to this increased cost of dispersing further from the edge.
Habitat turnover has long been known to favour dispersal. This is because the newly created patches will be, on average, colonized by the individuals that are better dispersers (Olivieri et al. 1995; Poethke et al. 2003). While long-lived, isolated patches will lead to reduced dispersal strategies (Schtickzelle et al. 2006). Because changes in the dispersal strategies at the range margins are often found along environmental gradients, it is usually impossible to differentiate environmental effects on dispersal distances from range position effects. However, in a Pacific coast plant of North America, Darling et al. (2008) showed increased dispersal distances away from the range core both north and south in a fairly static range, thus making it very unlikely that dispersal traits are correlated with environment. They attribute this increase in dispersal to habitat instability and a shift in the mating system. The results in figure 2 show that habitat turnover does indeed lead to longer dispersal distances, but that the effect is only strong when the core dispersal distances are short. This means that we should expect to see the results of Darling et al. (2008) more often in the species with short-distance dispersal.
The simulations presented here show that optimal dispersal strategies (dispersal kernels) may vary almost continuously across the range. This is especially seen in the high-cost realizations where the evolved dispersal distance is short. There are two possible reasons for this: the first is that there is more opportunity for local adaptation because individuals move less, thus preventing the swamping of strategies evolved for the margin by the strategies from the core (Kirkpatrick & Barton 1997; Bridle & Vines 2006); the second reason is that short-distance dispersal leads to a high level of kin competition, so the tension between costs and benefits of dispersal is more pronounced. These simulations only considered one rate and scale of mutation, although clearly the penetration of the effect of the margin will be determined, in part, by the rate of mutation.
Phillips et al. (2008) reviewed possible approaches to the simulation of expanding range fronts. The modelling presented here is for ‘dumb’, undirected dispersal, such as wind dispersal in plants. There is no opportunity for intelligent, or directed, dispersal, nor for the trading of resource allocation between, for example, dispersal distance and fecundity through investment in structures such as pappi to aid dispersal. Clearly, there are many animal species that do disperse intelligently or plants that rely on animal vectors for directed dispersal (for an example of coevolution of plant and vector see Purves & Dushoff 2005). In other species, potential fecundity is sacrificed for dispersal ability (e.g. Hughes et al. 2003). Such species may exhibit different patterns of dispersal distances across the range from those using only dumb dispersal. Similarly, there may be interactions between the processes setting the range limits, which have not been included here, and which produce idiosyncratic responses in the dispersal strategy. Progress towards consideration of some of the possible interacting forces affecting dispersal has been made by Lou (2008).
I have modelled only unconditional dispersal here, when the distance dispersed is determined only by the parent's dispersal strategy. However, it is widely expected that dispersal distances should be conditional on local environment (Zera & Denno 1997). For example, to avoid competition, dispersal distances should be generally longer in the crowded areas (Travis et al. 1999; Poethke & Hovestadt 2002; Kun & Scheuring 2006). Dispersal can also be condition-dependent, where a decision to engage in dispersal behaviour is mediated by food reserves, hunting success or reproductive condition (e.g. Bonte et al. 2007). Interspecific interactions may also affect dispersal strategies. Competition and predation may both increase evolved dispersal distance as individuals seek to avoid predators and competitors or seek prey. However, if the interaction is a mutualistic one, there may be a spreading of the range limit (Travis et al. 2005) or general reduction in the dispersal strategy as partners are required to move together (Brooker et al. 2007).
Here, I have considered only static ranges with limits set by a variety of demographic and environmental factors. In an era of climate change, we have to consider how species ranges will respond to changes in habitat quality or distribution. Range expansion is facilitated by increased dispersal at the margins; therefore, a species that already has increased dispersal at a static margin will be at a considerable advantage and be able to respond much more adroitly than the one with reduced dispersal strategies at the margins. Therefore, I suggest that, all other things being equal, species where the ranges are limited by habitat turnover, reduction in habitat quality or birth rate will be much more likely to respond to climate change than those that have higher costs of dispersal at the margin, or have abrupt edges. Consideration of the interactions between the processes that set range limits, the potential for the evolution of dispersal and the rate of climate change could considerably improve the predictive power of future models.
Acknowledgments
Two anonymous reviewers made many constructive comments on an earlier version of this manuscript.
Footnotes
One contribution of 17 to a Special Issue ‘Geographic range limits of species’.
References
- Baskett M.L., Weitz J.S., Levin S.A. The evolution of dispersal in reserve networks. Am. Nat. 2007;170:59–78. doi: 10.1086/518184. doi:10.1086/518184 [DOI] [PubMed] [Google Scholar]
- Bonte D., Van Belle S., Maelfait J.-P. Maternal-care and reproductive state-dependent mobility determine initial natal dispersal in a wolf spider. Anim. Behav. 2007;74:63–69. doi:10.1016/j.anbehav.2006.06.021 [Google Scholar]
- Bridle J.R., Vines T.H. Limits to evolution at range margins: when and why does adaptation fail? Trends Ecol. Evol. 2006;22:140–147. doi: 10.1016/j.tree.2006.11.002. doi:10.1016/j.tree.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Brooker R.W., Travis J.M.J., Clark E.J., Dytham C. Modelling species' range shifts in a changing climate: the impacts of biotic interactions, dispersal distance and the rate of climate change. J. Theor. Biol. 2007;245:59–65. doi: 10.1016/j.jtbi.2006.09.033. doi:10.1016/j.jtbi.2006.09.033 [DOI] [PubMed] [Google Scholar]
- Cody M.L., Overton J.M. Short-term evolution of reduced dispersal in island plant populations. J. Ecol. 1996;84:53–61. doi:10.2307/2261699 [Google Scholar]
- Cousens R., Dytham C., Law R. Oxford University Press; Oxford, UK: 2008. Dispersal in plants: a population perspective. [Google Scholar]
- Cwynar L.C., MacDonald G.M. Geographic variation of lodgepole pine in relation to population history. Am. Nat. 1987;129:463–469. doi:10.1086/284651 [Google Scholar]
- Darling E., Samis K.E., Eckert C.G. Increased seed dispersal potential towards geographic range limits in a Pacific coast dune plant. New Phytol. 2008;178:424–435. doi: 10.1111/j.1469-8137.2007.02349.x. doi:10.1111/j.1469-8137.2007.02349.x [DOI] [PubMed] [Google Scholar]
- Duckworth R.A. Adaptive dispersal strategies and the dynamics of range expansion. Am. Nat. 2008;172:S4–S17. doi: 10.1086/588289. doi:10.1086/588289 [DOI] [PubMed] [Google Scholar]
- Dytham C. How landscapes affect the evolution of dispersal behaviour in reef fishes: results from an individual-based model. J. Fish Biol. 2003;63:213–225. doi:10.1111/j.1095-8649.2003.00231.x [Google Scholar]
- Gillespie D.T. Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 1977;81:2340–2361. doi:10.1021/j100540a008 [Google Scholar]
- Gros A., Poethke H.J., Hovestadt T. Evolution of local adaptations in dispersal strategies. Oikos. 2006;114:544–552. doi:10.1111/j.2006.0030-1299.14909.x [Google Scholar]
- Holt R.D. On the evolutionary ecology of species' ranges. Evol. Ecol. Res. 2003;5:159–178. [Google Scholar]
- Hughes C.L., Hill J.K., Dytham C. Evolutionary trade-offs between reproduction and dispersal in populations at expanding range boundaries. Biol. Lett. 2003;270:S147–S150. doi: 10.1098/rsbl.2003.0049. doi:10.1098/rsbl.2003.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C.L., Dytham C., Hill J.K. Modelling and analysing evolution of dispersal in populations at expanding range boundaries. Ecol. Ent. 2007;32:437–445. doi:10.1111/j.1365-2311.2007.00890.x [Google Scholar]
- Kirkpatrick M., Barton N.H. Evolution of a species' range. Am. Nat. 1997;150:1–23. doi: 10.1086/286054. doi:10.1086/286054 [DOI] [PubMed] [Google Scholar]
- Kun A., Scheuring I. The evolution of density-dependent dispersal in a noisy spatial population model. Oikos. 2006;115:308–320. doi:10.1111/j.2006.0030-1299.15061.x [Google Scholar]
- Lou Y. Some challenging mathematical problems in evolution of dispersal and population dynamics. Tutorials Math. Biosci. IV: Evol. Ecol. 2008;1922:171–205. doi:10.1007/978-3-540-74331-6_5 [Google Scholar]
- McInerny G., Travis J.M.J., Dytham C. Range shifting on a fragmented landscape. Ecol. Informatics. 2007;2:1–8. doi:10.1016/j.ecoinf.2006.12.001 [Google Scholar]
- McPeek M.A., Holt R.D. The evolution of dispersal in spatially and temporally varying environments. Am. Nat. 1992;140:1010–1027. doi:10.1086/285453 [Google Scholar]
- Murrell D.J., Travis J.M.J., Dytham C. The evolution of dispersal distance in spatially-structured populations. Oikos. 2002;97:229–236. doi:10.1034/j.1600-0706.2002.970209.x [Google Scholar]
- Olivieri I., Michalakis Y., Gouyon P.-H. Metapopulation genetics and the evolution of dispersal. Am. Nat. 1995;146:202–228. doi:10.1086/285795 [Google Scholar]
- Päivinen J., Grapputo A., Kaitala V., Komonen A., Kotiaho J.S., Saarinen K., Wahlberg N. Negative density–distribution relationships in butterflies. BMC Biol. 2005;3:5. doi: 10.1186/1741-7007-3-5. doi:10.1186/1741-7007-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B.L., Brown G.P., Webb J.K., Shine R. Invasion and the evolution of speed in toads. Nature. 2006;439:803. doi: 10.1038/439803a. doi:10.1038/439803a [DOI] [PubMed] [Google Scholar]
- Phillips B.L., Chipperfield J.D., Kearney M.R. The toad ahead: challenges of modelling the range and spread of an invasive species. Wildl. Res. 2008;35:222–234. doi:10.1071/WR07101 [Google Scholar]
- Poethke H.J., Hovestadt T. Evolution of density- and patch-size-dependent dispersal rates. Proc. R. Soc. B. 2002;269:637–643. doi: 10.1098/rspb.2001.1936. doi:10.1098/rspb.2001.1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethke H.J., Hovestadt T., Mitesser O. Local extinction and the evolution of dispersal rates: causes and correlations. Am. Nat. 2003;161:631–640. doi: 10.1086/368224. doi:10.1086/368224 [DOI] [PubMed] [Google Scholar]
- Purves D.W., Dushoff J. Directed seed dispersal and metapopulation response to habitat loss and disturbance: application to Eichhornia paniculata. J. Ecol. 2005;98:658–669. doi:10.1111/j.1365-2745.2005.00988.x [Google Scholar]
- Renshaw E. Cambridge University Press; Cambridge, UK: 1991. Modelling biological populations in space and time. [Google Scholar]
- Roff R.A., Fairbairn D.J. The genetic basis of dispersal and migration, and its consequences for the evolution of correlated traits. In: Clobert J., Danchin E., Dhondt A.A., Nichols J.D., editors. Dispersal. Oxford University Press; Oxford, UK: 2001. pp. 191–202. [Google Scholar]
- Schtickzelle N., Mennechez G., Baguette M. Dispersal depression with habitat fragmentation in the bog fritillary butterfly. Ecology. 2006;87:1057–1065. doi: 10.1890/0012-9658(2006)87[1057:ddwhfi]2.0.co;2. doi:10.1890/0012-9658(2006)87[1057:DDWHFI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Simmons A.D., Thomas C.D. Changes in dispersal during species range expansions. Am. Nat. 2004;164:378–395. doi: 10.1086/423430. doi:10.1086/423430 [DOI] [PubMed] [Google Scholar]
- Slepoy A., Thompson A.P., Plimpton S.J. A constant-time kinetic Monte Carlo algorithm for simulation of large biochemical reaction networks. J. Chem. Phys. 2008;128:205101. doi: 10.1063/1.2919546. doi:10.1063/1.2919546 [DOI] [PubMed] [Google Scholar]
- Stefan A.S. To fly or not to fly? Colonization of Baltic islands by winged and wingless carabid beetles. J. Biogeogr. 1984;11:413–426. doi:10.2307/2844805 [Google Scholar]
- Travis J.M.J., Dytham C. The evolution of dispersal in a metapopulation: a spatially explicit, individual-based model. Proc. R. Soc. B. 1998;265:17–23. doi:10.1098/rspb.1998.0258 [Google Scholar]
- Travis J.M.J., Dytham C. Habitat persistence, habitat availability and the evolution of dispersal. Proc. R. Soc. B. 1999;266:723–728. doi:10.1098/rspb.1999.0696 [Google Scholar]
- Travis J.M.J., Dytham C. Dispersal evolution during invasions. Evol. Ecol. Res. 2002;4:1119–1129. [Google Scholar]
- Travis J.M.J., Murrell D.J., Dytham C. The evolution of density-dependent dispersal. Proc. R. Soc. B. 1999;266:1837–1842. doi:10.1098/rspb.1999.0854 [Google Scholar]
- Travis J.M.J., Brooker R.W., Dytham C. The interplay of positive and negative species interactions across an environmental gradient: insights from an individual-based simulation model. Biol. Lett. 2005;1:5–8. doi: 10.1098/rsbl.2004.0236. doi:10.1098/rsbl.2004.0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- With K.A., Crist T.O. Critical thresholds in species' responses to landscape structure. Ecology. 1995;76:2446–2459. doi:10.2307/2265819 [Google Scholar]
- With K.A., Cadaret S.J., Davis C. Movement responses to patch structure in experimental fractal landscapes. Ecology. 1999;80:1340–1353. doi:10.1890/0012-9658(1999)080[1340:MRTPSI]2.0.CO;2 [Google Scholar]
- Zera A.J., Denno R.F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 1997;42:207–230. doi: 10.1146/annurev.ento.42.1.207. doi:10.1146/annurev.ento.42.1.207 [DOI] [PubMed] [Google Scholar]