Abstract
Kin selection theory predicts that, in species where progeny members compete for limiting parental care, individual offspring should be more prone to monopolize parental resources as their genetic relatedness to brood competitors decreases. Mixed parentage among broodmates may arise as a consequence, for example, of extra-pair fertilization or brood parasitism events. In this experimental study of barn swallows (Hirundo rustica), we reciprocally partially cross-fostered hatchlings between broods and compared the behaviour of pairs of related and unrelated broodmates in a competitive context, both under normal food provisioning regime and after mild food deprivation. We found that scramble competition for food mediated by visual and vocal solicitation displays (begging) is inversely related to relatedness among competitors, independent of their level of satiation. Nestlings may modulate their competitive behaviour according to vocal cues that vary with their origin and allow kin recognition. We also uncover direct fitness costs to both parents and offspring arising from mixed parentage in a brood, in terms of increased parental workload and reduced survival of the nestlings. Such previously neglected costs may select for reduced frequency of extra-pair fertilizations and brood parasitism in species with extensive parental care.
Keywords: begging, brood parasitism, extra-pair fertilization, kin selection, parental care, sibling competition
1. Introduction
Theoretical models of intrafamilial conflicts predict that the extent of competition among broodmates should be constrained by the indirect fitness cost of subtracting critical resources from related competitors (Hamilton 1964; Clutton-Brock 1991; Godfray 1991, 1995; Mock & Parker 1997). The marginal direct fitness gain of acquiring additional parental resources declines as an individual's state and satiation improves. Thus, offspring in relatively poor condition can gain more from the same additional amount of food (Godfray 1991). Variable relatedness among broodmates, which results from extra-bond fertilizations and brood parasitism, affects the pay-off of monopolizing parental care, reducing the individual cost of selfishness as relatedness among competitors declines. Because offspring should favour their nest-mates only when the indirect fitness benefit of such an altruistic act exceeds the direct cost to the actor, they are predicted to secure a larger share of food at the expense of nest-mates as relatedness to them declines (Hamilton 1964; Godfray 1995).
Across bird species, decreasing average intrabrood relatedness depending on multiple paternities and intra- or interspecific brood parasitism has led to the evolution of intense ‘begging’ behaviour (sensu Kilner & Johnstone 1997; Wright & Leonard 2002) and higher growth rates, as adaptations to outcompete broodmates (Briskie et al. 1994; Royle et al. 1999; Remeš 2006; Boncoraglio & Saino 2008; Boncoraglio et al. 2009). However, the frequency of extra-pair offspring and brood parasites varies considerably among broods (Saino et al. 1997a; Birkhead & Møller 1998), and the inclusive fitness benefit of care monopolization is expected to vary according to the actual genetic relatedness of nest-mates. This should select for fine-tuned competitive behaviour, whereby any individual offspring is more prone to subtract resources from its broodmates when these are parasites or half- rather than full-sibs, and for kin discrimination among broodmates (Nakagawa & Waas 2004). Surprisingly, studies explicitly testing these hypotheses in vertebrates are rare.
The barn swallow (Hirundo rustica) is a socially monogamous, semi-colonial, insectivorous passerine bird (Møller 1994). Both parents attend the offspring that are totally dependent on parental food provisioning up to four to five weeks after hatching. Food available to the offspring is limiting (Saino et al. 1997b), and chicks compete for it using vocalizations, gaping and posturing (begging displays; Kilner & Johnstone 1997). As in other passerines, the intensity of begging increases with hunger, and parents adjust their provisioning to the current solicitation level of offspring, providing more food to the chicks that beg more (Saino et al. 2000; Boncoraglio et al. 2008). Food provisioning is intense, especially in weeks 2–3 after hatching (up to 500 feedings delivered to a brood per day; G. Boncoraglio 2006, personal observation); parents normally feed only one chick per visit (Møller 1994). Male chicks are able to prevail over their female broodmates in competition for food in the short run, but are more negatively affected by long-term adverse conditions during rearing (Boncoraglio et al. 2008; Saino et al. 2008). Approximately 50 per cent of broods contain at least one extra-pair chick, while approximately 2 per cent of eggs originate from intraspecific brood parasitism (Møller 1994; Saino et al. 1997a). Thus, intrabrood genetic relatedness can vary from r∼0 (as is the case between host offspring and parasitic chicks) to r∼0.25 (maternal half-sibs; paternal half-sibs are rare), up to r∼0.5 (full-sibs). Inbreeding in barn swallows is unlikely because of large genetic population size, high natal dispersal and temporal breeding segregation between yearlings and older individuals (Møller 1994). Genetic relatedness among mates and colony members is therefore likely to be close to zero.
In our experiment, we created broods of mixed origin by reciprocally swapping the same number of hatchlings between broods of the same age (n=32 broods and 16 ‘dyads’), thus producing nests with chicks that had r∼0 and 0.5 (assuming no extra-pair fertilizations have occurred). For each brood, we compared the begging behaviour of a pair of siblings and a pair of unrelated nest-mates while competing both under a normal feeding regime and after mild food deprivation. Parental feeding effort during feeding trials was estimated by measuring the body mass gain realized by the nestlings during the trial, which has been shown to reliably reflect the short-term food intake as measured by feeding rates in barn swallows (Boncoraglio et al. 2008). Vocalizations may mediate individual and kin recognition in birds and mammals (Hauber & Sherman 2001; Nakagawa & Waas 2004). We therefore recorded individual begging calls of each chick before feeding trials to test whether sonographic features of their calls varied with parentage in mixed broods, being possibly a cue for kin recognition among broodmates.
2. Material and methods
(a) General field and laboratory methods
This study was performed in eight barn swallow colonies (n=141 breeding pairs in total) east of Milan (northern Italy) during spring 2007. Nests were visited daily to record breeding events. Taking day of hatching as day 0, on day 1 we performed partial cross-fostering within dyads (n=16) of broods where the first egg hatched on the same morning, immediately after hatch completion of both broods (the average hatching spread in this population is approximately 26 hours; Boncoraglio & Saino 2008). After manipulation, each brood contained half resident (1–3) and half cross-fostered (1–3) hatchlings. Broods to be cross-fostered were chosen in order to minimize the difference in original brood size, which was not altered. Nestlings were individually marked at day 1. Nestlings to be cross-fostered were chosen randomly (for an alternative approach see Brinkhof et al. 1999). No significant difference in body mass was found at day 1 among resident and cross-fostered nestlings (F1,95.3=0.67, p=0.41). At day 7 after hatching, nestlings were ringed and sexed using molecular techniques (Boncoraglio et al. 2008). The same was done for 33 control broods whose composition had not been manipulated during the experiment. Control broods were handled as often as mixed broods, but nestling begging behaviour was not recorded due to time and equipment limitation. These broods did not differ from mixed broods in the mean and variance of clutch size, hatching date or brood size (t- and Levene tests, p always greater than 0.05). On day 12, body mass, tarsus length and third primary wing feather length were recorded and a standard immunological test reflecting T cell-mediated immune response (the phytohaemagglutination (PHA) test; Saino et al. 1997b) was performed. Three control and three experimental broods failed before day 12. Survival at day 15 was evaluated on a larger sample of 75 mixed and 65 control broods observed during spring 2005 and 2007 to increase the statistical power of the tests (see also Boncoraglio & Saino 2008). No difference existed between mixed and control broods from both years with respect to the mean and variance of clutch size, hatching date or brood size (t- and Levene tests, p always greater than 0.05).
(b) Audio and video recordings of focal pairs during feeding trials
On day 14 after hatching, for each brood we randomly chose a pair of nestlings, irrespective of their origin, and left them in the nest of rearing for a feeding trial while temporarily removing their nest-mates. Before the trials, nestlings of all pairs were recorded while alone in the nest during three consecutive feeding visits with a Sony ECM C-115 microphone connected to a Sony TCD-D7 DAT recorder, according to an established protocol (Boncoraglio et al. 2008). This operation took less than 10 min for the large majority of the chicks. Afterwards, nestlings were weighed, marked individually on their heads with small white spots and put back together in the nest. All feeding visits that occurred in the following 45 min were audio recorded with the same equipment as before, and video recorded with a Sony DCR-SR72E digital video camera positioned at 1.5 m from the nest. Nestlings were weighed again at the end of the feeding trials to record body mass change. The same protocol of recording was repeated on the focal nestlings after 1.5 hours of food deprivation, which simulated a short spell of reduced food provisioning naturally occurring during bad weather. During food deprivation, nestlings were kept in a safe and warm place. The following day (day 15), one of the focal nestlings used on day 14 was randomly chosen and paired with a sibling, if on day 14 it was tested with a non-sibling, or with a non-sibling, if on day 14 it was tested with a sibling. This second pair was tested using the same protocol as on day 14, including individual audio recording before the trials. The order of comparisons (i.e. related or unrelated pairs) to be performed for each brood on days 14 and 15, and the choice of considering either two resident or two transferred chicks for the trial of related nest-mates, were established in the morning of day 14 by tossing a coin. No bias was found in the assortment of our sample with respect to both factors (day 14 comparison: 17 related versus 12 unrelated pairs; binomial test, p=0.46; sibling comparison: 14 resident versus 15 transferred pairs; binomial test, p=1.00). The reason for reusing, on day 15, one of the nestlings that was already tested on day 14 is as follows. In order to avoid any bias (see above), we decided to randomize the choice of pairs of related nestlings to be tested with respect to them being resident or transferred. Thus, at least three nestlings of each origin would have been required within each brood in order to set up the planned comparisons without reusing any nestling on day 15. However, maximum brood size at hatching in barn swallows is generally six (exceptionally, seven) and the frequency of broods with six chicks at hatching is less than 14 per cent (G. Boncoraglio, M. Caprioli & N. Saino 2007, unpublished data). In addition, mortality occurred between hatching and day 14, when the tests were started. Hence, the chances of obtaining an appreciable sample size using dyads only including broods where hatching was completed on the same day with six hatchlings each and no mortality before day 14 were minimal. For similar reasons, we could not adopt a set-up with the experimental broods being composed exclusively of clusters of cross-fostered chicks.
Extra-pair fertilizations in our study farms account for 26 per cent of the nestlings, whereas the frequency of brood parasitic chicks is 2 per cent, as recently reported by Boncoraglio & Saino (2008). This implies that pairs of siblings (as we define them throughout the paper) actually included maternal half-sibs in some cases. The occurrence of extra-pair fertilizations, however, does not invalidate our experimental design because average relatedness within pairs of half-sibs was higher than that within pairs composed of one resident and one foreign, cross-fostered nestling. In addition, any occurrence of brood parasitism was negligible in our sample of 32 broods.
(c) Analysis of audio and video recordings
Mean syllable duration (ms) and relative amplitude (dB) of individual begging calls of each nestling were measured from individual recordings with Avisoft SAS Lab Pro, following Boncoraglio et al. (2008). For assessing the intensity of nestling begging behaviour during feeding trials, we randomly selected three feeding visits per trial. Feeding visits were randomly chosen over the entire length of the track for the recordings performed before food deprivation, and over the first half of the track for the recordings performed after food deprivation. This was done to prevent the dissipation of the effect of food deprivation on nestling begging behaviour, potentially resulting from the predicted increase of feeding effort by parents. No difference in the rank order and time elapsed from the onset of recordings was found among the feeding visits selected for related and unrelated focal pairs, respectively, both before and after food deprivation (Wilcoxon signed-rank test and paired t-tests, p always greater than 0.05). Mean begging amplitude (dB, corrected for background amplitude) reached by the focal pairs during visits was measured with Praat v. 4.4.04 (http://www.praat.org), according to Boncoraglio & Saino (2008), and was averaged within each trial. Higher values of mean begging amplitude could have depended on higher amplitude of calls uttered by begging nestlings, higher call rates (Leonard & Horn 2001) or lower distance between chicks' heads and microphone position, which was invariably located in the centre of the nest rim, i.e. the place from which parents preferentially feed (G. Boncoraglio 2007, personal observation). However, all these possibilities would reflect escalating competition between the nestlings, so we could interpret such a variable as a suitable index of scramble competition independently on the mechanism(s) that produced higher amplitude values. Repeatability of begging amplitude was significant within feeding trial (F1,115=5.80, p<0.001, R2=0.748). Total feeding events and the number of feedings obtained by each nestling were measured by inspecting 45 min video recordings using VLC Media Player 0.8.4a. Maximum intensity of the postural begging display of both nestlings was scored during each of the three visits on a four-level scale varying from 0 (chick not begging) to 3 (chick standing on its tarsi and begging with fully stretched neck towards the attending parent; Kilner 2002), and averaged within trial between the nestlings. Repeatability of postural scores assessed independently for a random subsample of 60 feeding events that were analysed twice was highly significant (F1,118=31.60, p<0.001, R2=0.969). All measures were performed blindly with respect to treatments.
(d) Statistical analyses
Generalized linear mixed model (GLMM) analyses of variance were used to investigate the effects of brood composition (mixed or untreated), kinship of the focal pair (‘siblings’ or ‘unrelated’) and foster status of the nestlings (resident or transferred; fixed effects) on begging calls, begging postures, morphological and immunological variables, body mass gain during feeding trials and nestling survival. Food deprivation (before or after) was included as a fixed factor when needed. In the models, depending on the variable under scrutiny, we included dyad of partially cross-fostered broods, and nest of origin and nest of rearing nested within dyad (random factors), together with the interactions of nest of rearing with all fixed factors and covariates. Chick identity was entered as a random factor whenever required. Nestling survival at day 15 was treated as a two-state response variable (1=surviving and 0=not surviving), assuming a binomial error distribution and a logit link function. Statistical analyses were run using the SAS (v. 9.0) statistical package. Parameter estimates were obtained by restricted maximum-likelihood method. Degrees of freedom were estimated by Satterthwaite's approximation; the same results were obtained using the between–within method of partitioning of degrees of freedom as implemented by SAS v. 9.0 (Littell et al. 1996). Residuals of the models were tested for prerequisite conditions of normality and homogeneity of variances; data transformation was never required.
3. Results
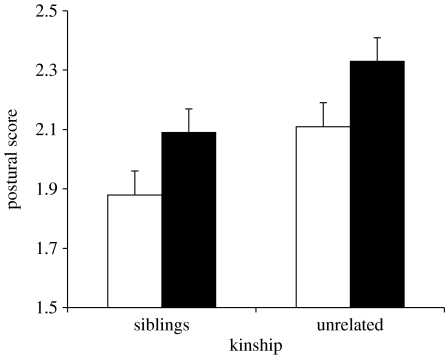
We first performed a GLMM for repeated measures on the intensity of postural begging displays, including food deprivation (before or after), kinship (siblings or unrelated nestlings) and their interaction as fixed factors, and dyad of partially cross-fostered broods, nest of rearing nested within dyad, and the interactions between nest of rearing and all fixed factors in the model as random factors. The nest of rearing was considered as the experimental unit subjected to repeated measures. Mean postural begging intensity of competing chicks varied according to nest of rearing (z=1.73, p=0.042) and was more intense in trials after than before food deprivation, and when nestlings were confronted with a non-sibling rather than a sibling, with no significant interaction effect between food deprivation and kinship (table 1; figure 1). Similarly, begging loudness during feeding events increased after food deprivation (F1,28=17.65, p=0.0002; mean loudness change: 3.31 dB ±1.10 s.e.), and when unrelated rather than related nestlings were simultaneously present in the nest (F1,56=7.24, p=0.0094; mean loudness difference: 1.81 dB ±0.97 s.e.; interaction: F1,56=2.52, p=0.118). Hence, nestlings scaled up their begging behaviour when competing with a familiar but unrelated broodmate rather than with a familiar sibling.
Table 1.
Effects of kinship (siblings versus unrelated nestlings) and food deprivation (before versus after) on the intensity of postural begging display measured at three feeding events (n=29 broods).
| z | F | d.f. | p-value | |
|---|---|---|---|---|
| dyad of cross-fostered broods | 0.00 | 1.0000 | ||
| brood of rearing (dyad) | 1.73 | 0.0421 | ||
| kinship | 7.76 | 1,28 | 0.0095 | |
| food deprivation | 16.21 | 1,56 | 0.0002 | |
| kinship×food deprivation | 0.01 | 1,56 | 0.9365 |
Figure 1.
Maximum intensity (+s.e.) of postural begging display in 29 pairs of barn swallow siblings and unrelated nest-mates before (white bars) and after (black bars) food deprivation.
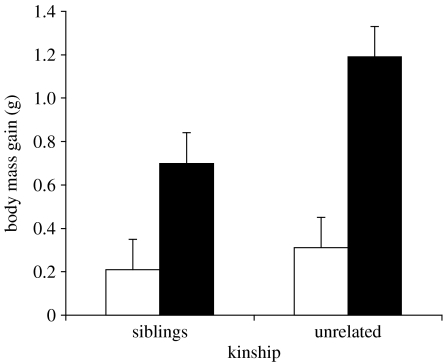
In a GLMM analysis with the same model as for postural begging, total body mass gain realized by the nestlings during feeding trials was higher when competing with an unrelated rather than a related nest-mate, and after than before food deprivation (table 2; figure 2). When considering the number of feeding visits during the trials, instead of total body mass gain of the nestlings, we found that parents increased their feeding rate after food deprivation (F1,56=24.45, p<0.001; before food deprivation: 10.37±1.22 s.e. visits per trial; after food deprivation: 14.53±1.22 s.e. visits per trial), while a marginally non-significant effect was found for kinship (F1,28=3.76, p=0.06; siblings: 11.28±1.29 s.e. visits per trial; unrelated: 13.61±1.29 s.e. visits per trial; interaction: F1,56=0.78, p=0.38).
Table 2.
Effects of kinship (siblings versus unrelated nestlings) and food deprivation (before versus after) on nestling body mass gain during feeding trials (n=29 broods).
| z | F | d.f. | p-value | |
|---|---|---|---|---|
| dyad of cross-fostered broods | 1.35 | 0.0890 | ||
| brood of rearing (dyad) | 0.25 | 0.4016 | ||
| kinship | 6.31 | 1,28 | 0.0181 | |
| food deprivation | 32.28 | 1,28 | <0.0001 | |
| kinship×food deprivation | 2.98 | 1,28 | 0.0954 |
Figure 2.
Nestling body mass gain (+s.e.) during feeding trials in 29 pairs of barn swallow siblings and unrelated nest-mates before (white bars) and after (black bars) food deprivation.
All the results presented above were neither affected by the sex composition of focal pairs (three-level fixed factor: both males, male and female, or both females) nor by sex in combination with food deprivation (p always greater than 0.05; details not shown). In addition, we tested whether the fact that pairs of siblings were resident in their original nest or cross-fostered affected the results by repeating the same analyses as above, while including a three-level fixed-effect factor nested within kinship, accounting for nestlings in a pair being siblings in their original brood, siblings in a foster brood or unrelated. The effect of such a factor was not significant in all models (p always greater than 0.05), and its inclusion did not alter the outcome of previous analyses for the other predictors (details not shown). Post hoc comparisons confirmed that no differences existed between pairs of siblings when they were in their original or in a foster brood with respect to begging behaviour, body mass gain or feedings received (p always greater than 0.05).
The intensity of postural begging display positively predicted individual body mass gain (F1,167=4.09, p=0.045; b=0.09±0.05 s.e.) and the number of feedings received during trials (F1,118=15.72, p<0.001; b=1.64±0.41 s.e.) in repeated-measures GLMMs performed while considering the nest of rearing as the experimental unit, food deprivation as a fixed factor (kinship effect was not significant and therefore removed from both models), individual body mass at the beginning of the trial (only in the analysis on body mass gain) and mean postural score during the trial as covariates, and dyad of partially cross-fostered broods, nest of rearing and nest of origin, both nested within dyad, chick identity and the interactions between nest of rearing and all fixed factors or covariates as random factors. Hence, nestlings that begged more intensely obtained more food and feedings. Parents did not differentially allocate food between their own and cross-fostered chicks, as nestling origin (resident or transferred) did not significantly affect body mass gain (F1,28=1.25, p=0.27) or number of feedings (F1,28=0.12, p=0.73).
Body mass, tarsus length (reflecting skeletal size), wing feather length (reflecting plumage development) and immune response as measured by the PHA test at day 12 of age did not differ between resident and cross-fostered chicks, nor among nestlings reared in mixed broods compared with control broods whose composition had not been manipulated during the experiment (p always greater than 0.05), in GLMMs including brood composition (mixed or control) and nestling origin (resident or transferred) as fixed factors, and dyad of partially cross-fostered broods, nest of rearing and nest of origin, both nested within dyad, as random factors. However, nestling survival at day 15 estimated in a larger sample of 140 broods and 622 hatchlings in total (see §2) was lower in mixed compared with control broods (F1,135=5.05, p=0.026; survival rate: mixed=94.15%±0.59 s.e.; control=98.68%±0.62 s.e.) in a GLMM performed while assuming a binomial distribution of error and a logit link function for the dependent variable, and including in the model brood composition (mixed or control) and nestling origin (resident or transferred) as fixed factors, and year, dyad of partially cross-fostered broods, nest of rearing and nest of origin nested within dyad as random factors. Survival in mixed broods was not significantly affected by nestling origin (F1,488=2.23, p=0.14). In addition, a GLMM on the same sample of 140 broods including year of breeding (random factor) and brood size at hatching (covariate) revealed that brood size at day 15 was smaller in mixed broods compared with control broods (F1,136=5.84, p=0.017; mixed=3.73±0.17 s.e. nestlings; control=4.15±0.18 s.e. nestlings). No differences existed between mixed and control broods from both years in nestling morphological variables at day 12 (GLMM analyses, p always greater than 0.05; see also above) nor in their variances (Levene tests, p always greater than 0.05).
Repeating all analyses while controlling for brood size and hatching date as covariates gave qualitatively identical results to those reported above for all the models; for brevity, further details are not reported here.
4. Discussion
We have experimentally shown that the intensity of scramble competition for depreciable care is inversely related to relatedness among barn swallow nestlings. In fact, both the postural and vocal components of begging behaviour escalated when pairs of familiar but unrelated nest-mates were competing for food. These findings did not depend on the level of satiation of the two competitors, as begging displays were more intense in pairs of mixed origin both before and after food deprivation. As individuals are selected to limit their selfishness in competitive interactions when relatives are faced with a fitness cost for their actions (Hamilton 1964; Godfray 1995), the inverse relationship between begging intensity and genetic relatedness conforms to the expectations of kin selection theory (Hamilton 1964; Godfray 1995; Mock & Parker 1997). In fact, since begging intensity directly affects individual food intake in this species and in other altricial birds (this study; Kilner & Johnstone 1997; Boncoraglio et al. 2008), and food provided by parents during the rearing period is limiting to barn swallow offspring (Saino et al. 1997b, 2000), individual nestlings should be prone to beg more in order to subtract food from a competitor, particularly when facing an unrelated chick. In addition, resident and transferred nestlings were equally able to discriminate between siblings and unrelated nest-mates (see §3) and no differences were found among them in morphology or immune response at day 12. Hence, worse matching of cross-fostered nestlings with their rearing environment compared with resident nestlings seems not to have occurred, and discrimination based on kin, or on maternal effects, remains the most likely explanation for the outcome of the experiment.
Intense begging in pairs of nestlings with mixed origin could have been the consequence of no genetic relatedness enhancing competitive behaviour, or, alternatively, of reduced food provisioning to such pairs (Godfray 1991; Kilner & Johnstone 1997). However, body mass gain realized by individual nestlings during feeding trials was higher in pairs of unrelated rather than related nest-mates. Because body mass gain reliably reflects the total amount of food obtained by the chicks in the short term, as estimated by feeding rates (Boncoraglio et al. 2008), the possibility that enhanced begging by unrelated pairs was due to reduced food intake can be ruled out. Rather, our findings show that, even if the differences we detected across groups on single components of begging display were apparently small, increased begging by pairs of nestlings of different origin prompted the adults to provide them with more food compared with pairs of related offspring, or at least covaried with other factors promoting increased food provisioning to such pairs.
Begging escalation by chicks confronted with unrelated competitors occurs also when mixed and control broods rather than nestling pairs are compared in barn swallows (Boncoraglio & Saino 2008). Surprisingly, while pairs of unrelated chicks obtained more food in the short run, chicks in mixed broods did not grow faster than untreated control broods whose original composition was not manipulated during this experiment. Escalation in competitive interactions among unrelated nestlings thus appeared to be wasteful of parental effort, possibly because an increase in food intake was counterbalanced by larger energetic costs of scrambling (Godfray 1995; Kilner & Johnstone 1997; Mock & Parker 1997). Barn swallow nestlings in the competitive environment of an enlarged brood, where scrambling for food is more intense (Saino et al. 1997b), increase their circulating corticosterone levels (Saino et al. 2003). The fact that mixed parentage among broodmates did not enhance body mass and size in mixed broods, despite resulting in higher food intake, could depend on the effect of increased levels of corticosterone on lipolysis and protein catabolism (von Holst 1998; Wingfield et al. 1998).
The lower survival of nestlings in mixed broods we observed could also reflect a harsher regime of scramble competition in mixed compared with untreated broods. Variation in the pay-off of altruistic versus selfish behaviour depending on parentage among nest-mates could have resulted in extreme monopolization of parental resources by competitively superior nestlings in mixed broods and, consequently, in augmentation of the risk of starvation of their nest-mates (Godfray 1995; Mock & Parker 1997). Moreover, reduced survival of related broodmates entailed the nestlings from mixed broods having an additional indirect fitness cost.
Because chicks reacted differentially according to variable relatedness to competitors, cues must exist that allow kin discrimination among them. We found that sonographic quality (i.e. mean syllable duration) of begging calls recorded before feeding trials varied with parentage between unrelated nestlings that shared the same rearing environment (see also Boncoraglio & Saino 2008). Our results also match the findings of another study of barn swallows, where it was shown that the effect size of parentage on variation in begging syllable duration is similar to the size of the effect of parentage on acoustic begging cues that are hypothesized to allow kin recognition among family members in a closely related colonial species, the cliff swallow (Hirundo pyrrhonota; Medvin et al. 1992). Thus, learning and common rearing conditions of unrelated family members did not overcome the differences in the quality of vocalizations resulting from genetic or early maternal effects through egg quality. Acoustic communication has been shown to mediate kin recognition in a number of animal species (Hauber & Sherman 2001; Nakagawa & Waas 2004). Variation in such traits could have therefore provided the basis for self-referent kin recognition (Hauber & Sherman 2001) and individual decisions of increasing competition in unrelated pairs. If variation in vocalizations according to parentage is not entirely due to early maternal effects, it may serve as a basis for kin recognition also among half-siblings. Accordingly, two comparative studies have shown that competition among nest-mates increases across species with the frequency of extra-pair fertilizations (Briskie et al. 1994; Royle et al. 1999). To the best of our knowledge, no experimental study has tested the prediction of kin discrimination among half-siblings. However, we acknowledge that the design of our experiment does not allow us to disentangle the effect of variation in genetic relatedness of the chicks from variation mediated by maternal effects. Further studies are thus required before our conclusions can be extended to competition among half-sibs.
Did parents also pay a cost for increased scramble competition among unrelated nest-mates? Begging escalation in unrelated pairs of chicks increased food provisioning, but this did not translate into enhanced phenotypic quality of the offspring in mixed broods. Resident nestlings did not benefit more than cross-fostered nest-mates from increased care allocation. In addition, nestlings from mixed broods experienced lower survival independent of their origin, implying that parents may suffer increased mortality of genetically related offspring when caring for a brood with mixed origin. Finally, an increase in parental effort significantly depresses long-term survival of attending adults in barn swallows (Saino et al. 1997b), as well as in several bird species and organisms (Clutton-Brock 1991; Stearns 1992).
When providing food, parents apparently could not recognize their own compared with cross-fostered nestlings. This finding is supported by the outcome of extensive research on parent–offspring recognition in semi-colonial and non-colonial swallow species (e.g. Medvin & Beecher 1986; Leonard et al. 1997). Differentially higher ability to recognize kin by offspring may have evolved because of the relatively low costs of alloparental care, compared with acting altruistically towards unrelated nest-mates (Clutton-Brock 1991; Stearns 1992; Whittingham et al. 2003; Holen & Johnstone 2007).
This study uncovered previously neglected costs of brood parasitism and perhaps extra-pair fertilizations to both adults and offspring in an avian species. Mixed brood parentage had no benefits for the offspring, because increased food provisioning by enhanced begging did not translate into higher phenotypic quality. Rather, more intense competition increased nestling mortality and probably reduced parental survival because of increased parental effort of both mothers and fathers (Saino et al. 1997b). All these costs for parents and offspring are likely to limit the net benefits of very widespread phenomena such as extra-pair fertilizations and intraspecific brood parasitism in species with extensive parental care (Birkhead & Møller 1998).
Acknowledgements
This study was performed according to Italian laws on animal research. We thank Marty Leonard, Anders Møller, Martina Muller and Nikolaus von Engelhardt for their comments on an earlier version of the manuscript; Salvatore Boncoraglio for producing most of the items required for the fieldwork; Roberta Martinelli for help in the laboratory; and Anthony Bertucci, Massimiliano Mori and Vincenzo Pignataro for their help in the fieldwork. Kate Lessells and two anonymous referees provided precious advice during the reviewing process. G.B. was funded by an Italian PhD grant from the Ministero della Ricerca ed Università, Italy.
References
- Birkhead T.R., Møller A.P. Academic Press; London, UK: 1998. Sperm competition and sexual selection. [Google Scholar]
- Boncoraglio G., Saino N. Barn swallow chicks beg more loudly when broodmates are unrelated. J. Evol. Biol. 2008;21:256–262. doi: 10.1111/j.1420-9101.2007.01441.x. doi:10.1111/j.1420-9101.2007.01441.x [DOI] [PubMed] [Google Scholar]
- Boncoraglio G., Martinelli R., Saino N. Sex-related asymmetry in competitive ability of sexually monomorphic barn swallow nestlings. Behav. Ecol. Sociobiol. 2008;62:729–738. doi:10.1007/s00265-007-0498-8 [Google Scholar]
- Boncoraglio G., Saino N., Garamszegi L.Z. Begging and cowbirds: brood parasites make hosts scream louder. Behav. Ecol. 2009;20:215–221. doi:10.1093/beheco/arn137 [Google Scholar]
- Brinkhof M.W.G., Heeb P., Kölliker M., Richner H. Immunocompetence of nestling great tits in relation to rearing environment and parentage. Proc. R. Soc. Lond. B. 1999;266:2315–2322. doi:10.1098/rspb.1999.0925 [Google Scholar]
- Briskie J.V., Naugler C.T., Leech S.M. Begging intensity of nestling birds varies with sibling relatedness. Proc. R. Soc. Lond. B. 1994;258:73–78. doi:10.1098/rspb.1994.0144 [Google Scholar]
- Clutton-Brock T.H. Princeton University Press; Princeton, NJ: 1991. The evolution of parental care. [Google Scholar]
- Godfray H.C.J. Signalling of need by offspring to their parents. Nature. 1991;352:328–330. doi:10.1038/352328a0 [Google Scholar]
- Godfray H.C.J. Signalling of need between parents and young: parent–offspring conflict and sibling rivalry. Am. Nat. 1995;146:1–24. doi:10.1086/285784 [Google Scholar]
- Hamilton W.D. The genetical evolution of social behavior, I and II. J. Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. doi:10.1016/0022-5193(64)90038-4 [DOI] [PubMed] [Google Scholar]
- Hauber M.E., Sherman P.W. Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends Neurosci. 2001;24:609–616. doi: 10.1016/s0166-2236(00)01916-0. doi:10.1016/S0166-2236(00)01916-0 [DOI] [PubMed] [Google Scholar]
- Holen Ø.H., Johnstone R.A. Parental investment with a superior alien in the brood. J. Evol. Biol. 2007;20:2165–2172. doi: 10.1111/j.1420-9101.2007.01426.x. doi:10.1111/j.1420-9101.2007.01426.x [DOI] [PubMed] [Google Scholar]
- Kilner R.M. Sex differences in canary (Serinus canaria) provisioning rules. Behav. Ecol. Sociobiol. 2002;52:400–407. doi:10.1007/s00265-002-0533-8 [Google Scholar]
- Kilner R.M., Johnstone R.A. Begging the question: are offspring solicitation behaviours signals of need? Trends Ecol. Evol. 1997;12:11–15. doi: 10.1016/s0169-5347(96)10061-6. doi:10.1016/S0169-5347(96)10061-6 [DOI] [PubMed] [Google Scholar]
- Leonard M.L., Horn A.G. Dynamics of calling by tree swallow (Tachycineta bicolor) nestmates. Behav. Ecol. Sociobiol. 2001;50:430–435. doi:10.1007/s002650100380 [Google Scholar]
- Leonard M.L., Horn A.G., Brown C.R., Fernandez N.J. Parent–offspring recognition in tree swallows, Tachycineta bicolor. Anim. Behav. 1997;54:1107–1116. doi: 10.1006/anbe.1997.0559. doi:10.1006/anbe.1997.0559 [DOI] [PubMed] [Google Scholar]
- Littell R.C., Milliken G.A., Stroup W.W., Wolfinger R.D. SAS Institute; Cary, NC: 1996. SAS system for mixed models. [Google Scholar]
- Medvin M.B., Beecher M.D. Parent–offspring recognition in the barn swallow (Hirundo rustica) Anim. Behav. 1986;34:1627–1639. doi:10.1016/S0003-3472(86)80251-2 [Google Scholar]
- Medvin M.B., Stoddard P.K., Beecher M.D. Signals for parent–offspring recognition: strong sib–sib call similarity in cliff swallows but not barn swallows. Ethology. 1992;90:17–28. [Google Scholar]
- Mock D.W., Parker G.A. Oxford University Press; New York, NY: 1997. The evolution of sibling rivalry. [Google Scholar]
- Møller A.P. Oxford University Press; Oxford, UK: 1994. Sexual selection and the barn swallow. [Google Scholar]
- Nakagawa S., Waas J.R. ‘O sibling, where art thou?’—a review of avian sibling recognition with respect to the mammalian literature. Biol. Rev. 2004;79:101–119. doi: 10.1017/s1464793103006249. doi:10.1017/S1464793103006249 [DOI] [PubMed] [Google Scholar]
- Remeš V. Growth strategies of passerine birds are related to brood parasitism by the brown-headed cowbird (Molothrus ater) Evolution. 2006;60:1692–1700. doi:10.1111/j.0014-3820.2006.tb00513.x [PubMed] [Google Scholar]
- Royle N.J., Hartley I.R., Owens I.P.F., Parker G.A. Sibling competition and the evolution of growth rates in birds. Proc. R. Soc. Lond. B. 1999;266:923–932. doi:10.1098/rspb.1999.0725 [Google Scholar]
- Saino N., Primmer C.R., Ellegren H., Møller A.P. An experimental study of paternity and tail ornamentation in the barn swallow (Hirundo rustica) Evolution. 1997a;51:562–570. doi: 10.1111/j.1558-5646.1997.tb02443.x. doi:10.2307/2411128 [DOI] [PubMed] [Google Scholar]
- Saino N., Calza S., Møller A.P. Immunocompetence of nestling barn swallows in relation to brood size and parental effort. J. Anim. Ecol. 1997b;66:827–836. doi:10.2307/5998 [Google Scholar]
- Saino N., Ninni P., Incagli M., Calza S., Sacchi R., Møller A.P. Begging and parental care in relation to offspring need and condition in the barn swallow (Hirundo rustica) Am. Nat. 2000;156:637–649. doi: 10.1086/316996. doi:10.1086/316996 [DOI] [PubMed] [Google Scholar]
- Saino N., Suffritti C., Martinelli R., Rubolini D., Møller A.P. Immune response covaries with corticosterone plasma levels under experimentally stressful conditions in nestling barn swallows (Hirundo rustica) Behav. Ecol. 2003;14:318–325. doi:10.1093/beheco/14.3.318 [Google Scholar]
- Saino N., de Ayala R.M., Martinelli R., Boncoraglio G. Male-biased brood sex ratio depresses average phenotypic quality of barn swallow nestlings under experimentally harsh conditions. Oecologia. 2008;156:441–453. doi: 10.1007/s00442-008-0971-8. doi:10.1007/s00442-008-0971-8 [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life-histories. [Google Scholar]
- von Holst D. The concept of stress and its relevance for animal behavior. In: Møller A.P., Milinski M., Slater P.J.B., editors. Stress and behavior. Academic Press; San Diego, CA: 1998. pp. 1–132. [Google Scholar]
- Whittingham L.A., Dunn P.O., Clotfelter E.D. Parental allocation of food to nestling tree swallows: the influence of nestling behaviour, sex and paternity. Anim. Behav. 2003;65:1203–1210. doi:10.1006/anbe.2003.2178 [Google Scholar]
- Wingfield J.C., Hunt K., Breuner C., Dunlap K., Fowler G.S., Freed L., Lepson J. Environmental stress, field endocrinology, and conservation biology. In: Clemmons J.R., Buchhoz R., editors. Behavioral approaches to conservation in the wild. Cambridge University Press; New York, NY: 1998. pp. 95–131. [Google Scholar]
- Wright J., Leonard M.L. Kluwer; Dordrecht, The Netherlands: 2002. The evolution of begging. [Google Scholar]