Abstract
Formal models have shown that diel variation in female mate searching is likely to have profound influence on daily signalling routines of males. In studies on acoustic communication, the temporal patterns of the receivers' signal evaluation should thus be taken into account when investigating the functions of signalling. In bird species in which diel patterns of signalling differ between males singing to defend a territory or to attract a mate, the diel patterns of mate and territory prospecting are suggested to depend on the sex of the prospector. We simulated newly arriving female nightingales (Luscinia megarhynchos) by translocating radio-tagged females to our study site. The mate-searching females prospected the area mostly at night, visiting several singing males. The timing of female prospecting corresponded to the period of the night when the singing activity of unpaired males was higher than that of paired males. In contrast to females, territory searching males have been shown to prospect territories almost exclusively during the dawn chorus. At dawn, both paired and unpaired males sang at high rates, suggesting that in contrast to nocturnal singing, dawn singing is important to announce territory occupancy to prospecting males. In the nightingale, the sex-specific timing of prospecting corresponded to the differential signalling routines of paired and unpaired males. The temporal patterns in the behaviour of signallers and receivers thus appear to be mutually adapted.
Keywords: signalling routines, timing of prospecting, nocturnal song, dawn chorus
1. Introduction
Almost all animals show behavioural traits that vary depending on the time of day (McNamara et al. 1987). Studies investigating animal behaviour around the clock often reveal surprising temporal flexibility; for example, young reed warblers (Acrocephalus scirpaceus) regularly show nocturnal movements well before migration (Mukhin et al. 2005) and golden hamsters (Mesocricetus auratus) are nocturnal in captivity but diurnal in nature (Gattermann et al. 2008). Most behaviour is energy and time consuming, and the optimal daily routine is thought to be an adaptive compromise (Hutchinson et al. 1993). Behavioural routines have often been studied with an emphasis on external factors such as light, temperature, food availability or predation risk (e.g. Olsson et al. 2000; Thomas & Cuthill 2002; Macleod et al. 2005). However, behaviour frequently occurs in social contexts, and interactions with conspecifics may strongly shape behavioural routines (Davidson & Menaker 2003; Helm et al. 2006). In the context of social interactions, daily routines can be predicted using formal models without necessarily including environmental factors (McNamara et al. 1987).
Social interactions often involve information transfer from signalling individuals to receivers (Bradbury & Vehrencamp 1998). It has been shown that signalling individuals adjust the timing of their signals in order to avoid acoustic interference with other signalling individuals on short temporal scales (Todt & Naguib 2000; Gerhardt & Huber 2002; Brumm 2006). Individuals are also likely to time their signalling to the period when intended receivers are present and ready to collect information. On the receiver side, individuals often gather information by actively prospecting an unfamiliar area or by visiting the territories of conspecifics (Reed et al. 1999). Receivers are expected to show prospecting activity at time periods when relevant information is available (Boulinier et al. 1996; Reed et al. 1999; Piper et al. 2006). Prospecting behaviour that would be independent of the behaviour of the signallers would be costly and inefficient and is therefore not likely to be adaptive (Kondoh & Ide 2003). Thus, patterns in the diel timing of signalling and prospecting are expected to be correlated.
Using formal models, it has been demonstrated that diel variation in female prospecting behaviour is likely to have profound influence on the timing of daily routines in males (Hutchinson et al. 1993). However, few studies investigated female mate searching directly by tracking individuals during the period of primary mate choice (i.e. during the initial selection of a social partner; Bensch & Hasselquist 1992; Draud et al. 2008). Recently, Jacot et al. (2008) reported one of the first studies on daily routines of signalling and information gathering, investigating the diel signalling activity of male field crickets (Gryllus campestris). Most females were captured near the males in the late afternoon, when singing activity of the males was highest and best reflected male nutritional condition. However, such a temporal pattern of capturing success could arise even if female prospecting activity shows no clear diel pattern: males signalling at particularly high rates could simply have attracted more females because the signalling males were more easily detected by the females. It thus remains to be shown whether, during the period of social mate choice, the timing of female prospecting activity correlates with male signalling patterns.
Among the several signal modalities, acoustic communication is particularly suitable to investigate diel variation in the timing of signalling and prospecting, because acoustic signals can be modulated within a short time period and often follow diel rhythms. A well-studied acoustic communication system is bird song, which is mostly used by territorial males, to repel competitors and to attract potential mates (Catchpole & Slater 2008). In some bird species, resource-holding males signal their quality to females mainly at dawn (Otter et al. 1997; Double & Cockburn 2000; Dalziell & Cockburn 2008; Murphy et al. 2008). In other species, dawn singing seems to be relatively unimportant in mate attraction, but males may address potential mates during other times of the day (Staicer 1996; Staicer et al. 1996).
Here, we investigated the association between the timing of male signalling and the timing of sex-specific prospecting in nightingales (Luscinia megarhynchos). Territorial male nightingales sing intensely at dawn, and mostly unpaired males sing also at night but stop nocturnal song after pair formation (Amrhein et al. 2002, 2004a). Nocturnal song was thus suggested to serve attracting females (Amrhein et al. 2002), and dawn singing seems to serve mainly to defend a territory (Amrhein et al. 2004b; Kunc et al. 2005). In an earlier study, it has been shown that non-territorial male nightingales prospect territories during the dawn chorus (Amrhein et al. 2004b). If nocturnal song of unpaired males serves to attract females, we predicted that female nightingales prospect for mates at night. In this study, we thus simulated unpaired females that prospect an unfamiliar area by translocating female nightingales to our study site. We used radio telemetry to observe their mate-searching behaviour during the first 2 days and nights after translocation. We then compared the diel prospecting patterns of females with the results obtained on males (Amrhein et al. 2004b). We found that the timing of prospecting is sex specific in the nightingale and is related to the timing of signalling in paired males and unpaired males.
2. Material and methods
The study took place over 2 years (2007 and 2008) at the Petite Camargue Alsacienne in the Upper Rhine Valley in France, where we had surveyed approximately 50 nightingale territories per year since 1994 (Amrhein et al. 2002). The identity of males and females were controlled by regular mist-netting, and the birds were individually marked with a metal ring and a unique combination of three colour rings. Pairing status of males was further controlled by examining the territories of males for female alarm calls. The first males started singing in their territories on 10 April 2007 and on 11 April 2008, and the first female was captured on 21 April 2007 and 20 April 2008.
(a) Translocation
We captured female nightingales 70 km north of our study site. At the capture site, we monitored singing activity of males by surveying singing males at midnight and in the early morning. When a resident male stopped singing at night, indicating the arrival of a female (Amrhein et al. 2002, 2004a), we placed mist nets in its territory and captured the female within 3 days after the male had stopped nocturnal song. From 21 April to 4 May 2007, we translocated 10 females from the capture site to our study site. We released the females with a transmitter glued to their back feathers at approximately 12.00 Central European Summer Time, within 5 hours of capture. All translocated females were tracked continuously during the first 42 hours at the release site. We used the total stretch of way a female covered per hour as our measure of prospecting activity. In cases in which the location of the females could not always be identified precisely because of a weak signal or because of particularly rapid and lengthy movements, the distance measurements taken during the corresponding hours were omitted from the analyses. The resulting sample sizes for each hour are given in figure 1. Translocated females that eventually settled within the study area were checked at least once within 3 days until the transmitters fell off or stopped signalling after approximately three to four weeks. We used telemetry equipment by Titley Electronics, Australia: three element Yagi antennae, Regal 2000 receivers and LT1 transmitters (equipment mass of 1.0 g=4.6% of the average mass of our subjects). We stopped translocating females as soon as we captured the first female carrying an egg at the capture site. The study plots and the methods used in the present study were the same as in an earlier translocation study on male nightingales (Amrhein et al. 2004b).
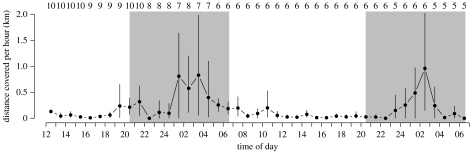
Figure 1.
Prospecting activity of female nightingales. Total distances covered per hour (mean and bootstrapped 95% confidence interval) are given for the 42 hours after translocation of females to the study site. Sample sizes are given at the top of the figure; grey shaded areas indicate nocturnal periods from sunset to sunrise.
(b) Singing activity
Singing activity of males was surveyed in a preliminary study from 2 to 6 May 2007, and during a more extended period from 11 April to 4 May 2008, 1 year after the female translocation experiment. We made nine rounds of inspection per night at our study site. The first round started at sunset (dusk round), and the last round started 75 min before sunrise (dawn round); the seven remaining rounds (N1–N7) were spaced out regularly between the dusk and the dawn round. The exact starting time of the rounds slightly changed from day to day due to the seasonal changes of sunset and sunrise; mean starting times for the rounds are given in figure 3. Rounds lasted between 50 and 65 min and followed a fixed route of 8.5 km length; the direction of the rounds was fixed for a particular night, but the direction was changed from one night to the next. Territories with singing males were excluded from the analyses if males deserted their territories during the study period, or if male song posts were further than 100 m (perpendicular distance) away from our route. We used the date at which we captured the first female at our study site to subdivide the study period into a period prior to female arrival, when all males were unpaired (11–20 April) and a period during female arrival when males started to get paired (21 April to 4 May). For each period (before the female arrival and during female arrival) and for each round (dusk round, N1–N7 and dawn round), we plotted singing activity in the figures as the proportion of rounds a male was recorded to sing. We also subdivided the males into paired and unpaired males (bachelors), depending on whether a male was paired at the end of the study period. Because we used the arrival date of the first female at the study site to define the period of female arrival for all males, several subsequently paired males were still unpaired at the beginning of the period of female arrival.
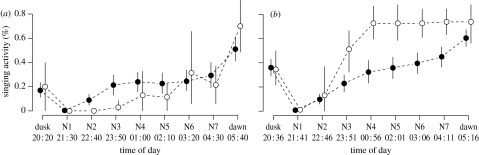
Figure 3.
Singing activity of 31 males as censused during nine rounds per night, starting at sunset and ending at sunrise, (a) prior to female arrival (11 April until 20 April) and (b) during the time of female arrival (21 April until 4 May). Singing activity of a male is expressed as the proportion of rounds it was heard singing (mean and bootstrapped 95% confidence interval). Filled circles indicate males that are paired or will be paired (n=25) and open circles indicate bachelors (n=6). Dusk and dawn rounds started at sunset and 75 min before sunrise, respectively; the remaining rounds (N1–N7) were spaced out regularly between the dusk and dawn round. Starting times of the rounds are given as averages for the respective periods of the breeding season.
(c) Statistical analyses
All statistical analyses were performed using the software R (v. 2.6.1; R Development Core Team 2007). All sample sizes refer to the number of individuals. Descriptive statistics in the text are given as mean±s.d. To account for the repeated sampling of the same individuals and, thus, the non-independence of data points, we used linear mixed-effect models (LMMs) with individual subject fitted as random factor, including individual-specific intercepts and slopes (Gelman & Hill 2007). To investigate temporally changing prospecting activities of females, we fitted the distance covered per hour as the response variable, the individual females as the random factor and the date of translocation and the hour (time of day) as covariates; to account for the daily cycles of prospecting activity, we modelled the hour as a sine-cosine function (Crawley 2007). We started with the full models including all variables, and then removed non-significant terms (Crawley 2007). To investigate the effect of pairing status on daily singing patterns, we used generalized linear mixed models (GLMMs) with a logistic link function (LME4 package; Bates & Sarkar 2006), because the response variable was binary (singing or not singing). We fitted the singing activity as the response variable, the rounds (numbered from 1 to 9; 1=dusk-round, 9=dawn-round) as the covariate, the pairing status (paired male or bachelor) and the seasonal period (before and during female arrival) as fixed factors, and the individual males as the random factor. To test for a possible curvilinear pattern in the nocturnally changing singing activity, we additionally fitted the quadratic term of the covariate round2 to the model. In the figures, we give the mean values of prospecting activity and singing activity with bootstrapped 95% confidence intervals (10 000 iterations; Crawley 2007).
3. Results
(a) Translocation
The prospecting activity of females, as indicated by the total distance covered per hour, showed a distinct diel pattern (LMM, hour: d.f.=2, p<0.001): the females covered short distances during the day, they were stationary in the hour between 22.00 and 23.00, but covered long distances during the second half of the night from approximately 01.00 to approximately 04.00 (figure 1). At dawn, the distances covered per hour dropped to low levels. The length of the distances covered per hour did not change as the season progressed (LMM, date of translocation: d.f.=1, p=0.84). The eight females for which we could determine the exact starting times started nocturnal movements between 23.50 and 03.38, and average starting times were 02.29 (±1.77 h; n=7) in the first night and 00.38 (±1.32 h; n=5) in the second night. The prospecting trips lasted from 13 to 235 min (103.23±76.14 min; n=7). In total, the females covered 0 to 4.71 km (1.12±1.67 km; n=7) during the first night and 0 to 6.17 km (1.49±2.67 km; n=5) during the second night. Note that the average distances covered at night are likely to be underestimated, because hours during which we lost track of the females were omitted from the analyses. Figure 2 shows the prospecting path of a female performed during the second night after translocation, visiting at least six males singing. Each female performed a prospecting trip at least during one of the two nights. Five of the 10 females finally settled in a territory of a male within our study area, three females returned to the capture site and the remaining two females left our study site but were not recorded back at the capture site. From the five females that left our study site, four left during the first night (after having visited several males within our study area) and one during the second night. Of the five females that settled and paired to a male within the study area, three settled in a territory of a male during the first night, one during the second night and one during the third night. After settlement in a male's territory, none of the females were located outside the territory again during our occasional checks. In 2008, at least one translocated female returned to the same territory in our study area in which it settled in 2007.
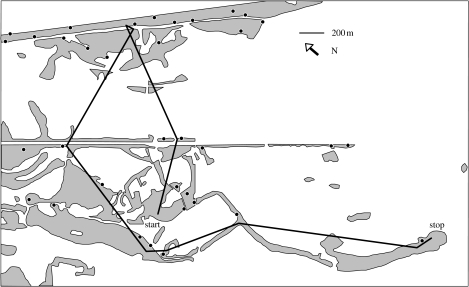
Figure 2.
The prospecting path of a single radio-tracked female in the second night after translocation. Between 23.50 and 03.30, she covered 5.8 km and visited at least six of the territorial males singing that night (black dots), spending between 5 and 35 min in the territories of the males. The female finally settled and paired to the male in the last territory visited that night (stop). Grey areas indicate reed, bushes or woods, white areas are mainly fields and meadows.
(b) Singing activity of paired males and bachelors
To test for an effect of study year on singing activity, we fitted a first GLMM using the data on singing activity of 34 males surveyed during a limited period in 2007 and of 31 males from the corresponding period in 2008. The changes of singing activity across our nocturnal rounds seemed not to differ between the 2 years (GLMM, interaction round×year: d.f.=1, p=0.634; interaction round2×year: d.f.=1, p=0.723), and the measures of average singing activity across nocturnal rounds were strongly correlated between years (Pearson's correlation, paired males: r=0.875, t=4.773, d.f.=7, p=0.002; bachelors: r=0.950, t=8.087, d.f.=7, p<0.001). Therefore, in all further analyses we used the data of 2008 only, when singing activity was surveyed continuously during the entire study period.
In 2008, we monitored the singing activity of 31 males that arrived at the study site on average on 16 April (±4.2 days). Later during the breeding season, 25 of those males were paired and six were bachelors. Male singing activity followed a distinct temporal pattern between dusk and dawn: singing activity dropped to zero in the first round after dusk, and thereafter increased continuously until dawn (figure 3). This general development of singing activity over the night was not found to differ between the period before and during female arrival (GLMM, interaction round×period: d.f.=1, p=0.132). However, singing activity differed between paired males and bachelors depending on the period before or during female arrival (GLMM, interaction period×status: d.f.=1, p<0.001) and on the time of night (GLMM, interaction round×status: d.f.=1, p<0.001). Before female arrival, subsequently paired males sang more than bachelors during the second and third nocturnal rounds (N2 and N3, as indicated by non-overlapping 95% CI in figure 3a). During the period of female arrival, during the rounds from about midnight (N3) until the last nocturnal round (N7; figure 3b), bachelors sang more than paired males (note that several subsequently paired males were still unpaired at the beginning of the period of female arrival, which explains why there was nocturnal singing activity of paired males during that period). No difference in singing activity between paired males and bachelors could be detected for the dusk or dawn rounds (figure 3).
4. Discussion
Translocated female nightingales covered the longest distances between 01.00 and 04.00 in the night. This is in contrast to the temporal pattern of territory prospecting in non-territorial male nightingales that made significant movements only during the dawn chorus in the 1 or 2 hours before sunrise, as shown in an earlier study using the same methods and the same study populations (Amrhein et al. 2004b). This sex-specific timing of prospecting corresponded to the singing activity of territorial males: females searching for mates showed prospecting activity mostly at night when mainly bachelors were singing, while territory searching males showed prospecting activity only at dawn when all territorial males were singing intensely, and vacant territories should become apparent (Amrhein et al. 2004b). Thus, in the nightingale, the sex-specific patterns in the diel timing of prospecting and the patterns in the diel timing of male signalling were correlated.
State-dependent models of daily singing routines have been used to simulate diel fluctuations in the birds' energy reserves (McNamara et al. 2001). These models accurately predicted the typical temperate zone passerine singing routines. However, male singing routines have also been suggested to depend on female behaviour if the male pairing propensity (i.e. the probability that a singing male pairs in a particular time interval) varies with time of day (Hutchinson et al. 1993). Therefore, the optimal singing routine of males may be strongly influenced by the temporally changing availability of females. In nightingales, unpaired males that are singing at night when females are prospecting for mates most likely have a higher pairing probability than unpaired males that would be singing exclusively at dawn. As predicted by the formal models (Hutchinson et al. 1993), this should shape the optimal singing routine of signalling males and may explain the occurrence of nocturnal song in the nightingale.
Nocturnal song of otherwise diurnal species is comparatively rare in western Palaearctic songbirds (Amrhein et al. 2002); in those species, nocturnal prospecting of mate-searching females could potentially explain nocturnal singing of the males. However, nocturnal prospecting by females seems to be adaptive only if there is nocturnal song by males. It still remains unclear whether nocturnal singing is a cause for or an effect of nocturnal prospecting by females, and how nocturnal song evolved in the first place. A possible scenario is that in nocturnally migrating species such as the nightingale, males that happened to sing at night had a higher pairing success because they more readily attracted females arriving from migration. This could have led to the evolution of nocturnal song in males, and of nocturnal prospecting in females. Owing to the mutual dependence of the timing of signalling and of the timing of signal evaluation by receivers, formal models of daily routines in both behaviours would need to include game theoretic approaches (McNamara et al. 2001).
Females usually base their choice of mate on reliable signals indicating male quality (Andersson 1994). Particularly in monogamous species, females are likely to evaluate males also with regard to their pairing status (Staicer 1996), and females are expected to trade between male quality and pairing status (Slagsvold & Drevon 1999). By prospecting at night, female nightingales may be able to infer both the pairing status and the quality of singing males. Clearly, nocturnal song is a good indicator of male pairing status, since it is mainly bachelors that sing at night (Amrhein et al. 2002, 2004a). However, if nocturnal song is costly in the nightingale (Thomas 2002), nocturnal singing may also serve as an honest signal of male quality. In this study, at the beginning of the season before the arrival of females, the singing activity of males that later in the breeding season successfully attracted a female was higher around midnight (rounds N2 and N3), as compared to the singing activity of males that later could not attract a female. An early start of nocturnal singing in the first hours of the night could thus indicate male quality. In contrast to the first hours of the night, the singing activity at dawn or dusk did not predict future pairing status of males. Thus, females may not base their choice of mate on dawn or dusk singing in the nightingale. This is in line with an increasing number of studies providing evidence that a main function of dawn singing is territory defence in several songbirds (Slagsvold et al. 1994; Liu 2004; Kunc et al. 2005; Amrhein & Erne 2006).
Our study showed that sex-specific timing of prospecting for territories or mates corresponded to the differential singing activity of paired and unpaired males in the nightingale. The temporal patterns in the behaviour of signallers and receivers thus appear to be mutually adapted.
Acknowledgments
Permission for the experiment was given by the Centre de Recherches sur la Biologie des Populations d'Oiseaux, Paris.
We thank Erica Van Rooji, Loïc Pillet, Béatrice Bouton, Rébecca Flück, Keri Langridge and Camille Rueff for assistance in the field. Fraenzi Korner-Nievergelt gave valuable comments during data analysis. The research was funded by the Swiss Association Pro Petite Camargue Alsacienne, the Treubel-Fonds, the Basler Stiftung für biologische Forschung, the Ornithologische Gesellschaft Basel and the Deutsche Forschungsgemeinschaft (NA 335/4 and 8).
References
- Amrhein V., Erne N. Dawn singing reflects past territorial challenges in the winter wren. Anim. Behav. 2006;71:1075–1080. doi:10.1016/j.anbehav.2005.07.023 [Google Scholar]
- Amrhein V., Korner P., Naguib M. Nocturnal and diurnal singing activity in the nightingale: correlations with mating status and breeding cycle. Anim. Behav. 2002;64:939–944. doi:10.1006/anbe.2002.1974 [Google Scholar]
- Amrhein V., Kunc H.P., Naguib M. Seasonal patterns of singing activity vary with time of day in the nightingale (Luscinia megarhynchos) Auk. 2004a;121:110–117. doi:10.1642/0004-8038(2004)121[0110:SPOSAV]2.0.CO;2 [Google Scholar]
- Amrhein V., Kunc H.P., Naguib M. Non-territorial nightingales prospect territories during the dawn chorus. Proc. R. Soc. Lond. B. 2004b;271:S167–S169. doi: 10.1098/rsbl.2003.0133. doi:10.1098/rsbl.2003.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, M. 1994 Sexual selection Monographs in behavior and ecology. Princeton, NJ: Princeton University Press.
- Bates, D. & Sarkar, D. 2006 LME4: linear mixed-effects models using S4 classes. R package, v. 0.9975-10. See http://cran.r-project.org
- Bensch S., Hasselquist D. Evidence for active female choice in a polygynous warbler. Anim. Behav. 1992;44:301–311. doi:10.1016/0003-3472(92)90036-9 [Google Scholar]
- Boulinier T., Danchin E., Monnat J.Y., Doutrelant C., Cadiou B. Timing of prospecting and the value of information in a colonial breeding bird. J. Avian Biol. 1996;27:252–256. doi:10.2307/3677230 [Google Scholar]
- Bradbury J.W., Vehrencamp S.L. Sinauer; Sunderland, MA: 1998. Principles of animal communication. [Google Scholar]
- Brumm H. Signalling through acoustic windows: nightingales avoid interspecific competition by short-term adjustment of song timing. J. Comp. Physiol. A. 2006;192:1279–1285. doi: 10.1007/s00359-006-0158-x. doi:10.1007/s00359-006-0158-x [DOI] [PubMed] [Google Scholar]
- Catchpole C.K., Slater P.J.B. Cambridge University Press; Cambridge, UK: 2008. Bird song. [Google Scholar]
- Crawley M. Wiley; London, UK: 2007. The R book. [Google Scholar]
- Dalziell A.H., Cockburn A. Dawn song in superb fairy-wrens: a bird that seeks extrapair copulations during the dawn chorus. Anim. Behav. 2008;75:489–500. doi:10.1016/j.anbehav.2007.05.014 [Google Scholar]
- Davidson A.J., Menaker M. Birds of a feather clock together—sometimes: social synchronization of circadian rhythms. Curr. Opin. Neurobiol. 2003;13:765–769. doi: 10.1016/j.conb.2003.10.011. doi:10.1016/j.conb.2003.10.011 [DOI] [PubMed] [Google Scholar]
- Double M., Cockburn A. Pre-dawn infidelity: females control extra-pair mating in superb fairy-wrens. Proc. R. Soc. Lond. B. 2000;267:465–470. doi: 10.1098/rspb.2000.1023. doi:10.1098/rspb.2000.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draud M.J., Verga J.N., Haley M.P., Itzkowitz M. Mate inspection patterns in the female beaugregory damselfish (Stegastes leucostictus) Acta Ethol. 2008;11:6–15. doi:10.1007/s10211-007-0036-8 [Google Scholar]
- Gattermann R., et al. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol. Lett. 2008;4:253–255. doi: 10.1098/rsbl.2008.0066. doi:10.1098/rsbl.2008.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A., Hill J. Cambridge University Press; Cambride, UK: 2007. Data analysis using regression and multilevel/hierarchical models. Analytical methods for social research. [Google Scholar]
- Gerhardt H.C., Huber F. University of Chicago Press; Chicago, IL: 2002. Acoustic communication in insects and anurans: common problems and diverse solutions. [Google Scholar]
- Helm B., Piersma T., Van der Jeugd H. Sociable schedules: interplay between avian seasonal and social behaviour. Anim. Behav. 2006;72:245–262. doi:10.1016/j.anbehav.2005.12.007 [Google Scholar]
- Hutchinson J.M.C., McNamara J.M., Cuthill I.C. Song, sexual selection, starvation and strategic handicaps. Anim. Behav. 1993;45:1153–1177. doi:10.1006/anbe.1993.1139 [Google Scholar]
- Jacot A., Scheuber H., Holzer B., Otti O., Brinkhof M.W.G. Diel variation in a dynamic sexual display and its association with female mate-searching behaviour. Proc. R. Soc. B. 2008;275:579–585. doi: 10.1098/rspb.2007.1500. doi:10.1098/rspb.2007.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh M., Ide J.Y. Evolution of periodicity in insect mate-seeking behaviour: a male–female coevolutionary game model. Anim. Behav. 2003;65:1013–1020. doi:10.1006/anbe.2003.2147 [Google Scholar]
- Kunc H.P., Amrhein V., Naguib M. Seasonal variation in dawn song characteristics in the common nightingale. Anim. Behav. 2005;70:1265–1271. doi:10.1016/j.anbehav.2005.02.010 [Google Scholar]
- Liu W.C. The effect of neighbours and females on dawn and daytime singing behaviours by male chipping sparrows. Anim. Behav. 2004;68:39–44. doi:10.1016/j.anbehav.2003.06.022 [Google Scholar]
- Macleod R., Gosler A.G., Cresswell W. Diurnal mass gain strategies and perceived predation risk in the great tit Parus major. J. Anim. Ecol. 2005;74:956–964. doi:10.1111/j.1365-2656.2005.00993.x [Google Scholar]
- McNamara J.M., Mace R.H., Houston A.I. Optimal daily routines of singing and foraging in a bird singing to attract a mate. Behav. Ecol. Sociobiol. 1987;20:399–405. doi:10.1007/BF00302982 [Google Scholar]
- McNamara J.M., Houston A.I., Collins E.J. Optimality models in behavioral biology. SIAM Rev. 2001;43:413–466. doi:10.1137/S0036144500385263 [Google Scholar]
- Mukhin A., Kosarev V., Ktitorov P. Nocturnal life of young songbirds well before migration. Proc. R. Soc. B. 2005;272:1535–1539. doi: 10.1098/rspb.2005.3120. doi:10.1098/rspb.2005.3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.T., Sexton K., Dolan A.C., Redmond L.J. Dawn song of the eastern kingbird: an honest signal of male quality? Anim. Behav. 2008;75:1075–1084. doi:10.1016/j.anbehav.2007.08.020 [Google Scholar]
- Olsson O., Wiktander U., Nilsson S.G. Daily foraging routines and feeding effort of a small bird feeding on a predictable resource. Proc. R. Soc. Lond. B. 2000;267:1457–1461. doi: 10.1098/rspb.2000.1164. doi:10.1098/rspb.2000.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter K., Chruszcz B., Ratcliffe L. Honest advertisement and song output during the dawn chorus of black-capped chickadees. Behav. Ecol. 1997;8:167–173. doi:10.1093/beheco/8.2.167 [Google Scholar]
- Piper W.H., Walcott C., Mager J.N., Perala M., Tischler K.B., Harrington E., Turcotte A.J., Schwabenlander M., Banfield N. Prospecting in a solitary breeder: chick production elicits territorial intrusions in common loons. Behav. Ecol. 2006;17:881–888. doi:10.1093/beheco/arl021 [Google Scholar]
- R Development Core Team 2007 R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing.
- Reed J., Boulinier R., Danchin E., Oring L. Informed dispersal: prospecting by birds for breeding sites. Curr. Ornithol. 1999;15:189–259. [Google Scholar]
- Slagsvold T., Drevon T. Female pied flycatchers trade between male quality and mating status in mate choice. Proc. R. Soc. Lond. B. 1999;266:917–921. doi:10.1098/rspb.1999.0724 [Google Scholar]
- Slagsvold T., Dale S., Saetre G.P. Dawn singing in the great tit (Parus major): mate attraction, mate guarding or teritorial defense. Behaviour. 1994;131:115–138. doi:10.1163/156853994X00244 [Google Scholar]
- Staicer C.A. Honest advertisement of pairing status: evidence from a tropical resident wood-warbler. Anim. Behav. 1996;51:375–390. doi:10.1006/anbe.1996.0036 [Google Scholar]
- Staicer C.A., Spector D.A., Horn A.G. The dawn chorus and other diel patterns in acoustic signalling. In: Kroodsma D.E., Miller E.H., editors. Ecology and evolution of acoustic communication in birds. Cornell University Press; Ithaca, NY: 1996. pp. 426–453. [Google Scholar]
- Thomas R.J. The costs of singing in nightingales. Anim. Behav. 2002;63:959–966. doi:10.1006/anbe.2001.1969 [Google Scholar]
- Thomas R.J., Cuthill I.C. Body mass regulation and the daily singing routines of European robins. Anim. Behav. 2002;63:285–292. doi:10.1006/anbe.2001.1926 [Google Scholar]
- Todt D., Naguib M. Vocal interactions in birds: the use of song as a model in communication. Adv. Study Behav. 2000;29:247–296. doi:10.1016/S0065-3454(08)60107-2 [Google Scholar]