Abstract
Within the Arthropoda, morphologies of neurons, the organization of neurons within neuropils and the occurrence of neuropils can be highly conserved and provide robust characters for phylogenetic analyses. The present paper reviews some features of insect and crustacean brains that speak against an entomostracan origin of the insects, contrary to received opinion. Neural organization in brain centres, comprising olfactory pathways, optic lobes and a central neuropil that is thought to play a cardinal role in multi-joint movement, support affinities between insects and malacostracan crustaceans.
Keywords: brain organization, malacostracan, insect, evolution, olfactory lobe
1. Introduction
In 1916, Nils Holmgren published what is now recognized as the first serious consideration of the phylogenetic relationships among the Arthropoda, based not upon their external morphology but on shared characters of their nervous systems. Holmgren declared the arthropods to be monophyletic, as did his pupil Hanström (1926). In so doing, these Swedish researchers founded an area of study that some today refer to as neurophylogeny, a catch-all term that is used both for conventional neuroanatomical comparisons and for parsimony analysis based on discrete neural characters. Both of these Swedish scientists were comparative anatomists, and one may assume that their focus on the nervous system was the recognition that through geological time brain organization shows great stability. An example of this is seen in scorpions that have been geographically isolated for 225 Myr, yet they show near-identical brain organization whether they come from Saharan Africa or Arizona's Sonoran Desert. Mutations of higher brain circuits rarely confer a selective advantage. A mutation in the fruitfly that causes failure of midline fusion of a brain region called the central complex results in an animal that walks efficiently in a straight line but is unable to negotiate curves (Strauss 2002). In nature, the life of such a fly would be brief indeed. However, there are counter-examples: a comparable but evolutionarily acquired modification of the central complex typifies the brains of aquatic Hemiptera that employ rowing actions for locomotion but which are inept at walking on land (Strausfeld 1999).
The reliability of neural characters for multi-character analyses has been discussed in depth by Buschbeck (2000). Her conclusion that these maintain phylogenetic information derives from a study of neuron shapes and relationships within a retinotopic neuropil, called the medulla, in the optic lobes of dipterous insects. Across a wide range of species, neuron shapes, dispositions and relationships are highly conserved, whereas at a deeper level of the system, in the lobula complex, interspecific differences can be ascribed to taxon-specific behaviours (Buschbeck & Strausfeld 1996, 1997).
Neurophylogeny must also consider the phenomenon of convergence. On an interphyletic scale, a much-cited example is that of the olfactory lobes (for a review, see Strausfeld & Hildebrand (1999)). Receptor mechanisms have clearly evolved independently in insects and chordates, but, in both, olfactory receptor axons sort out to discrete glomeruli according to their odorant receptor identities (review: Benton 2006). Axons establish connections within a network that in vertebrates and insects involves comparable cell morphologies and synaptic connections (Christensen & White 2000; Eisthen 2002). Might such profound functional and structural similarities advise against using neural characters for questioning evolutionary relationships? Two considerations may be helpful. First, constraints imposed by the physical and chemical characteristics of ancient biotopes have led to the evolution of panphyletic components of the nervous system. Many authors make the point that from the fundamental components of the first nervous systems have arisen common computational elements: mapped representation of sensory fields; circuits that increase the signal-to-noise ratio; enhancement of spatial resolution; and the detection of coincidence (see Farris 2008). Such characters alone are worthless for establishing taxonomic relationships. Second, 540 Myr of experimentation has resulted in the optimization of brain regions in organisms belonging to divergent evolutionary trajectories. The olfactory system, cited above, is just one example. Another is the convergence of insect, vertebrate and cephalopod visual systems, recognized by Ramon y Cajal, who famously remarked in his autobiography that on seeing such similarities his faith in Darwinism was almost shattered, but after brief reflection, so he claimed, his confidence in evolution was restored (Cajal 1937).
These examples give pause for thought: if profound similarities occur across phyla, then surely they occur at higher resolution and at a smaller scale at the intraphyletic level. In these levels, however, neural characters can provide reliable indicators of relationships. Illustrative of this is the recent demonstration that it is the brain organization of the fabulously named Godzilliognomus frondosus, a remipede long assumed to be basal to the Crustacea, that reveals a modified decapod (Fanenbruck & Harzsch 2005). Furthermore, a phylogeny based on neural characters will be robust when its considered taxa, though not necessarily all of them, have the same degree of relatedness on the basis of shared anatomical and molecular characters.
Thus far, one published neural phylogeny for the Arthropoda shows such resilience while also challenging received opinion regarding the position of the Onychophora (Strausfeld et al. 2006). Significantly, this study generally agrees with molecular phylogenies in demonstrating that entomostracans (Cephalocarida, Maxillopoda and Branchiopoda: autapomorphies include ring-shaped abdominal segments lacking limbs; see Walossek 1999) and malacostracans (Leptostraca+Eumalacostraca: autapomorphies include 14 thoracic segments with limbs; see Olesen & Walossek 2000) belong to a clade called the Tetraconata (Dohle 2001) that includes the hexapods (Schultz & Regier 2000). A cladistic analysis based on optic lobe anatomy of the Tetraconata goes further in inferring a malacostracan ancestry of the insects (Strausfeld 2005). This contests molecular studies that place the insects closer to the entomostracans (Regier et al. 2005; Glenner et al. 2006; Mallatt & Giribet 2006). Given that so many datasets are still incomplete, such differences of interpretation put to the test whether neural characters can provide insights, or at least added depth, to inferences that originate from molecular studies alone.
Here, I review three systems of the arthropod brain that are relevant to such proof of principle. All three are independent of each other. Two systems serve the sensory modalities of olfaction and vision. The third system is a midline neuropil common to the Tetraconata.
2. Olfactory lobes and higher olfactory centres
The second head segment of crustaceans and insects is similarly equipped with a pair of uniramous appendages (Boxhall 2004). For crustaceans, these are known as the ‘antennules’ (or first antennae), and for the insects they are referred to as the ‘antennae’; an unfortunate appellation because the second pair of head appendages in crustaceans, which are absent in insects, are also referred to as the antennae (or second antennae). In malacostracan crustaceans, olfactory receptor neurons in specialized sensilla called aesthetascs, situated in the last segment of each antennule, supply a pair of lobes (the ‘antennular’ or olfactory lobe) situated in the brain's second segment, the deutocerebrum (Schmidt & Ache 1992). The second antennae supply numerous mechanoreceptor axons mainly to a pair of striate and columnar neuropils belonging to the third cerebral segment, the tritocerebrum (figure 1). In insects, the single pair of antennules (the antennae) provides mechanosensory axons from their first and second segments (the scape and pedicel) to comparable striate and columnar neuropils in the tritocerebrum. Sensilla on the distal segment of each antenna (the flagellum) are equipped with olfactory receptor neurons that supply axons to the paired olfactory lobes (the ‘antennal’ lobes) in the deutocerebrum (figure 1).
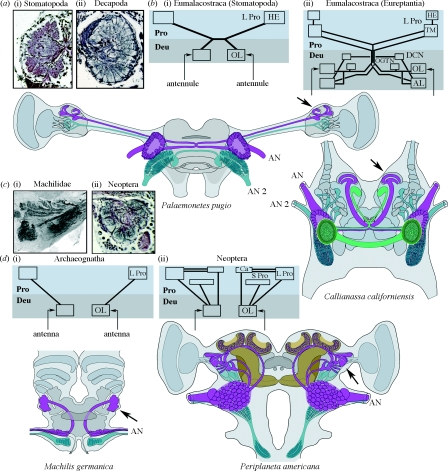
Figure 1.
Comparisons of olfactory centres and pathways in eumalacostracans and insects. Outlines of two eumalacostracan brains (the glass shrimp Palaemonetes pugio and the eureptantian Callianassa californiensis) show the olfactory lobes (OL) and their central projections (carmine) supplied by the antennules (AN), and mechanosensory neuropils of the tritocerebrum (blue-green) supplied by the second antennae (AN 2). Callianassa californiensis is shown with its accessory lobes (AL, bright green). (a) A basal eumalacostracan olfactory lobe ((i) the stomatopod Pseudosquilla ciliata) demonstrates islet-like glomeruli. (ii) A typical decapod olfactory lobe comprising columnar subunits (P. pugio). (b) The distribution of outputs from (i) stomatopod and (ii) eureptantian OL is summarized. The brain's deuto- and protocerebral segments (Deu, Pro) are indicated in two shades of grey. Insect olfactory pathways are shown in brain outlines of the archaeognathan Machilis germanica and the neopteran Periplaneta americana. In the latter, the mushroom bodies are coloured brown. Inputs to both olfactory and mechanosensory neuropils are provided by the antennules. Mechanosensory axons (blue-green) target centres in the tritocerebrum. (c) Islet-like glomeruli typifying the OL of monocondylic and dicondylic insects ((i) M. germanica and (ii) Musca domestica). (d) The distribution of outputs from the OL of (i) archaeognathans and (ii) neopteran insects is summarized. Outputs from reptantian OL are more elaborate than those of basal eumalacostracans: in addition to projections to the lateral protocerebrum (L Pro), they target centres in the deutocerebrum, including the AL (see Sandeman et al. 1992; Sullivan & Beltz 2005). A similar trend typifies olfactory pathways in insects: in the Archaeognatha, OL are connected to the lateral protocerebrum alone; in the Neoptera, outputs from the OL additionally supply several protocerebral neuropils, including the mushroom body calyces (Ca). However, in all eumalacostracans and insects, the primary output from the OL targets the lateral protocerebrum (indicated at brain outlines by arrows). DCN, deutocerebral commissure neuropil; OGTN, olfactory globular tract neuropil; HE, hemiellipsoid body; TM, terminal medulla of the protocerebrum; S Pro, superior protocerebrum.
Olfactory lobes occur within the deutocerebra of insects and malacostracan crustaceans, but they have not been identified in entomostracan crustaceans. Typically, olfactory lobes are divided into discrete subunits, columns or glomeruli, a feature that has led to the suggestion that the lobes of malacostracans and insects are homologous (Schachtner et al. 2005). However, differences in their neuronal organization could also suggest independent origins, and thus convergent evolution. For example, in most insects (other than anosmic species where there has been a secondary loss of the antennal lobes; Strausfeld et al. 2009), each glomerular subunit is an islet of neuropil, bordered by glial processes, that is supplied by its own identically coded olfactory receptor axons (Fishilevich & Vosshall 2005). Other than stomatopods, in eumalacostracan olfactory lobes, olfactory receptor neurons arborize to one or more of many dozens of contiguous columnar subunits (Schmidt & Ache 1992); however, there is no evidence that axons converge from each type of odorant receptor neuron to a specific column of the lobe.
In most insects, each glomerulus is visited by the confined dendritic trees of between two and eight relay neurons, called uniglomerular projection neurons. There is also a small population of projection neurons, the dendrites of which visit several glomeruli. By contrast, there are no reports of eumalacostracans having uniglomerular projection neurons. Rather, multiglomerular projection neurons are the norm; their dendrites visit many if not all of the columnar subunits (Schmidt & Ache 1997). A further distinction between the eumalacostracan and insect olfactory systems is revealed by the passage of axons from their olfactory lobes into the protocerebrum, the most rostral segment of the brain. In eumalacostracans, the axons of projection neurons bifurcate in the protocerebrum, each tributary extending laterally out to circumscribed neuropils flanking the protocerebrum (figure 1). Thus, second-order olfactory centres in the protocerebrum are provided with information from both antennules (Sullivan & Beltz 2001, 2005). In eureptantian decapods, but not in other eumalacostracans, the olfactory lobes are also connected to a satellite centre in the deutocerebrum, called the accessory lobe, as well as to several other discrete neuropils in that segment (Sandeman et al. 1992). This organization differs from that in pterygote insects, where, depending on species, the olfactory lobe provide as many as five ascending tracts to the protocerebrum (Kirschner et al. 2006; Lai et al. 2008). Projection neurons supply protocerebral centres on the same side of the brain as the lobe from which they originate (figure 1). One or more of these tracts supplies collateral input to the distal neuropil (calyces) of the paired mushroom bodies (Homberg et al. 1988), centres defined by many thousands of parallel fibres, which have been implicated in a number of higher functions including olfactory learning and memory (Heisenberg 2003).
It would appear, then, that the malacostracan and insect olfactory lobes are similar insofar as both serve the homologous pair of appendages, the antennules, equipped with olfactory receptor neurons. In both eumalacostracans and insects, the lobes supply secondary olfactory neuropils in the lateral protocerebrum. However, despite the distinctions outlined above, the olfactory system of one group of insects suggests close affinities to basal Eumalacostraca. This group is the Archaeognatha, flightless ‘bristletails’ that are considered Mid-Devonian relics (Labandeira et al. 1988) and whose mandibles have only one point of articulation, thus monocondylic, with the head capsule. Today, this group is represented by four families, the Machilidae providing the most species. Although machilids have glomerular olfactory lobes, they entirely lack mushroom bodies (figure 1). Projection neurons from their olfactory lobes extend out to the lateral protocerebrum where they provide an extensive volume of layered neuropil, the architecture of which is reminiscent of protocerebral olfactory neuropils (the terminal medulla and hemiellipsoid bodies) of eumalacostracan olfactory systems (Sullivan & Beltz 2001; Harzsch & Hansson 2008). Another similarity between insect and malacostracan olfactory systems is that in stomatopod crustaceans, which are classified as basal eumalacostracans (Abele 1991), the antennular lobes comprise discrete glomeruli, not columns. However, it is not yet known whether different molecular species of olfactory receptor neurons sort out to these glomeruli, as occurs in insects. Projection neurons arising from stomatopod glomeruli predominately terminate in the hemiellipsoid body (Sullivan & Beltz 2001). There is no evidence, either from selective impregnation (the Golgi method) or labelling with antisera, that the hemiellipsoid bodies are structurally equivalent to insect mushroom bodies. Would there be such a centre in a eumalacostracan, this might be apparent in species where protocerebral neuropils are condensed in the head proper, with all parts in proximity. Such a brain is provided by the reptantian Callianassa californiensis, or ghost shrimp, which lacks eyestalks. Projection neurons from the olfactory lobes supply a discrete neuropil in the lateral protocerebrum (figure 1), but as in the machilids there is no evidence for a neuropil remotely similar to a mushroom body.
In summary, the absence of olfactory lobes in entomostracans, but their presence in basal insects and eumalacostracans connected to discrete neuropils of the lateral protocerebrum, suggests closer affinities of insects to the Malacostraca than to the Entomostraca (sensu Walossek); that is, if it is assumed that olfactory lobes have not evolved convergently.
3. Unpaired midline neuropils
The absence of mushroom bodies in malacostracans and archaeognathans, and the projections of olfactory relays to comparable areas of their protocerebra, are alone insufficient to advocate a malacostracan origin of the insects. However, other parts of the brain suggest that archaeognathan and malacostracans indeed share common organization, independent of their olfactory systems.
The protocerebrum of dicondylic insects (Zygentoma+Palaeoptera+Neoptera) is equipped with a prominent neuropil at the midline known as the ‘central body’. This is one component of a wider system of interconnected protocerebral neuropils called the ‘central complex’. The central body consists of two contiguous elements, the fan-shaped body and, immediately caudal to it, the ellipsoid body (figure 2). In the Palaeoptera and Neoptera, these are partitioned into mirror symmetric assemblages of 16 folia-like modules. These receive a stereotypic organization of interweaving axons that originate from a rostral midline neuropil called the protocerebral bridge (Williams 1975). The three midline neuropils are connected to other paired regions of the protocerebrum from where they receive their inputs or to where they provide outputs. Present evidence supports the notion that the central body and the ellipsoid body contain representations of the visual and mechanosensory surround and that one of their functions is to plan and mediate multi-joint limb movements, such as directional walking or song production (Martin et al. 1999; Heinrich et al. 2001; Heinze & Homberg 2007). Disruptions of these centres, either through surgical or genetic lesion, result in ataxic motor defects much as do lesions of the mammalian cerebellum (Strauss 2002; Morton & Bastian 2007; Ridgel et al. 2007).
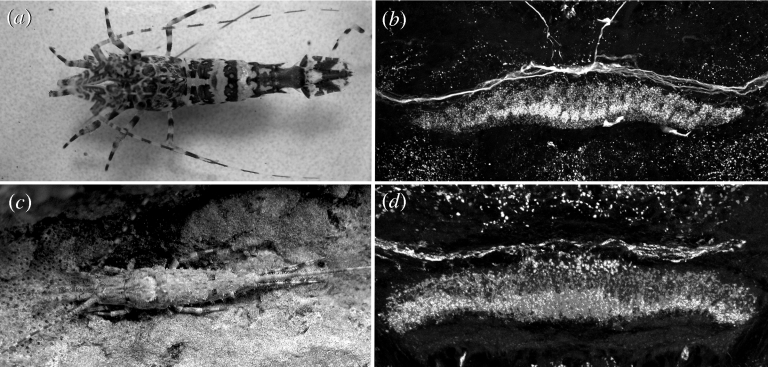
Figure 2.
Midline neuropils of the central complex of pterygote insects, exemplified by (a,c,d) the odonate Pachydiplax longipennis and (b,e) the phasmatid Extatosoma tiaratum. Note the modular architecture of the protocerebral bridge (PB) and central body (CB), defined both by their (c,d) intrinsic neurons and (e) efferents.
Malacostracan and entomostracan crustaceans also possess a midline central body, though this is much less prominent than in dicondylic insects. In entomostracans, this is a small reniform centre, in which systems of tangential neurons intermingle and are connected laterally to a pair of satellite neuropils. By contrast, the eumalacostracan midline neuropil is a robust spindle-shaped centre that is bilayered (Loesel et al. 2002). It is connected to satellite neuropils on either side, some of which have been suggested to correspond to centres associated with the central complex of pterygote insects (Uttig et al. 2000). Whereas, in the Palaeoptera and Neoptera, the three midline neuropils of the protocerebral bridge, fan-shaped body and ellipsoid body are characteristically divided into discrete folia (Williams et al. 2005; Boyan et al. 2008), there are no obvious folia in the central complexes of most malacostracans, although these centres are connected rostrally to a small girder-like neuropil that, because of its location, may be equivalent to the palaeopteran/neopteran protocerebral bridge. The modular and elaborately multistratified central complexes of insects (figure 2) are distinct from the spindle-shaped central body of malacostracan crustaceans (figure 3a,b). However, there is again an exception. With regard to its overall shape and internal organization, the central complex of the Archaeognatha is almost indistinguishable from that of a decapod crustacean (figure 3c,d), suggesting that this part of the Machilidae brain may have changed little from that of a malacostracan-like ancestor, unless such similarities are another instance of convergent evolution. The question arises whether the elaboration of the central complex in the Palaeoptera and Neoptera, with additional components such as the noduli, discrete modules and the elaboration of the protocerebral bridge, are evolutionary ‘add-ons’ that reflect the more intricate motor repertoires demanded by life on land. Indeed, some support for this is suggested by the central complexes of agile shore crabs, such as Hemigrapsus oregonensis, and littoral isopods, such as Ligia occidentalis, which are distinctly modular (Loesel et al. 2002). Even in the Zygentoma, apterygote dicondylic insects and a sister group of the pterygotes (Grimaldi & Engel 2005), the organization of the central complex shows traits more similar to those of pterygotes than malacostracans (Loesel et al. 2002).
Figure 3.
Comparable organization of the central body of (a,b) the caridid decapod Lebbeus groenlandicus and (c,d) the archaeognathan M. germanica. In (b,d), the central body is bilayered and has an isomorphic organization across its spindle-like extent.
4. Visual neuropils and chiasmata
Shaw & Varney (1999) established that in the bristletail Petrobius, a species of Archaeognatha, haemolymph has direct access to the compound eye. This lack of a blood–retina barrier is typical of crustaceans but not of dicondylic insects. While Shaw & Varney's (1999) study is an intriguing inference for a crustacean–insect relationship, it leaves open whether insects might be closer to the Malacostraca than to the Entomostraca. Nonetheless, an extensive dataset pertaining to deeper levels of the visual system supports a malacostracan origin.
The suggestion that insects and malacostracan crustaceans are sister groups is not new. Recent proponents (Osorio & Bacon 1994) emphasize similarities between optic lobe neuropils of these two groups whose compound eyes are served by four nested retinotopic neuropils. Three of these are linked by successive chiasmata, whereas the fourth receives a system of uncrossed fibres. The first chiasma horizontally reverses in the second optic neuropil (the medulla) the order of retinotopic columns in the first optic neuropil (the lamina). The second chiasma carries retinotopic neurons to a third neuropil (the lobula) where the horizontal order of columns is again reversed. By contrast, entomostracan crustaceans possess just two retinotopic neuropils: a lamina that is connected by uncrossed axons to a tectum-like neuropil linked to the brain's protocerebrum by systems of large diameter output neurons (figure 4). Across insects and all crustaceans, the lamina has a comparable retinotopic organization of photoreceptor endings and second-order relay neurons, the monopolar cells (Strausfeld & Nässel 1980). However, beneath this level, the optic lobes of malacostracans and insects share multiple characters that are absent from entomostracans. Prominent among these are the chiasmata, medulla and lobula (figure 4). Golgi impregnations show that many morphological types of neurons in the medulla of malacostracans have the same shapes and relative dispositions as in insects (Strausfeld & Nässel 1980).
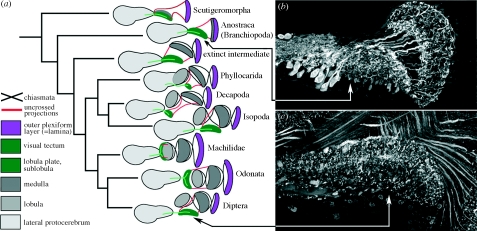
Figure 4.
(a) Four nested optic lobe neuropils (lamina, medulla, lobula and lobula plate) and two successive chiasmata are shared by malacostracan and insect optic lobes. (b) Entomostracans possess only a lamina and a tectum, linked by uncrossed fibres (from Sinakevitch et al. 2003). An intermediate morphology that consists of a second plexiform layer (the medulla) but still lacking a lobula has not been seen in any extant taxon other than in the secondarily reduced visual system of the Zygentoma (Strausfeld 2005). (c) The homologue of the entomostracan tectum in malacostracans and insects is the lobula plate, here from the fly Phaenicia (from Sinakevitch et al. 2003), which receives uncrossed axons from the inner face of the medulla and from the lobula.
Malacostracans and insects possess in common one retinotopic neuropil that receives uncrossed axons. This neuropil lies deep in the optic lobes and is supplied by axons from the inner surface of the medulla. Why does a system of uncrossed projections reside peripherally in the entomostracan optic lobe but deep within the malacostracan–insect optic lobes? Observations of the growth of the lamina and medulla in insects describe both centres arising orthogonally from two adjacent sets of precursor cells, called the outer optic anlagen (Meinertzhagen & Hanson 1993). In entomostracans, there is only one such set of precursors. It has been proposed that the second set in insects and malacostracans originated from an ancestral duplication of the single cell lineage originally providing the entomostracan lamina (Meinertzhagen 1991). The duplication provides a second retinotopic neuropil, the development of which, synchronized with that of the lamina, provides sequential connections that give rise to the first chiasma. Thus, in the brains of insects and malacostracans, the medulla is essentially the evolutionary progeny of the entomostracan lamina (Strausfeld 2005). In both groups, uncrossed connections are retained. In entomostracans, they extend from the inner face of the lamina to supply the second (and only other) retinotopic neuropil. In malacostracans, they extend from the inner surface of the medulla, an entomostracan derivative, to supply what was ancestrally the second retinotopic neuropil: namely, the tectum-like region that persists today in extant entomostracans. In malacostracans and insects, this neuropil is called the lobula plate.
The lobula plate was originally identified by Cajal & Sánchez (1915) in dipterous insects. It exists as a separate retinotopic neuropil in all members of this group as well as in Lepidoptera, Coleoptera and several other insect orders. The Spanish authors also identified its homologue in honeybees, where the equivalent neuropil lies beneath the lobula, as it does in the most primitive insects, the Machilidae (figure 4). The recent discovery of a lobula plate in isopod crustaceans, and its subsequent recognition in other eumalacostracans, demonstrates both the ubiquity and antiquity of this neuropil (Sinakevitch et al. 2003; Sztarker et al. 2005). A medulla and a reniform lobula occur only in malacostracans and insects, however (figure 4). Their considerable elaboration in stomatopods and reptantians, as well as in certain insects, may have been driven by increasingly complex visual environments.
How likely is it that the presence of a medulla and lobula in insects and malacostracans is the consequence of parallel evolution? Current studies on insect and crustacean optic lobes focus on the shapes and dispositions of identified neurons. Observations of Golgi-impregnated medullas of the crab H. oregonensis and a variety of insect species identify certain neurons in the medulla that share similar morphologies and layer relationships as those described from dipterous insects, which have been shown to maintain phylogenetic information (Buschbeck 2000). That such neurons are common to insects and malacostracans suggests phenotypic stability.
If an entomostracan origin for the insects and malacostracans is proposed from molecular studies, this would imply independent and parallel evolution in insects and malacostracans not only of their medullae, lobulae and chiasmata, but also parallel evolution of a number of neuronal cell types that have comparable morphologies and layer relationships in these taxa (figure 5a). An entomostracan origin for insects and malacostracans would suggest independent and parallel evolution of the malacostracan and insect olfactory systems. However, comparisons of monocondylic insects and eumalacostracans support a shared groundplan: a glomerular olfactory lobe and from it connections to circumscribed regions in the lateral protocerebrum. A malacostracan origin of the insects is also supported by common organization of the central complex in malacostracans and monocondylic insects.
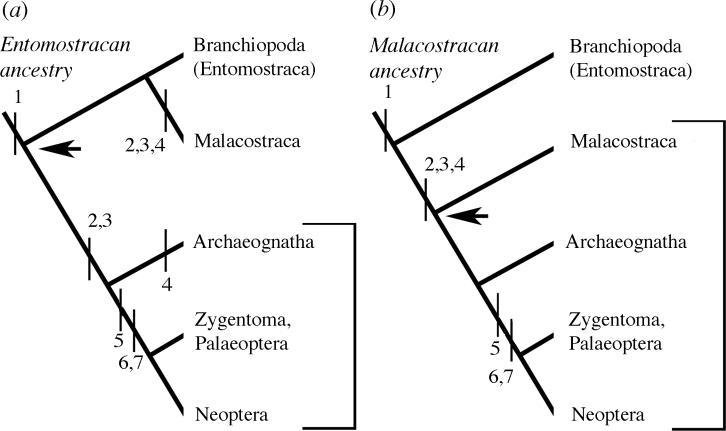
Figure 5.
Neuroanatomical characters considered in this paper support (b) a malacostracan origin of the insects. (a) Invoking an entomostracan ancestor of the Malacostraca and Insecta requires independent origins and convergent evolution of four nested optic neuropils, glomerular antennal lobes and the bilayered central complex. 1, Lamina+tectum (lobula plate); 2, lamina, medulla, lobula and chiasmata; 3, glomerular olfactory lobe connected to lateral protocerebrum; 4, stratified spindle-like central complex; 5, modular PB/central complex; 6, mushroom body; 7, olfactory lobe projections to mushroom body and lateral protocerebrum.
This parsimonious interpretation of the neuroanatomical data (figure 5b) comes from observations of just three brain regions. However, other cerebral attributes, such as connections between the protocerebrum and labral neuropils in the tritocerebrum, provide additional support for insect–malacostracan affinities. This is not to deny some intriguing enigmas, not least of which refer to the mushroom bodies. These are present in dicondylic insects, having no counterpart in machilids or crustaceans. Yet there are comparably organized centres in chelicerates, chilopods, polychaete annelids and polyclad turbellarians (Strausfeld et al. 1998). While these examples of convergence are fascinating in their own right, other shared neural characters nevertheless provide a perspective of insect evolution that may have escaped resolution by more conventional molecular and morphological means.
Acknowledgments
This paper originates from an invitation to contribute to an issue of Biology Letters containing a special feature on ‘Brain evolution’ (doi:10.1098/rsbl.2008.0687), honouring the 150-year anniversary of the publication of ‘On the Origin of Species’. Owing to its length, the paper is published here. I thank Tom Smulders for the original invitation. I am grateful to the University of Washington's Marine Biology Laboratories at Friday Harbor and its staff for facilities and support. Material for figure 3b,d was kindly provided by Rudi Loesel, University of Aachen. This study was supported by funds from the John D. and Catherine T. McArthur, from the Alexander von Humboldt Foundation, and a grant from the AFOSR (FFA9550-07-1-0165). Susan Fahrbach, Wake Forest University, helpfully commented on an earlier draft of the manuscript. I especially thank my wife, Camilla Strausfeld, for reading and critiquing the final text.
References
- Abele L.G. Comparison of morphological and molecular phylogeny of the Decapoda. Mem. Queensl. Mus. 1991;31:101–108. [Google Scholar]
- Benton R. On the origin of smell, odorant receptors in insects. Cell. Mol. Life Sci. 2006;63:1579–1585. doi: 10.1007/s00018-006-6130-7. doi:10.1007/s00018-006-6130-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxhall G.A. The evolution of arthropod limbs. Biol. Rev. 2004;79:253–300. doi: 10.1017/s1464793103006274. doi:10.1017/S1464793103006274 [DOI] [PubMed] [Google Scholar]
- Boyan G.S., Williams J.L.D., Herbert Z. Fascicle switching generates a chiasmal neuroarchitecture in the embryonic central body of the grasshopper Schistocerca gregaria. Arthropod Struct. Dev. 2008;37:539–544. doi: 10.1016/j.asd.2008.07.005. doi:10.1016/j.asd.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Buschbeck E.K. Neurobiological constraints and fly systematics: how different types of neural characters can contribute to a higher level dipteran phylogeny. Evolution. 2000;54:888–898. doi: 10.1111/j.0014-3820.2000.tb00089.x. doi:10.1554/0014-3820(2000)054[0888:NCAFSH]2.3.CO;2 [DOI] [PubMed] [Google Scholar]
- Buschbeck E.K., Strausfeld N.J. Visual motion-detection circuits in flies, small-field retinotopic elements responding to motion are evolutionarily conserved across taxa. J. Neurosci. 1996;16:4563–4578. doi: 10.1523/JNEUROSCI.16-15-04563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschbeck E.K., Strausfeld N.J. The relevance of neural architecture to visual performance. Phylogenetic conservation and variation in dipteran visual systems. J. Comp. Neurol. 1997;383:282–304. doi:10.1002/(SICI)1096-9861(19970707)383:3<282::AID-CNE2>3.0.CO;2-# [PubMed] [Google Scholar]
- Cajal, S. R. 1937 Recuerdos de Mi Vida (transl. Recollections of my life, E. Horne Craigie). Cambridge, MA; London, UK: MIT Press.
- Cajal S.R., Sánchez D. Contribución al conocimiento de los centros nerviosos de los insectos. Parte I Retina y centros opticos. Trab. Lab. Invest. Biol. Univ. Madrid. 1915;13:1–168. [Google Scholar]
- Christensen T.A., White J. Representation of olfactory information in the brain. In: Finger T.E., Silver W.L., Restrepo D., editors. The neurobiology of taste and smell. 2nd edn. Wiley-Liss, Inc; New York, NY: 2000. pp. 201–232. [Google Scholar]
- Dohle W. Are the insects terrestrial crustaceans? A discussion of some new facts and arguments and the proposal of the proper name ‘Tetraconata’ for the monophyletic unit Crustacea+Hexapoda. Ann. Soc. Entomol. France. 2001;37:85–103. [Google Scholar]
- Eisthen H.L. Why are olfactory systems of different animals so similar? Brain Behav. Evol. 2002;59:273–293. doi: 10.1159/000063564. doi:10.1159/000063564 [DOI] [PubMed] [Google Scholar]
- Fanenbruck M., Harzsch S. A brain atlas of Godzilliognomus frondosus Yager, 1989 (Remipedia, Godzilliidae) and comparison with the brain of Speleonectes tulumensis Yager, 1987 (Remipedia, Speleonectidae): implications for arthropod relationships. Arthropod Struct. Dev. 2005;34:343–378. doi:10.1016/j.asd.2005.01.007 [Google Scholar]
- Farris S.M. Structural, functional and developmental convergence of the insect mushroom bodies with higher brain centres of vertebrates. Brain Behav. Evol. 2008;72:1–15. doi: 10.1159/000139457. doi:10.1159/000139457 [DOI] [PubMed] [Google Scholar]
- Fishilevich E., Vosshall L.B. Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. doi:10.1016/j.cub.2005.07.066 [DOI] [PubMed] [Google Scholar]
- Glenner H., Thomsen P.F., Hebsgaard M.B., Sorensen M.V., Willerslev E. The origin of insects. Science. 2006;314:1883–1884. doi: 10.1126/science.1129844. doi:10.1126/science.1129844 [DOI] [PubMed] [Google Scholar]
- Grimaldi D., Engel M.S. Cambridge University Press; New York, NY: 2005. Evolution of the insects. [Google Scholar]
- Hanström, B. 1926 Vergleichende Anatomie des Nervensystems der wirbellosen Tiere Amsterdam, The Netherlands: A. Asher. (Facsimile reprint.)
- Harzsch S., Hansson B. Brain architecture in the terrestrial hermit crab Coenobita clypeatus (Anomura, Coenobitidae), a crustacean with a good aerial sense of smell. BMC Neurosci. 2008;9:1–35. doi: 10.1186/1471-2202-9-58. doi:10.1186/1471-2202-9-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R., Wenzel B., Elsner N. Pharmacological brain stimulation releases elaborate stridulatory behaviour in gomphocerine grasshoppers—conclusions for the organization of the central nervous control. J. Comp. Physiol. A. 2001;182:155–169. doi: 10.1007/s003590100188. doi:10.1007/s003590100188 [DOI] [PubMed] [Google Scholar]
- Heinze S., Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science. 2007;315:995–997. doi: 10.1126/science.1135531. doi:10.1126/science.1135531 [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. doi:10.1038/nrn1074 [DOI] [PubMed] [Google Scholar]
- Holmgren N. Zur vergleichenden Anatomie des Gehirns von Polychaeten, Onychophoren, Xiphosuren, Arachniden, Crustaceen, Myriapoden, und Insekten. K. Svenska Vetensk. Akad. Handl. 1916;56:1–303. [Google Scholar]
- Homberg U., Montague R.A., Hildebrand J.G. Anatomy of antennocerebral pathways in the brain of the sphinx moth Manduca sexta. Cell Tissue Res. 1988;254:255–281. doi: 10.1007/BF00225800. doi:10.1007/BF00225800 [DOI] [PubMed] [Google Scholar]
- Kirschner S., Kleineidam C.J., Zube C., Rybak J., Grünewald B., Rössler W. Dual olfactory pathway in the honeybee, Apis mellifera. J. Comp. Neurol. 2006;499:933–952. doi: 10.1002/cne.21158. doi:10.1002/cne.21158 [DOI] [PubMed] [Google Scholar]
- Labandeira C.C., Beal B.S., Hueber F.M. Early insect diversification: evidence from a Lower Devonian bristletail from Quebec. Science. 1988;242:913–916. [Google Scholar]
- Lai S.L., Awasaki T., Ito K., Lee T. Clonal analysis of Drosophila antennal lobe neurons: diverse neuronal architectures in the lateral neuroblast lineage. Development. 2008;135:2883–2893. doi: 10.1242/dev.024380. doi:10.1242/dev.024380 [DOI] [PubMed] [Google Scholar]
- Loesel R., Nässel D.R., Strausfeld N.J. Common design in a unique midline neuropil in the brains of arthropods. Arthropod Struct. Dev. 2002;31:77–91. doi: 10.1016/S1467-8039(02)00017-8. doi:10.1016/S1467-8039(02)00017-8 [DOI] [PubMed] [Google Scholar]
- Mallatt J., Giribet G. Further use of nearly complete, 28S and 18S rRNA genes to classify Ecdysozoa, 37 more arthropods and a kinorhynch. Mol. Phylogen. Evol. 2006;40:772–794. doi: 10.1016/j.ympev.2006.04.021. doi:10.1016/j.ympev.2006.04.021 [DOI] [PubMed] [Google Scholar]
- Martin J., Raabe T., Heisenberg M. Central complex sub-structures are required for the maintenance of locomotor activity in Drosophila melanogaster. J. Comp. Physiol. A. 1999;185:277–288. doi: 10.1007/s003590050387. doi:10.1007/s003590050387 [DOI] [PubMed] [Google Scholar]
- Meinertzhagen, I. A. 1991 Evolution of the cellular organization of the arthropod compound eye and optic lobe. In Vision and visual dysfunction, vol. 2 (eds. J. R. Cronly-Dillon & R. L. Gregory). Evolution of the Eye and Visual System, pp. 341–362. London, UK: MacMillan Press.
- Meinertzhagen I.A., Hanson T.A. The development of the optic lobes. In: Bate M., Arias A.M., editors. The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; New York, NY: 1993. pp. 1363–1491. [Google Scholar]
- Morton S.M., Bastian A.J. Mechanisms of cerebellar gait ataxia. Cerebellum. 2007;6:79–86. doi: 10.1080/14734220601187741. doi:10.1080/14734220601187741 [DOI] [PubMed] [Google Scholar]
- Olesen J., Walossek D. Limb ontogeny and trunk segmentation in Nebalia bipes (Crustacea, Malacostraca, Leptostraca) Zoomorphology. 2000;120:47–64. doi:10.1007/s004350000024 [Google Scholar]
- Osorio D., Bacon J.P. A good eye for arthropod evolution. Bioassays. 1994;16:419–424. doi: 10.1002/bies.950160610. doi:10.1002/bies.950160610 [DOI] [PubMed] [Google Scholar]
- Regier J.C., Shulz J.W., Kambic R.E. Pancrustacean phylogeny, hexapods are terrestrial crustaceans and maxillopods are not monophyletic. Proc. R. Soc. B. 2005;272:395–401. doi: 10.1098/rspb.2004.2917. doi:10.1098/rspb.2004.2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgel A.L., Alexander B.E., Ritzmann R.E. Descending control of turning behavior in the cockroach, Blaberus discoidalis. J. Comp. Physiol. A. 2007;193:385–402. doi: 10.1007/s00359-006-0193-7. doi:10.1007/s00359-006-0193-7 [DOI] [PubMed] [Google Scholar]
- Sandeman D., Sandeman R., Derby C., Schmidt M. Morphology of the brain of crayfish, crabs, and spiny lobsters, A common nomenclature for homologous structures. Biol. Bull. 1992;183:304–326. doi: 10.2307/1542217. doi:10.2307/1542217 [DOI] [PubMed] [Google Scholar]
- Schachtner J., Schmidt M., Homberg U. Organization and evolutionary trends of primary olfactory brain centres in Tetraconata (Crustacea plus Hexapoda) Arthropod Struct. Dev. 2005;34:257–299. doi:10.1016/j.asd.2005.04.003 [Google Scholar]
- Schmidt M., Ache B.W. Antennular projections to the midbrain of the spiny lobster. 2. Sensory innervation of the olfactory lobe. J. Comp. Neurol. 1992;318:291–303. doi: 10.1002/cne.903180306. doi:10.1002/cne.903180306 [DOI] [PubMed] [Google Scholar]
- Schmidt M., Ache B.W. Immunocytochemical analysis of glomerular regionalization and neuronal diversity in the olfactory deutocerebrum of the spiny lobster. Cell Tissue Res. 1997;287:541–563. doi: 10.1007/s004410050778. doi:10.1007/s004410050778 [DOI] [PubMed] [Google Scholar]
- Schultz J.W., Regier J.C. Phylogenetic analysis of arthropods using two nuclear protein-encoding genes supports a crustacean plus hexapod clade. Proc. R. Soc. B. 2000;267:1011–1019. doi: 10.1098/rspb.2000.1104. doi:10.1098/rspb.2000.1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S.R., Varney L.P. Primitive, crustacean-like state of blood–brain barrier in the eye of the apterygote insect Petrobius (Archaeognatha) determined from uptake of fluorescent tracers. J. Neurobiol. 1999;41:452–470. doi:10.1002/(SICI)1097-4695(199912)41:4<452::AID-NEU2>3.0.CO;2-5 [PubMed] [Google Scholar]
- Sinakevitch I., Douglass J.K., Scholtz G., Loesel R., Strausfeld N.J. Conserved and convergent organization in the optic lobes of insects and isopods, with reference to other crustacean taxa. J. Comp. Neurol. 2003;467:150–172. doi: 10.1002/cne.10925. doi:10.1002/cne.10925 [DOI] [PubMed] [Google Scholar]
- Strausfeld N.J. A brain region in insects that supervises walking. Prog. Brain Res. 1999;123:273–284. doi: 10.1016/s0079-6123(08)62863-0. doi:10.1016/S0079-6123(08)62863-0 [DOI] [PubMed] [Google Scholar]
- Strausfeld N.J. The evolution of crustacean and insect optic lobes and the origins of chiasmata. Arthropod Struct. Dev. 2005;34:235–256. doi:10.1016/j.asd.2005.04.001 [Google Scholar]
- Strausfeld N.J., Hildebrand J.G. Olfactory systems, common design, uncommon origins? Curr. Opin. Neurobiol. 1999;9:634–639. doi: 10.1016/S0959-4388(99)00019-7. doi:10.1016/S0959-4388(99)00019-7 [DOI] [PubMed] [Google Scholar]
- Strausfeld, N. J. & Nässel, D. R. 1980 Neuroarchitectures serving compound eyes of Crustacea and insects. In Handbook of sensory physiology, vol. VII/68 (ed. H. Autrum), pp. 1–132. Berlin, Germany: Springer.
- Strausfeld N.J., Hansen L., Li Y.S., Gomez R.S., Ito K. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn. Mem. 1998;5:11–37. [PMC free article] [PubMed] [Google Scholar]
- Strausfeld N.J., Strausfeld C.M., Loesel R., Rowell D., Stowe S. Arthropod phylogeny, onychophoran brain organization suggests an archaic relationship with a chelicerate stem lineage. Proc. Biol. Sci. 2006;273:1857–1866. doi: 10.1098/rspb.2006.3536. doi:10.1098/rspb.2006.3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld N.J., Sinakevitch I., Brown S.M., Farris S.M. Ground plan of the insect mushroom body: functional and evolutionary implications. J. Comp. Neurol. 2009;513:265–291. doi: 10.1002/cne.21948. doi:10.1002/cne.21948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr. Opin. Neurobiol. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. doi:10.1016/S0959-4388(02)00385-9 [DOI] [PubMed] [Google Scholar]
- Sullivan J.M., Beltz B.S. Neural pathways connecting the deutocerebrum and the lateral protocerebrum in the brains of decapod crustaceans. J. Comp. Neurol. 2001;441:9–22. doi: 10.1002/cne.1394. doi:10.1002/cne.1394 [DOI] [PubMed] [Google Scholar]
- Sullivan J.M., Beltz B.S. Integration and segregation of inputs to higher-order neuropils of the crayfish Brain. J. Comp. Neurol. 2005;481:118–126. doi: 10.1002/cne.20346. doi:10.1002/cne.20346 [DOI] [PubMed] [Google Scholar]
- Sztarker J., Strausfeld N.J., Tomsic D. Organization of optic lobes that support motion detection in a semiterrestrial crab. J. Comp. Neurol. 2005;493:396–411. doi: 10.1002/cne.20755. doi:10.1002/cne.20755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttig M., Agricola H.-J., Sandeman R., Sandeman D. Central complex in the brain of crayfish and its possible homology with that of insects. J. Comp. Neurol. 2000;416:245–261. doi:10.1002/(SICI)1096-9861(20000110)416:2<245::AID-CNE9>3.0.CO;2-A [PubMed] [Google Scholar]
- Walossek, D. 1999 On the Cambrian diversity of Crustacea. In Crustaceans and the biodiversity crisis (eds F. R. Schram & J. C. von Vaupel Klein), Proc. 4th Internat. Crust. Congress, vol. 1, pp. 3–27. Leiden, The Netherlands: Brill Academic Publishers.
- Williams J.L.D. Anatomical studies of the insect central nervous system, a ground-plan of the midbrain and an introduction to the central complex in the locust, Schistocerca gregaria (Orthoptera) J. Zool. Lond. 1975;176:67–86. [Google Scholar]
- Williams J.L.D., Guntner M., Boyan G.S. Building the central complex of the grasshopper Schistocerca gregaria, temporal topology organizes the neuroarchitecture of the w, x, y, z tracts. Arthropod Struct. Dev. 2005;34:97–110. doi:10.1016/j.asd.2004.11.001 [Google Scholar]