Abstract
Post-hatchling loggerhead turtles (Caretta caretta) in the northern Pacific and northern Atlantic Oceans undertake transoceanic developmental migrations. Similar migratory behaviour is hypothesized in the South Pacific Ocean as post-hatchling loggerhead turtles are observed in Peruvian fisheries, yet no loggerhead rookeries occur along the coast of South America. This hypothesis was supported by analyses of the size-class distribution of 123 post-hatchling turtles in the South Pacific and genetic analysis of mtDNA haplotypes of 103 nesting females in the southwest Pacific, 19 post-hatchlings stranded on the southeastern Australian beaches and 22 post-hatchlings caught by Peruvian longline fisheries. Only two haplotypes (CCP1 93% and CCP5 7%) were observed across all samples, and there were no significant differences in haplotype frequencies between the southwest Pacific rookeries and the post-hatchlings. By contrast, the predominant CCP1 haplotype is rarely observed in North Pacific rookeries and haplotype frequencies were strongly differentiated between the two regions (Fst=0.82; p=<0.00001). These results suggest that post-hatchling loggerhead turtles emerging from the southwest Pacific rookeries are undertaking transoceanic migrations to the southeastern Pacific Ocean, thus emphasizing the need for a broader focus on juvenile mortality throughout the South Pacific to develop effective conservation strategies.
Keywords: Caretta caretta, marine turtles, conservation genetics, mtDNA, molecular markers, post-hatchling
1. Introduction
Long-distance migration in animals is a fascinating syndrome of interacting behavioural, physiological, morphological and life-history traits (Dingle 1996). As successful migration is critical to the biology of several species at the risk of extinction (Dingle 1996), understanding migration in an endangered species will assist in the development of effective conservation and management strategies for that organism. In particular, understanding the migration routes of an endangered animal provides the spatial information relevant for improving their management and conservation by ensuring effort is directed at the geographical regions used by individuals throughout their life history.
Conventionally, animal tracking studies have used extrinsic markers or tags that require the subsequent re-sighting or recapture of individuals. These methods have provided valuable insight into the migration patterns of many animals, such as the multiple migrations undertaken by mature loggerhead turtles between the same feeding and nesting locations throughout its reproductive lifetime (e.g. Limpus & Limpus 2003b). However, this approach is not suitable for some organisms, especially for small animals and many that live in the marine environment. Recent developments in remote-sensing techniques and the use of intrinsic markers such as stable isotopes and genetic markers have provided another tracking tool for researchers. Together these tools compliment each other and allow a more comprehensive picture of an animal's migration to be obtained. Recent improvements to sophisticated telemetry and molecular markers have provided an appreciation of the spatial dynamics of a number of threatened marine species, including great white sharks (Bonfil et al. 2005), turtles in the Northern Hemisphere (Bolten et al. 1998) and some bird species (Phillips et al. 2005). Knowledge about the scope and patterns of some of these population's large-scale migrations has provided an opportunity to refine management strategies of these endangered marine species.
Molecular markers have been particularly valuable for revealing the movements of post-hatchling loggerhead turtles from their natal rookeries. mtDNA sequencing and mixed stock analyses confirmed that post-hatchling loggerhead turtles in the Azores and Madeira undergo transatlantic migrations from rookeries in the southern United States and Mexico (Bolten et al. 1998), and post-hatchling loggerhead turtles occupying waters offshore from Baja, California migrate across the Pacific Ocean from Japanese rookeries (Bowen et al. 1995). Additionally, preliminary genetic analysis of three pelagic loggerheads captured on the southern coast of Peru in 2002 indicate that these turtles are of Australian nesting stock (Alfaro-Shigueto et al. 2004). Such studies have provided valuable insight into the cryptic post-hatchling life stage, which prior to the use of molecular markers, have been speculative and based on size distributions and the geographical positions of rookeries (Carr 1986). Traditional tagging techniques are problematic for tracking post-hatchling migrations, due to high mortality rates, the need for a permanent tag (such as removing a scute or skin grafting) and broad dispersal that typically results in low densities of post-hatchlings in the open ocean (but see Witherington 2002). To date, our knowledge on this cryptic life stage is limited to the Northern Hemisphere, and movements of young turtles in the Southern Hemisphere remains hypothetical.
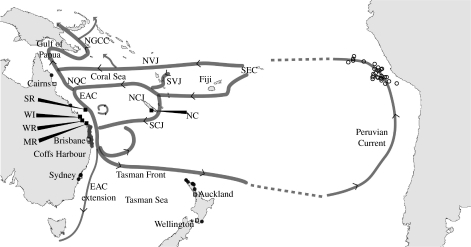
Throughout the Pacific, nesting aggregations of loggerhead turtles are restricted to the western side of the ocean basin, occurring in northern Japan (Kamezaki et al. 2003), eastern Australia and New Caledonia (Limpus & Limpus 2003b). In the southwest Pacific, small post-hatchlings occasionally strand along Australia's eastern coast and along the northern New Zealand coastline (Limpus et al. 1994), while in the southeast Pacific, larger post-hatchlings are captured in longline fisheries operating in the waters off the coasts of northern Chile and southern Peru (Donoso et al. 2000; Kelez et al. 2003; Alfaro-Shigueto et al. 2004; figure 1). The distribution of nesting locations on the western margin of the South Pacific Ocean allows for the possibility that loggerhead post-hatchlings may undertake transoceanic migrations as seen in post-hatchlings of this species in the Northern Hemisphere.
Figure 1.
The distribution of loggerhead (Caretta caretta) post-hatchling turtle records for the southern Pacific region relative to primary oceanic surface currents and the location of rookeries sampled for genetic characterization. Filled squares, sampled rookeries; filled circles, stranded post-hatchlings; and open circles, post-hatchlings captured in longline fisheries.The rookeries sampled included WR, Wreck Rock; SR, Swain Reefs; WI, Wreck Island; MR, Mon Repos; and NC, New Caledonia. The annotations refer to the southern equatorial current (SEC) that flows into the coral sea where it divides into a number of jets: the North Vanuatu Jet (NVJ); the South Vanuatu Current (SVJ); the North Caledonian Jet (NCJ); and the South Caledonian Jet (SCJ). These jets are the source of the current systems off eastern Australia, the East Australian Current (EAC), the North Queensland Current (NQC) and the New Guinea Coastal Current (NGCC).
Over the past three decades, the annual nesting population of loggerhead turtles in eastern Australia has experienced an 86 per cent reduction (Limpus & Limpus 2003a), which under the IUCN classification would qualify this population as being critically endangered (IUCN 2001). At present, the species is listed as endangered by the IUCN and by legislation of the Australian Commonwealth and Queensland governments. Rookery declines have largely been attributed to previous habitat loss and mortality in coastal trawl fisheries (Chaloupka 2003), although this situation has improved with habitat protection and the compulsory use of turtle excluder devices in all trawl nets operating in Queensland waters. Given the precarious status of the southwest Pacific loggerhead turtles, an understanding of habitat use and potential sources of mortality for all life-history stages is essential for developing more comprehensive management strategies. This is particularly true because of the potential link between post-hatchlings being captured in longline fisheries in the southeast Pacific and a poor understanding of the post-capture survival of turtles in fisheries (Hays et al. 2003; Chaloupka et al. 2004a,b).
In this study, we use multiple sources of evidence to gain insight into the migration syndrome of the loggerhead turtle by focusing on the movement of the post-hatchling life stage in the South Pacific Ocean. First, we expand on the characterization of the genetic composition of loggerhead turtle nesting populations in the South Pacific Ocean previously reported by FitzSimmons et al. (2003), as a baseline for comparison with post-hatchling data collected. Second, we analyse the genetic structure of post-hatchling loggerhead turtles that strand on beaches in the southwest Pacific region and of those captured in fisheries operating in the southeast Pacific Ocean. Third, we compare the genetic results with the body size and spatial location of loggerhead post-hatchling sea turtles in relation to primary rookeries and currents in the southern Pacific Ocean. We specifically aim to test whether the loggerhead post-hatchling turtles observed in the southeast Pacific have their origins in the southwest Pacific and use the outcome of this to reflect upon global migration for this species.
2. Material and methods
(a) Post-hatchling loggerhead turtle distribution in the southwest Pacific Ocean
Information on the spatial and size distribution of post-hatchling loggerhead turtles in the southwest Pacific Ocean was collated from two sources that represent the majority of public reports for post-hatching turtles in the region; New Zealand's Department of Conservation (Herpetofauna Division, Napier), and StrandNet (http://www.epa.qld.gov.au), the marine wildlife stranding database of the Queensland Environmental Protection Agency. The term post-hatchling refers to the life stage during which a sea turtle is a juvenile in the pelagic habitat (Bolten 2003). Accordingly, this study used knowledge of the size at which loggerhead turtles recruit into coastal feeding grounds in the southwest Pacific (mean curved carapace length (CCL)=78.6 cm; range 66.7–93.9 cm; table 1; Limpus & Limpus 2003a) and the turtle's occupied habitat to classify a turtle as a post-hatchling. For example, a turtle well below the minimum recruitment size found in a coastal habitat (such as stranded on a beach) was considered a post-hatchling, as was a turtle that was within the range of sizes found to be recruiting into coastal waters, but found in an oceanic habitat. The relationship between post-hatchling size (mean CCL) and their distance from the primary Queensland rookery (Mon Repos) was calculated with a linear regression test in Xlstat v. 2008.
Table 1.
Size-range distribution of post-hatchling loggerhead (Caretta caretta) turtles recorded throughout the South Pacific Ocean (adapted from Limpus et al. 2005). Numbers in parentheses in the ‘n’ column refer to records for which no carapace length data was recorded.
| curved carapace length (cm) | |||||
|---|---|---|---|---|---|
| mean (mode) | s.d. | range | n | reference | |
| post-hatchlings: western South Pacific Ocean | |||||
| East Australia (stranded) | 6.10 (5) | 2.0 | 4.5–14.4 | 114 (13) | EPA stranding and mortality database |
| New Zealand (stranded) | 14.99 (10) | 9.9 | 8.6–33.0 | 9 (2) | Gill 1997; EPA stranding and mortality database |
| post-hatchlings: eastern South Pacific Ocean | |||||
| southern Peru & northern Chile (longline fishery by catch) | 56.5 (57) | 7.6 | 38.0–72.5 | 42 (1) | Kelez et al. 2006 |
| 54.3 (53) | 11.1 | 26.0–65.5 | 23(8) | Alfaro-Shigueto et al. 2004 | |
| 71 | 1 | Donoso et al. 2000 | |||
| carapaces in southern Peru | 61 | 3.1 | 57.8–63.9 | 3 | Kelez et al. 2003 |
| size at recruitment to residency in south Queensland coastal waters | |||||
| southern Queensland coastal waters | 78.6 | 4.0 | 66.7–93.9 | 108 | Limpus & Limpus 2003a |
(b) Genetic sample collection
Samples for genetic analysis of loggerhead nesting populations in the South Pacific Ocean were collected from the primary loggerhead rookeries in eastern Australia (Mon Repos, n=37; Swain Reefs, n=37; Wreck Island, n=23; Wreck Rock, n=6) from 1991 to 1996 and New Caledonia (La Roche Percee; n=29) during the 2005 nesting season (figure 1). Nesting beyond these locations is sporadic and only occurs in low densities (Limpus 2004). Samples were collected from nesting female turtles after egg deposition either by removing a small (approx. 0.5 mm2) piece of skin from the upper shoulder region or by taking 0.5–1.0 ml of blood from the dorsal cervical sinus using a 21 gauge, 38 mm needle. For the sampling of some Australian rookeries, tissue was taken from non-sibling hatchlings, from either a dead hatchling or an embryo. Tissue samples were collected from 19 post-hatchlings that were stranded along the east Australian coast between 1996 and 2004 (figure 1). A small piece of skin was removed from the underside of the pelvic region of dead turtles, or a small notch (approx. 5 mm2) of carapace was taken from the outer edge of the 10th or 11th marginal scute of live turtles. Tissue samples (n=22) were also collected from turtles captured in longline fishing vessels operating off the central and southern coast off Peru during the years 2002–2005 (figure 1), by removing a piece of skin (approx. 2 cm2) from the shoulder region. After tissue sampling, morphometric data collections were made following standard Queensland Turtle Conservation Programme methods (Limpus et al. 1983).
(c) DNA sequence analysis
Genomic DNA was isolated from the blood and tissue samples collected from rookeries and stranded post-hatchlings by proteinase K digestion in a lysis buffer (10 mM Tris, 1 mM EDTA, 10 mM NaCl, 0.1% SDS) and a salting out procedure in 2.5 M ammonium acetate followed by ethanol precipitation. For samples collected from post-hatchlings that were captured by the longline fishery, genomic DNA was isolated using a QIAGEN DNeasy blood and tissue kit, as per the manufacturer's directions. Direct polymerase chain reaction sequencing was completed by amplification of the 5′ end of the control region (for full methods see the electronic supplementary material). DNA sequences of 1120 bp were truncated to a directly comparable 380 bp region for comparison with previously described haplotypes for loggerhead turtles stored in GenBank (National Centre for Biotechnology Information, http://www.ncbi.nlm.nih.gov), the Archie Carr Centre for Sea Turtle Research (http://accstr.ufl.edu/genetics.html) and from FitzSimmons (2003). Estimates of haplotype and nucleotide diversity were calculated using Arlequin (Excoffier et al. 2005) based on Kimura 2P parameters, as determined from ModelTest 3.7 (Posada & Crandall 1998). Population pairwise Fst was calculated in Arlequin (Excoffier et al. 2005) to assess the genetic structure between the Pacific Ocean rookeries, post-hatchling populations and North versus South Pacific regions. Maximum-likelihood (ML) estimates for the origin populations of the post-hatchlings were derived from SPAM (ADF&G 2003) based on haplotype frequencies of rookeries in the Pacific Ocean.
3. Results
(a) Post-hatchling distribution
Documentation on the occurrence of post-hatchlings in the South Pacific region was scarce prior to the 1980s, with only 13 records, the first dating back to 1922. After 1980, recording of post-hatchling observations through StrandNet became more regular. For this study, there were records of 123 loggerhead post-hatchlings available for the southwest Pacific region (table 1). These numbers were derived from StrandNet, and included New Zealand records and those that had been reported in previous literature (Limpus et al. 1994). The majority of records for post-hatchling loggerhead turtles in the southwest Pacific were for animals that had become stranded along Australia's eastern coast between Fraser Island (25.25° S, 153.167° E) in southern Queensland, southwards to the mid-New South Wales coast (n>101), with a few records (n=9) for northern New Zealand beaches (table 1). Loggerhead post-hatchlings were also reported in the southeast Pacific, where 131 turtles had been recorded as captured in longline fisheries operating in the waters off the coasts of Peru and northern Chile (table 1).
Post-hatchling loggerhead turtles recorded along Australia's eastern Pacific coast ranged in size from 4.5 cm (i.e. neonates) up to 14.4 cm CCL, with the majority (90%) of the individuals measuring less than 9.0 cm CCL (table 1). The mean size of loggerhead post-hatchlings increased with distance from the primary rookery locations in the direction of the South Pacific subtropical gyre (R2=0.959, F=70.3, p=0.014; figure 1). The mean CCL measurements were 6.10 cm (mode=5 cm) along the east Australian coast, 14.99 cm (mode=10 cm) on the New Zealand coast and 54.3–71.0 cm (mode=53–57 cm) in the waters offshore from Peru and Chile (table 1). Post-hatchling loggerhead turtles were observed stranding along Australia's eastern seaboard throughout the year; however, most (90%) occurred from March to May, following the time of hatchling emergence, with the remaining 10 per cent occurring between June and November.
(b) Rookery genetic structure
Sequencing of the mtDNA control region revealed the presence of two haplotypes (CCP1 and CCP5) within the South Pacific rookeries. These two haplotypes have been reported previously for this region (FitzSimmons et al. 2003, CCP1 reported as haplotype A by Bowen et al. 1995) and are distinguished by one polymorphic site. Investigation into an extended sequence length (1120 versus 380 bp) did not uncover any further haplotypes, nor did it reveal any finer resolution between the two haplotypes (CCP1 and CCP5) from that already determined in 380 bp. CCP1 was the dominant haplotype, occurring in 98 per cent (n=101) of the eastern Australian samples and in 93 per cent (n=27) of the New Caledonian samples, with the remaining turtles being genotyped as haplotype CCP5. This genetic composition of the southwest Pacific rookeries made them distinct from Japanese rookeries (Fst=0.82; p=0.00001), but not distinct from one another (Fst=−0.019; p=0.19).
(c) Post-hatchling genetic structure
All 19 loggerhead post-hatchlings genotyped from the southwest Pacific carried the CCP1 haplotype. Out of the 22 post-hatchling turtles sampled in the longline fishery in the southeast Pacific, 21 (95%) had CCP1 haplotype and one (5%) had the CCP5 haplotype. The haplotype frequencies in the two post-hatchlings populations were not significantly different from each other (Fst=−0.007; p=0.99), nor were they significantly different from the southwest Pacific rookeries (Fst=−0.016; p=0.99). ML analysis determined that all post-hatchlings were derived from the South Pacific rookeries (s.e.±0.00).
(d) Haplotype and nucleotide diversity
Loggerhead turtle populations in the South Pacific Ocean possessed very low haplotype and nucleotide diversity. Estimated haplotype diversity for the eastern Australian rookeries was 0.095 (s.d.=0.028), which was similar to the value of 0.133 (s.d.=0.081) estimated for the New Caledonian rookery. Stranded post-hatchlings had a haplotype diversity value of 0.000 (s.d.=0.000) and the oceanic juvenile loggerhead aggregation had a haplotype diversity of 0.159 (s.d.=0.095).
4. Discussion
(a) Rookery and post-hatchling haplotypes
Southwest Pacific loggerhead turtle rookeries are characterized by low haplotype and nucleotide diversity, with two haplotypes present, one of which is dominant. The detection of only two haplotypes in the southwest Pacific rookeries is consistent with previous findings that used smaller sample sizes (Bowen et al. 1995; FitzSimmons et al. 2003). This low haplotype diversity is replicated in the northern Pacific Ocean where 99.6 per cent of the sampled nesting population are comprised of two haplotypes (Hatase et al. 2002). The haplotypes that comprise the rookeries in the northern Pacific rookeries are distinct from those in the southern Pacific rookies, with the exception of the very low occurrence (0.4%, n=1) of the dominant southern haplotype (CCP1) at the Japanese rookery (Hatase et al. 2002). This high level of heterogeneity between loggerhead turtle rookeries in the southern and northern Pacific Ocean shows a clear genetic disjunction between the populations in the two basins, with little-to-no gene flow between them. Furthermore, there was no evidence to suggest that Japanese post-hatchlings are migrating into the southern Pacific waters. All post-hatchlings genotyped were the South Pacific haplotypes, and ML analysis determined that the North Pacific rookeries have no contribution to the post-hatchling populations in the South Pacific Ocean. Thus, the small post-hatchlings that strand along the eastern Australian and northern New Zealand coastlines, and the larger post-hatchlings captured in longline fisheries off the coasts of Peru and Chile, represent different cohorts from either the eastern Australian or New Caledonian populations.
(b) Post-hatchling distribution and the role of currents
Size, and temporal and spatial distributions of post-hatchling loggerheads throughout the South Pacific Ocean suggest an association of these turtles with the South Pacific gyre. As post-hatchlings swim offshore after emerging from the southern Pacific rookeries, they will encounter the southward flowing western boundary current of the South Pacific gyre—the east Australian current (EAC). The distribution of post-hatchling turtles in a southward direction away from the southwest Pacific rookeries indicates the EAC's influence on the initial migration route of small post-hatchlings. It is expected that post-hatchlings that were stranded along Australia's east coast also include turtles hatched at offshore rookeries (e.g. New Caledonia and Vanuatu) that would become entrained within the EAC by way of the southern equatorial current (SEC), which flows westwards past these archipelagos towards the Australian coast (figure 1). Unfortunately, the observed lack of heterogeneity between the southwest Pacific rookeries prevents this from being confirmed and suggests the need to test this with highly variable microsatellite loci.
After the EAC swings away from the Australian coast, post-hatchlings using this current for transportation will most likely be directed eastwards into the Tasman Front (figure 1). If they remain with the Tasman Front, post-hatchlings would travel past the Lord Howe Island and to the north of New Zealand, across the southern Pacific Ocean and past Peru and Chile via the Peru current (also known as the Humboldt Current; figure 1, Burrage 1993). Assuming young post-hatchlings exhibit true ‘drifting’ behaviour, oceanographic particle tracking models and drifter trajectories may show the possible range of drift scenarios for loggerhead hatchlings in the South Pacific, akin to the use of this technique in the North Atlantic (Hays & Marsh 1997). Such techniques may also provide input, along with carapace size data, on the length of time juvenile loggerhead turtles spend within oceanic waters in the South Pacific Ocean.
If larger pelagic juveniles maintain their association with the South Pacific gyre, it could be assumed that they return to coastal Australia waters via the SEC. However, this return route cannot be substantiated until pelagic animals are found along this route, or until satellite trackers are deployed on pelagic turtles in the southeastern Pacific.
The relationship that southern Pacific loggerhead post-hatchling turtles have with the South Pacific gyre during their developmental migration is in accordance with tracking studies in the North Pacific, which found that larger pelagic loggerhead turtles associate with currents and their frontal systems (Polovina et al. 2000, 2003, 2006). Additionally, dietary studies have reported that juvenile pelagic turtles consume a range of organisms in the Pacific Ocean, which are indicative of the habitat generated along the borders of oceanic currents (Parker et al. 2005; Boyle & Limpus 2008).
(c) The juvenile developmental migration syndrome
To date, the only loggerhead populations for which the migratory routes of their post-hatchlings have been resolved are for rookeries located on the western side of ocean basins, i.e. the northwestern Atlantic Ocean (Mexico, southeastern USA), and the northwestern (Japan) and southwestern Pacific Ocean (present study). Despite the consistency of post-hatchling transoceanic migrations across these three populations, the lack of evidence of post-hatchling turtles in other pelagic locations where we would expect them suggests that transoceanic migrations are not the rule for all loggerhead populations. For example, if hatchlings emerging from east African rookeries were undertaking transoceanic migrations, we would expect some records of larger pelagic turtles in the southeast Indian Ocean. However, there is no evidence of hatchlings from these rookeries entering the Indian Ocean gyres. Instead, recoveries of small, notched post-hatchlings from Tongaland reveal that most are swept southwards after entering the Agulhas Current, with some rounding the Cape and entering the Atlantic Ocean (Baldwin et al. 2003). Likewise, records do not exist of hatchlings emerging from the Brazilian coast and entering the South Atlantic subtropical gyre. Although the lack of sightings of pelagic juvenile loggerhead turtles could be interpreted as evidence that transoceanic migrations are restricted to the three populations discussed, our current knowledge is based on opportunistic observations from fishing fleets operating in oceanic waters. Or in the case of northeast Atlantic, the fortuitous location of islands (Azores and Madeira) in the path of currents that act as an observation platform. Further efforts are needed to determine whether the absence of evidence of transoceanic migrations by post-hatchling loggerhead turtles from other populations is indeed, evidence of absence.
Although the migration routes of post-hatchling loggerhead turtles are determined (at least initially) by the flow direction of major oceanic surface currents (Luschi & Hays 2003), they are able to adjust their swimming behaviour relative to geomagnetic positioning (Lohmann & Lohmann 2003), thus suggesting the action of selective pressures on behavioural traits. One factor driving the evolution of migratory routes taken by post-hatchling loggerhead turtles may be the distribution of abundant food resources. For example, the upwelling off the Peruvian and Californian continental shelves are the most biologically productive upwelling systems in the world (Fiedler et al. 1991), and young loggerhead turtles foraging off the Baja coast of Mexico take advantage of large aggregations of pelagic red crabs in this region (Peckham & Nichols 2002). Similarly, loggerhead post-hatchlings from the western North Atlantic use the rich foraging grounds of the Azores and to some extent, the Mediterranean Sea (Bolten 2003). If migrations of the post-hatchling loggerhead turtles are resource driven and evolved to take advantage of high-value food resources, then loggerhead hatchlings emerging from rookeries on the eastern side of the Atlantic Ocean, for example (i.e. West Africa, Mediterranean Sea), where these regions of higher productivity are, will not embark on transoceanic migrations but instead travel to more localized feeding areas. Future research that elucidates the migratory routes of other populations, in particular those of juvenile loggerhead turtles derived from rookeries on eastern continental margins, will provide greater insight into the driving factors of these developmental migrations.
(d) Conservation and management implications
The present study has provided evidence of the genetic connectivity between loggerhead turtles in the southwest Pacific rookeries and in southeast Pacific feeding grounds. This connectivity clearly demonstrates the ocean basin-wide geographical scale at which the understanding of population dynamics, threats and conservation management have to be addressed for loggerhead turtles in the South Pacific Ocean. The trans-national nature of the juvenile turtles studied here further reiterates the importance of international collaborations when developing management strategies for migratory species. The need for international dialogue and combined management efforts is particularly pertinent in light of the reduced population numbers of loggerhead turtles at eastern Australian rookeries.
Acknowledgments
This research was undertaken in accordance with animal ethics permits Environmental Australia MS2003-2002/AQIS200303424/JCUA807_03.
The establishment of StrandNet and the records of post-hatchling turtles held within were made possible through an extensive network of individuals and organizations. In particular, the authors wish to thank the Queensland Environment Protection Agency, Underwater World, Sea World, New South Wales Parks and Wildlife Service and The New Zealand Department of Conservation. We thank the volunteers of the Queensland Turtle Research Programme for their help in obtaining samples. We extend special thanks to C. Manrique, S. Sanchez and all the APECO team, the fishing companies that allowed observers on the vessels, especially Mr Visani, Orgullo de Mar, Miss Changana and Ramos family; the captains, crews and owners of the vessels: ‘Juana Rosa I’; ‘Cesaro’; ‘Mi milagritos II’; ‘Melissa’; and ‘Don Venero’; Mr Julio ‘lagarto’; Mr Miguel and Gisela Chilca; the observers: O. Infante; J. M. Balta; E. Arones; R. Atencia; R. Leon; and M. A. Cornejo; R. Blas for facilitating the IBT laboratory, equipment and supplies. Thanks to G. Iannacone and E. Iglesias for the help with DNA analysis. The sequencing and genotyping facility of the University of Puerta Rico-Rìo Piedras is supported in part by the following agencies: NCRR-AABRE grant no. P20 RR16470, NIH-SCORE grant no. S06 GM08102, University of Puerto-Rico Biology Department, NSF-CREST grant no. 0206200 and NINDS-SNRP USA NS39405. Funding contributions came from CMS, NMFS, and Queensland's Smart State Funding Programme. IdeaWild provided some fieldwork equipment.
Supplementary Material
Full methods for genetic analysis of nesting and loggerhead turtles in the southwest Pacific
References
- ADF&G. 2003 SPAM v. 3.7: statistics program for analysing mixtures Alaska Department of Fish and Game, Commercial Fisheries Division, Gene Conservation Lab.
- Alfaro-Shigueto J., Dutton P.H., Mangel J., Vega D. First confirmed occurrence of loggerhead turtles in Peru. Mar. Turtle Newsl. 2004;103:7–11. [Google Scholar]
- Baldwin R., Hughes G.R., Prince R. Loggerhead turtles in the Indian ocean. In: Bolten A.B., Witherington B.E., editors. Loggerhead sea turtles. Smithsonian Books; Washington, DC: 2003. pp. 218–232. [Google Scholar]
- Bolten A.B. Active swimmers—passive drifters: the oceanic juvenile stage of loggerheads in the Atlantic system. In: Bolten A.B., Witherington B.E., editors. Loggerhead sea turtles. Smithsonian Books; Washington, DC: 2003. pp. 63–78. [Google Scholar]
- Bolten A.B., Bjorndal K.A., Martins H.R., Dellinger T., Biscoito M.J., Encalada S.E., Bowen B.W. Transatlantic developmental migrations of loggerhead sea turtles demonstrated by mtDNA sequence analysis. Ecol. Appl. 1998;8:1–7. doi:10.1890/1051-0761(1998)008[0001:TDMOLS]2.0.CO;2 [Google Scholar]
- Bonfil R., Meyer M., Scholl M.C., Johnson R., O'Brien S., Oosthuizen H., Swanson S., Kotze D., Paterson M. Transoceanic migration, spatial dynamics, and population linkages of white sharks. Science. 2005;310:100–103. doi: 10.1126/science.1114898. doi:10.1126/science.1114898 [DOI] [PubMed] [Google Scholar]
- Bowen B.W., Abreugrobois F.A., Balazs G.H., Kamezaki N., Limpus C.J., Ferl R.J. Trans-Pacific migrations of the loggerhead turtle (Caretta caretta) demonstrated with mitochondrial DNA markers. Proc. Natl Acad. Sci. USA. 1995;92:3731–3734. doi: 10.1073/pnas.92.9.3731. doi:10.1073/pnas.92.9.3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M.C., Limpus C.J. The stomach contents of post-hatchling green and loggerhead sea turtles in the southwest Pacific: an insight into habitat association. Mar. Biol. 2008;155:233–241. doi:10.1007/s00227-008-1022-z [Google Scholar]
- Burrage D.M. Coral sea currents. Corella. 1993;17:135–145. [Google Scholar]
- Carr A.F. Rips, FADS, and little loggerheads. BioScience. 1986;36:92–100. doi:10.2307/1310109 [Google Scholar]
- Chaloupka M. Stochastic simulation modelling of loggerhead sea turtle population dynamics given exposure to competing mortality risks in the western south Pacific region. In: Bolten A.B., Witherington B.E., editors. Loggerhead sea turtles. Smithsonian Books; Washington, DC: 2003. pp. 274–294. [Google Scholar]
- Chaloupka M., Parker D.M., Balazs G. Modelling post-release mortality of loggerhead sea turtles exposed to the Hawaii-based pelagic longline fishery. Mar. Ecol. Prog. Ser. 2004a;208:285–293. doi:10.3354/meps280285 [Google Scholar]
- Chaloupka M., Parker D.M., Balazs G. Tracking turtles to their death: reply to Hays et al. Mar. Ecol. Prog. Ser. 2004b;283:301–302. doi:10.3354/meps283301 [Google Scholar]
- Dingle H. Oxford University Press; New York, NY: 1996. Migration: the biology of life on the move. [Google Scholar]
- Donoso M., Dutton P., Serra R., Britomontero J.L. Sea turtles found in the waters off Chile. In: Kalb H.J., Wibbels T., editors. Proc. 19th Annu. Symp. on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-443; Miami, FL: 2000. pp. 218–219. [Google Scholar]
- Excoffier L., Laval G., Schneider S. Arlequin v. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinf. Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Fiedler P.C., Philbrick V., Chavez F.P. Oceanic upwelling and productivity in the eastern tropical Pacific. Limnol. Oceanogr. 1991;36:1834–1850. [Google Scholar]
- FitzSimmons, N. N., Farrington, L. W., McCann, M. J., Moritz, C. & Limpus, C. J. 2003 Genetic identification of Australian marine turtle stocks and their representation at feeding grounds and in regional harvests. Report to Environment Australia.
- Gill B.J. Records of turtles and sea snakes in New Zealand, 1837–1996. NZ J. Mar. Freshwater Res. 1997;31:477–486. [Google Scholar]
- Hatase H., et al. Population structure of loggerhead turtles, Caretta caretta, nesting in Japan: bottlenecks on the Pacific population. Mar. Biol. 2002;141:299–305. [Google Scholar]
- Hays G.C., Marsh R. Estimating the age of juvenile loggerhead sea turtles in the North Atlantic. Can. J. Zool. 1997;75:40–46. doi:10.1139/z97-005 [Google Scholar]
- Hays G.C., Broderick A.C., Godley B.J., Luschi P., Nichols W.J. Satellite telemetry suggests high levels of fishing-induced mortality in marine turtles. Mar. Ecol. Prog. Ser. 2003;262:305–309. doi:10.3354/meps262305 [Google Scholar]
- IUCN 2001 IUCN red list categories and criteria: v. 3.1 (9 February 2000): IUCN—The World Conservation Union. Gland, Switzerland.
- Kamezaki N., et al. Loggerhead turtles nesting in Japan. In: Bolten A.B., Witherington B.E., editors. Loggerhead sea turtles. Smithsonian Books; Washington, DC: 2003. pp. 210–217. [Google Scholar]
- Kelez S., Velez-Zuazo X., Manrique C. New evidence on the loggerhead sea turtle Caretta caretta (Linnaeus 1758) in Peru. Ecol. Appl. 2003;2:141–142. [Google Scholar]
- Kelez S., Manrique C., Velez-Zuazo X. Shark longline fishery and sea turtles in Peruvian waters. In: Frick M., Panagopoulou A., Rees A.F., Williams K., editors. Book of abstracts. 26th Annu. Symp. on Sea Turtle Biology and Conservation. International Sea Turtle Society; Island of Crete, Greece: 2006. pp. 262–263. [Google Scholar]
- Limpus C.J. A biological review of Australian marine turtles. I. Loggerhead turtle, Caretta caretta (Linneaus) Queensland Environmental Protection Agency; Brisbane, Queensland, Australia: 2004. p. 72. [Google Scholar]
- Limpus C.J., Limpus D.J. Biology of the loggerhead turtle in western South Pacific Ocean foraging areas. In: Bolten A.B., Witherington B.E., editors. Loggerhead sea turtles. Smithsonian Books; Washington, DC: 2003a. pp. 93–113. [Google Scholar]
- Limpus C.J., Limpus D.J. Loggerhead turtles in the equatorial and southern Pacific Ocean: a species in decline. In: Bolten A.B., Witherington B.E., editors. Loggerhead sea turtles. Smithsonian Books; Washington, DC: 2003b. pp. 199–209. [Google Scholar]
- Limpus C.J., Parmenter C.J., Baker V., Fleay A. The Crab Island sea turtle rookery in the north-eastern Gulf of Carpentaria. Aust. Wildl. Res. 1983;10:173–184. doi:10.1071/WR9830173 [Google Scholar]
- Limpus C.J., Walker T.A., West J. Post-hatchling sea turtle specimens and records from the Australian Region. In: James R., editor. Proc. Marine Turtle Conservation Workshop. ANCA; Canberra, Australia: 1994. pp. 95–100. [Google Scholar]
- Limpus, C. J., Limpus, D. J., Arthur, K. E. & Parmenter, C. J. 2005 Monitoring green turtle population dynamics in Shoalwater Bay: 2000–2004. Research Publication no. 83
- Lohmann K.J., Lohmann C.M.F. Orientation mechanisms of hatchling loggerheads. In: Bolten A.B., Witherington B.E., editors. Loggerhead sea turtles. Smithsonian Books; Washington, DC: 2003. pp. 44–62. [Google Scholar]
- Luschi P., Hays G.C. A review of long-distance movements by marine turtles, and the possible role of ocean currents. Oikos. 2003;103:293–302. doi:10.1034/j.1600-0706.2003.12123.x [Google Scholar]
- Parker D.M., Cooke W.J., Balazs G.H. Diet of oceanic loggerhead sea turtles (Caretta caretta) in the central North Pacific. Fish. Bull. 2005;103:142–152. [Google Scholar]
- Peckham H., Nichols W.J. Why did the turtle cross the ocean? Pelagic red crabs and loggerhead turtles along the Baja California coast. In: Seminoff J.A., editor. Proc. 22nd Annu. Symp. on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-503; Miami, FL: 2002. pp. 47–48. [Google Scholar]
- Phillips R.A., Silk J.R.D., Croxall J.P., Afanasyev V., Bennett V.J. Summer distribution and migration of nonbreeding albatrosses: individual consistencies and implications for conservation. Ecology. 2005;86:2386–2396. doi:10.1890/04-1885 [Google Scholar]
- Polovina J.J., Kobayashi D.R., Parker D.M., Seki M.P., Balazs G.H. Turtles on the edge: movement of loggerhead turtles (Caretta caretta) along oceanic fronts, spanning longline fishing grounds in the central North Pacific, 1997–1998. Fish. Oceanogr. 2000;9:71–82. doi:10.1046/j.1365-2419.2000.00123.x [Google Scholar]
- Polovina J.J., Howell E., Parker D.M., Balazs G.H. Dive-depth distribution of loggerhead (Carretta carretta) and olive ridley (Lepidochelys olivacea) sea turtles in the central North Pacific: might deep longline sets catch fewer turtles? Fish. Bull. 2003;101:189–193. [Google Scholar]
- Polovina J.J., Uchida I., Balazs G.H., Howell E., Parker D.M., Dutton P. The Kuroshio extension bifurcation region: a pelagic hotspot for juvenile loggerhead sea turtles. Deep-Sea Res. II. 2006;53:326–339. doi:10.1016/j.dsr2.2006.01.006 [Google Scholar]
- Posada D., Crandall K.A. ModelTest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. doi:10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Witherington B.E. Ecology of neonate loggerhead turtles inhabiting lines of downwelling near a Gulf Stream front. Mar. Biol. 2002;140:843–853. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full methods for genetic analysis of nesting and loggerhead turtles in the southwest Pacific