Abstract
The influence of environmental complexity on brain development has been demonstrated in a number of taxa, but the potential influence of social environment on neural architecture remains largely unexplored. We investigated experimentally the influence of social environment on the development of different brain parts in geographically and genetically isolated and ecologically divergent populations of nine-spined sticklebacks (Pungitius pungitius). Fish from two marine and two pond populations were reared in the laboratory from eggs to adulthood either individually or in groups. Group-reared pond fish developed relatively smaller brains than those reared individually, but no such difference was found in marine fish. Group-reared fish from both pond and marine populations developed larger tecta optica and smaller bulbi olfactorii than individually reared fish. The fact that the social environment effect on brain size differed between marine and pond origin fish is in agreement with the previous research, showing that pond fish pay a high developmental cost from grouping while marine fish do not. Our results demonstrate that social environment has strong effects on the development of the stickleback brain, and on the brain's sensory neural centres in particular. The potential adaptive significance of the observed brain-size plasticity is discussed.
Keywords: brain size, brain architecture, group living, nine-spined stickleback, phenotypic plasticity, Pungitius
1. Introduction
Several forms of plasticity in brain architecture have been demonstrated at different neuroanatomical levels and life stages in numerous taxa, including mammals, birds and fishes (e.g. Diamond et al. 1966; Rosenzweig & Bennett 1969; Kempermann et al. 1997; Tramontin & Brenowitz 2000; Zupanc 2001; Draganski & May 2008). During the past few decades, experimental studies have shed light on the effects of abiotic and biotic environmental complexities on the development of neural architecture (reviewed in Van Praag et al. 2000; Mohammed et al. 2002). For instance, rodents kept in stimulus-rich environments increased their brain size (Diamond et al. 1966; Rosenzweig & Bennett 1969), had more hippocampal neurons (Kempermann et al. 1997) and showed an elevated level of neurogenesis (Kempermann et al. 1997; Nilsson et al. 1999) as compared with those kept in a stimulus-poor environment. Chinook salmon (Oncorhynchus tshawytscha) reared in overly simplified hatchery conditions developed smaller bulbi olfactorii and telencephala as compared with wild conspecifics (Kihslinger et al. 2006). Kihslinger & Nevitt (2006) demonstrated that simply adding a few rocks in the rearing tanks resulted in increased cerebellum size in salmon (Oncorhynchus mykiss) alevins, while structural complexity of the abiotic environment affected the rate of cell proliferation in the telencephalon of juvenile coho salmon (Oncorhynchus kisutch; Lema et al. 2005). Structurally enriched environment has also been shown to increase foraging skills and learning ability in Atlantic salmon (Salmo salar; Brown et al. 2003). Obviously, brain architecture and behaviour are expected to be correlated both within and between species. In line with that expectation, Burns & Rodd (2008) have demonstrated a negative correlation between ‘hastiness’ and telencephalon size in guppies (Poecilia reticulata). On a larger scale, several comparative studies have revealed that behavioural and neural complexity appears to evolve in concert (e.g. Lefebvre et al. 1997; Reader & Laland 2002; Gonzalez-Voyer et al. 2009).
Social environment is also implicated as an important factor in shaping the ontogeny and evolution of brain architecture. For instance, the large brains of primates are thought to be a consequence of living in complex societies (Dunbar & Shultz 2007). Coevolution of sociality and brain size has also been demonstrated in other mammals (Perez-Barberia et al. 2007), while recent studies have started to uncover the importance of parental care-type and pair-bonding in the brain-size evolution of fishes (Pollen et al. 2007; Gonzalez-Voyer et al. 2009). However, apart from these interspecific comparative studies, only very few experimental studies have investigated the effects of sociality on brain architecture. Social isolation was found to decrease the number of new neurons in the dentate gyrus of prairie voles (Microtus ochrogaster; Fowler et al. 2002), while greater social complexity increased neuronal recruitment in birds (Lipkind et al. 2002; Adar et al. 2008).
Adult neurogenesis is limited to a few areas of the brain in mammals (Gould et al. 1999; Hastings et al. 2000, 2001), yet several studies have demonstrated its more widespread occurrence in birds (e.g. Reh & Fischer 2001). In comparison with mammals and birds, neurogenesis persists longer into adulthood in reptiles (Font et al. 2001) and fishes (Zupanc & Horschke 1995; Zupanc 2001, 2006), contributing to lifelong growth of brain size and thereby the potential for plastic responses to environmental heterogeneity (Birse et al. 1980; Raymond & Easter 1983; Zupanc & Horschke 1995). Hence, fishes provide an excellent model for neural plasticity studies. The effect of abiotic environment on the development of brain architecture in salmonid fishes has been demonstrated (e.g. Kihslinger & Nevitt 2006; Kihslinger et al. 2006). However, despite the widespread occurrence of group living in numerous fish taxa (e.g. Pitcher & Parrish 1993; Krause & Ruxton 2002), no studies of the potential effects of social environment on brain architecture in fishes have been conducted. Likewise, studies investigating the possibility that genetically based population-level differences in brain development are due to sociality are as yet to be conducted. Such differences could be expected to occur if the costs and benefits of grouping differ among populations residing in different selective environments.
The aim of this study was to investigate the long-term effects of social environment on brain development of nine-spined sticklebacks (Pungitius pungitius), and to compare the effects between populations originating from contrasting environments. This was done by comparing the relative size of brains and five different brain regions of adult fish subjected to different social environment treatments in the laboratory from hatching until adulthood. We were interested in addressing the following questions. (i) Is there any difference in relative brain size of nine-spined sticklebacks reared either individually or in groups? (ii) Which parts of the brain are the most affected by these conditions? (iii) Are there any population or habitat-specific differences in the detected patterns? The latter could be expected because the study populations originated from two contrasting environments (viz. marine and pond environments) where the costs and benefits of grouping are different. Pond fish grow faster (G. Herczeg, A. Gonda & J. Merilä, unpublished data), are more aggressive, are bolder, have higher drive to feed (Herczeg et al. 2009a), and probably, as a consequence, display a higher cost of grouping (Herczeg et al. 2009b) than their marine conspecifics. Hence, we could formulate two main hypotheses. First, we hypothesize that there should be a habitat-specific treatment effect on relative brain size due to the habitat-specific differences in the costs of grouping (i.e. pond fish should have smaller brains when reared in groups than when reared individually). Second, we hypothesize about habitat-independent treatment effects on brain parts involved in communication or, more generally, in perception of the social environment. Here, we expected that the tectum opticum (the visual centre) will be enlarged in group-reared fish as compared with individual-reared conspecifics.
2. Material and methods
(a) Field sampling and study populations
Adult nine-spined sticklebacks were collected from late May to early June of 2007, immediately before the peak of the breeding season, with the aid of minnow traps and seine nets from four populations representing two contrasting habitat types. Although it is known that sampling can introduce a bias towards bolder-than-average fish (Biro & Dingemanse 2009), this effect is hard to avoid, and we believe that the role of this possible bias is negligible in our case. These were two marine populations from the Baltic Sea near Helsinki (Finland) and the White Sea in Levin Navolok Bay (Russia), and two pond populations from Bynästjärnen (Sweden) and Pyöreälampi (Finland; figure 1). The marine sampling sites were shallow coastal bays close to creek inlets (Baltic Sea being a brackish water environment), representing low-salinity sea habitats. Even though we could sample only two replicate populations per habitat type, the large geographical (above 500 km) and genetic (based on highly polymorphic microsatellite markers; T. Shikano, G. Herczeg & J. Merilä, unpublished data) distance made them truly independent. The surface area of ponds was less than 5 ha and their maximum depth around 10 m. The habitats differ in several respects: nine-spined stickleback is the only fish species in the ponds apart from a small number of recently introduced small-bodied whitefish (Coregonus lavaretus) in Pyöreälampi. Based on diet analyses (e.g. Kahilainen et al. 2004), these whitefish are a potential competitor but not a predator of the nine-spined sticklebacks. It is noteworthy that we never caught a single whitefish among the thousands of sticklebacks during our extensive sampling in Pyöreälampi. Thus, pond sticklebacks experience no fish predation and no (or negligible) interspecific competition. By contrast, marine sticklebacks face several types of predatory fishes and interspecific competitors. These differences have resulted in entirely different evolutionary constraints of group living in the populations used here: pond fish suffer from reduced growth when kept in groups while marine fish do not (Herczeg et al. 2009b).
Figure 1.
Map showing the location of the study populations. BAS, Baltic Sea, Finland; WHS, White Sea, Russia; PYÖ, Pyöreälampi, Finland; BYN, Bynästjärnen, Sweden. Open circles, small isolated ponds; filled circles, marine populations.
(b) Breeding conditions and experimental design
After collection, adult fish were transported to the aquaculture facilities of the University of Helsinki and kept at 17°C under permanent light and fed with frozen bloodworms (Chironomidae sp.) until a sufficient number of fish had attained reproductive condition. Both wild-caught adults and all their offspring (see below) were kept and raised in freshwater. Five artificial crosses per population were made in the last week of June. The clutches were placed into 1.4 l tanks of two Allentown Zebrafish Rack Systems (hereafter ‘rack’; Aquaneering Inc., San Diego, CA, USA). Racks had closed water circulating systems with multi-level filtering (physical, chemical, biological and UV filters) and inbuilt thermostats. Unfertilized eggs were removed daily. After hatching, 10 fish per family (i.e. 50 fish per population) were placed individually and randomly into the 1.4 l tanks of the two racks (hereafter individual treatment). The transparent plastic tanks were separated from each other with white panels to block visual contact between neighbours. Chemical contact could not be blocked due to the closed water system.
Of the remaining fish, in the second treatment (hereafter group treatment), a maximum of 80 individuals (depending on the size of the family) per family were divided into two replicates and placed in well-aerated 10 l plastic tanks. After three to four weeks, fish were transported to similar 10 l tanks with mosquito nets at the sides, and these tanks were placed in larger plastic tanks (76×54×40 cm, length, width and height, respectively; eight 10 l tanks in each) set with an open, one-way water flow. The 10 l tanks were placed randomly into the large tanks, and replicates within family were placed into different large tanks. After another three to four weeks (depending on the day of fertilization) 20 fish per family were chosen (replicates equally represented) and pooled within populations, resulting in pools of 100 fish per population. From the Baltic population, equal family representation and reaching n=100 were impossible because of the low number of individuals in the original families and the subsequent mortality. Here, 93 fish were pooled (26, 24, 21, 16 and 6 per family, respectively). Each new population pool was divided into two replicates. The replicates were placed randomly into halves of the larger (76×54×40 cm) plastic tanks halved by mosquito net and set with an open, one-way water flow. The replicates within populations were placed into different tanks. The water volume was set to 140 l in the larger tanks; hence, the per capita water volume (1.4 l), or in other words the fish density, was similar between treatments from this point onwards. In short, only chemosensory clues of conspecifics were present in the individual treatment, while visual, chemosensory and tactile cues were all present in the group treatment.
In both treatments, the temperature was set to 17°C throughout the experiment. We changed from a 24-hour light (natural at high latitudes in summer) cycle to a 12 L : 12 D periodism gradually during the course of one week after week 12. Owing to the latitudinal differences between the populations (figure 1), we did not attempt to mimic the natural light regimes any more closely. Fish were ad libitum fed two times per day. Feeding was started with live brine shrimp (Artemia salina); as the fish grew, we switched to frozen copepeods (Cyclops sp.) and then to frozen bloodworms. No gravel or other physical structures were presented in the rearing environments.
(c) Brain measurements
At the age of five months, when fish reached adult size (standard length from the tip of the nose to the tail base=4–7 cm; e.g. Bănărescu & Paepke 2001), 15 individuals from every population and every treatment were killed by an overdose of MS 222 (tricaine methanesulphonate). Individuals from the individual treatments represented families equally, and individuals from the group treatment were selected randomly from the mixed population pools (replicates represented evenly). After over-anaesthetizing the fish, their body weight was measured to the nearest 0.01 g with a digital balance and their standard length to the nearest 0.01 mm with a digital calliper. Then the brains of the fish were dissected and put into a 4 per cent formalin–0.1 M phosphate-buffered saline solution for 48 hours of fixation. After that, digital photographs were taken of the brains from three viewpoints (dorsal, right lateral and ventral) with a digital camera (Canon EOS 10D, Canon Inc., Tokyo, Japan) through a connected dissecting microscope (Wild M5A, Heerbrugg, Switzerland). A scale was positioned in each photograph for later measurements. Brains were positioned symmetrically and in a horizontal position by eye. We estimated the repeatability of our measurements based on three repeated independent measures of a subsample of brains (n=20) and found that all measurements (see below) were highly repeatable (all r>0.8).
The size of the brain and five different brain parts—bulbus olfactorius, telencephalon, tectum opticum, cerebellum and hypothalamus—were measured from the digital photographs with tpsDig v. 1.37 software (Rohlf 2002). The width, height and length of each structure were taken and defined as the greatest distance enclosed by the given structure. The measures were perpendicular to the midline in the case of width, parallel to the projection of the brain in the case of length and perpendicular to the projection of the brain in the case of height. A detailed description of the measurements is given by Pollen et al. (2007), whose measurement procedures we followed. The volume of the total brain and the different brain parts was estimated according to the ellipsoid model (e.g. Huber et al. 1997; Pollen et al. 2007). This model might not account for fine-scale changes in brain shape, but it should be suitable for the purpose of our study as we compared populations of the same species where large shape changes are not expected. These estimates were validated by Pollen et al. (2007), who found that they provided consistent volume estimates of different brain regions. The volume (V) of the different brain parts was calculated as
| (2.1) |
where L, W and H denote the length, width and height of the given structure, respectively. For paired structures we used a doubled volume estimate of right side measurements. The total volume of the brain was estimated in two different ways. First, we used the equation
| (2.2) |
(Pollen et al. 2007); and, second, we simply summed the volumes of the different parts. The method of calculation did not influence the results qualitatively. Hence, only the results from the ellipsoid model are reported. We note that this method did not allow us to analyse fine-scale structural differences within brain parts, but significant differences at measures used would indeed indicate large treatment and/or population effects.
(d) Data analyses
All morphological variables were log transformed to correct for the allometric relationship between brain size and body size (Northcutt et al. 1978) and to achieve a linear relationship between them. The transformed values were used in all analyses. A general linear mixed model (GLMM) was used to test for the habitat and treatment effects on brain size. Because we found a marginally significant difference in the body weight–standard length relationship between the populations (GLM ANCOVA: F3,112=2.38, p=0.073), we corrected for both body weight and standard length in our analyses. In the GLMM, brain volume was the dependent variable, treatment and habitat fixed factors, body weight and standard length covariates, and population nested in habitat type a random factor.
Since the different parts of the brain were not independent, a multivariate GLM was conducted to test for the treatment effects at the population level. In this analysis (MANCOVA), the size of bulbus olfactorius, telencephalon, tectum opticum, cerebellum and hypothalamus were defined as dependent variables, treatment and population as fixed factors and body weight, standard length and brain volume as covariates.
In all models, we included the interaction between the fixed factors. Analyses were carried out with the SPSS v. 16.0 for Windows (SPSS Inc., Chicago, IL) software package.
3. Results
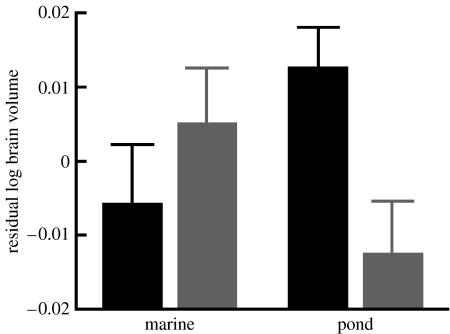
After correcting for size effects (body weight: F1,112.55=49.991, p<0.0001; standard length: F1,113.09=34.866, p<0.0001), a habitat-specific treatment effect on brain size was found (habitat×treatment interaction: F1,112.16=7.816, p=0.006; figure 2). The main effects of treatment (F1,112.34=2.035, p=0.157) and habitat (F1,2.07=0.146, p=0.738) were insignificant, as was the effect of population within habitat type (Z=0.978, p=0.328). Pond fish grew smaller brains in the group treatment than in the individual treatment, while no such effect was observed in marine fish (figure 2).
Figure 2.
Social environmental effect on brain volume (mean+s.e.). Significant habitat-dependent treatment effect was found. Black bars, individual treatment; grey bars, group treatment.
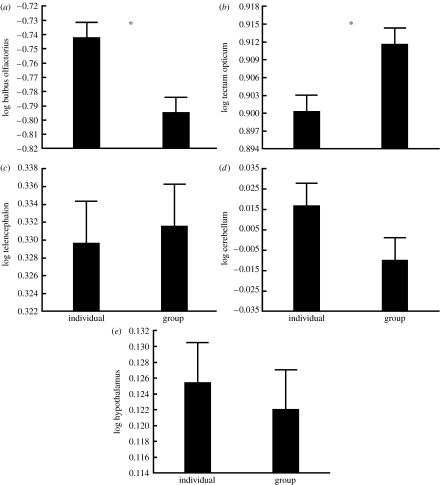
After correcting for size effects (body weight: Wilks's λ5,105=0.922, p=0.124; standard length: Wilks's λ5,105=0.921, p=0.117; brain volume Wilks's λ5,105=0.145, p<0.0001), multivariate GLM revealed a significant treatment (Wilks's λ5,105=0.828, p=0.001) and population effects (Wilks's λ15,290=0.39, p<0.0001) on different brain parts. The treatment×population interaction was insignificant (Wilks's λ15,290=0.92, p=0.86). Univariate analyses of the data revealed significant treatment effect on two brain parts, the bulbus olfactorius (F1,109=11.22, p=0.001; figure 3) and the tectum opticum (F1,109=7.72, p=0.006; figure 3). The bulbus olfactorius was significantly larger, while the tectum opticum was smaller in fish from the individual treatment than in fish from the group treatment. The treatments did not affect the size of telencephalon (F1,109=0.16, p=0.69), cerebellum (F1,109=2.52, p=0.115) or hypothalamus (F1,109=0.288, p=0.593). The treatment-independent population differences are not in the focus of the present paper and will be discussed elsewhere.
Figure 3.
(a–e) Least-squares mean (+s.e.) size of brain parts in different social environmental treatments in different populations. Significant treatment effects are marked with asterisks.
4. Discussion
Our results demonstrate that social environment can have marked effects on the development of the nine-spined stickleback's brain. Fish originating from pond populations developed smaller brains when reared in groups than when reared alone, while fish originating from marine populations showed a (insignificant) trend towards the opposite. According to our knowledge, this is the first time an interpopulation difference in brain-size plasticity during ontogenesis has been demonstrated. The fact that the difference in the level of plasticity was habitat- and not population-specific suggests that habitat-specific natural selection is the likely cause of the observed difference (cf. Clarke 1975; Endler 1986; McGuigan et al. 2005). We further discovered that social environment affected the development of different brain regions (viz. bulbus olfactorius and tectum opticum) in a similar manner in all populations. Individually reared fish receiving information from their conspecifics only via chemical cues developed significantly larger bulbi olfactorii than fish grown in groups. By contrast, group-reared fish subject to visual, chemical and tactile sensory inputs from conspecifics grew significantly larger tecta optica than individually reared fish. Size of telencephalon, cerebellum and hypothalamus appeared to be unaffected by social environment.
Population differences in learning and memorizing abilities (e.g. Mackney & Hughes 1995; Nelson et al. 1995; Girvan & Braithwaite 1998; Brown & Braithwaite 2005) and in brain architecture have been demonstrated in some taxa (Garamszegi & Eens 2004; Pravosudov et al. 2006; Brown et al. 2007; Burns & Rodd 2008; A. Gonda, G. Herczeg & J. Merilä, unpublished data). However, we are not aware of any study that would have investigated interpopulation variation in neural plasticity. In the present study, population differences in plasticity in response to social environment occurred between populations from two markedly different habitats in which the cost of sociality is expected and known to differ (Herczeg et al. 2009a,b).
Because marine nine-spined sticklebacks are under heavy fish predation throughout their lifespan, grouping can be beneficial in reducing predator-caused mortality and, assuming that food is patchier in marine than in pond environments, in increasing foraging efficiency too (Pitcher & Parrish 1993; Krause & Ruxton 2002). By contrast, intraspecific competition is expected to be one of the main biotic selective forces in pond sticklebacks. In fact, fish from ponds are more aggressive, bolder and have higher drive to feed (Herczeg et al. 2009a) than their marine conspecifics. Furthermore, and probably as a consequence, pond fish face high costs of grouping in terms of growth even when constraints originating from food limitation, predation, parasitism or reproduction are ruled out (Herczeg et al. 2009b). This happens irrespective of the fact that pond fish occur in high densities (G. Herczeg & A. Gonda, personal observation) and that, under stress, both pond and marine fish tend to group (Herczeg et al. 2009b). Therefore, group living or permanent contact with conspecifics can be considered to better reflect the natural situation for both marine and pond fish than living in isolation (which is hard to imagine in the studied habitat types), but also more stressful for pond than for marine fish. Considering that the brain is the most expensive tissue to develop and maintain (e.g. Aiello & Wheele 1995), the results showing that pond fish had smaller relative brain size when kept in groups than when kept alone, while marine fish showed some tendency towards opposite patterns, are not unexpected. However, it is interesting that the cost of grouping could manifest as a reduction in brain size in a situation where—due to the increased need during social interactions—one could actually expect larger brains to be developed. The fact that we used laboratory-reared fish suggests that the among-population patterns are likely to have a genetic basis, even though the possibility of maternal effects cannot be ruled out in our design. Furthermore, we found repeated, habitat-specific, population-independent differences that strongly support the role of natural selection in shaping the pattern (e.g. Clarke 1975; Endler 1986; Schluter & Nagel 1995; Foster 1999; McGuigan et al. 2005). We suggest that selection did not act directly on brain plasticity, but rather on the causes behind the differences in grouping costs between habitat types (e.g. behaviour), manifested as energetic constraints on brain development.
Previous studies in brain development have demonstrated that those parts of the brain that are likely to be important in a particular context develop more than those of less importance (Kihslinger & Nevitt 2006; Kihslinger et al. 2006; Lisney et al. 2007). It has also been shown that changes in demand alter the number and size of component elements, making the relative size of different brain parts a reliable predictor of their importance for the organism in question (Kotrschal et al. 1998). In our experiment, individually reared fish could only get information from their conspecifics by chemical cues, while visual, chemical and tactile cues were all available for group-reared fish. Our treatments were extremely simple in terms of abiotic complexity (we applied empty plastic tanks). Hence, one could expect that olfactory centres will be enlarged in the individual treatment, while visual centres will be enlarged in the group treatment. Our results are in line with these expectations: individually reared fish had larger bulbi olfactorii coupled with smaller tecta optica than their group-reared conspecifics, irrespectively of population origin. Evolutionary trade-offs between olfactory and visual centres of the primate brain have been shown at the interspecific level (Barton et al. 1995; Barton & Harvey 2000), but not in fish (Van Staaden et al. 1995; Huber et al. 1997). Our results support the existence of such a trade-off at the ontogenetic level: fish in a certain treatment not only enhanced the growth of the more-used structure, but also reduced the less-used one. These responses make sense considering the extremely high cost of developing and maintaining brain tissue (Aiello & Wheele 1995).
In summary, the results demonstrate that social environment—i.e. solitude versus membership of a group of conspecifics—has a marked effect on the development of the nine-spined stickleback brain. Individually reared pond fish developed relatively larger brains than their group-reared conspecifics from the same populations, while no such effect (or rather a tendency towards the opposite) was detected in marine fish. This pattern might arise from the higher costs of sociality in pond fish than in marine fish, originating in the lower benefits of grouping and higher drive for intraspecific competition in pond than in marine nine-spined sticklebacks (Herczeg et al. 2009a,b). Furthermore, we found that individually kept fish developed larger bulbi olfactorii but smaller tecta optica than fish kept in groups, irrespective of population origin. This finding supports the contention that the relative size of certain brain parts is related to their relative importance. Our study provides the first evidence for habitat-specific difference in brain-size plasticity, and emphasizes the importance of social environment in shaping brain architecture, with special emphasis on the main neural sensory centres.
Acknowledgments
The experiments were done under the licence of the Helsinki University Animal Experimentation Committee.
We thank Victor Berger, Göran Englund, Tuomas Leinonen, Daniel Lussetti and Pirkko Siikamäki for helping us in organizing and executing the field sampling, and John Loehr for correcting English. Special thanks to the Oulanka Research Station and White Sea Biological Station for their help and support. We are highly indebted to Hans Hofmann and Pertti Panula for helping with practical issues related to the preparation and measurement of brains. A.G. was supported by a fellowship from CIMO (www.cimo.fi). G.H. and J.M. were supported by the Academy of Finland.
References
- Adar E., Lotem A., Barnea A. The effect of social environment on singing behavior in the zebra finch (Taeniopygia guttata) and its implication for neuronal recruitment. Behav. Brain Res. 2008;187:178–184. doi: 10.1016/j.bbr.2007.09.011. doi:10.1016/j.bbr.2007.09.011 [DOI] [PubMed] [Google Scholar]
- Aiello L.C., Wheele P. The expensive-tissue hypothesis: the brain and digestive system in human and primate evolution. Curr. Anthropol. 1995;36:199–221. doi:10.1086/204350 [Google Scholar]
- Bănărescu P., Paepke H.J. The freshwater fishes of Europe. Vol. 5/III. AULA-Verlag; Wiebelsheim, Germany: 2001. [Google Scholar]
- Barton R.A., Harvey P.H. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. doi:10.1038/35016580 [DOI] [PubMed] [Google Scholar]
- Barton R.A., Purvis A., Harvey P.H. Evolutionary radiation of visual and olfactory brain systems in primates, bats and insectivores. Phil. Trans. R. Soc. Lond. B. 1995;348:381–392. doi: 10.1098/rstb.1995.0076. doi:10.1098/rstb.1995.0076 [DOI] [PubMed] [Google Scholar]
- Biro P.A., Dingemanse N.J. Sampling bias resulting from animal personality. Trends Ecol. Evol. 2009;24:66–67. doi: 10.1016/j.tree.2008.11.001. doi:10.1016/j.tree.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Birse S.C., Leonard R.B., Coggeshall R.E. Neuronal increase in various areas of the nervous system of the guppy, Lebistes. J. Comp. Neurol. 1980;194:291–301. doi: 10.1002/cne.901940202. doi:10.1002/cne.901940202 [DOI] [PubMed] [Google Scholar]
- Brown C., Braithwaite V.A. Effects of predation pressure on the cognitive ability of the poeciliid Brachyraphis episcope. Behav. Ecol. 2005;16:482–487. doi:10.1093/beheco/ari016 [Google Scholar]
- Brown C., Davidson T., Laland K. Environmental enrichment and prior experience improve foraging behaviour in hatchery-reared Atlantic salmon. J. Fish Biol. 2003;63:187–196. doi:10.1111/j.1095-8649.2003.00208.x [Google Scholar]
- Brown C., Western J., Braithwaite V.A. The influence of early experience on, and inheritance of, cerebral lateralization. Anim. Behav. 2007;74:231–238. doi:10.1016/j.anbehav.2006.08.014 [Google Scholar]
- Burns J.G., Rodd H. Hastiness, brain size and predation regime affect the performance of wild guppies in a spatial memory task. Anim. Behav. 2008;76:911–922. doi:10.1016/j.anbehav.2008.02.017 [Google Scholar]
- Clarke B.C. The contribution of ecological genetics to evolutionary theory: detecting the direct effects of natural selection on particularly polymorphic loci. Genetics. 1975;79:101–113. [PubMed] [Google Scholar]
- Diamond M.C., Law F., Rhodes H., Lindner B., Rosenzweig M.R., Krech D., Bennett E.L. Increases in cortical depth and glia numbers in rats subjected to enriched environment. J. Comp. Neurol. 1966;128:117–126. doi: 10.1002/cne.901280110. doi:10.1002/cne.901280110 [DOI] [PubMed] [Google Scholar]
- Draganski B., May B. Training-induced structural changes in the adult human brain. Behav. Brain Res. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. doi:10.1016/j.bbr.2008.02.015 [DOI] [PubMed] [Google Scholar]
- Dunbar R.I.M., Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. doi:10.1126/science.1145463 [DOI] [PubMed] [Google Scholar]
- Endler J.A. Princeton University Press; Princeton, NJ: 1986. Natural selection in the wild. [Google Scholar]
- Font E., Desfilis E., Pérez-Cañellas M.M., Garcıa-Verdugo J.M. Neurogenesis and neuronal regeneration in the adult reptilian brain. Brain Behav. Evol. 2001;58:276–295. doi: 10.1159/000057570. doi:10.1159/000057570 [DOI] [PubMed] [Google Scholar]
- Foster S.A. The geography of behaviour: an evolutionary perspective. Trends Ecol. Evol. 1999;14:190–195. doi: 10.1016/s0169-5347(98)01577-8. doi:10.1016/S0169-5347(98)01577-8 [DOI] [PubMed] [Google Scholar]
- Fowler D.C., Liu Y., Ouimet C., Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J. Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. doi:10.1002/neu.10042 [DOI] [PubMed] [Google Scholar]
- Garamszegi L.Z., Eens M. Brain space for a learned task: strong intraspecific evidence for neural correlates of singing behavior in songbirds. Brain Res. Rev. 2004;44:187–193. doi: 10.1016/j.brainresrev.2003.12.001. doi:10.1016/j.brainresrev.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Girvan J.R., Braithwaite V.A. Population differences in spatial learning in three-spine sticklebacks. Proc. R. Soc. Lond. B. 1998;265:913–918. doi:10.1098/rspb.1998.0378 [Google Scholar]
- Gonzalez-Voyer A., Winberg S., Kolm N. Social fishes and single mothers: brain evolution in African cichlids. Proc. R. Soc. B. 2009;276:161–167. doi: 10.1098/rspb.2008.0979. doi:10.1098/rspb.2008.0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E., Tanapat P., Hastings N.B., Shors T.J. Neurogenesis in adulthood: a possible role in learning. Trends Cogn. Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. doi:10.1016/S1364-6613(99)01310-8 [DOI] [PubMed] [Google Scholar]
- Hastings N.B., Tanapat P., Gould E. Comparative views of adult neurogenesis. Neuroscientist. 2000;6:315–325. [Google Scholar]
- Hastings N.B., Tanapat P., Gould E. Neurogenesis in the adult mammalian brain. Clin. Neurosci. Res. 2001;1:175–182. doi:10.1016/S1566-2772(01)00003-2 [Google Scholar]
- Herczeg G., Gonda A., Merilä J. Predation mediated population divergence in complex behaviour of nine-spined stickleback (Pungitius pungitius) J. Evol. Biol. 2009a;22:544–552. doi: 10.1111/j.1420-9101.2008.01674.x. doi:10.1111/j.1420-9101.2008.01674.x [DOI] [PubMed] [Google Scholar]
- Herczeg G., Gonda A., Merilä J. The social cost of shoaling covaries with predation risk in nine-spined stickleback (Pungitius pungitius) populations. Anim. Behav. 2009b;77:575–580. doi:10.1016/j.anbehav.2008.10.023 [Google Scholar]
- Huber R., van Staaden M., Kaufman L.S., Liem K.F. Microhabitat use, trophic patterns and the evolution of brain structure in African cichlids. Brain Behav. Evol. 1997;50:167–182. doi: 10.1159/000113330. doi:10.1159/000113330 [DOI] [PubMed] [Google Scholar]
- Kahilainen K., Malinen T., Tuomaala A., Lehtonen H. Diel and seasonal habitat and food segregation of three sympatric Coregonus lavaretus forms in a subarctic lake. J. Fish. Biol. 2004;64:418–434. doi:10.1111/j.0022-1112.2004.00307.x [Google Scholar]
- Kempermann G., Kuhn H.G., Gage F.H. More hippocampal neurons in adult mice living in enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. doi:10.1038/386493a0 [DOI] [PubMed] [Google Scholar]
- Kihslinger R.L., Nevitt G.A. Early rearing environment impacts cerebellar growth in juvenile salmon. J. Exp. Biol. 2006;209:504–509. doi: 10.1242/jeb.02019. doi:10.1242/jeb.02019 [DOI] [PubMed] [Google Scholar]
- Kihslinger R.L., Lema S.C., Nevitt G.A. Environmental rearing conditions produce forebrain differences in wild Chinook salmon Oncorhynchus tshawytscha. Comp. Biochem. Physiol. A. 2006;145:145–151. doi: 10.1016/j.cbpa.2006.06.041. doi:10.1016/j.cbpa.2006.06.041 [DOI] [PubMed] [Google Scholar]
- Kotrschal K., van Staaden M.J., Huber R. Fish brains: evolution and environmental relationships. Rev. Fish. Biol. Fish. 1998;8:373–408. doi:10.1023/A:1008839605380 [Google Scholar]
- Krause J., Ruxton G.D. Oxford University Press; Oxford, UK: 2002. Living in groups. [Google Scholar]
- Lefebvre L., Whittle P., Lascaris E., Finkelstein A. Feeding innovation and forebrain size in birds. Anim. Behav. 1997;53:549–560. doi:10.1006/anbe.1996.0330 [Google Scholar]
- Lema S.C., Hodges M.J., Marchetti M.P., Nevitt G.A. Proliferation zones in the salmon telencephalon and evidence for environmental influence on proliferation rate. Comp. Biochem. Physiol. A. 2005;141:327–335. doi: 10.1016/j.cbpb.2005.06.003. doi:10.1016/j.cbpb.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Lipkind D., Nottebohm R., Rado R., Barnea A. Social change affects the survival of new neurons in the forebrain of adult songbirds. Behav. Brain Res. 2002;133:31–43. doi: 10.1016/s0166-4328(01)00416-8. doi:10.1016/S0166-4328(01)00416-8 [DOI] [PubMed] [Google Scholar]
- Lisney T.J., Bennett M.B., Collin S.P. Volumetric analysis of brain areas indicates a shift in sensory orientation during development in the deep-sea grenadier Coryphaenoides armatus. Raffl. Bull. Zool. 2007;14:7–15. [Google Scholar]
- Mackney P.A., Hughes R.N. Foraging behaviour and memory window in sticklebacks. Behaviour. 1995;132:1241–1253. doi:10.1163/156853995X00559 [Google Scholar]
- McGuigan K., Chenoweth S.F., Blows M.W. Phenotypic divergence along lines of genetic variance. Am. Nat. 2005;165:32–43. doi: 10.1086/426600. doi:10.1086/426600 [DOI] [PubMed] [Google Scholar]
- Mohammed A.H., Zhu S.W., Darmopil S., Hjerling-Leffler J., Ernfors P., Winblad B., Diamond M.C., Eriksson P.S., Bogdanovic N. Environmental enrichment and the brain. Prog. Brain Res. 2002;138:109–133. doi: 10.1016/S0079-6123(02)38074-9. doi:10.1016/S0079-6123(02)38074-9 [DOI] [PubMed] [Google Scholar]
- Nelson D.A., Marler P., Palleroni A. A comparative approach to vocal learning: intraspecific variation in the learning process. Anim. Behav. 1995;50:83–97. doi:10.1006/anbe.1995.0223 [Google Scholar]
- Nilsson M., Perfilieva E., Johansson U., Orwar O., Eriksson P.S. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. doi:10.1002/(SICI)1097-4695(19990615)39:4<569::AID-NEU10>3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- Northcutt R.G., Neary T.J., Senn D.G. Observations on the brain of coelacanth Latimeria chalumnae: external anatomy and quantitative analysis. J. Morph. 1978;155:181–192. doi: 10.1002/jmor.1051550205. doi:10.1002/jmor.1051550205 [DOI] [PubMed] [Google Scholar]
- Perez-Barberia F.J., Shultz S., Dunbar R.I.M. Evidence for coevolution of sociality and relative brain size in three orders of mammals. Evolution. 2007;61:2811–2821. doi: 10.1111/j.1558-5646.2007.00229.x. doi:10.1111/j.1558-5646.2007.00229.x [DOI] [PubMed] [Google Scholar]
- Pitcher, T. J. & Parrish, J. K. 1993 Functions of shoaling behaviour in teleosts. In Behaviour of teleost fishes (ed. T. J. Pitcher ), pp. 363–439, 2nd edn. London, UK: Chapman & Hall.
- Pollen A.A., Dobberfuhl A.P., Scace J., Igulu M.M., Renn S.C.P., Shumway C.A., Hofmann H.A. Environmental complexity and social organization sculpt the brain in Lake Tanganyikan cichlid fish. Brain Behav. Evol. 2007;70:21–39. doi: 10.1159/000101067. doi:10.1159/000101067 [DOI] [PubMed] [Google Scholar]
- Pravosudov V.V., Kitaysky A.S., Omanska A. The relationship between migratory behaviour, memory and the hippocampus: an intraspecific comparison. Proc. R. Soc. B. 2006;273:2641–2649. doi: 10.1098/rspb.2006.3624. doi:10.1098/rspb.2006.3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond P.A., Easter S.S., Jr Postembryonic growth of the optic tectum in goldfish. I. Location of germinal cells and numbers of neurons produced. J. Neurosci. 1983;3:1077–1091. doi: 10.1523/JNEUROSCI.03-05-01077.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader S.M., Laland K.M. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl Acad. Sci. USA. 2002;99:4436–4441. doi: 10.1073/pnas.062041299. doi:10.1073/pnas.062041299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh T.A., Fischer A.J. Stem cells in the vertebrate retina. Brain Behav. Evol. 2001;58:296–305. doi: 10.1159/000057571. doi:10.1159/000057571 [DOI] [PubMed] [Google Scholar]
- Rohlf, F. J. 2002 tpsDig, digitize landmarks and outlines, version 1.37. Department of Ecology and Evolution, State University of New York at Stony Brook.
- Rosenzweig M.R., Bennett E.L. Effects of differential environments on brain weights and enzyme activities in gerbils, rats, and mice. Dev. Psychobiol. 1969;2:87–95. doi: 10.1002/dev.420020208. doi:10.1002/dev.420020208 [DOI] [PubMed] [Google Scholar]
- Schluter D., Nagel L.M. Parallel speciation by natural selection. Am. Nat. 1995;146:292–301. doi:10.1086/285799 [Google Scholar]
- Tramontin A.D., Brenowitz E.A. Seasonal plasticity in adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. doi:10.1016/S0166-2236(00)01558-7 [DOI] [PubMed] [Google Scholar]
- Van Praag H., Kempermann G., Cage F.H. Neural consequences of environmental enrichment. Nat. Rev. 2000;1:191–198. doi: 10.1038/35044558. doi:10.1038/35044558 [DOI] [PubMed] [Google Scholar]
- Van Staaden M., Huber R., Kaufman L., Liem K. Brain evolution in cichlids of the African Great Lakes: brain and body size, general patterns and evolutionary trends. Zoology. 1995;98:165–178. [Google Scholar]
- Zupanc G.K.H. Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Brain Behav. Evol. 2001;58:250–275. doi: 10.1159/000057569. doi:10.1159/000057569 [DOI] [PubMed] [Google Scholar]
- Zupanc G.K.H. Neurogenesis and neuronal regeneration in the adult fish brain. J. Comp. Physiol. A. 2006;192:649–670. doi: 10.1007/s00359-006-0104-y. doi:10.1007/s00359-006-0104-y [DOI] [PubMed] [Google Scholar]
- Zupanc G.K.H., Horschke I. Proliferation zones in the brain of adult gymnotiform fish—a quantitative mapping study. J. Comp. Neurol. 1995;353:213–233. doi: 10.1002/cne.903530205. doi:10.1002/cne.903530205 [DOI] [PubMed] [Google Scholar]