Abstract
Evolutionary responses to the long-term exploitation of individuals from a population may include reduced growth rate, age at maturation, body size and productivity. Theoretical models suggest that these genetic changes may be slow or impossible to reverse but rigorous empirical evidence is lacking. Here, we provide the first empirical demonstration of a genetically based reversal of fishing-induced evolution. We subjected six populations of silverside fish (Menidia menidia) to three forms of size-selective fishing for five generations, thereby generating twofold differences among populations in mean weight and yield (biomass) at harvest. This was followed by an additional five generations during which size-selective harvest was halted. We found that evolutionary changes were reversible. Populations evolving smaller body size when subjected to size-selective fishing displayed a slow but significant increase in size when fishing ceased. Neither phenotypic variance in size nor juvenile survival was reduced by the initial period of selective fishing, suggesting that sufficient genetic variation remained to allow recovery. By linear extrapolation, we predict full recovery in about 12 generations, although the rate of recovery may taper off near convergence. The recovery rate in any given wild population will also depend on other agents of selection determined by the specifics of life history and environment. By contrast, populations that in the first five generations evolved larger size and yield showed little evidence of reversal. These results show that populations have an intrinsic capacity to recover genetically from harmful evolutionary changes caused by fishing, even without extrinsic factors that reverse the selection gradient. However, harvested species typically have generation times of 3–7 years, so recovery may take decades. Hence, the need to account for evolution in managing fisheries remains.
Keywords: fisheries management, fishery-induced evolution, life-history evolution, contemporary evolution
1. Introduction
It is now widely recognized that wild populations may evolve rapidly in response to human-induced sources of selection such as fishing (Jørgensen et al. 2007; Allendorf et al. 2008). Such changes may cause a shift to smaller body sizes and diminished productivity and resilience of populations (Hutchings 2005; Walsh et al. 2006). Assuming these changes are not merely phenotypic but are genetically based, a crucial unanswered question remains: are such evolutionary changes irreversible, or at least very slow to reverse, if fishing ceases (Law & Grey 1989; Law 2000; de Roos et al. 2006; Dieckmann & Heino 2007; Jørgensen et al. 2007; Kuparinen & Merilä 2007)?
Evidence indicative of rapid evolutionary changes in life history caused by size-selective harvesting has come from numerous recent studies encompassing fish (Haugen & Vøllestad 2001; Olsen et al. 2004; Edeline et al. 2007; Swain et al. 2007), mammal (Coltman et al. 2003) and plant populations (Law & Salick 2005). In many of these cases, harvesters either preferentially exploit large individuals or, as in some fisheries, do so because minimum size regulations require it. For harvested fishes, the life-history changes include earlier size and age at maturity, slower growth rate and other physiological changes that induce smaller overall body sizes and lower population productivity. Because many traits such as fecundity and survival are positively correlated with body size, decreases in size driven by harvest selection generally cause reductions in absolute fitness and are therefore detrimental to population growth rate (Hutchings 2005; Walsh et al. 2006).
Theoretical modelling work (de Roos et al. 2006; Dunlop et al. 2007) and numerous time-series analyses of exploited fish populations (Haugen & Vøllestad 2001; Olsen et al. 2004; Edeline et al. 2007; Swain et al. 2007) suggest that only a few generations of selection are required to cause evolutionary change. New statistical approaches that account for environmental variation have greatly improved the inferences that can be drawn from retrospective analyses of harvested stocks (Dieckmann & Heino 2007), but still such studies lack the necessary proof that observed phenotypic changes are truly genetic and not the result of some unknown environmental factor. This gap has been filled, however, by experimental studies on captive (Conover & Munch 2002) and field populations (Reznick et al. 1997; Biro & Post 2008), which, by controlling for environmental variation, provide proof that rapid genetic change is possible.
Numerous authors have speculated that rapid bouts of evolutionary (i.e. genetic) change driven by harvest selection may either be irreversible or slow to reverse (Law & Grey 1989; Law 2000; de Roos et al. 2006; Dieckmann & Heino 2007; Jørgensen et al. 2007; Kuparinen & Merilä 2007). In general, this is because, the mere cessation of selective harvest does not ensure that extrinsic sources of natural selection in the environment (e.g. predators, competitors, pathogens) will be sufficiently intense to drive life-history traits rapidly (within a few generations) back to their original states. However, even in the absence of extrinsic factors that reverse the selection gradient, there are intrinsic sources of selection affecting relative fitness that could favour larger fish once fishing ceases (e.g. size-dependent fecundity, reproductive success, competition or survival). In addition, negative genetic correlations with other traits could cause a character to rebound when directional selection is relaxed (Hill & Caballero 1992; Roff 1992; Teotonio & Rose 2000). Whether such mechanisms operate in fish populations is unknown.
Given that the goal of fisheries management is to provide a sustainable yield in perpetuity, the question of reversibility is crucial. Because size selection imposed by fishing may oppose natural selection (Carlson et al. 2007), fishing can cause evolutionary changes in traits that become maladaptive once fishing ceases (Walsh et al. 2006). If phenotypic changes that reduce yield or fitness were to become permanent, this would contradict the precautionary approach to resource management, which holds that avoidance of irreversible and undesirable change is paramount (Francis et al. 2007).
Evidence of reversibility has thus far been circumstantial. Several case studies of wild fisheries show evidence of trait reversals following harvest closures (Olsen et al. 2004; Edeline et al. 2007; Fukuwaka & Morita 2008) while another does not (Swain et al. 2007). Despite the improved statistical approaches to account for confounding environmental influences (Dieckmann & Heino 2007), none of these field studies can rule out phenotypic plasticity driven by environmental factors rather than evolution as the cause. There are no empirical data that directly measure genetic reversibility of evolutionary change in size-exploited fishes.
To fill this knowledge gap, we subjected six captive populations of a commercially harvested marine species to five generations of either large-, small- or random size-selective harvest (i.e. two populations per treatment). This caused rapid genetic divergence in body size, growth rate, harvestable biomass and a suite of other physiological, behavioural and morphological traits (Conover & Munch 2002; Walsh et al. 2006). We then halted size-selective fishing for all six populations and tracked the resultant evolutionary trajectories over generations 6–10. We reasoned that if fishing-induced evolution is irreversible, or very slow to reverse, the populations should retain their newly evolved character states after fishing ceased. Alternatively, the large- and small-harvested populations may display convergence back towards their original character states. In this paper, we report the final outcome of this 10-year experiment.
The subject of our study was the Atlantic silverside, Menidia menidia Linnaeus, a marine harvested species common to the east coast of North America. This species has proven to be an excellent empirical model of the selective processes that influence the evolution of growth rate in the wild (Conover & Present 1990; Munch & Conover 2003, 2004; Munch et al. 2003), and its short generation time (1 year) and the relative ease of culturing large populations in captivity enable selection experiments that would be impossible in most other marine fishes.
Our results provide the first empirical measurement of the intrinsic capacity for reversal of evolutionary changes caused by fishing. We show that large-harvested populations, which initially evolved slower growth rates, smaller body sizes and reduced yield, displayed reverse evolution back towards their original state after size-selective fishing was relaxed, although at a slower rate. There was no reversal in the small-harvested populations, however, indicating an asymmetry in the response to relaxation of directional selection. Our results offer hope for the slow recovery of large body size and productivity in fisheries that selectively harvest large fish. Our findings have important implications for fisheries management and highlight the need for long-term evolutionary consequences of harvest to be considered.
2. Material and methods
(a) Selection and maintenance of experimental populations
Six populations of M. menidia were maintained independently throughout the experiment, with each generation of each population raised simultaneously under identical environmental and density conditions using previously published protocols (Conover & Present 1990; Conover & Munch 2002). The identical common garden conditions ensured that the observed differences during both the size-selective and non-size-selective periods must be genetic rather than environmental (Conover & Munch 2002). All six populations originated from a common pool of approximately 700 wild-collected M. menidia from Great South Bay, NY, USA, where their natural genetic growth rates are intermediate for the species (Conover & Present 1990). In each generation, the six experimental populations were reared in two ‘phases’ consisting of groups of progeny spawned over 10–20 days. The phases represented duplicates of each population within each generation. The phases were reared in parallel in independent sea-water systems as a precaution in case of culture system failure or disease. Prior to spawning, the two phases within each population were combined to produce two new duplicate phases of offspring for the next generation.
All fish were provided unlimited food. Embryos and larvae were reared initially in 19 l tanks at 21°C for 15 d and then at 15°C throughout the temperature-sensitive sex-determining period (15–90 d post-fertilization), which ensures a 1 : 1 sex ratio in M. menidia originating from New York (Conover & Heins 1987). On day 90 of every generation, mean length of fish was determined in each phase and population, and 550 juveniles from each phase and population were transferred to 700 l cylindrical tanks (i.e. a total of 1100 juveniles per population split across two tanks). Temperatures were then raised to 27°C gradually over a two-week period. When the average age of fish in each phase was 190 d (post-fertilization), approximately 500 fish were still alive. The total length of all individuals was measured before applying the appropriate harvest regime.
During generations 1–5, ‘large-harvested’ populations (n=2) were harvested of all fish with lengths exceeding the phase's 10th percentile (i.e. the largest 90% were removed), a practice that mimics minimum size regulations imposed in many fisheries. In ‘small-harvested’ populations (n=2), all fish below the 90th percentile were harvested (i.e. the smallest 90%). Higher mortality of smaller fish is frequently imposed by natural forces of selection. The control populations (n=2) were harvested at a 90 per cent rate, but randomly with respect to size.
During generations 6–10, a 90 per cent harvest rate was maintained, but size-selective harvest was relaxed so that all removals were random with respect to size in all populations. The harvest rate was therefore 90 per cent for all populations throughout, with harvest survivors becoming the spawning stock for the next generation. The two phases of surviving fish within each population were recombined at the end of every generation and maturation was induced by photoperiod manipulation. Adult fish (n=100 per population) freely spawned on a daily basis over egg substrates that were later removed to start the next generation.
(b) Statistical analysis
All statistical analyses were performed using a general linear model to account for the effects of generation and phase.
3. Results
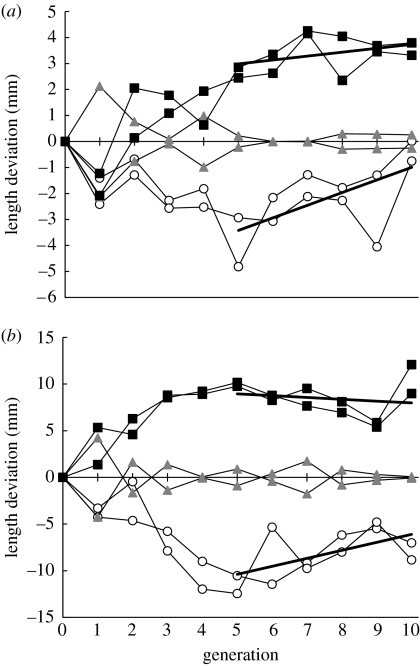
In the large-harvested populations that experienced dramatic declines in mean size and population biomass during the first five generations, we found evidence of a slow but significant recovery. Mean length at day 90 increased significantly between generations 6 and 10, nearly attaining full recovery (figure 1a). Mean length at day 190 also increased significantly over generations 6–10 (figure 1b), recovering about half of the original loss in mean length caused by the evolutionary response to fishing. By contrast, small-harvested populations that evolved increased size and population biomass showed no significant reversal of size at days 90 (figure 1a) or 190 (figure 1b) over generations 6–10.
Figure 1.
Trends in mean length across five generations with size-selective fishing followed by five generations without selective fishing. (a) Mean length at day 90 (end of the larval stage). (b) Mean length at day 190 (harvest). Squares represent the small-size harvested populations, triangles are the randomly harvested controls and circles are the large-size harvested populations. Connecting lines represent the six separate populations with two replicates per treatment. Plotted are mean lengths as differences from the mean for the randomly harvested lines within each generation. Regression analysis (solid thick lines) showed that length at days 90 and 190 increased significantly after selective fishing ceased in the large-size harvested lines (day 90 slope=0.49, s.e.m.=0.18, two-tailed t-test, n=12, p=0.03; day 190 slope=0.86, s.e.m.=0.36, two-tailed t-test, n=12, p=0.04) but did not change significantly in the small-size harvested lines (day 90 slope=0.15, s.e.m.=0.11, two-tailed t-test, n=12, p=0.19; day 190 slope=-0.19, s.e.m.=0.30, two-tailed t-test, n=12, p=0.36).
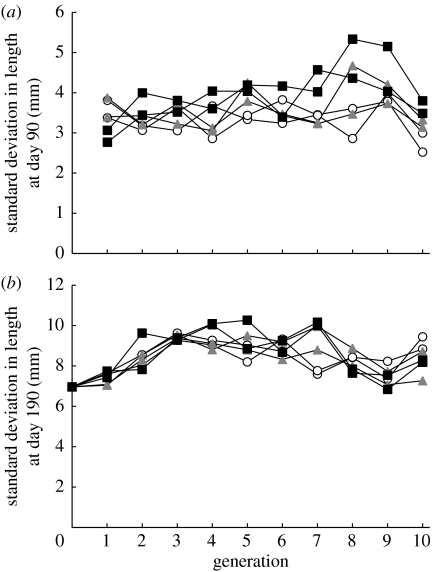
An additional concern about the evolutionary impact of fishing is the loss of genetic variation caused by intense selection, which may reduce phenotypic diversity and thereby the capacity for evolutionary change (Roff 1992). However, there was no evidence of loss in size variation as the standard deviation in length at day 90 or 190 did not decline (figure 2) in any of the lines and the coefficient of variation in length actually increased in all treatments.
Figure 2.
Trends in the standard deviation (s.d.) of mean length for the six populations throughout the experiment. (a) Day 90 (end of the larval period). (b) Day 190 (harvest). Symbols are as defined in figure legend 1.
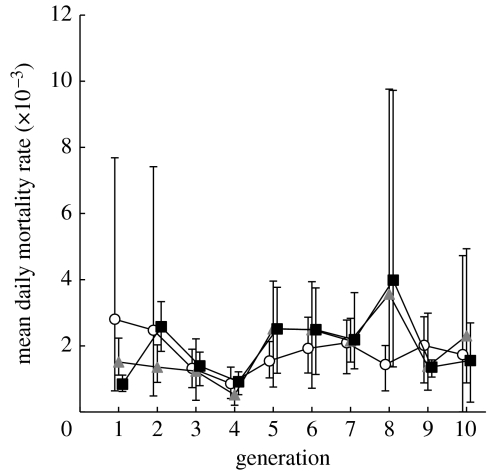
Selection for small size over multiple generations also could lead to the accumulation of detrimental genes if, for example, small fish are genetically inferior in general. If so, this would reduce viability and thereby yield as well as the capacity for rebound. To test for systematic trends in viability, we examined post-larval survival (days 90–190) across the 10 generations of the experiment. We found no evidence of an overall decline in post-larval survival (figure 3) that might reflect loss of genetic variability or inbreeding in any populations. Apparently, there was sufficient genetic variation remaining after five generations of harvest selection to allow reverse evolution.
Figure 3.
Mean daily mortality during days 90–190 in the six populations throughout the experiment. Symbols are as defined in figure legend 1. Vertical lines represent the range of the two replicates within each treatment.
In small populations, an important source of genetic divergence is expected to be genetic drift. The number of spawners in our experimental populations (approx. 100 fish per generation) was very low compared with most marine fishes in the wild and drift could therefore have confounded the effects of selection. Were that the case, we would have expected replicate lines within the same treatment to have diverged substantially from each other, especially after selection due to fishing ceased. The close tracking of replicate lines within each treatment (figure 1), however, indicates that selection was a more potent force than drift throughout both the size-selective (generations 1–5) and non-size-selective (generations 6–10) harvest periods.
4. Discussion
We found clear evidence of an evolutionary rebound from the reductions in size caused by five generations of intense size-selective fishing. While the ultimate cause of reverse evolution in our experiment is not certain, we offer the following explanation.
Extensive prior research has demonstrated that growth rate in wild M. menidia populations is finely tuned to the environmental gradients that co-occur with latitude and does so because stabilizing selection optimizes growth at any given location (Conover & Present 1990; Munch et al. 2003; Munch & Conover 2003, 2004). Severe size-selective winter mortality selects for rapid growth in northern populations and large size also provides the benefits of increased fecundity and reproductive success. Further south, however, where winter is mild, a trade-off occurs because rapidly growing fish are more vulnerable to predators, so southern populations evolve slower growth rates. A complex co-adapted suite of physiological, behavioural and morphological characters are genetically correlated with these growth changes (Conover & Present 1990; Munch et al. 2003; Munch & Conover 2003, 2004; Walsh et al. 2006; Chiba et al. 2007). Our experiment introduced locally co-adapted wild genotypes into a benign environment that was relatively neutral with respect to extrinsic selective factors, except for the presence or absence of size-selective fishing mortality. The large-harvested populations evolved not only slower growth but also changes in a suite of covarying traits that were dragged along through genetic and phenotypic correlations with size. These included changes in physiology, (e.g. lower food consumption rate, food conversion efficiency), behaviour (less boldness in foraging) and reproductive success (e.g. lower fecundity, egg and larval sizes, larval growth and survival; Walsh et al. 2006). Ordinarily, higher values for these traits increase fitness, so when fishing ceased, there was both selection pressure due to the size dependency of relative fitness and the genetic correlations among traits that drove evolution in reverse relative to the random lines, which experienced no such disruption of co-adapted genes. Hence, the rebound was a result of factors intrinsic to the population. In directional selection experiments on other species, genetic covariances with other traits (Roff 1992) have been invoked to explain evolutionary reversals following relaxation of selection (Hill & Caballero 1992; Teotonio & Rose 2000). However, the occurrence of reversal in these studies was highly trait dependent, and none focused on recovery following cessation of size-selective harvest regimes as those imposed here.
How do we explain the lack of reversal in the small-harvested populations that evolved larger size and faster growth? Apparently, after fishing ceased, these populations experienced stabilizing selection on size. This means that the intrinsic factors that might select for still larger size, such as increased fecundity, were countervailed by factors that select against large size such as the cost of rapid growth. In M. menidia, the cost of growth is nonlinear: when growth rate exceeds a threshold, it becomes negatively correlated with swimming performance, leading to higher vulnerability to predators (Munch et al. 2003; Munch & Conover 2003, 2004). In the absence of predators in our systems, selection against rapid growth was probably too weak to overcome selection for increased size, and therefore insufficient to cause an evolutionary reversal. Hence, the net effect of stabilizing selection was apparently neutral.
Our experimental results demonstrate that harvested populations have an intrinsic capacity for evolutionary recovery from the harmful effects of fishing that is genetically based. Although the usefulness of such experiments for understanding fisheries has been questioned (Hilborn 2006), the strength of selection experiments as ours is that they: (i) disentangle genetic and environmental influences, (ii) demonstrate whether a putative selective agent is capable of generating an evolutionary response, (iii) measure the evolvability of multiple traits, (iv) assess the extent to which genetic correlations influence evolution, and (v) control for genetic drift (Fuller et al. 2005). Studies of wild fisheries cannot provide such information with certainty.
In fact, proof that fishing causes evolutionary change in wild populations is exceedingly difficult because fishing represents a massive, uncontrolled experiment in an environment that changes constantly (Rijnsdorp 1993; Law 2000). Several recent studies provide compelling evidence of rapid life-history divergence in response to fishing (Haugen & Vøllestad 2001; Olsen et al. 2004; Edeline et al. 2007; Swain et al. 2007), but whether or not the phenotypic changes have a genetic basis in these cases remains uncertain. Our experimental approach removes any doubt that the phenotypic response to the switching on and off of harvest is genetic but raises questions about applicability to the wild. With the intersection of these two approaches realized, however, the weight of evidence tilts strongly in favour of fisheries-induced evolution.
Our results provide the first empirical measurement of the intrinsic capacity for reversal of evolutionary changes caused by fishing. Extrapolating from figure 1b, and assuming a linear trajectory, we predict that full recovery of fish size would require approximately 12 generations after harvest ceases, although it is likely that the rate of recovery will slow as size converges near its original state. This is good news for fisheries managers because it means that evolutionary reversals are possible and not dependent solely on extrinsic selective factors. Of course, the actual recovery rate for populations in the wild will differ because of the influence of additional factors not simulated by our experiment. The first one is the severity of selection and the magnitude of resultant evolutionary change. On this point, our estimates are probably conservative, as the selection we imposed was more severe and occurred more quickly than that in most fisheries. The second one is the effect of life history. Recovery might occur more quickly, for example, in semelparous species such as Menidia than in species with overlapping generations. Finally, wild populations are subject to numerous changes in the extrinsic environment that affect the fitness landscape such as shifts in the abundance of prey, predators or competitors, the climate regime, density-dependent factors and others (Carlson et al. 2007; Edeline et al. 2007). It will be difficult to predict recovery time for any given wild population without knowledge of all such selective factors.
While evidence of any intrinsic capacity for recovery is encouraging, generation time in many harvested species is generally approximately 3–7 years, meaning that, if our estimates are correct, recovery would take roughly three to eight decades given a size decline in the magnitude we induced. The predicted slowness of evolutionary recovery may be playing a role in the well-known failure of several cod stocks to return to historical levels of abundance and body size once the fishery was closed (Hutchings 2005; Swain et al. 2007). A precautionary approach to fisheries management therefore requires that evolutionary consequences of harvest be considered.
Acknowledgments
All animal care and research was carried out in accordance with the policies of the Stony Brook University Institutional Animal Care and Use Committee.
We thank Bill Chamberlain and Chris Knakal for managing our fish culture system and Walter Burak, Susumu Chiba, Lora Clarke, Damien Drisco, Keith Dunton, Tara Duffy, Amy Fenwick, Joanna Gyory, Lyndie Hice, Adrian Jordaan, John Maniscalco, Sean Moser, Glenn Wagner and Matt Walsh for their assistance. This work was supported primarily by a grant from the Institute for Ocean Conservation Science at Stony Brook University with additional support from the US National Science Foundation.
References
- Allendorf F.W., England P.R., Luikart G., Ritchie P.A., Ryman N. Genetic effects of harvest on wild animal populations. Trends Ecol. Evol. 2008;23:327–337. doi: 10.1016/j.tree.2008.02.008. doi:10.1016/j.tree.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Biro P.A., Post J.R. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc. Natl Acad. Sci. USA. 2008;105:2919–2922. doi: 10.1073/pnas.0708159105. doi:10.1073/pnas.0708159105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S.M., Edeline E., Vøllestad L.A., Haugen T., Winfield I.J., Fletcher J.M., Ben J.J., Stenseth N.C. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius) Ecol. Lett. 2007;10:512–521. doi: 10.1111/j.1461-0248.2007.01046.x. doi:10.1111/j.1461-0248.2007.01046.x [DOI] [PubMed] [Google Scholar]
- Chiba S., Arnott S.A., Conover D.O. Coevolution of foraging behavior with intrinsic growth rate: risk-taking in naturally and artificially selected growth genotypes of Menidia menidia. Oecologia. 2007;154:237–246. doi: 10.1007/s00442-007-0825-9. doi:10.1007/s00442-007-0825-9 [DOI] [PubMed] [Google Scholar]
- Coltman D.W., O'Donoghue P., Jorgenson J.T., Hogg J.T., Strobeck C., Festa-Bianchet M. Undesirable evolutionary consequences of trophy hunting. Nature. 2003;426:655–658. doi: 10.1038/nature02177. doi:10.1038/nature02177 [DOI] [PubMed] [Google Scholar]
- Conover D.O., Heins S.W. Adaptive variation in environmental and genetic sex determination in a fish. Nature. 1987;326:496–498. doi: 10.1038/326496a0. doi:10.1038/326496a0 [DOI] [PubMed] [Google Scholar]
- Conover D.O., Present T.M.C. Countergradient variation in growth rate: compensation for length of the growing season among Atlantic silversides from different latitudes. Oecologia. 1990;83:316–324. doi: 10.1007/BF00317554. doi:10.1007/BF00317554 [DOI] [PubMed] [Google Scholar]
- Conover D.O., Munch S.B. Sustaining fisheries yields over evolutionary time scales. Science. 2002;297:94–96. doi: 10.1126/science.1074085. doi:10.1126/science.1074085 [DOI] [PubMed] [Google Scholar]
- de Roos A.M., Boukal D.S., Persson L. Evolutionary regime shifts in age and size at maturation of exploited fish stocks. Proc. R. Soc. B. 2006;273:1873–1880. doi: 10.1098/rspb.2006.3518. doi:10.1098/rspb.2006.3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann U., Heino M. Probabilistic maturation reaction norms: their history, strengths, and limitations. Mar. Ecol. Prog. Ser. 2007;335:253–269. doi:10.3354/meps335253 [Google Scholar]
- Dunlop E.S., Shuter B.J., Dieckmann U. Demographic and evolutionary consequences of selective mortality: predictions from an eco-genetic model for smallmouth bass. Trans. Am. Fish. Soc. 2007;136:749–765. doi:10.1577/T06-126.1 [Google Scholar]
- Edeline E., Carlson S.M., Stige L.C., Winfield I.J., Fletcher J.M., James J.B., Haugen T.O., Vøllestad L.A., Stenseth N.C. Trait changes in a harvested population are driven by a dynamic tug-of-war between natural and harvest selection. Proc. Natl Acad. Sci. USA. 2007;104:15799–15804. doi: 10.1073/pnas.0705908104. doi:10.1073/pnas.0705908104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R.C., Hixon M.A., Clarke M.E., Murawski S.A., Ralston S. Fisheries management—ten commandments for ecosystem-based fisheries scientists. Fisheries. 2007;32:217–233. doi:10.1577/1548-8446(2007)32[217:TCFBFS]2.0.CO;2 [Google Scholar]
- Fukuwaka M., Morita K. Increase in maturation size after the closure of a high seas gillnet fishery on hatchery-reared chum salmon Oncorhynchus keta. Evol. Appl. 2008;1:376–387. doi: 10.1111/j.1752-4571.2008.00029.x. doi:10.1111/j.1752-4571.2008.00029.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R.C., Baer C.F., Travis J. How and when selection experiments might actually be useful. Integr. Comp. Biol. 2005;45:391–404. doi: 10.1093/icb/45.3.391. doi:10.1093/icb/45.3.391 [DOI] [PubMed] [Google Scholar]
- Haugen T.O., Vøllestad L.A. A century of life-history evolution in grayling. Genetica. 2001;112–113:475–491. doi:10.1023/A:1013315116795 [PubMed] [Google Scholar]
- Hilborn R. Faith-based fisheries. Fisheries. 2006;31:554–555. [Google Scholar]
- Hill W.G., Caballero A. Artificial selection experiments. Ann. Rev. Ecol. Syst. 1992;23:287–310. doi:10.1146/annurev.es.23.110192.001443 [Google Scholar]
- Hutchings J.A. Life history consequences of overexploitation to population recovery in Northwest Atlantic cod (Gadus morhua) Can. J. Fish. Aquat. Sci. 2005;62:824–832. doi:10.1139/f05-081 [Google Scholar]
- Jørgensen C., et al. Ecology: managing evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. doi:10.1126/science.1148089 [DOI] [PubMed] [Google Scholar]
- Kuparinen A., Merilä J. Detecting and managing fisheries-induced evolution. Trends Ecol. Evol. 2007;22:652–659. doi: 10.1016/j.tree.2007.08.011. doi:10.1016/j.tree.2007.08.011 [DOI] [PubMed] [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. ICES J. Mar. Sci. 2000;57:659–668. doi:10.1006/jmsc.2000.0731 [Google Scholar]
- Law R., Grey D.R. Evolution of yields from populations with age-specific cropping. Evol. Ecol. 1989;3:343–359. doi:10.1007/BF02285264 [Google Scholar]
- Law W., Salick J. Human-induced dwarfing of Himalayan snow lotus Saussurea laniceps (Asteraceae) Proc. Natl Acad. Sci. USA. 2005;102:10218–10220. doi: 10.1073/pnas.0502931102. doi:10.1073/pnas.0502931102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch S.B., Conover D.O. Rapid growth results in increased susceptibility to predation in Menidia menidia. Evolution. 2003;57:2119–2127. doi: 10.1111/j.0014-3820.2003.tb00389.x. doi:10.1111/j.0014-3820.2003.tb00389.x [DOI] [PubMed] [Google Scholar]
- Munch S.B., Conover D.O. Nonlinear growth cost in Menidia menidia: theory and empirical evidence. Evolution. 2004;58:661–664. doi:10.1111/j.0014-3820.2004.tb01689.x [PubMed] [Google Scholar]
- Munch S.B., Mangel M., Conover D.O. Quantifying natural selection on body size from field data with an application to winter mortality in Menidia menidia. Ecology. 2003;84:2168–2177. doi:10.1890/02-0137 [Google Scholar]
- Olsen E.M., Heino M., Lilly G.R., Morgan M.J., Brattey J., Ernande B., Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. doi:10.1038/nature02430 [DOI] [PubMed] [Google Scholar]
- Reznick D.N., Shaw F.H., Rodd H.F., Shaw R.G. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata) Science. 1997;275:1934–1937. doi: 10.1126/science.275.5308.1934. doi:10.1126/science.275.5308.1934 [DOI] [PubMed] [Google Scholar]
- Rijnsdorp A.D. Fisheries as a large-scale experiment on life-history evolution: disentangling phenotypic and genetic effects in changes in maturation and reproduction of North Sea plaice, Pleuronectes platessa L. Oecologia. 1993;96:391–401. doi: 10.1007/BF00317510. doi:10.1007/BF00317510 [DOI] [PubMed] [Google Scholar]
- Roff D.A. Chapman & Hall; New York, NY: 1992. The evolution of life histories: theory and analysis. [Google Scholar]
- Swain D.P., Sinclair A.F., Hanson J.M. Evolutionary response to size-selective mortality in an exploited fish population. Proc. R. Soc. B. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. doi:10.1098/rspb.2006.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teotonio H., Rose M.R. Variation in the reversibility of evolution. Nature. 2000;408:463–466. doi: 10.1038/35044070. doi:10.1038/35044070 [DOI] [PubMed] [Google Scholar]
- Walsh M.R., Munch S.B., Chiba S., Conover D.O. Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecol. Lett. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. doi:10.1111/j.1461-0248.2005.00858.x [DOI] [PubMed] [Google Scholar]